Abstract
Poorly managed gestational diabetes can lead to severe complications for mother and child including fetal overgrowth, neonatal hypoglycemia and increased autism risk. Use of metformin to control it is relatively new and promising. Yet safety concerns regarding gestational metformin use remain, as its long-term effects in offspring are unclear. In light of beneficial findings with metformin for adult mouse social behavior, we hypothesized gestational metformin treatment might also promote offspring sociability. To test this, metformin was administered to non-diabetic, lean C57BL/6J female mice at mating, with treatment discontinued at birth or wean. Male offspring exposed to metformin through birth lost social interaction preference relative to controls by time in chambers, but not by sniffing measures. Further, prenatal metformin exposure appeared to enhance social novelty preference only in females. However due to unbalanced litters and lack of statistical power, firm establishment of any sex-dependency of metformin’s effects on sociability was not possible. Since organic cation transporter 3 (OCT3) transports metformin and is dense in placenta, social preferences of OCT3 knock-out males were measured. Relative to wild-type, OCT3 knock-outs had reduced interaction preference. Our data indicate gestational metformin exposure under non-diabetic conditions, or lack of OCT3, can impair social behavior in male C57BL6/J mice. Since OCT3 transports serotonin and tryptophan, impaired placental OCT3 function is one common mechanism that could persistently impact central serotonin systems and social behavior. Yet no gross alterations in serotonergic function were evident by measure of serotonin transporter density in OCT3, or serotonin turnover in metformin-exposed offspring brains. Mechanisms underlying the behavioral outcomes, and if with gestational diabetes the same would occur, remain unclear. Metformin’s impacts on placental transporters and serotonin metabolism or AMPK activity in fetal brain need further investigation to clarify benefits and risks to offspring sociability from use of metformin to treat gestational diabetes.
Keywords: 1,1-dimethylbiguanide; autism; pregnancy; SLC22A3; serotonin; social behavior
Graphical abstract
1. Introduction
Gestational diabetes appears to be associated with elevated risk of autism and other developmental disabilities in offspring [1,2,3,4]. In light of this, reports that metformin controls gestational diabetes better than the “gold-standard”, insulin -- with improved pregnancy outcomes including less maternal obesity and hypertension, reduced neonatal hypoglycemia and fewer infant seizures [5,6,7,8,9,10,11,12] -- raises the exciting possibility that it might also reduce autism incidence in this population. However use of metformin in pregnancy is still new, so its long-term impacts on exposed offspring are largely unknown. Since metformin readily crosses the placenta and may concentrate in cord blood, the potential exists for adverse developmental effects [13,14,15]. Evaluation of relative fetal risks and benefits from gestational metformin use is confounded by complications arising from gestational diabetes, other interventions used, adherence to them, and the extent of glycemic control [16, 17, 18].
Mouse studies provide conflicting evidence about acute impacts of fetal metformin exposure, and rarely address persistent effects. For example, one study found blastocyst defects in embryos exposed to 100 μg/ml metformin; a level 20 times greater than what occurs clinically [19]. Another saw delayed neural tube closure in 10% of embryos exposed to 150-1800 μg/ml metformin [20]. Neural tube defects are attributed to activation of adenosine monophosphate activated protein kinase (AMPK), which is one of metformin’s main actions, raising safety concerns for its use in pregnancy [12,15]. Yet when in vitro (1 mM) vs. in utero (120 mg/kg/d) metformin exposures were compared, investigators found AMPK activation and neural tube-related Pax3 inhibition in cell culture, but not in embryos exposed in vivo [21]. With diet-induced gestational diabetes, neural tube defects occurred in 11% of mouse offspring, but metformin (200 mg/kg) treatment of pregnant dams attenuated this [22]. Prenatal metformin exposure also protected offspring from fatty diet-induced obesity and glucose intolerance [23]. Hence metformin exposure during gestation appears to benefit offspring of dams on high fat diet or with gestational diabetes, while adverse impacts seem limited to high concentrations in vitro.
However there is a dearth of controlled long-term studies of the effects of gestational metformin exposure on offspring social and repetitive behaviors. To address this, we administered metformin to lean C57BL/6J dams throughout pregnancy, stopping the treatment at birth or at wean, to establish its effects on autism-relevant behaviors in adolescent offspring. Based on the study that gave metformin to pregnant dams in drinking water [22] and similar findings [21,23], we hypothesized in utero metformin exposure might promote offspring social behavior as it did for adult fragile X syndrome mice [24]. Since metformin is transported by organic cation transporters (OCT3) that are highly expressed in placenta [25, 26], the social behavior of OCT3 knock-out mice was examined to set the stage for their use in future studies of gestational metformin exposure.
2. Materials and Methods
2.1. Animals
Twenty five adolescent (4 week-old) C57BL/6J female mice and 9 males were purchased from the Jackson Laboratory (Sacramento, CA, USA). Upon arrival they were same-sex group housed in 484 cm2 ventilated cages with chipped wood bedding (Teklad sani-chip #7090 Envigo, East Millstone, NJ, USA). They acclimated for two weeks to 23.8 ± 1°C and 14:10 light cycle, with ad libitum access to rodent diet (Teklad 7912 irradiated, Envigo) and water (reverse osmosis, acidified to pH 2.5-3 with HCl). Their diet was switched to an L-amino acid open source control diet (Research Diets, New Brunswick, NJ #A11022501) when they were randomly assigned to one of nine cages (3 per treatment) and mated (2-3 females per male).
Sixteen each of 3 month-old male organic cation transporter 3 (OCT3) knock-out and wild-type mice (C57BL/6J background, bred in house from colony founders provided by Dr. Kim Tieu, Plymouth University, UK), 32 in total, were used in behavior tests. The OCT3 knock-out line, devoid of OCT3 expression in all tissues, was generated by homologous recombination; genotyping was performed at wean as described previously [27, 28, 29]. With ear tag identifiers, mice were group housed (3-5 per cage) by sex in 484 cm2 ventilated cages with chipped wood bedding at room temperature 23.8 ± 1°C on a 14:10 light cycle, and ad libitum access to rodent diet (Teklad 7912 irradiated, Envigo) and water. All procedures involving live mice were approved by the Institutional Animal Care and Use Committee, and were consistent with the National Institutes of Health Guide for the Humane Treatment of Animals [30], and ARRIVE Guidelines [31].
2.2. Metformin administration, tissue content and maternal glucose tolerance
The day before males were introduced, 16 female mice were given 0.5 mg/ml metformin (1,1-dimethylbiguanide hydrochloride, #D150959 Sigma-Aldrich, St. Louis, MO) dissolved in drinking water, and nine were given drinking water sans drug as controls. Water bottle treatments were freshened weekly. Mice were weighed daily, and water consumption per cage measured. On average (± SEM) female mice weighed 14 ± 0.8 g at the start of the experiment, and reached a peak weight of 26.6 ± 1.3 g late in pregnancy. Over the course of the study metformin-treated mice drank 5.8 ± 0.3 ml and control mice drank 5.1 ± 0.6 ml per day. Female mice took in an estimated maximum of 250 mg/kg/d metformin at the start of the study (when they weighed less), and minimum of 130 mg/kg/d at their peak pregnancy weights.
On their 14-15th day of pregnancy, 3 representative metformin drinking dams and 2 controls were euthanized so their trunk blood serum, brains and fetal brains could be collected, weighed and stored at −80°C for HPLC/MS/MS measures. Metformin standard and phenformin internal standard (# P7045 Sigma-Aldridge, St. Louis, MO) were prepared in Milli-Q water at 1 mg/mL and stored at −80°C. Metformin was quantified in mouse serum or whole brain homogenates prepared in 75% MeOH and centrifuged to remove protein. Calibration standards were prepared by spiking serum or supernatants of brain homogenates from control dams to achieve final concentrations of 0, 15.6, 62.5, 250, 1000, 4000 ng/mL. Then, 100 μL of calibrator and unknown samples were mixed with 5 μL of 10 μg/mL phenformin and 1.5 mL of acetonitrile. The samples were vortexed on high for 2 min, and then centrifuged at 16,060 g for 5 min at 23°C. Supernatants were transferred to 10 X 75 mm glass tubes and dried under a stream of nitrogen. The residues were then re-dissolved in 150 μL mobile phase, transferred to 1.5 mL microfilterfuge tubes and centrifuged at 16,060 g for 1 minute. The final extracts were transferred to autosampler vials and 25 μL was injected into the LC/MS/MS.
The HPLC system consisted of a Shimadzu SCL-10A Controller, LC-10AD pump with a FCV-10AL mixing chamber, SIL-10AD autosampler, and an AB Sciex API 3200 tandem mass spectrometer with turbo ion spray. The analytical column was a Grace Alltima C18 (4.6 × 150 mm, 5 micron, Alltech, Deerfield, IL) maintained at 60°C by column oven (Shimadzu CTO-10A). The isocratic mobile phase was 0.1% formic acid dissolved in acetonitrile:water (50:50). The mobile phase flow rate was 0.5 mL/min. Metformin transition was detected at 130.0 → 71.0 Da and phenformin transition at 206.2 → 60.1 Da. The ratio of the metformin peak area to that of phenformin (response ratio) for each unknown sample was compared against a linear regression of calibrator response ratios. This yielded mean dam serum metformin levels 1.27 ± 0.12 mg/L (≈10 μM, within therapeutic range [32]) and mean brain levels of 161 ± 20 pg/mg in dams (N = 3) and 158 ± 10 pg/mg in their fetuses (N = 4 per dam, 12 total). The number of dams in each treatment group, pregnancy outcomes and experimental use of pups is in Table 1.
Table 1.
