Abstract
Bluetongue (BT) is a non-contagious disease affecting domestic and wild ruminants. Outbreaks of BT can cause serious economic losses. To investigate the distribution characteristics of bluetongue virus (BTV), two large-scale censuses of BTV prevalence in Xinjiang, China were collected. Spatial autocorrelation analysis, including global spatial autocorrelation and local spatial autocorrelation, was performed. Risk areas for BTV occurrence in Xinjiang were detected using the presence-only maximum entropy model. The global spatial autocorrelation of BTV distribution in Xinjiang in 2012 showed a random pattern. In contrast, the spatial distribution of BTV from 2014 to 2015 was significantly clustered. The hotspot areas for BTV infection included Balikun County (p < 0.05), Yiwu County (p < 0.05) and Hami City (p < 0.05) in 2012. These three regions were also hotspot areas during 2014 and 2015. Sheep distribution (25.6% contribution), precipitation seasonality (22.1% contribution) and mean diurnal range (16.2% contribution) were identified as the most important predictors for BTV occurrence in Xinjiang. This study demonstrated the presence of high-risk areas for BTV infection in Xinjiang, which can serve as a tool to aid in the development of preventative countermeasures of BT outbreaks.
Keywords: Spatial distribution, Bluetongue, Spatial autocorrelation, MaxEnt
Introduction
Bluetongue (BT) is a non-contagious disease affecting ruminant and camelid species (Hofmann et al., 2008). BT, caused by Bluetongue virus (BTV), is a vectorborne disease transmitted between ruminant hosts by blood-feeding midges of the Culicoides spp. Hosts of BTV infection are domestic and wild ruminants, including sheep, goats, cattle and deer. Among these, goats and cattle are often considered as asymptomatic reservoir hosts (Maclachlan, 1994) or sub-clinically affected (Maclachlan et al., 2009). Severe clinical signs are often seen in certain breeds of sheep, European fine wool and mutton breeds for example (Maclachlan et al., 2009). The most commonly observed clinical signs include fever, hyperemia in nasal and oral mucosa, edema in the lip, ulcers of the oral mucosa, cyanosis of the tongue, and skeletal muscle deformation. Cyanotic tongues are the most obvious characteristic that aid in differentiation from other diseases.
Within the Orbivirus genus in the Reoviridae family, BTV is the prototype member (Coetzee et al., 2012). Currently, 26 serotypes of BTV (BTV 1 to BTV 26) have been serologically identified (Maan et al., 2011). The BTV genome consists of 10 linear dsRNA genome segments (Verwoerd, Louw & Oellermann, 1970), which encode seven structural (VP1-7), and five non-structural proteins (NS1, NS2, NS3, NS3/A and NS4) (Belhouchet et al., 2011; Van Dijk & Huismans, 1988). Differences of the outer capsid proteins, particularly VP2 (Huismans & Erasmus, 1981), determine which of the 26 serotypes the virus belongs.
Late in the 18th century, BT was first reported officially in Cape of Good Hope, South Africa. After a systematic clinical study, Spreull named the disease “BT” for the first time in 1905, with reference to the characteristic cyanotic tongues of the infected sheep (Spreull, 1905). In 1943, an outbreak of BT occurred in Cyprus, which is believed to be the first occurring outside of Africa (Gambles, 1949). Since that time, BT has subsequently occurred in many regions of the world, with Antarctica being the only continent free of BTV infection for now (Maclachlan et al., 2009). In China, BTV has become widely distributed throughout the mainland since it was first reported in Yunnan Province in 1979. At present, 11 serotypes of BTV have been isolated in China (Yang et al., 2016; Zhang et al., 1999), with BTV-1 and BTV-16 being the most commonly isolated serotypes (Lee et al., 2011; Zhang et al., 1999).
Outbreaks of BT can create serious economic consequences. Extensive measures are required to control the spread of the virus among infected and cohoused livestock. It is estimated that economic losses resulting from a BT outbreak in 1996 totaled more than $3 billion USD worldwide (Tabachnick, 1996). Thus, BT was included on the World Organisation of Animal Health (OIE) list of notifiable diseases in the mid 1960s, and is classified as a Class A disease of concern in China as well. At present, there are no effective treatments for the disease. Thus, measures to prevent and control BT outbreaks are of critical importance.
In this study, data from two recent large-scale sampling investigations (2012 and 2014–2015) in Xinjiang Province, China were further analyzed. To investigate the distribution characteristics of BTV, a global and local spatial autocorrelation analysis was performed. Risk areas for BTV occurrence in Xinjiang were detected using the presence-only maximum entropy (MaxEnt) model. This work presents the risk zones of BT in Xinjiang Province, which may subsequently provide useful information for the development of effective strategies for the prevention and control of BT outbreaks.