Treatment Groups and Pregnancy Outcomes for Dams and Offspring.
| Treatment Group | Dam | Female Offspring | Male Offspring |
|---|---|---|---|
| Control | C1 | 2 | 2 |
| C2 | 3 | 2 | |
| C3 | 1 | 3 | |
| C4 | 3 | 2 | |
| C5 | 0 | 3 | |
| C6 | 1 | 2 | |
| C7 | 0 | 2 | |
| C8 | ED 14-15 tissues | ED 14-15 tissues | |
| C9 | ED 14-15 tissues | ED 14-15 tissues | |
| Total | 10 | 16 | |
| Metformin → Birth | MB1 | 0 | 2 |
| MB2 (5 dead PND1) | 0 | 0 | |
| MB3 | 2 | 2 | |
| MB4 | 3 | 3 | |
| MB5 | 3 | 1 | |
| MB6 (2 dead PND1) | 0 | 0 | |
| MB7 | ED 14-15 tissues | ED 14-15 tissues | |
| MB8 | ED 14-15 tissues | ED 14-15 tissues | |
| Total | 8 | 8 | |
| Metformin → Wean | MW1 (4 dead PND1) | 0 | 0 |
| MW2 (not pregnant) | 0 | 0 | |
| MW3 | 4 | 0 | |
| MW4 | 1 | 3 | |
| MW5 (1 dead PND1) | 0 | 0 | |
| MW6 | 2 | 3 | |
| MW7 | 3 | 2 | |
| MW8 | ED 14-15 tissues | ED 14-15 tissues | |
| Total | 10 | 8 | |
Numbers are pups surviving to wean, weaned offspring were ear tagged and same-sex group housed until behavior tests, C = control, MB = metformin treatment → birth, MW = metformin treatment → wean, M = male, F = female
Glucose tolerance tests were performed in 5 control and 7 metformin-treated dams between gestational day (GD) 12 and 16, since peak pancreatic β cell mass and islet cell function peaks at this time in pregnant mice [33]. Dams were fasted overnight for 16 h and injected at 10:00 am with glucose (1 g/kg, i.p.), and blood samples were collected from a tail vein nick at baseline and 15, 30, 60, 90, and 120 min after glucose injection. It was not possible to test all pregnant mice because for some dams the GD 12-16 window was missed, and for one control the glucose injection was incomplete. Blood glucose concentrations were measured using TRUEresult glucose meter and TRUEtest strips (Nipro Diagnostics, Ft. Lauderdale, FL). Glucose levels at each time-point and area under the curve were compared by two-way repeated measures analysis of variance (ANOVA) and Sidak’s multiple comparison, while area under the curve was compared by t-test using Prism (v. 7, Graphpad Software, La Jolla, CA).
When pups were born, mothers and litters were given their own cages. Nestlet squares were provided and metformin administration in drinking water was stopped in half of the cages, but continued in the other half to tease apart impacts of gestational metformin exposure from continued exposure to metformin in mothers’ milk through wean at postnatal day 25. At wean pups were ear-tagged and housed in same sex groups of 2-5 mice per cage. Weight of pups was recorded just before behavior tests at 6-7 weeks of age, and analyzed by two-way ANOVA. For behavior tests following gestational exposures to metformin vs. controls, the individual pups are the units of measure in the study. While 3 or more litters per group were tested, a limitation of this study is that some pups in test groups were siblings from the same litter (Table 1).
2.3. Autism-relevant behavior tests
2.3.1. Social interaction and social novelty preference
Three chamber tests are widely used in autism research for testing social interaction and social novelty preference in mice. These were conducted in 7 week-old male and female offspring, with each sex tested on different days, as previously described [34]. Tests were performed under dim red light between 1000 and 1400 h after mice acclimated to the room for 30 minutes in home cages. Each mouse subject was introduced to the test arena to acclimate to the center chamber with side chamber doors closed for 10 min. Then side chamber doors were opened so the mouse could explore all three empty chambers for 10 min.
For the social interaction preference test phase, subject mice are returned to the center chamber with doors closed and a cup cage is introduced into both end chambers. One empty cup cage serves as a novel stimuli, the other contains an age and sex-matched stranger of a different strain (129S1/SvlmJ). Position of the novel stimuli and stranger cages is randomized. To start the test while a video-camera recorded, the doors are opened to allow subject mice to explore the entire arena and both stimuli for 10 minutes. The whole arena is recorded so time spent in each chamber, chamber entries, as well as time spent sniffing either the novel or social stimuli cup cage can later be measured by treatment-blind observers.
Before the social novelty preference test phase, subject mice are returned to the center chamber and doors closed. A novel stranger mouse from a different home cage than the original stranger is added to the empty cup cage of the previous test phase, and the original stranger in its cup cage is moved slightly. Again the camera is turned on and doors are open so the experimental mouse can explore and interact with either the original stranger mouse, or the novel mouse for 10 min. Subjects and strangers were returned to holding or home cages, and fecal boli were tallied. Arenas were cleaned with 70% ethanol and paper towels between each test session. From the video-recordings a treatment blind observer measured time in chamber and time sniffing to assess and compare social novelty preference among groups.
Time spent in chambers, time spent sniffing stimuli, and chamber entries were measured by treatment-blind observers. Preferences (interaction = stranger time – novel object time; novelty = new stranger time – old stranger time) were compared among offspring groups by analysis of variance (ANOVA) or unpaired t-test for comparing behaviors of OCT3 genotypes. For each offspring sex in the metformin study, two-way (TREATMENT × PREFERENCE) ANOVA, with Dunnett’s or Tukey’s multiple comparison test were performed, while on OCT3 data Sidak’s was performed to obtain 95% CI and mean differences with Prism 7 (Graphpad, La Jolla, CA). Within -group sociability preferences were determined post-hoc by Tukey’s or Sidak’s multiple comparison tests. Since some offspring in the metformin study were siblings (Table 1), within each treatment preferences were also compared among litters using ANOVA.
2.3.2. Marble burying
Repetitive manipulation (burying) of inanimate objects by mice is translationally relevant to stereotypic or self-stimulatory behaviors characteristic of autism. Clean rat housing cages were filled to a depth of 8-10 cm with sani-chip bedding. Fifteen flat blue glass marbles were spaced evenly across the surface of the bedding in 5 rows of 3. Immediately after sociability tests, mice were placed alone in covered testing cages to interact with marbles on bedding undisturbed for 30 minutes. At test end marbles more than 2/3 covered with bedding were counted, and groups were compared by either two-way ANOVA or unpaired t-test.
2.3.3. Whole brain serotonin turnover
Whole brains collected from adolescent offspring at the completion of behavior testing were flash frozen on dry ice and stored at −80°C. A subset of 3-4 brains per group (each from a different litter) were weighed and homogenized in 75% MeOH and centrifuged to remove protein. Aliquots of supernatants were then injected into the HPLC system using an isocratic mobile phase and an Alltima C18 analytical column. Peak areas of analytes were compared against a linear regression of calibrators using Empower software (Waters Corp), and analyzed for levels of serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA). These measures will allow us to compare the relative amount of 5-HT turnover occurring in mice from each metformin treatment group. Mean neurochemical levels between groups were analyzed by 2-way ANOVA.
2.3.4. Serotonin transporter density in organic cation transporter3 (OCT3) knock-out mice
Naïve 3 month-old wild-type and knock out males (6 each) were euthanized by cervical dislocation and decapitation without anesthesia to preserve binding site integrity. Brains were removed and rinsed in ice cold saline, placed on a paper towel for 5 sec to dry and then frozen on powdered dry ice. Brains were stored in labeled bags at −80 °C until sectioning at −18-20 °C in a cryostat (Leica, Buffalo Grove, IL). Coronal sections (20 μm) were thaw mounted onto gelatin coated microscope slides, desiccated for 18-24 h at 4°C and frozen at −80°C until use. Serotonin transporter (SERT) binding was performed using [3H] citalopram (Perkin-Elmer, NEN). Briefly sections were defrosted for 10 min at 4°C, pre-washed in 50 mM Tris-HCl, 120 mM NaCl, 5 mM KCl pH 7.4 for 1h at room temperature (25°C). Incubation with 10 nM [3H] citalopram at 25°C in pH 7.4 buffer was carried out for 1 h. Sertraline (5 μM) was used to define non-specific binding on separate slides. Sections were washed twice for 10 min each in 4°C pH 7.4 buffer, dipped for 2 s in 4°C deionized water, and dried on a slide warmer at a moderate heat setting (4) for 20 min. Sections on slides were opposed to Kodak Biomax MR film for 6 weeks along with [3H] standards calibrated to 3H labeled brain mash to obtain units of fmol/mg protein [35]. Autoradiographic images were captured on a stabilized fluorescent light box (Northern Lights, Imaging Research, Ontario, CA), CCD monochrome camera (CFW-1612M, Scion, Frederick, MD) and copy stand, Kaiser RS-1, White Plains, NY), calibrated using a linear function and density was measured with Image J (http://rsb.info.nih.gov/ij/download.html). Since multiple regions were measured, wild-type and OCT3 knock-out means were compared by MANOVA using Statistica (’98 edition, Statsoft, Tulsa, OK).
3. Results
3.1. Maternal glucose tolerance mid-gestation
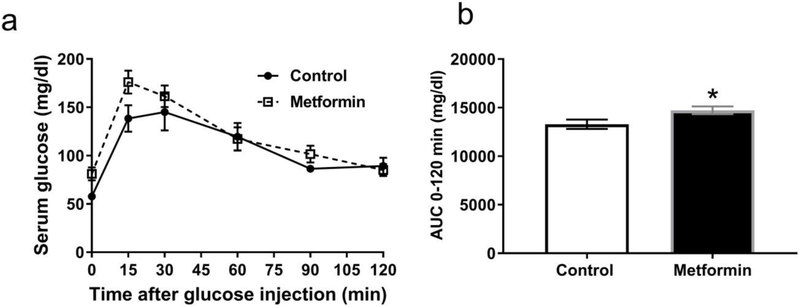
Fasting serum glucose and response to glucose challenge at each time was not significantly different between metformin-treated and control dams at GD 12-16 (Fig. 1a). However area under the curve (AUC) ± SEM for 0-120 min was slightly yet significantly higher in metformin treated dams (14724 ± 403, n = 7) versus controls (13290 ± 486, n = 5; unpaired t(10) = 2.28, p < 0.05). This indicates glucose clearance was slower in the metformin treated dams at mid-gestation versus controls (Fig. 1b). However neither dam group had peak blood glucose levels in excess of 250 mg/dl in the fasting glucose tolerance test, the threshold criteria for glucose intolerance in mouse models of gestational diabetes [36].
Fig. 1. Glucose tolerance of pregnant dams is slightly impaired by metformin treatment.
A. Glucose tolerance test results from mid-gestation (ED 12-16) dams after 16 h (overnight) fast at baseline and 15 −120 min after injection of glucose (1 g/kg) did not reveal any differences between control (black circles, N = 5) or metformin (open boxes, N = 7) treatments at any specific time points. B. Comparison of area under curve (AUC) values from the glucose tolerance test revealed a slight but significant increase in metformin-treated dams relative to vehicle controls (* p < 0.05). Data are presented as means ± SEM.
3.2. Similar body weights in control and metformin exposed offspring
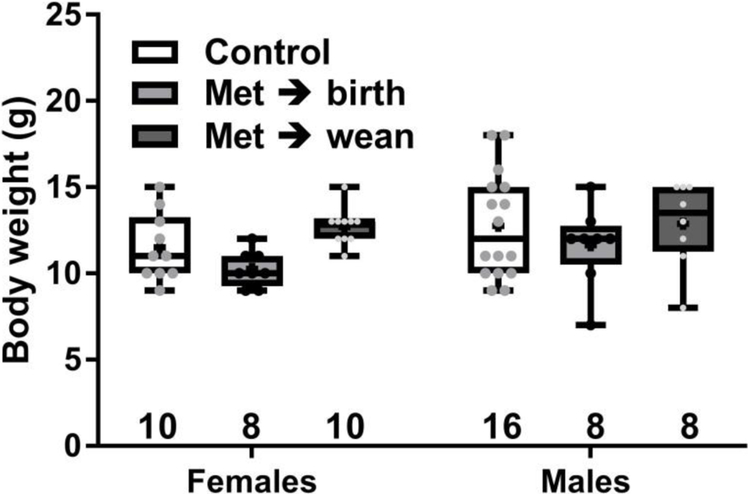
Adolescent female and male offspring (N = 8-16) from at least three different mothers per metformin treatment group were assessed in all behavior tests. When mice reached 6-7 weeks old, there was no significant difference in body weights between sexes (F (1, 54) = 2.44, p = 0.12) or among metformin treatments and controls (F (2, 54) = 2.93, p = 0.06), and no interaction (F(2,54) = 0.39). Weights and group sample sizes are shown in Fig. 2. Weights of OCT3 wild-type and knock-outs were mean ± sem, 28 ± 0.7 and 28 ± 0.9 g, N=16.
Fig. 2. Adolescent offspring weights at time of behavior tests.
Dam treatments were metformin in drinking water (0.5 mg/ml) stopped at birth (Met → birth), at wean (Met → wean) or control. Offspring weights (6-7 wk old) did not differ significantly. Boxes show 25th to 75th percentiles, center line is median, plus signs are means, whiskers are minimum to maximum weight. Weights for individuals are small points, all mice were used in the behavior tests, group size appears under boxes.
3.3. Social interaction vs. novel object preferences in metformin-exposed and control offspring
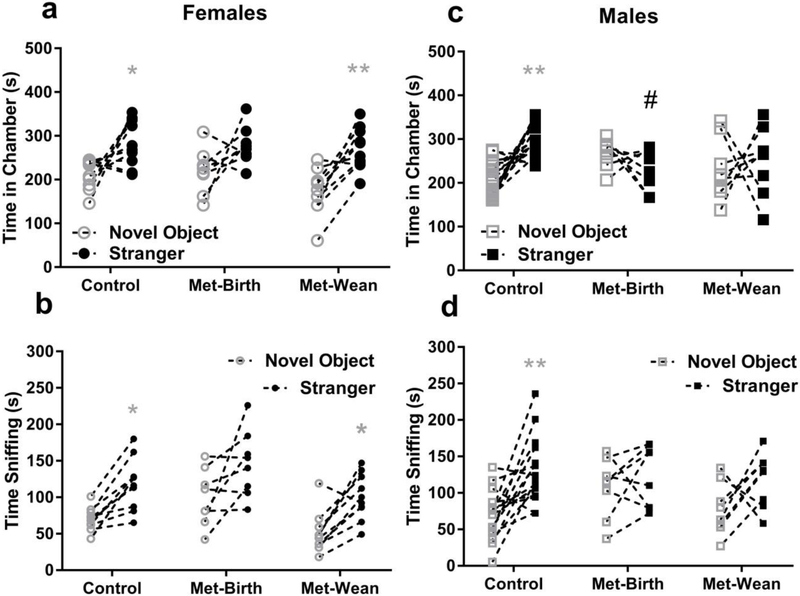
3.3.1. Female offspring social interaction preferences
Among female offspring, there were no significant differences between prenatal metformin exposure groups in social interaction preference (stranger – novel object), for which mean ± SEM (95% CI of mean) = control 71 ± 26 (11 to 130) sec, met → birth 60 ± 31 (−13 to 131) sec and met → wean 101 ± 19 (57 to 143) sec by measure of chamber times (F (2, 25) = 0.72, p = 0.49, Fig. 3a). There were also no differences in female offspring interaction preference from sniffing time, for which mean ± SEM (95% CI) = control 48 ± 9 (28 to 69) sec, met → birth 46 ± 20 (−1 to 93) sec and met → wean 49 ± 11 (24 to 74) sec (F (2, 25) = 0.013, p = 0.99, Fig. 3b).
Fig. 3. Gestational metformin exposure reduced male preference for social interaction.
There were no significant differences in female offspring sociability preference between groups. Within groups, a) time spent in stranger chambers (filled circles) vs. novel objects (open circles) was greater in female offspring of control dams (mean ± sem = 283 ± 17 vs. 212 ± 11 sec,*p < 0.05, N = 10) and dams treated with metformin through wean (Met-wean = 271 ± 15 vs. 170 ± 16 sec,**p<0.01, N = 10), but not from dams given metformin through birth (Met-birth = 279 ± 15 vs. 219 ± 18 sec, N = 8). b) Time spent sniffing stranger mice vs. novel objects was also greater in female offspring of control dams (119 ± 11 vs. 70 ± 5 sec,* p <0.05) and Met-wean (100 ± 10 vs. 51 ± 9 sec, *p < 0.05), but not Met-birth (146 ± 16 vs. 100 ± 14) exposed females. Male offspring from Met-birth dams spent significantly more time in novel object chambers and less time with strangers (# p < 0.05 in c) and exhibited a loss of social interaction preference in chamber entries (* p <0.05), but not in sniffing (d). Within groups c) time spent in stranger chambers (filled squares) vs. novel objects (open squares) was greater in male offspring of controls (mean ± sem = 301 ± 10 vs. 209 ± 10 sec,**p < 0.01, N = 16) but not in male offspring from Met - birth (231 ± 17 vs. 268 ± 11 sec, N = 8), or Met- wean (250 ± 28 vs. 231 ± 25 sec, N = 8) dams. d) In sniffing male offspring of controls displayed a preference for strangers vs. objects (130 ± 11 vs. 68 ± 8 sec, ** p <0.01) male offspring from Met-birth (123 ± 15 vs. 107 ± 15 sec) or Met-wean (118 ± 13 vs. 78 ± 13 sec) dams did not. Data are presented as symbols for novel object and stranger dwelling or sniffing times with a connecting line between measures of each individual animal.
Regarding within group female offspring sociability preferences based on time in chambers, two-way (TREATMENT × CHAMBER) ANOVA found that at least one group had a chamber preference (F (1, 50) = 37, p <0.001), with no significant interactions (F (2, 50) = 0.95, p = 0.39) or treatment effects (F (2, 50) = 2.2, p =0.12). Tukey’s post-hoc analysis revealed preferences for stranger vs. novel object chamber times in control (mean difference −70.9, 95% CI = −133.5 to −8.306) and metformin → wean (mean difference −101, 95% CI =163.5 to −38.31) groups. For female offspring sniff a two-way (TREATMENT × CHAMBER) ANOVA was also run to reveal significant chamber preferences (F (1, 50) = 29, p <0.0001) and metformin effects (F (2, 50) = 9, p < 0.001) with no interactions (F (2, 50) = 0.01, p=0.99) among groups. Significant within group preferences for stranger vs. novel object sniffing were found in control (mean difference −48.4, 95% CI = −92.06 to −4.741) and metformin → wean groups (mean difference −49.4, 95% CI = −93.06 to −5.741) indicated by * for p <0.05 or **p <0.01 in Fig 3a and 3b. The treatment effect was due to the metformin → birth spending more time sniffing novel objects than the metformin → wean group (mean difference 49, 95% CI = 2.192 to 94.81, p <0.05).