Materials and Methods
Study area
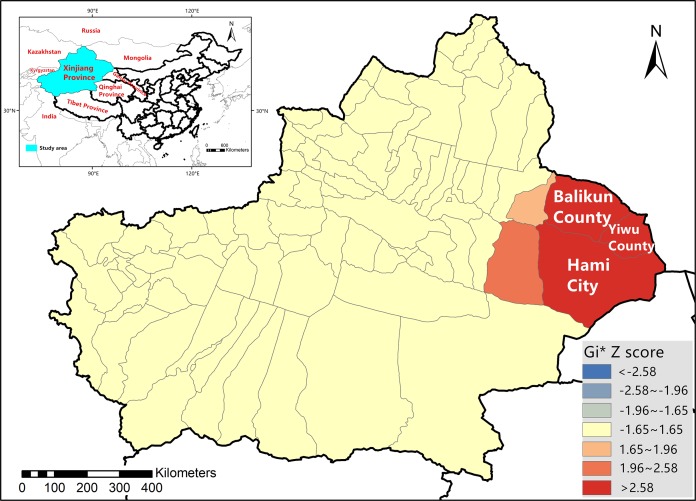
The Xinjiang Uygur Autonomous Region is located along the northwestern border of China (Fig. 1), extending from latitude 34°22′ to 49°10′N and longitude 73°40′ to 96°23′E. The region has a temperate continental climate. According to the data released by the National Bureau of Statistics of the People’s Republic of China (http://www.stats.gov.cn/), Xinjiang is the second largest province in terms of sheep and goat production in China.
Figure 1. Location of Xinjiang Province on the map of China and bluetongue virus hotspot analysis in Xinjiang Province in 2012.
Collection and processing of samples
In 2012, a total of 1,441 blood samples were collected from sheep and goats among seven prefectures (Bayingolin Mongolian Autonomous Prefecture, Hotan Prefecture, Aksu Prefecture, Kashi Prefecture, Changji Hui Autonomous Prefecture, Ili Kazak Autonomous Prefecture, Hami City) including seven counties (cities) (Yuli County, Hotan County, Wensu County, Taxkorgan County, Mori County, Qapqal County, Hami City) in Xinjiang, China. Between 2014 and 2015, a total of 2,135 sheep and goat blood samples were collected among eight prefectures (Kumul Prefecture, Changji Hui Autonomous Prefecture, Urumchi, Turpan Prefecture, Ili Kazakh Autonomous Prefecture, Bayingolin Mongol Autonomous Prefecture, Hotan Prefecture, Kashgar Prefecture) including 15 counties (cities) (Balikun County, Yiwu County, Hami City, Qitai County, Jimsar County, Urumqi City, Shanshan County, Turpan City, Toksun County, Yining County, Hejing County, Heshuo County, Yuli County, Hotan City, Taxkorgan County) in Xinjiang, China. All samples were tested at the Institute of Veterinary Medicine, Xinjiang Academy of Animal Science. Competitive enzyme linked immunosorbent assay (c-ELISA) was used to test for serological evidence of BTV infection. The serum test kit for BTV was kindly provided by the Yunnan Key Laboratory of Tropical and Subtropical Animal Virus Diseases.
The instability caused by variance of BTV apparent prevalence might over inflate the estimate of BTV prevalence. To account for this, Empirical Bayes smoothing was performed in openGeoDa (Anselin & Mccann, 2009) before mapping (Barro et al., 2015; Leyland & Davies, 2005).
Environmental data collection
To characterize the environmental requirements for BTV to be present, 21 environmental factors that may influence BT occurrence were initially selected (García-Bocanegra et al., 2018; Remya, Ramachandran & Jayakumar, 2015). Environmental factors included 19 bioclimatic variables (Bio 1-Bio 19) and gridded sheep and goat densities (SD and GD). The 19 bioclimatic variables obtained from WorldClim (http://www.worldclim.org/), represent annual trends, seasonality and limiting or extreme environmental factors. Two animal distribution variables were obtained from Livestock Geo-Wiki (https://livestock.geo-wiki.org/home-2/), and represent sheep and goat densities. All the environmental parameters were converted to ASCII raster grids and preprocessed to a spatial resolution of 30 arc-seconds (ca. one km2 at ground level).
Spatial autocorrelation analysis
The distribution of a phenomenon is presented as either a clustered, dispersed, or random pattern within a given space. Spatial autocorrelation analysis was used to investigate geographic patterns of BTV distribution in the Xinjiang Province. BTV positive rate was taken as the attribute value. Both local and global spatial autocorrelations were used to analyze the datasets.
Global spatial autocorrelation analysis
A global spatial autocorrelation was applied to analyze the distribution of BTV where all counties were seen as a whole. Global Moran’s I measured the spatial autocorrelation of county locations and the BTV positive rate. Global Moran’s I ranges from −1 to 1, which correspond to highly dispersed and highly clustered distributions, respectively. These parameters were calculated as follows (Cliff & Ord, 1973):
where:
Xi = the BTV positivity rate in the ith county;
= the mean of the BTV positivity rate in all counties within Xinjiang Province;
Xj = the BTV positivity rate in the jth county;
Wij = a weight parameter for the pair of counties i and j that represents proximity;
n = the number of counties in Xinjiang Province.
Local spatial autocorrelation analysis
Local spatial autocorrelation was applied to explore the BTV distribution mode within a particular county. The local Getis-Ord Gi* statistic (Hinman, Blackburn & Curtis, 2006) and its Z-value were calculated to test for statistical significance of BTV local autocorrelation values. If Gi* > 0 and Z > 1.96, the county would be considered as a hotspot area, indicating that BTV distribution within this province were spatially clustered with a significance level of 99% (p < 0.01). Getis-Ord Gi* was calculated as follows (Getis & Ord, 2010):
where:
Xi = the BTV positivity rate in the jth county;
Wij = a weight parameter for the pair of counties i and j that represents proximity;
= the mean of the BTV positivity rate in all counties within Xinjiang;
n = the number of counties in Xinjiang;
S = the standard deviation.
Maximum entropy modeling
Risk areas for BTV infection in Xinjiang were detected using the presence-only MaxEnt ecological niche model (Phillips, Anderson & Schapire, 2006). BTV positive livestock samples were used as proxies for the vector-borne BTV to model the virus presence. Correlation among environmental factors was assessed using Spearman’s rank correlation coefficient to avoid variable multicollinearity that can result in model over-fitting (Graham, 2003). A cross-correlation value less than 0.75 was used as the cut-off threshold to exclude highly correlated variables (Zhang et al., 2012). Variables considered co-linear were excluded and nine variables were selected as evaluator variables.
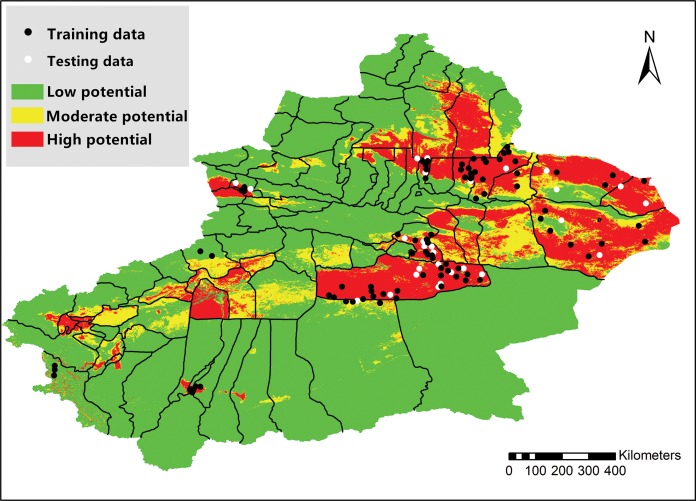
The MaxEnt model used BTV positive locations in 2012, 2014 and 2015 as presence data and 10,000 randomly chosen background points as “Pseudo-Absence” data. To reduce environmental bias resulting from sampling bias introduced from spatially clustered occurrences, all the locality data of 164 BTV positive samples were rarefied at one km2. A total of 112 occurrence locality data remained for model development after rarefying the database using the Spatially Rarefy Occurrence Data for SDMs tool (Brown, Bennett & French, 2017). A total of 75% of the BTV occurrence locations were used as the training set for model calibration, and 25% were used as the testing set for model evaluation. A regularization value of one was used to avoid over fitting of the test data. Area under the curve (AUC) of the receiver operating characteristic plot was calculated to evaluate the produced model (Phillips, Anderson & Schapire, 2006). AUC ranges from zero to one, with one indicating perfect discrimination (Fielding & Bell, 1997). The importance of each variable was assessed using the Jackknife test and percent contribution. The potential species distribution map had a series of values from zero to one which indicated low potential to high potential. These values were regrouped into four classes of potential habitats with high potential (>0.421), moderate potential (0.296–0.421) and low potential (<0.296) based on 10th percentile presence threshold (0.296) (Hannah & Edwin, 2012) and the maximum training sensitivity plus specificity (0.421) (Bean, Stafford & Brashares, 2012).
Maximum entropy models were developed using MaxEnt version 3.2.0. Final outputs of the model predictions were visualized in ArcGIS 10.2.
Ethics statement
Ethics Committee approval was obtained from the Laboratory Animal Ethics Committee of Northeast Agricultural University to the commencement of the study.
Results
C-ELISA for BTV
During 2012, a total of 1,441 sheep and goat blood samples were collected to assess the prevalence of BT. A total of 19 samples were determined to be positive for BTV (1.32%). The rate of BTV positive samples was the highest in Yuli County, at 3.33%. Qapqal County was found to be free of BTV in 2012. The detailed data are presented in Table 1.
Table 1. Result of c-ELISA for BTV in Xinjiang in 2012.
| Prefectures | Counties (cities) | Number of samples | Number of positive samples | Positive rate% |
|---|---|---|---|---|
| Bayingolin Mongolian Autonomous Prefecture | Yuli County | 120 | 4 | 3.33 |
| Hotan Prefecture | Hotan County | 137 | 3 | 2.19 |
| Aksu Prefecture | Wensu County | 218 | 2 | 0.92 |
| Kashi Prefecture | Taxkorgan County | 273 | 6 | 2.20 |
| Changji Hui Autonomous Prefecture | Mori County | 200 | 2 | 1.00 |
| Ili Kazak Autonomous Prefecture | Qapqal County | 100 | 0 | 0.00 |
| Hami City | Hami City | 393 | 2 | 0.51 |
| In total | 1,441 | 19 | 1.32 |
In 2014 and 2015, A total of 2,135 sheep and goat blood samples were collected to determine the prevalence of BT. There were 145 samples found to be BTV positive (6.79%). The rate of BTV positive animals in Hejing County was the highest at 15.33%. Turpan Prefecture was observed to be free of BTV. The detail information is shown in Table 2.