3.3.2. Male offspring social interaction preferences
Between male offspring treatment groups, there was a significant difference in social interaction preference (F (2, 29) = 5.11, p <0.05) by measure of time in chambers.. Specifically met → birth male offspring had reduced social interaction preference relative to controls (mean difference = 129, 95% CI = 32 to 226, Dunnett’s p < 0.05, # in Fig 3c). Social interaction preference by chamber time mean ± SEM (95% CI) = control 92 ± 18 (53 to 131) sec, met → birth −37 ± 27 (−100 to 26) sec and met → wean 19 ± 51 (−101 to 140) sec. However social interaction preference by sniff revealed no significant differences among male offspring groups, for which mean ± SEM (95% CI) = control 62 ± 15 (31 to 93) sec, met → birth 16 ± 19 (−29 to 60)sec and met → wean 40 ± 22 (−13 to 93) sec (F (2, 29) = 1.11, p = 0.20, Fig. 3d). Hence in male offspring, gestational exposure to metformin stopped at birth resulted in a loss of social interaction preference evident in chamber time, but not in sniffing time.
Within male offspring groups, a two-way (TREATMENT X CHAMBERS) ANOVA revealed a significant interaction between these variables (F (2, 58) = 9, p < 0.001). Tukey’s post-hoc analysis revealed that only control offspring spent more time with strangers than novel objects by chamber time (mean difference = −92, 95% CI= −144 to −39, Tukey’s p < 0.001, * Fig. 3c). By contrast, male offspring from Met → wean dams spent significantly less time in stranger chambers than controls (Mean difference = 70, 95% CI =5 to 134, Tukey’s p < 0.05, # in Fig 3c). For male offspring social sniff, the TREATMENT × CHAMBERS ANOVA revealed a significant chamber preference (F (1, 58) = 14, p <0.001), with with no significant interactions (F (2, 58) = 1.9, p = 0.16) or treatment effects (F (2, 58) = 1.06, p = 0.35). Yet only male offspring from control dams spent significantly more time sniffing strangers than novel objects (Mean difference = −62, 95% CI =−103 to −21, Tukey’s p < 0.001 ** in Fig 3d).
3.3.3. Maternal effects on social interaction preference
Since siblings were included in behavior measures, we looked for litter effects (with all male and female offspring together) within each metformin exposure group on social interaction preference using ANOVA. No significant litter effects were evident for chamber entries in the control group (seven dams F (6,19) = 1.46, p = 0.24), metformin - birth group (four dams F (3,12) = 1.65, p = 0.23), or metformin - wean group (four dams F (3,14) = 1.29, p = 0.32). For sniff there were no significant litter effects for control (F (6,19) =1.10, p = 0.40) or metformin - wean groups (F (3,14) = 1.82, p = 0.19). However, there was a significant litter effect in the metformin – birth group (F (3,12) = 5.06, p = 0.02) in sniffing. This appears to be due to litter MB3, which had greater sniff preference for social interaction than MB1 (mean difference = −110.5, 95% CI= 215.6 to −5.395, Tukey’s p < 0.05) or MB4 (mean difference = 90.67, 95% CI =12.33 to 169, p <0.05). Litter MB3 had 2 female offspring with sniff interaction preferences of 77 and 160 sec, and two males 35 and 96 sec.
3.3.4. Unclear if metformin’s effect on male offspring interaction preference is sex-dependent
Because no litter effects were evident in chamber time interaction preferences, a SEX by TREATMENT two-way ANOVA was subsequently performed with this data. While the effect of SEX was significant (F(1,54) = 5.08, p < 0.05), as was effect of TREATMENT (F(2,54) = 3.14, p = 0.05), the SEX by TREATMENT interaction didn’t meet criteria for significance (F (2,54) = 2.81, p = 0.065). Thus it could not be firmly established if the effects of gestational metformin exposure on social interaction preference were indeed sex-dependent from this small preliminary study.
3.4. Social novelty preferences in metformin-exposed and control offspring
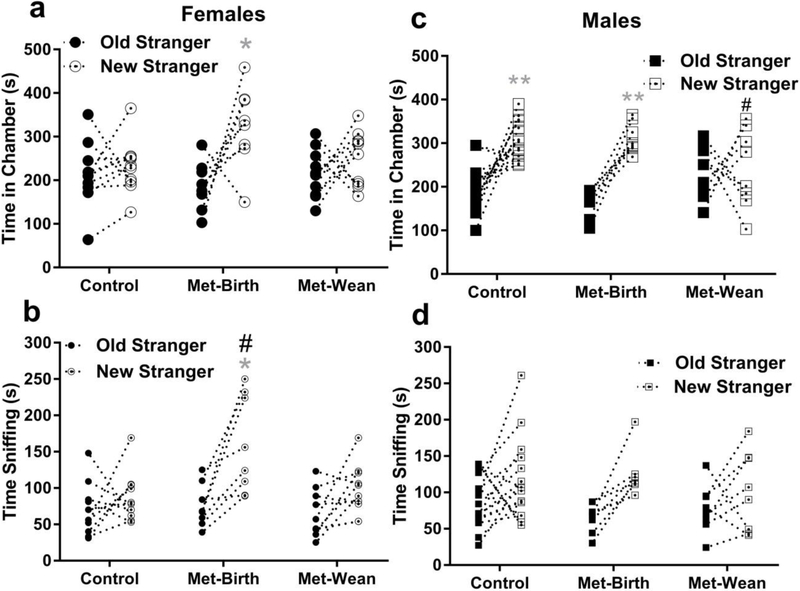
3.4.1. Female offspring social novelty preferences
Between female offspring groups, there were no significant differences in social novelty preference (new stranger time – old stranger time), from time in chambers for which mean ± SEM (95% CI of mean) = control 16 ± 27 (−44 to 77) sec, Met → birth 138 ± 52 (15 to 260) sec and Met → wean 37 ± 35 (−42 to 115) sec (F (2, 25) = 2.8, p = 0.08, Fig. 4a). However female offspring novelty preference differed between groups by measure of sniffing, for which mean ± SEM (95% CI): Met → birth 84 ± 22 (32 to 136) sec, but not Met → wean 34 ± 14 (3 to 66) sec, was significantly greater than control 17 ± 19 (−26 to 60) sec (F (2, 25) = 3.4, p = 0.05, mean difference = −67, 95% CI = −128 to −5, Dunnett’s # p = 0.05, Fig 4b).
Fig. 4. Gestational metformin exposures had mixed effects on social novelty preference.
Considering all offspring, metformin exposure through birth enhanced or maintained within group social novelty preference, whereas exposure to metformin until wean impaired it. a) No within-group preference for new (dot in center circle) vs. old (filled circle) stranger chambers was evident in female offspring from control dams (mean ± sem = 230 ± 19 vs. 214 ± 24 sec, N = 10) or metformin stop at wean (Met-wean 252 ± 19 vs. 215 ± 18 sec, N = 10), whereas the metformin stopped at birth (Met-birth) group (325 ± 33 vs. 187 ± 20 sec, *p < 0.05, N = 8) preferred new strangers. b) Female offspring from Met-birth dams exhibited a significant preference for new strangers in sniffing time (159 ± 24 vs. 75 ± 10 sec, #(between) and *(within groups) p <0.05), whereas control (88 ± 11 vs. 70 ± 12 sec) or Met-wean female offspring (98 ± 11 vs. 64 ±10 sec) did not. c) Control and Met-birth male offspring spent more time in chambers with new (dot in center squares) vs. old (filled squares) strangers (control 303 ± 11 vs. 199 ± 13; N= 16, Met-birth 312 ± 12 vs. 162 ± 11 N = 8; *p < 0.05), but Met-wean male offspring (242 ± 32 vs. 234 ± 23, N = 8) did not. d). However based on sniffing, there was no preference for novelty exhibited by male offspring from control (115 ± 14 vs. 87 ± 9), met-birth (122 ± 11 vs. 65 ± 7) or met-wean (101 ± 19 vs. 77 ± 12) dams. Data are presented as symbols showing individual values with a connecting line between new and old stranger times for each individual mouse.
To resolve social novelty preferences within female offspring groups a two way TREATMENT × CHAMBERS ANOVA of chamber times revealed a significant interaction (F (2, 50)= 3.9, p < 0.05) and chamber preference (F (1, 50) = 12, p <0.001) among groups, but no significant treatment effect (F (2, 50) = 1.1, p = 0.33). Tukey’s post-hoc analysis demonstrated no female offspring within-group preferences for new vs. old stranger chambers were evident in control (mean difference −16.3, 95% CI = −106 to 75) or Met → wean (mean difference −37, 95% CI = −126 to 54) groups. Yet Met → birth female offspring spent more time with new vs. old strangers (mean difference −138, 95% CI = −237 to −37, Tukey’s p <0.05) shown by * in Fig 4a. For sniff of new vs. old strangers, the TREATMENT × CHAMBERS ANOVA revealed a significant interaction (F (2, 50) = 3.2, p <0.05), treatment effect (F (2, 50) = 5, p <0.05) and chamber preferences (F (1, 50) = 18, p <0.0001) among female offspring groups. This was because control (mean difference −17, 95% CI = −70 to 36), and Met → wean (mean difference −34, 95% CI = −87 to 19) had no preferences among the two strangers, whereas Met → birth (mean difference −84, 95% CI = −143 to −25, p <0.05, *Fig 4b) sniffed the novel stranger more than the old stranger.
3.4.2. Male offspring social novelty preferences
Among male offspring, in chamber time measures there was a significant difference in social novelty preference (F (2, 29) = 4.08, p <0.05) between metformin exposed groups, but neither differed from control. Met → wean males had reduced social interaction preference relative to Met → birth (mean difference = 141, 95% CI = 15 to 266, Tukey’s p < 0.05, # in Fig 4c). Social novelty preference mean ± SEM (95% CI) = control 104 ± 22 (56-151) sec, met → birth 149 ± 19 (103-95) sec and met → wean 9 ± 53 (−117-135) sec by male offspring chamber times. Yet by measure of sniff for male offspring there were no between group differences in novelty preference (F (2, 29) = 0.81, p = 0.45), mean ± SEM (95% CI) = control 29 ± 16 (−5-62) sec, met → birth 57 ± 13 (26-88) sec and met → wean 24 ± 21 (−27-74) sec, Fig. 4d.