Table 2. Result of c-ELISA for BTV in Xinjiang from 2014 to 2015.
| Prefectures | Counties (cities) | Number of samples | Number of positive samples | Positive rate% |
|---|---|---|---|---|
| Kumul Prefecture | Balikun County | 115 | 5 | 4.35 |
| Yiwu County | 98 | 4 | 4.08 | |
| Hami City | 156 | 8 | 5.13 | |
| Changji Hui Autonomous Prefecture | Qitai County | 125 | 14 | 11.20 |
| Jimsar County | 133 | 8 | 6.02 | |
| Urumchi | Urumqi City | 140 | 12 | 8.57 |
| Turpan Prefecture | Shanshan County | 120 | 0 | 0.00 |
| Turpan City | 136 | 0 | 0.00 | |
| Toksun County | 122 | 0 | 0.00 | |
| Ili Kazakh Autonomous Prefecture | Yining County | 132 | 7 | 5.30 |
| Bayingolin Mongol Autonomous Prefecture | Hejing County | 137 | 21 | 15.33 |
| Heshuo County | 215 | 19 | 8.84 | |
| Yuli County | 235 | 33 | 14.04 | |
| Hotan Prefecture | Hetian City | 127 | 11 | 8.66 |
| Kashgar Prefecture | Taxkorgan County | 144 | 3 | 2.08 |
| In total | 2,135 | 145 | 6.79 |
Spatial autocorrelation analysis
Global spatial autocorrelation analysis
The results of the global spatial autocorrelation analysis are presented in Table 3. The 2012 distribution of BTV in Xinjiang followed a random pattern, but was significantly clustered in 2014–2015.
Table 3. Global spatial autocorrelation analysis of BTV in the Xinjiang Province.
| Year | Global Moran’s I | Z score | p-value |
|---|---|---|---|
| 2012 | 0.0002 | 0.3708 | 0.7108 |
| 2014–2015 | 0.0526 | 1.8150 | 0.0695 |
Local spatial autocorrelation analysis
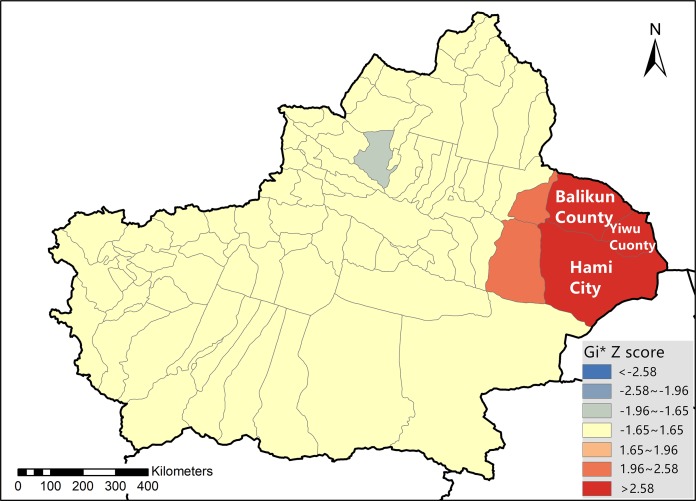
The results of the 2012 BTV hotspot analysis in Xinjiang Province are presented in Fig. 1 and Table S1. As is demonstrated in the figure, Balikun County (Z = 3.1256, p = 0.0018), Yiwu County (Z = 4.7139, p < 0.001) and Hami City (Z = 4.1515, p < 0.001) were hotspot areas for BTV infection in 2012. Figure 2 showed the result of the hotspot analysis of BTV distribution in Xinjiang from 2014 to 2015. As shown in the figure, during 2014 and 2015, Balikun County (Z = 4.0818, p = 0.0018), Yiwu County (Z = 5.6000, p < 0.001) and Hami City (Z = 4.9151, p < 0.001) were considered to be BTV infection hotspot areas. Table S1 presented Gi* Z scores and p-values of the hotspot areas.
Figure 2. Bluetongue virus hotspot analysis in Xinjiang Province during 2014 and 2015.
MaxEnt modeling
Area under the curve score for the training data was 0.880, indicating that the approach fit the training data fairly well. AUC score for the test data was 0.876 (SD = 0.029), also indicating that the model performed well. As is shown in Table 4, five variables, including sheep distribution (25.6% contribution), precipitation seasonality (22.1% contribution), mean diurnal range (16.2% contribution) and isothermality (16.9% contribution), provided over 80% of model contribution. Sheep distribution, precipitation seasonality and mean diurnal range were identified as the most important predictors for BTV occurrence in Xinjiang. Results of Jackknife test and response curves were shown in Figs. S1 and S2. Figure 3 was a representation of the MaxEnt model for BTV. Warmer colors showed areas with better predicted conditions for BTV occurrence, and these areas were identified as “high-risk” areas.
Table 4. Environmental variables used in maximum entropy model for bluetongue virus occurrence.
| Abbreviation | Description of variables | Unit | Percent contribution | Permutation importance |
|---|---|---|---|---|
| Bio 2 | Mean diurnal range | °C | 16.2 | 22 |
| Bio 3 | Isothermality | – | 16.9 | 18.6 |
| Bio 4 | Temperature seasonality | (coeff. of variation °C) | 8.4 | 10.2 |
| Bio 6 | Minimum temperature of coldest month | °C | 5.6 | 3 |
| Bio 12 | Annual precipitation | mm | 1.8 | 4.6 |
| Bio 15 | Precipitation seasonality | (coeff. of variation; %) | 22.1 | 11.9 |
| Bio 19 | Precipitation of coldest quarter | mm | 3.1 | 7.8 |
| SD | Sheep density | Heads/km2 | 25.6 | 21.3 |
| GD | Goats density | Heads/km2 | 0.2 | 0.7 |
Figure 3. Map of Xinjiang Province showing high-risk areas for bluetongue virus occurrence.
Discussion
The first case of BT in China was identified in Yunnan Province in 1979, and BTV was isolated after the outbreak. Soon thereafter, cases of BT in Hubei, Anhui, Sichuan, Gansu and Shanxi Provinces were reported. Concurrently, BTV seropositive animals were found in 29 provinces throughout China, including Guangdong, Guangxi, Jiangsu, Xinjiang, and others, indicating a rapid spread of BT throughout the country. A previously published study covering 27 provinces throughout China between 1987 and 1989 indicated a nationwide BTV seroprevalence rate in sheep and goats of 4.73%. In a similar study, the highest seroprevalence was observed in Guangxi Province in 2001, with 31.7% of sheep and goats testing positive for BTV infection. In Inner Mongolia, the BTV seropositivity rates were 11.75% in 2014 and 11.27% in 2015. It has been demonstrated that BT has become widely established in China for several decades.
In Xinjiang Province, China, three large-scale epidemiological surveys were conducted since the initial cases of BTV were confirmed. The first epidemiological survey analyzed a total of 160,671 blood samples collected from sheep, goats, cattle, yaks and deer from 1988 to 1989 to determine rates of seroconversion to BTV. The result confirmed for the first time that BT was widespread in Xinjiang Province, with goats exhibiting the highest rate of seroconversion to BTV. The second epidemiological survey was conducted in 2012. In total, 1,441 blood samples from sheep and goats, as well as 701 from cattle were collected. The observed rate of BTV positive samples was 1.32% (19 in 1,441) in sheep and goats and 0% (zero in 701) in cattle. However, it has been demonstrated that the rate of BTV positive sera collected in Southern Xinjiang is higher than was observed in Northern Xinjiang. The third and most recent epidemiological survey covered the period from 2014 to 2015. During these two years, 2,135 blood samples collected from sheep and goats were tested, and the rate of BTV positive sera was 6.79% (145 in 2,135). The investigation indicated that the epidemic status of BT in Xinjiang remains a significant concern.
Spatial epidemiology plays an important role in the study of infectious diseases in the field of public health. One such method, spatial autocorrelation, has been used widely in epidemic studies (Al-Ahmadi & Al-Zahrani, 2013; Ma et al., 2017; Ratovonirina et al., 2017). According to our research, in 2012, the distribution of BTV was observed to follow a random pattern, considering Xinjiang Province as a whole. However, local spatial autocorrelation analysis showed that Balikun County (p = 0.0018), Yiwu County (p < 0.001) and Hami City (p < 0.001) were hotspots of BTV infection. It has been proposed by others that case clusters which occur randomly also have an effect on the spread of an infectious disease (Jeefoo, Tripathi & Souris, 2011). During 2014 and 2015, both global and local spatial autocorrelation analyses demonstrated a significantly clustered distribution of BTV in Xinjiang, and that Balikun County (p < 0.001), Yiwu County (p < 0.001) and Hami City (p < 0.001) were also hotspot areas.
In 2011, 48 Culicoides species (Diptera: Ceratopogonidae) were recorded in Xinjiang, China (Tian, Liu & Jia, 2011). Adult female haematophagous midges of the Culicoides spp. are the only known vectors and the only known mode of transmission through which BTV can spread between susceptible ruminant hosts (Toit, 1944). As such, the epidemic distribution of BT is closely related to the activity of the midge vector (Tabachnick, 2004), which is generally distributed between 40°N and 35°S latitude (Gibbs & Greiner, 1994). However, the hotspot areas for BTV infection observed during the study period lie outside of this geographical range, with the northernmost hotspot being located at 45°N latitude. Additionally, the MaxEnt model identified BTV high-risk areas north to 47°N (Fig. 3) in Xinjiang Province. It is possible that this observation is the result of the expansion of the habitat range of the midge vector due to climate change. It has been reported that the distribution of Culicoides are predicted to move northwards up to 53°N latitude with changing climatic and environmental conditions (Zuliani et al., 2015).
Maximum entropy calculates the relationship between the presence data and some environmental predictors which were known to be related to the disease (García-Bocanegra et al., 2018). This study successfully built a presence-only MaxEnt model relying on climatic and environmental data. Sheep distribution, precipitation seasonality and mean diurnal range were identified as the most important predictors for BTV occurrence. Areas with sheep density higher than 500 heads/km2 were found to be areas of high risk for BTV (Fig. S2). It has been reported that sheep were most susceptible to BTV infection among all the small ruminants (Coetzee et al., 2012). Moreover, a decrease in the variation coefficient of precipitation seasonality resulted in lower risk of BTV presence. Seasonality of BTV occurrence has been investigated, and the infection regularity in different seasons was observed (Ward, 1996). In this study, the relationship between precipitation seasonality and BTV occurrence may be influenced by the extremely arid climate throughout a whole year in Xinjiang Province. The response curve of Bio 2 showed that areas with mean diurnal range of 14 °C were found to be areas of high risk for BTV. The effect of temperature and precipitation on BTV infection has been investigated (Brand & Keeling, 2017), as well as the effects of seasonal and meteorological parameters on the Culicoides existence (Ander, Meiswinkel & Chirico, 2012; Racloz et al., 2008; Sanders et al., 2011). However, influence of annual mean temperature and precipitation on BTV occurrence was not observed in our research. As we know, both biological factors (vegetation, human and animal activities etc.) and natural environmental factors (light, temperature, atmospheric gases etc.) have major impacts on the spread of Culicoides spp, which is the major transmission mode (Blanda et al., 2018). Thus, these factors can have a significant influence on the geographical distribution of BTV. For example, an association between BT disease diffusion and some landscape features has been reported (Guis et al., 2007).