Male offspring within-group preferences for new vs. old strangers were evident from a two-way (TREATMENT × CHAMBERS) ANOVA through a significant interaction (F (2, 58) = 7.2, p < 0.05) and chamber preference (F (1, 58) = 38, p < 0.0001) but not a metformin treatment effect (F (2, 58) = 0.5, p =0.6). Tukey’s post-hoc analyses showed greater sniffing of the novel stranger by male offspring from control (mean difference −104, 95% CI −159 to −47) and Met → birth (mean difference −149, 95% CI −229 to −70) dams (Tukey’s p < 0.001 indicated by ** in Fig 4c), but not Met→ wean (mean difference = −9, 95% CI −89 to 71). By measure of sniffing there was only a chamber preference (F (1, 58) = 11, p < 0.005), without interaction (F (2, 58) = 0.76, p = 0.47) or metformin treatment effect (F (2, 58) = 0.47, p = 0.63). Yet no within-group male offspring preference for new versus old strangers was evident for control (mean difference −29, 95% CI −74 to 17), Met → birth (mean difference −57, 95% CI −121 to 7), or Met → wean (mean difference −24, 95% CI −88 to 40) groups could be found, as shown in Fig 4d.
3.4.3. No maternal effects on social novelty preference
Within each metformin exposure group we also looked for litter effects on social novelty preference using ANOVA. No significant litter effects were evident for chamber entries in the control group (seven dams F (6,19) = 0.45, p = 0.83), metformin - birth group (four dams F (3,12) = 1.13, p = 0.38), or metformin - wean group (four dams F (3,14) = 0.69, p = 0.57). For sniff there were also no significant litter effects for control (F (6,19) =1.23, p = 0.33), metformin – birth (F(3,14) = 0.78, p = 0.53) or metformin - wean groups (F (3,14) = 0.21, p = 0.89).
3.5. Social Interaction and Novelty Preferences of Organic Cation Transporter 3 Male Mice
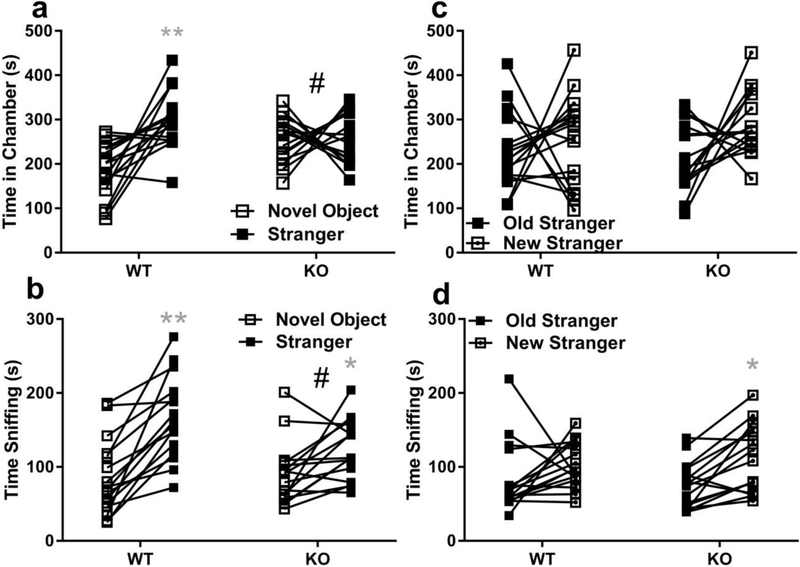
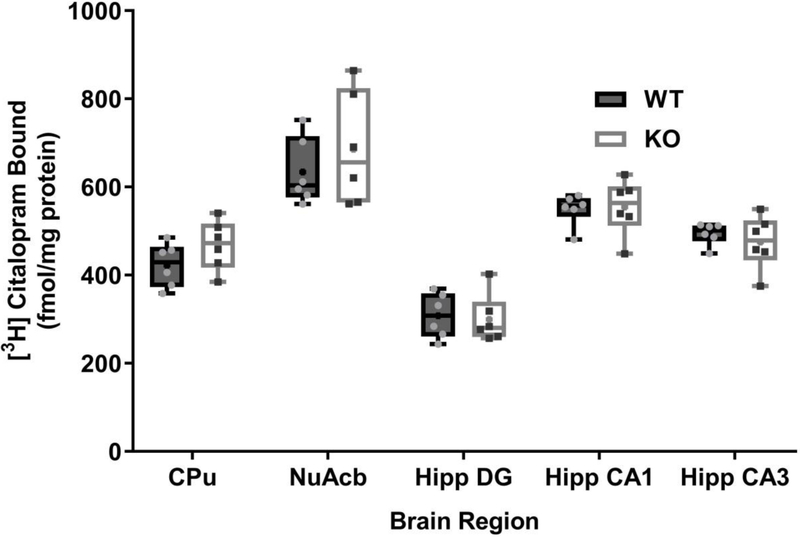
Because organic cation transporter 3 (OCT3) are involved in trans-placental transport of metformin, monoamines and amino acids [37], male OCT3 knock-out (KO) sociability was characterized versus wild-types (WT). Social interaction preference (stranger – novel object time) was compared between OCT3 wild-type (WT) and knock-out (KO) by two-tailed t-test. OCT3 KO had significantly lower social interaction preference than WT by chamber time (mean difference = −115 ± 37, 95% CI = −191 to −39, t(30) = 3.09, p <0.01, Fig 5a), and by sniffing time (mean difference = −47 ± 18, 95% CI = −84 to −11, t(30) = 2.64, p <0.05, Fig 5b) measures. Post-hoc Sidak’s multiple comparisons found KO spent more time in novel object chambers than WT (mean difference = −70, 95% CI −127 to −13, p <0.05), but found no differences in their stranger chamber times (mean difference = 44, 95% CI= −12 to 101). Within-groups, WT stranger chamber time was greater than novel object chamber time (mean difference −116, 95% CI −173 to 60, **p <0.001, Fig 5a), whereas KO exhibited no real preference (mean difference = – 2, 95% CI = −58 to 55, p = 0.99). Accordingly, WT tended to spend more time sniffing stranger mice than KO (mean difference 43, 95% CI −1 to 88, p = 0.058), whereas novel object sniffing did not differ among OCT3 genotypes (mean difference = −4, 95% CI −48 to 41, p = 0.99).
Fig. 5. Organic cation 3 knock-out mice do not exhibit social interaction preference.
Male wild-type (WT, N = 16) and OCT3 knock-out (KO, N = 16) mouse social preferences were compared, and a) Social interaction preference by measure of time in chambers was significantly lower in OCT3 KO mice # p <0.05). Only WT spent more time in chambers with strangers (filled square) vs. novel objects (open square) WT mean ± sem = 301 ± 16 vs. 185 ± 16 sec, KO 257 ± 14 vs. 256 ± 13 sec (**p <0.01). b) By measure of sniff OCT3 KO social interaction preference was also significantly less than WT (# p <0.05), WT mean ± sem = 166 ± 14 vs. 86 ± 13 sec, KO 122 ± 10 vs. 89 ± 11 sec (**p <0.01; *p<0.05). c) No significant differences were observed between OCT3 genotypes for social novelty preference wherein time in chambers with new (square with dot in center) vs. old (filled square) strangers differences were compared. WT mean ± sem = 252 ± 26 vs. 228 ± 22 sec, KO 287 ± 18 vs. 211 ± 19 sec. d)By measure of time sniffing WT and KO did not differ in novelty preference, WT mean ± sem = 103 ± 8 vs. 84 ± 12, KO 113 ± 11 vs. 74 ± 8, but within KO group more time was spent sniffing new vs. old strangers (*p<0.05). Data are presented as symbols showing individual values with a connecting line between them for each individual mouse.
No significant differences in social novelty preference (new – old stranger time) were found between OCT3 WT and KO by chamber time (mean difference = 51 ± 55, 95% CI = −61.93 to 163.6, t(30) = 0.9, p = 0.36, Fig 5c), or by sniffing (mean difference = 19.25 ± 17.09, 95% CI = −15.65 to 54.15, t(30)=1.1, p = 0.27, Fig 5d). Exploratory post-hoc comparison of novelty preference within-groups showed OCT3 KO spent more time sniffing new strangers (Fig 5d, mean difference = −38, 95% CI −75 to −1, *p < 0.05), whereas WT did not.
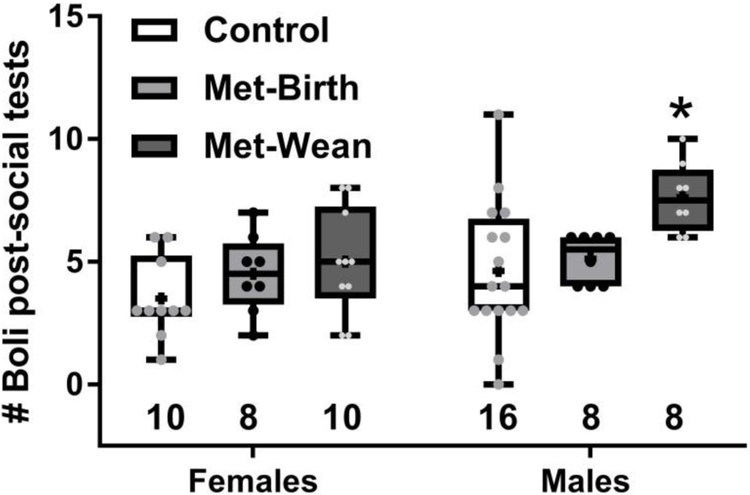
Fecal boli dropped during sociability preference tests
Counts of fecal boli dropped in the middle chamber of the test arena during social interaction plus social novelty tests demonstrated that males in the metformin exposure discontinued at wean group dropped more boli than their controls (SEX F (1, 54) = 7.34, p =0.009; TREATMENT F (2, 54) = 6.438, p=0.0031; no interaction, Sidak’s p < 0.05, Fig. 6). Tests were consistently performed at the same time of day, so this was not a likely influence on fecal count. OCT3 wild-type (mean ± sem = 3.5 ± 0.7 boli) and knock-outs (4.9 ± 7 boli) did not differ significantly in number of fecal boli dropped during sociability tests (t(30) =1.36, p=0.18).