As is stated above, data of BTV positive blood samples were used as the input data for the MaxEnt model. Using livestock samples to represent vector-borne BTV may cause the result of niche models and cluster maps to not agree. Future studies should be conducted considering more predictors, including vector, vegetation and other environmental and climatic variables unavailable in this study. Furthermore, factors affecting the distribution of haematophagous midges require much consideration.
Although we believe our research is comparably reliable, limitations still exist. The apparent prevalence may differ from the true prevalence caused by serological tests. A low number of positives with a low number of tests will over inflate the estimates of BTV prevalence, although Empirical Bayes smoothing was performed. The sensitivity and specificity of the test should be performed in the future study. Furthermore, the result of MaxEnt and the hotspot maps do not agree perfectly. Maybe it is because BTV positive cases were used as a proxy for the vector when developing niche models. And the sampling biases, including animal movement and low sample numbers, may also lead to different results.
Conclusions
The global spatial autocorrelation data on the distribution of BTV in Xinjiang in 2012 exhibited a random pattern, which became markedly clustered in 2014–2015. The hotspot areas for BTV infection included Balikun County, Yiwu County and Hami City in 2012. These three regions were also hotspot areas during 2014 and 2015. A BTV suitability map was generated to show the high-risk areas for BTV occurrence in Xinjiang. This study can serve as a tool for the development of preventative countermeasures for future BT outbreaks.
Supplemental Information
Raw data.
Acknowledgments
We would like to thank the Institute of Veterinary Medicine, Xinjiang Academy of Animal Science, and all of the veterinarians who contributed to this study. We also thank the Heilongjiang Key Laboratory for Laboratory Animals and Comparative Medicine, and the Key Laboratory of the Provincial Education Department of Heilongjiang for Common Animal Disease Prevention and Treatment.
Funding Statement
This work was supported by the National Key R & D Program of China (No. 2016YFD0501106) and the National S&T Program of China (No. 2015BAD12B05). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Jun Ma conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper.
Xiang Gao performed the experiments.
Boyang Liu performed the experiments.
Hao Chen contributed reagents/materials/analysis tools, prepared figures and/or tables.
Jianhua Xiao authored or reviewed drafts of the paper, approved the final draft.
Hongbin Wang contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Ethical approval was obtained from the Laboratory Animal Ethics Committee of Northeast Agricultural University.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in a Supplemental File.
References
- Al-Ahmadi & Al-Zahrani (2013).Al-Ahmadi K, Al-Zahrani A. Spatial autocorrelation of cancer incidence in Saudi Arabia. International Journal of Environmental Research and Public Health. 2013;10(12):7207–7228. doi: 10.3390/ijerph10127207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ander, Meiswinkel & Chirico (2012).Ander M, Meiswinkel R, Chirico J. Seasonal dynamics of biting midges (Diptera: Ceratopogonidae: Culicoides), the potential vectors of bluetongue virus, in Sweden. Veterinary Parasitology. 2012;184(1):59–67. doi: 10.1016/j.vetpar.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Anselin & Mccann (2009).Anselin L, Mccann M. OpenGeoDa, open source software for the exploration and visualization of geospatial data. Proceedings of the 17th ACM SIGSPATIAL International Conference on Advances in Geographic Information Systems–GIS ‘09; Seattle. 2009. pp. 550–551. [DOI] [Google Scholar]
- Barro et al. (2015).Barro AS, Kracalik IT, Malania L, Tsertsvadze N, Manvelyan J, Imnadze P, Blackburn JK. Identifying hotspots of human anthrax transmission using three local clustering techniques. Applied Geography. 2015;60:29–36. doi: 10.1016/j.apgeog.2015.02.014. [DOI] [Google Scholar]
- Bean, Stafford & Brashares (2012).Bean WT, Stafford R, Brashares JS. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography. 2012;35(3):250–258. doi: 10.1111/j.1600-0587.2011.06545.x. [DOI] [Google Scholar]