Fig. 6. Males exposed to metformin through wean dropped more fecal boli during sociability testing.
Boli in the center chamber of the three chamber arena were tallied after social preference tests ended, and males in the metformin through wean (Met-wean) group dropped significantly more than control males (*p < 0.05). Boxes show 25th to 75th percentiles, center line is median, plus signs are means, whiskers are minimum to maximum fecal boli, # boli per individual are small symbols, sample sizes are shown as numbers under each box.
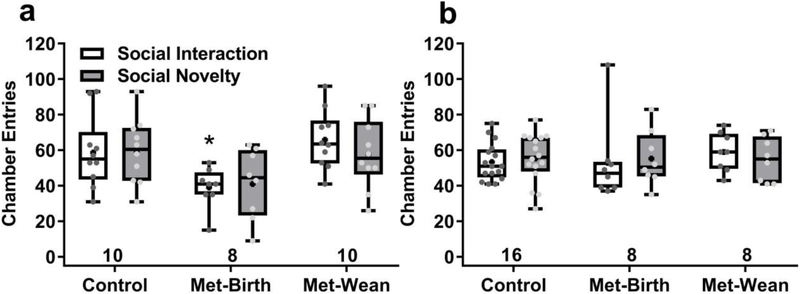
3.5. Chamber entries and time in arena center
During sociability tests total number of chamber entries was tallied, and female offspring exposed to metformin through birth made fewer entries during social interaction (F (2, 50) = 7.456, p = 0.0015, Sidak’s p <0.05 Fig. 7a), but not social novelty tests. Gestationally exposed males exhibited no differences in number of chamber entries among treatment groups (Fig. 7b). OCT3 wild-type and knock-out mice did not differ in number of chamber entries, either in social interaction (mean ± sem = 39 ± 3 vs 38 ± 4) or social novelty (45 ± 17 vs. 35 ± 15) tests.
Fig. 7. Chamber entries by metformin-exposed offspring during sociability tests.
(a) Female mice exposed to metformin through wean made significantly fewer entries during social interaction tests than controls (* p <0.05). (a) Male mice made comparable chamber entries across treatment groups in both test phases. Boxes show 25th to 75th percentiles, center lines are medians, plus signs are means, whiskers are minimum to maximum entries, entries of individuals are small symbols, sample sizes are shown as numbers under each box.
Time spent in the middle chambers did not differ among gestational metformin treatment groups or sex in social interaction (mean ± sem 215 ± 15 sec) or in social novelty (54 ± 5 sec). Time spent in middle chamber also did not differ significantly for OCT3 genotypes in the social interaction (220 ± 14 sec) or social novelty (40 ± 4 sec) tests.
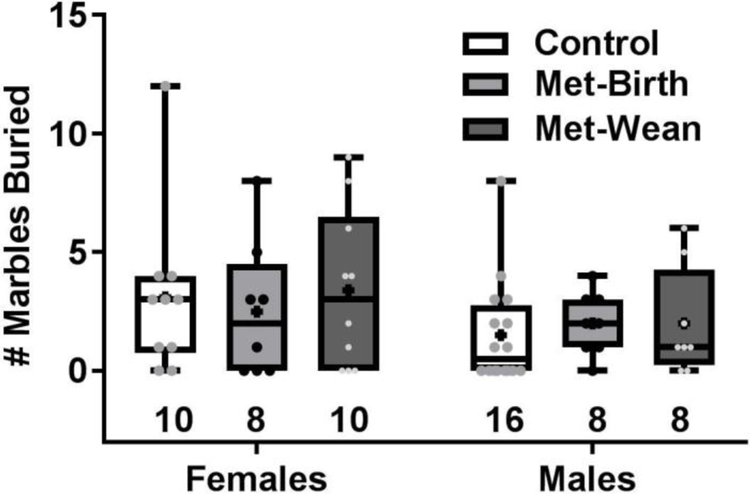
3.6. Marble burying
The number of marbles buried by mice after 30 min in a cage with 15 evenly spaced marbles over bedding did not differ significantly among offspring sexes (F (1, 54) = 2.7, p = 0.11) or metformin treated dam groups (F (2, 54) = 0.15, p = 0.86) (Fig. 8). Also, there was no significant difference (t(30) = 0.37, p = 0.71) between the number of marbles buried by wild-type (mean ± sem = 6 ± 1) or OCT3 knock-out mice (5 ± 1).
Fig. 8. Marble burying behavior was unchanged by gestational metformin exposure.
Neither females nor males exhibited any difference in the number of marbles buried in bedding over 30 min. Boxes show 25th to 75th percentiles, center lines are medians, plus signs are means, whiskers are minimum to maximum buried, and number buried per individual are small symbols, sample sizes are shown as numbers under each box.
3.8. Serotonin turnover following behavior tests
Since changes in serotonin (5-HT) neurotransmission have been associated with social interaction preference differences in prior studies [38], we decided to measure serotonin turnover in a subset of the brains collected from the juvenile mice from the behavior tests. Whole brain 5-hydroxyindoleacetic acid (5-HIAA) content was divided by 5-HT content for each brain to obtain % 5-HT turnover levels, which did not differ (SEX F(1,14) = 0.22, p = 6, TREATMENT F(2,14) = 0.05, p = 0.94, no interaction) among sexes or dam metformin treatment groups, as shown in Table 2.
Table 2.
Whole brain serotonin levels and turnover in metformin- exposed offspring.
| Treatment Group | 5-HT (mg/g) | 5-HIAA (mg/g) | %5-HT turnover |
|---|---|---|---|
| Female control | 686 ± 22 | 1351 ± 26 | 199 ± 9 |
| Female met-birth | 619 ± 22 | 1120 ± 46 | 181 ± 2 |
| Female met-wean | 628 ± 28 | 1300 ± 182 | 204 ± 23 |
| Male control | 640 ± 16 | 1172 ± 38 | 182 ± 17 |
| Male met-birth | 612 ± 41 | 1285 ± 48 | 208 ± 19 |
| Male met-wean | 690 ± 33 | 1202 ± 90 | 174 ± 8 |
Data shown are mean ± SEM, N=3-4 randomly selected from group for measures, no significant differences found.
3.9. Serotonin transporter density in terminal fields of wild-type and OCT3 knock-out mice
Specific binding of [3H] citalopram to brain regions including the caudate putamen, nucleus acumbens core, and hippocampal dentate gyrus, CA1 and CA3 regions were measured and compared between OCT3 wild-type and knock-out mice (Fig. 9). No significant differences were found (Wilk’s λ (5,6) = 0.44, p =0.31).
Fig. 9. Serotonin transporter density in wild-type and OCT3 knock-out mouse terminal fields.
No significant differences were observed between genotypes in the brain regions measured. CPu = caudate putamen (striatum), Nu Acb = nucleus accumbens core, Hipp = hippocampus, DG = dentate gyrus, CA1 = CA1 region, CA3 = CA3 region. Boxes show 25th to 75th percentiles, center lines are medians, plus signs are means, whiskers are minimum to maximum densities, and individual density measures are small symbols , N = 6 per genotype.
4. Discussion
The primary finding of this pilot study is that gestational metformin exposure discontinued at birth reduced social interaction preference relative to controls in adolescent male offspring by one measure (time spent in chambers) but not by a second (social sniffing). The social behavior findings from gestational metformin exposures should be interpreted cautiously, as they are largely inconclusive due to large confidence intervals (high variability), small sample sizes and study design limitations including behaviors measured from more than one offspring per litter. These preliminary outcomes did not support our initial hypotheses that gestational metformin exposure might enhance offspring social behavior. On the other hand, female offspring exposed to metformin through wean retained social interaction preference. Also female offspring exposed to metformin until birth exhibited an increase in social novelty preference by measure of social sniff. It must be emphasized that the mouse dams in this study were not diabetic or on high fat diet, in contrast to prior studies of the effects of gestational metformin exposure [21,22,23]. Few studies have reported impacts of pre or perinatal exposure to metformin on offspring behaviors, but this was of great interest because of the potential for metformin use to prevent the reported increase in autism incidence in offspring from mothers with gestational diabetes [1-4].
Gestational diabetes affects 18% of pregnancies [36], and better treatments than insulin alone are needed since insulin resistance is a natural state of pregnancy. High doses (0.5 – 2.5 g/day) of metformin are required to manage gestational diabetes as its half-life is only 6 hours, and metformin readily cross placenta and blood-brain barriers [5, 7-9]. One population of women in whom metformin use was studied prior to and throughout pregnancy are those diagnosed with polycystic ovarian syndrome (PCOS). Few adverse outcomes from metformin use in PCOS offspring were reported [39]. Yet one study found larger head circumference in metformin-exposed PCOS offspring correlated with maternal obesity [40]. Another found metformin-exposed PCOS children were overweight relative to untreated PCOS controls [41]. Excessive release of androgens underlies most cases of PCOS, and the condition itself is associated with an elevated risk of autism [42], again making it difficult to tease apart effects of gestational exposure to metformin on offspring social behavior with this condition.
The tentative loss of social interaction preference in male mouse offspring following gestational metformin exposure, if it proves to be robust with further study -- is concerning, and the mechanisms underlying it remain unclear. Gestational metformin use can reduce birth weight, and low birth weight is also considered an autism risk factor [9, 15, 16]. Offspring birth weights were not systematically monitored in this study, and may have differed from controls earlier in development. However we did not find lower weights in adolescent mouse offspring exposed to metformin in utero (Fig. 2). We also did not examine fetal mice for neural tube defects that were reported in other studies [12, 15, 19, 20, 21]. Yet surviving litter sizes were comparable, and motor development and home cage behaviors of metformin-exposed pups and dams did not appear overtly different from controls. Metformin treatment may influence maternal care, and this should be examined in detail by cross-fostering pups in future studies.
Metformin inhibits gluconeogenesis and boosts insulin sensitivity [43]. It may do so largely by inhibiting complex I of the mitochondrial respiratory electron transport chain (reducing ATP/AMP ratios among other actions), not by fueling insulin release as sulfonylureas do [11, 12, 23]. Metformin also reduces lipid and cholesterol synthesis by activating AMP-activated protein kinase (AMPK), among other kinases [12, 43]. Since metformin is hydrophilic, its entry into liver cells, and thus its efficacy, is dependent upon organic cation transporter (OCT) subtype 1 (SLC22A1), as evidenced by poor metformin performance in individuals with gene polymorphisms impairing OCT1 expression or function [11, 12, 14, 43 - 46].
Metformin is also a substrate of OCT3 (SLC22A3) [47, 48, 49], which is highly expressed in placenta and brain [25, 26, 48, 49 ,50, 51]. Metformin may indeed pose fetal risk via AMPK activation, but to have this action it must first enter placentas and fetal brain cells during prenatal development. Insulin signaling promotes expression of OCTs [52, 53]. So in non-diabetic pregnancy, late in gestation as insulin levels rise and placental OCT3 expression peaks [26], maternal metformin treatments may more freely cross the placenta via upregulated OCT3. If this occurs at high enough concentrations, metformin may be able to impair social behavior through excessive activation of fetal AMPK [13, 19, 42], as has been reported to occur in the valproic acid pharmacological mouse model of autism [54]. Measures of ED 14-15 brain levels of metformin in dams (161 ± 20 pg/mg) and fetuses (158 ± 10 pg/mg) were essentially the same, suggesting similar metformin uptake levels in mother and fetus at this stage.
However, early in pregnancy the embryo and young fetus may be less sensitive to adverse effects from metformin use because OCT3 and other placental transport mechanisms for metformin (such as the serotonin transporter [44]) are either not yet or more sparsely expressed [26, 51]. Indeed in a clinical study metformin use in the first trimester only posed no apparent risk to offspring [55]. Hence catching gestational diabetes early and treating early with metformin, but possibly reducing dose or discontinuing treatment in favor of insulin at mid-gestation may better protect the fetal brain from excessive metformin induced AMPK activity. Furthermore if placental and fetal insulin receptors are under-expressed, as they appear to be in one human GDM study [56], insulin-dependent OCT3 expression is likely to be reduced in the placenta, thereby reducing the risk of later metformin use adversely over-activating AMPK. Ongoing longitudinal studies report a lack of overt ill effects from gestational metformin exposure, and have tracked the growth and development of children exposed to metformin from infancy through early adolescence [57, 58]. This is because metformin’s AMPK activation has anti-inflammatory effects that counter the actions of TNFα in a genetic mouse model of gestational diabetes (Tally-Ho), and this property may be neuroprotective for mothers and offspring in diabetic pregnancies [58, 59]. A clinical offshoot of the “Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU)” study compared neurophysiological responses of 45 12 yr old children from diabetic pregnancies exposed in utero to metformin (23) or insulin (22) vs. controls (12 children) and found metformin did not worsen salivary cortisol deficits, reduced cortical excitability or attenuated neuroplasticity found to be associated with maternal insulin resistance in the insulin treated group [59]. However this study was small and not designed to capture changes in relative risk for developmental disorders, so larger scale studies are warranted.
It is noteworthy that the constitutive OCT3 knock-out mouse is deficient in preference for social interaction relative to wild-types (Fig. 5). Prior behavior studies in OCT3 knock-out mice are conflicted, one found increased stress and anxiety related behaviors [60], while another found the opposite [61]. However deficits in mouse tests of social preference and sociability deficits in autism spectrum disorders do not necessarily stem from increased anxiety [63]. This study finding of a lack of social interaction preference in OCT3 knock-outs provides additional evidence that OCT3 probably plays critical roles in shaping social behavior either via changes in placental transport or during early brain development. Since OCT3 expression is high in placenta and brain [25,26,50,31], and OCTs can transport serotonin [63], and apparently also tryptophan [64] placental OCT3 expression becomes highly relevant to serotonergic hypotheses of autism etiology [38, 65]. Under conditions of GDM, if insulin signaling in the placenta is compromised by hyperglycemia and/or insulin insensitivity, OCT3 expression may be reduced compromising serotonin and/or tryptophan transport [52, 56, 63, 64].
In fetal brain serotonin (5-HT) and the tryptophan used to synthesize it play many essential roles. For example 5-HT receptors mediate neuron growth, differentiation, pruning and synapse formation, and 5-HT transporters tightly control how long and how much 5-HT is available for neurotransmission [65, 66, 67]. Interestingly, 5-HT receptor and transporter proteins function in fetal brains even before neurons producing 5-HT are formed, highlighting their importance in directing neurodevelopment [67, 68]. Studies in mice demonstrate that most of the 5-HT orchestrating early fetal development comes from maternal stores of tryptophan relative to platelet-bound maternal serotonin [69, 70]. Tryptophan is also converted to kynurenine by indoleamine 2,3-dioxygenase (IDO) which is richly expressed in the placenta to establish maternal immunosuppression early in pregnancy and to suppress vasoconstrictive activity of 5-HT late in pregnancy [71]. In mice carrying an allogenic fetus, suppression of IDO caused pre-eclampsia, showing the importance of this pathway for suppressing maternal immune responses during pregnancy [72].
Serotonin (5-HT) is an endogenous substrate of OCT3, and metformin can competitively inhibit in vitro 5-HT uptake at 5-10 mM concentrations in human cell lines overexpressing it [44, 63]. However, with daily 850 mg/kg doses depending on timing since last intake, human metformin content in maternal blood ranges from 0.86 - 13.73 μM and in umbilical vein from 2.81 – 12.4 μM [73]. With orders of magnitude differences between therapeutic and effective uptake blocking concentrations, it seems unlikely that metformin could impact fetal development by competitively inhibiting bulk 5-HT uptake via OCT3 alone. Tryptophan also appears to be a substrate of OCT1, OCT2 (and potentially OCT3), but again with an unlikely IC50 to inhibit organic cation uptake in cells expressing them at the range 6 mM [64].
In mice OCT3 and vesicular monoamine transporter (VMAT) are expressed together in specialized syncytiotrophoblast cells within the labyrinth of the placenta to transport 5-HT synthesized locally from maternal tryptophan or yolk sac stores to the fetus [74]. Also epigenetic imprinting governs expression of the insulin growth factor 2 cluster, which OCT1, 2, and 3 genes belong to, introducing variable expression or function of these proteins in mouse and human placentas [75, 76]. Given the complexities of placental microstructure and its insulin-dependent 5-HT transporter regulation [56] in gestational diabetes, localized deficient transporter expression may diminish transfer of serotonin or tryptophan from placenta to fetus sufficiently at critical stages to persistently disrupt brain development and social behavior.
Postnatally, there is evidence that metformin is transported into mothers milk at concentrations lower than maternal serum levels [77]. So far only organic cation/carnitine transporters (OCTNs), which are closely related and similar in structure to OCTs, were found to upregulate in human breast tissue, and OCTNs are responsible for transport of carnitine (which metabolizes fatty acids) into breast milk [78, 79, 80]. In mice OCT1 is upregulated in nursing mammary glands, and in OCT1 and OCT2 double knock-outs milk to plasma ratio of metformin was diminished, indicating they also transport metformin into milk [81]. Reductions in chamber exploration (Fig. 7a) and increased social novelty preference exhibited by female offspring from metformin treatment stopped at birth not evident in female offspring stopped at wean(Fig. 4) could have precipitated from abrupt cessation of metformin exposure at birth.
More fecal boli dropped by males exposed to metformin until wean after social interaction and novelty preference tests (Fig. 6) corresponded with avoidance of novel stranger mice. Interestingly, male rats similarly exposed to metformin engaged less in sexual behavior than controls [82]. Metformin was shown to have endocrine disrupting properties in fish [83], and such effects may have contributed to changes in exposed male mouse behaviors. Alternatively, adaptive changes to metformin exposure may have persisted in the gastrointestinal tract of metformin exposed mice, possibly through changes in microbiome [84].
In summary, prenatal metformin exposure reduced preference for social interaction in a small group of male offspring from lean C57BL/6J dams, some of which were siblings. By contrast female offspring social behavior was enhanced in female offspring exposed to metformin if treatment ceased at birth. Organic cation transporters (OCTs) are most likely responsible for entry of metformin into the placenta and fetus, and to a lesser extent into maternal milk. It remains unclear how prenatal metformin exposure impacts the social behavior of offspring, be it via changes in transport capacity for substrates of OCTs including 5-HT and tryptophan, or through intercellular mechanisms such as functional inhibition of mitochondrial respiratory complex I and AMPK activation. Use of mouse models to study the interactions of maternal metabolic conditions such as obesity and gestational diabetes with metformin exposure in offspring will aid in teasing apart how both conditions and metformin treatments impact offspring social behavior.