- Belhouchet et al. (2011).Belhouchet M, Jaafar FM, Firth AE, Grimes JM, Mertens PP, Attoui H. Detection of a fourth orbivirus non-structural protein. PLOS ONE. 2011;6(10):e25697. doi: 10.1371/journal.pone.0025697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanda et al. (2018).Blanda V, Blanda M, Russa FL, Scimeca R, Scimeca S, D’Agostino R, Auteri M, Torina A. Geo-statistical analysis of Culicoides spp. distribution and abundance in Sicily, Italy. Parasites & Vectors. 2018;11(1):78. doi: 10.1186/s13071-018-2658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand & Keeling (2017).Brand SP, Keeling MJ. The impact of temperature changes on vector-borne disease transmission: Culicoides midges and bluetongue virus. Journal of the Royal Society Interface. 2017;14(128):20160481. doi: 10.1098/rsif.2016.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, Bennett & French (2017).Brown JL, Bennett JR, French CM. SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ. 2017;5:e4095. doi: 10.7717/peerj.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff & Ord (1973).Cliff AD, Ord JK. Spatial autocorrelation. Trends in Ecology & Evolution. 1973;14:196. [Google Scholar]
- Coetzee et al. (2012).Coetzee P, Stokstad M, Venter EH, Myrmel M, Van Vuuren M. Bluetongue: a historical and epidemiological perspective with the emphasis on South Africa. Virology Journal. 2012;9(1):198. doi: 10.1186/1743-422x-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding & Bell (1997).Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation. 1997;24(1):38–49. doi: 10.1017/s0376892997000088. [DOI] [Google Scholar]
- Gambles (1949).Gambles RM. Bluetongue of sheep in Cyprus. Journal of Comparative Pathology & Therapeutics. 1949;59:176–190. doi: 10.1016/s0368-1742(49)80018-x. [DOI] [Google Scholar]
- García-Bocanegra et al. (2018).García-Bocanegra I, Belkhiria J, Napp S, Cano-Terriza D, Jiménez-Ruiz S, Martínez-López B. Epidemiology and spatio-temporal analysis of West Nile virus in horses in Spain between 2010 and 2016. Transboundary & Emerging Diseases. 2018;65(2):567–577. doi: 10.1111/tbed.12742. [DOI] [PubMed] [Google Scholar]
- Getis & Ord (2010).Getis A, Ord JK. The analysis of spatial association by use of distance statistics. Geographical Analysis. 2010;24(3):189–206. doi: 10.1111/j.1538-4632.1992.tb00261.x. [DOI] [Google Scholar]
- Gibbs & Greiner (1994).Gibbs EP, Greiner EC. The epidemiology of bluetongue. Comparative Immunology Microbiology & Infectious Diseases. 1994;17(3–4):207–220. doi: 10.1016/0147-9571(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Graham (2003).Graham MH. Confronting multicollinearity in ecological multiple regression. Ecology. 2003;84(11):2809–2815. doi: 10.1890/02-3114. [DOI] [Google Scholar]
- Guis et al. (2007).Guis H, Tran A, De LRS, Baldet T, Gerbier G, Barragué B, Biteau-Coroller F, Roger F, Viel JF, Mauny F. Use of high spatial resolution satellite imagery to characterize landscapes at risk for bluetongue. Veterinary Research. 2007;38(5):669–683. doi: 10.1051/vetres:2007025. [DOI] [PubMed] [Google Scholar]
- Hannah & Edwin (2012).Hannah S, Edwin M. Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PLOS ONE. 2012;7(2):e32202. doi: 10.1371/journal.pone.0032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman, Blackburn & Curtis (2006).Hinman SE, Blackburn JK, Curtis A. Spatial and temporal structure of typhoid outbreaks in Washington, D.C., 1906–1909: evaluating local clustering with the Gi* statistic. International Journal of Health Geographics. 2006;5:1–17. doi: 10.1186/1476-072X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann et al. (2008).Hofmann MA, Renzullo S, Mader M, Chaignat V, Worwa G, Thuer B. Genetic characterization of Toggenburg orbivirus, a new bluetongue virus, from goats, Switzerland. Emerging Infectious Diseases. 2008;14(12):1855–1861. doi: 10.3201/eid1412.080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huismans & Erasmus (1981).Huismans H, Erasmus BJ. Identification of the serotype-specific and group-specific antigens of bluetongue virus. Onderstepoort Journal of Veterinary Research. 1981;48:51–58. [PubMed] [Google Scholar]
- Jeefoo, Tripathi & Souris (2011).Jeefoo P, Tripathi NK, Souris M. Spatio-temporal diffusion pattern and hotspot detection of dengue in Chachoengsao Province, Thailand. International Journal of Environmental Research and Public Health. 2011;8(1):51–74. doi: 10.3390/ijerph8010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2011).Lee F, Ting L-J, Lee M-S, Chang W-M, Wang F-I. Genetic analysis of two Taiwanese bluetongue viruses. Veterinary Microbiology. 2011;148(2–4):140–149. doi: 10.1016/j.vetmic.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Leyland & Davies (2005).Leyland AH, Davies CA. Empirical Bayes methods for disease mapping. Statistical Methods in Medical Research. 2005;14(1):17–34. doi: 10.1191/0962280205sm387oa. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2017).Ma J, Xiao J, Gao X, Liu B, Chen H, Wang H. Spatial pattern of foot-and-mouth disease in animals in China, 2010–2016. PeerJ. 2017;5:e4193. doi: 10.7717/peerj.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan et al. (2011).Maan S, Maan NS, Nomikou K, Batten C, Antony F, Belaganahalli MN, Samy AM, Reda AA, Al-Rashid SA, Batel ME, Oura CAL, Mertens PPC. Novel bluetongue virus serotype from Kuwait. Emerging Infectious Diseases. 2011;17(5):886–889. doi: 10.3201/eid1705.101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclachlan (1994).Maclachlan NJ. The pathogenesis and immunology of bluetongue virus infection of ruminants. Comparative Immunology, Microbiology and Infectious Diseases. 1994;17(3–4):197–206. doi: 10.1016/0147-9571(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Maclachlan et al. (2009).Maclachlan NJ, Drew CP, Darpel KE, Worwa G. The pathology and pathogenesis of bluetongue. Journal of Comparative Pathology. 2009;141(1):1–16. doi: 10.1016/j.jcpa.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Phillips, Anderson & Schapire (2006).Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190(3–4):231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- Racloz et al. (2008).Racloz V, Venter G, Griot C, Stärk K. Estimating the temporal and spatial risk of bluetongue related to the incursion of infected vectors into Switzerland. BMC Veterinary Research. 2008;4(1):42. doi: 10.1186/1746-6148-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratovonirina et al. (2017).Ratovonirina NH, Rakotosamimanana N, Razafimahatratra SL, Raherison MS, Refrégier G, Sola C, Rakotomanana F, Razanamparany VR. Assessment of tuberculosis spatial hotspot areas in Antananarivo, Madagascar, by combining spatial analysis and genotyping. BMC Infectious Diseases. 2017;17(1):562. doi: 10.1186/s12879-017-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remya, Ramachandran & Jayakumar (2015).Remya K, Ramachandran A, Jayakumar S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecological Engineering. 2015;82:184–188. doi: 10.1016/j.ecoleng.2015.04.053. [DOI] [Google Scholar]
- Sanders et al. (2011).Sanders CJ, Shortall CR, Gubbins S, Burgin L, Gloster J, Harrington R, Reynolds DR, Mellor PS, Carpenter S. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. Journal of Applied Ecology. 2011;48(6):1355–1364. doi: 10.1111/j.1365-2664.2011.02051.x. [DOI] [Google Scholar]
- Spreull (1905).Spreull J. Malarial catarrhal fever (bluetongue) of sheep in South Africa. Journal of Comparative Pathology & Therapeutics. 1905;18:321–337. doi: 10.1016/s0368-1742(05)80073-6. [DOI] [Google Scholar]
- Tabachnick (1996).Tabachnick WJ. Culicoides variipennis and bluetongue-virus epidemiology in the United States. Annual Review of Entomology. 1996;41(1):23–43. doi: 10.1146/annurev.en.41.010196.000323. [DOI] [PubMed] [Google Scholar]
- Tabachnick (2004).Tabachnick WJ. Culicoides and the global epidemiology of bluetongue virus infection. Veterinaria Italiana. 2004;40:144. [PubMed] [Google Scholar]
- Tian, Liu & Jia (2011).Tian Y, Liu ZJ, Jia L. Fauna of Ceratopogonidae in Xinjiang. Chinese Journal of Hygienic Insecticides & Equipments. 2011;4:270–273. [Google Scholar]
- Toit (1944).Toit RMD. The Transmission of Blue-tongue and Horse-sickness by Culicoides. Onderstepoort Journal of Veterinary Science. 1944;7:16–390. [Google Scholar]
- Van Dijk & Huismans (1988).Van Dijk AA, Huismans H. In vitro transcription and translation of bluetongue virus mRNA. Journal of General Virology. 1988;69(3):573–581. doi: 10.1099/0022-1317-69-3-573. [DOI] [PubMed] [Google Scholar]
- Verwoerd, Louw & Oellermann (1970).Verwoerd DW, Louw H, Oellermann RA. Characterization of bluetongue virus ribonucleic acid. Journal of Virology. 1970;5:1–7. doi: 10.1128/jvi.5.1.1-7.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward (1996).Ward MP. Seasonality of infection of cattle with bluetongue viruses. Preventive Veterinary Medicine. 1996;26(2):133–141. doi: 10.1016/0167-5877(95)00525-0. [DOI] [Google Scholar]
- Yang et al. (2016).Yang H, Xiao L, Wang J, Meng J, Lv M, Liao D, Song J, Gao L, Xiong H, He Y, Niu B, Chuang X, Li H. Phylogenetic characterization genome segment 2 of bluetongue virus strains belonging to serotypes 5, 7 and 24 isolated for the first time in China During 2012 to 2014. Transboundary & Emerging Diseases. 2016;64(4):1317–1321. doi: 10.1111/tbed.12479. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (1999).Zhang N, Maclachlan NJ, Bonneau KR, Zhu J, Li Z, Zhang K, Zhang F, Xia L, Xiang W. Identification of seven serotpes of bluetongue virus from the People’s Republic of China. Veterinary Record. 1999;145(15):427–429. doi: 10.1136/vr.145.15.427. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2012).Zhang MG, Zhou ZK, Chen WY, Slik JWF, Cannon CH, Raes N. Using species distribution modeling to improve conservation and land use planning of Yunnan, China. Biological Conservation. 2012;153:257–264. doi: 10.1016/j.biocon.2012.04.023. [DOI] [Google Scholar]
- Zuliani et al. (2015).Zuliani A, Massolo A, Lysyk T, Johnson G, Marshall S, Berger K, Cork SC. Modelling the northward expansion of Culicoides sonorensis (Diptera: Ceratopogonidae) under future climate scenarios. PLOS ONE. 2015;10(8):e0130294. doi: 10.1371/journal.pone.0130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in a Supplemental File.