Acknowledgements
This study was financially supported by a grant from the John L. Santikos Charitable Foundation of the San Antonio Area Foundation, a Congressionally Directed Medical Research Program Autism Idea Award (AR110109) and by National Institutes of Health Grants R21HD081261 (GGG), and R01MH093320 (LCD). VRG was supported by a National Institute on Aging training grant (T32AG021890) to Dr. Nicolas Musi. CMS was supported by the South Texas Advanced Research Training Undergraduate Program (R25MH092919) to Drs. Alan Frazer & David Weiss. ARN and WQZ were supported by the Summer Physiology Undergraduate Research Program (SPUR) at the University of Texas Health Science Center at San Antonio. We thank Senior Research Associates Melissa Vitela for mouse genotyping and Greg Friesenhahn for LC/MS/MS measurements of metformin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None of the authors have any conflicts of interest to report.
References
- [1].Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I I, Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 129 (2012) e1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, Buchanan TA, Coleman KJ, Getahun D, Association of maternal diabetes with autism in offspring. JAMA. 313 (2015) 1425–34. [DOI] [PubMed] [Google Scholar]
- [3].Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, Caruso D, Pearson C, Kiang S, Dahm JL, Hong X, Wang G, Wang MC, Zuckerman B, Wang X, The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics. 137 (2016) e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang C, Geng H, Liu W, Zhang G, Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine. 96 (2017) e6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators, Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 358 (2008) 2003–15. [DOI] [PubMed] [Google Scholar]
- [6].Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM, Metformin in gestational diabetes: offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care. 34 (2011) 2279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balani J, Hyer SL, Rodin DA, Shehata H, Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: a case–control study. Diabetic Medicine. 26 (2009) 798–802. [DOI] [PubMed] [Google Scholar]
- [8].Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S, Metformin compared with insulin in the management of gestational diabetes mellitus: A randomized clinical trial. Diabetes Research and Clinical Practice. 98 (2012) 422–429. [DOI] [PubMed] [Google Scholar]
- [9].Ballas J, Moore TR, Ramos GA, Management of diabetes in pregnancy. Current diabetes reports. 12 (2012) 33–42. [DOI] [PubMed] [Google Scholar]
- [10].Zhao LP, Sheng XY, Zhou S, Yang T, Ma LY, Zhou Y, Cui YM, Metformin vs. insulin for gestational diabetes mellitus: A meta-analysis. Br J Clin Pharmacol. 80 (2015) 1224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gray SC, McGuire TM, Cohen N, Little PJ, The emerging role of metformin in gestational diabetes mellitus. Diabetes Obes Metab. 19 (2017) 765–772. [DOI] [PubMed] [Google Scholar]
- [12].Rena G, Pearson ER, Sakamoto K, Molecular mechanism of action of metformin: old or new insights? Diabetologia. 56 (2013) 1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brown FM, Wyckoff J, Rowan JA, Jovanovic L, Sacks DA, Briggs GG, Metformin in pregnancy its time has not yet come. Diabetes Care. 29 (2006) 485–6. [DOI] [PubMed] [Google Scholar]
- [14].Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong J, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Clinical pharmacokinetics of metformin. Clinical pharmacokinetics. 50 (2011) 81–98. [DOI] [PubMed] [Google Scholar]
- [15].Simmons D, Safety considerations with pharmacological treatment of gestational diabetes mellitus. Drug Saf. 38 (2015) 65–78. [DOI] [PubMed] [Google Scholar]
- [16].Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G, Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol. 206 (2012) 314.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Langer O, Oral hypoglycemic agents in pregnancy: their time has come, The Journal of Maternal-Fetal & Neonatal Medicine. 12 (2002) 376–83. [DOI] [PubMed] [Google Scholar]
- [18].Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine. 352 (2005) 2477–86. [DOI] [PubMed] [Google Scholar]
- [19].Bedaiwy MA, Miller KF, Goldberg JM, Nelson D, Falcone T, Effect of metformin on mouse embryo development. Fertil. Steril. 76 (2001)1078–1079. [DOI] [PubMed] [Google Scholar]
- [20].Denno KM, Sadler TW, Effects of the biguanide class of oral hypoglycemic agents on mouse embryogenesis. Teratology. 49 (1994) 260–266. [DOI] [PubMed] [Google Scholar]
- [21].Lee HY, Wei D, Loeken MR, Lack of metformin effect on mouse embryo AMPK activity: implications for metformin treatment during pregnancy. Diabetes Metab Res Rev. 30 (2014) 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P, Cellular Stress, Excessive Apoptosis, and the Effect of Metformin in a Mouse Model of Type 2 Diabetic Embryopathy. Diabetes. 64 (2015) 2526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Salomäki H, Heinäniemi M, Vähätalo LH, Ailanen L, Eerola K, Ruohonen ST, Pesonen U, Koulu M, Prenatal metformin exposure in a maternal high fat diet mouse model alters the transcriptome and modifies the metabolic responses of the offspring. PLoS One. 9 (2014) e115778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gantois I, Khoutorsky A, Popic J, Aguilar-Valles A, Freemantle E, Cao R, Sharma V T, Pooters A Nagpal A Skalecka VT Truong S Wiebe IA Groves SM Jafarnejad C Chapat EA McCullagh K Gamache K Nader JC Lacaille CG Gkogkas N Sonenberg, Metformin ameliorates core deficits in a mouse model of fragile X syndrome. Nat Med. 23 (2017) 674–677. [DOI] [PubMed] [Google Scholar]
- [25].Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V, Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. Journal of Biological Chemistry. 273 (1998) 15971–9. [DOI] [PubMed] [Google Scholar]
- [26].Lee N, Hebert MF, Prasad B, Easterling TR, Kelly EJ, Unadkat JD, Wang J, Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab Dispos. 41 (2013) 2225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP, Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol. 21(2001):4188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K, The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 106(2009):8043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mayer FP, Schmid D, Owens WA, Gould GG, Apuschkin M, Kudlacek O, Salzer I, Boehm S, Chiba P, Williams PH, Wu HH, Gether U, Koek W, Daws LC, Sitte HH. An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology. 2018. April 6. doi: 10.1038/s41386-018-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].National Research Council, Guide for the care and use of laboratory animals. National Academies Press, 2010. [Google Scholar]
- [31].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology. 8 (2010) e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kajbaf F, De Broe ME, Lalau JD, Therapeutic concentrations of metformin: A systematic review. Clin Pharmacokinet. 55 (2016) 439–59. [DOI] [PubMed] [Google Scholar]
- [33].Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS, Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 16 (2010) 804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang M, Silverman JL, Crawley JN, Automated three-chambered social approach task for mice. Current protocols in neuroscience. (2011) 8–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Geary WA, Toga AW, Wooten GF, Quantitative film autoradiography for tritium: methodological considerations. Brain Res. 337 (1985) 99–108. [DOI] [PubMed] [Google Scholar]
- [36].Pennington KA, van der Walt N, Pollock KE, Talton OO, Schulz LC, Effects of acute exposure to a high-fat, high-sucrose diet on gestational glucose tolerance and subsequent maternal health in mice. Biol Reprod. 96 (2017) 435–445. [DOI] [PubMed] [Google Scholar]
- [37].Lee N, Hebert MF, Wagner DJ, Easterling TR, Liang CJ, Rice K, Wang J, Organic cation transporter 3 facilitates fetal exposure to metformin during pregnancy. Mol Pharmacol. 94(2018):1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Muller CL, Anacker AMJ, Veenstra-VanderWeele J, The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 3 (2016) 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Matsuzaki T, Tungalagsuvd A, Iwasa T, Munkhzaya M, Yano K, Mayila Y, Tokui T, Yanagihara R, Matsui S, Kato T, Kuwahara A, Irahara M, Clinical outcome of various metformin treatments for women with polycystic ovary syndrome. Reprod Med Biol. 16 (2017) 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hjorth-Hansen A, Salvesen Ø, Engen Hanem LG, Eggebø T, Salvesen KÅ, Vanky E, Ødegård R, Fetal growth and birth anthropometrics in metformin-exposed offspring born to mothers With PCOS. J Clin Endocrinol Metab. 103 (2018) 740–747. [DOI] [PubMed] [Google Scholar]
- [41].Engen Hanem LG, Stridsklev S, Júlíusson PB, Salvesen Ø, Roelants M, Carlsen SM Ødegård R, Vanky E, Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age; follow-up of two RCTs. J Clin Endocrinol Metab. 103 (2018) 1612–21. [DOI] [PubMed] [Google Scholar]
- [42].Lee BK, Arver S, Widman L, Gardner RM, Magnusson C, Dalman C, Kosidou K, Maternal hirsutism and autism spectrum disorders in offspring. Autism Res. 10 (2017) 1544–1546. [DOI] [PubMed] [Google Scholar]
- [43].Turban S, Stretton C, Drouin O, Green CJ, Watson ML, Gray A, Ross F, Lantier L, Viollet B, Hardie DG, Marette A, Hundal HS, Defining the contribution of AMP-activated protein kinase (AMPK) and protein kinase C (PKC) in regulation of glucose uptake by metformin in skeletal muscle cells. J Biol Chem. 287(2012):20088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen L, Shu Y, Liang X, Chen EC, Yee SW, Zur AA, Li S, Xu L, Keshari KR, Lin MJ, Chien HC, OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proceedings of the National Academy of Sciences. 111 (2014) 9983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yee SW, Lin L, Merski M, Keiser MJ, Gupta A, Zhang Y, Chien HC, Shoichet BK, Giacomini KM, Prediction and validation of enzyme and transporter off-targets for metformin. J Pharmacokinet Pharmacodyn. 42 (2015) 463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zamek-Gliszczynski MJ, Giacomini KM, Zhang L, Emerging clinical importance of hepatic organic cation transporter 1 (OCT1) in drug pharmacokinetics, dynamics, pharmacogenetic variability, and drug interactions. Clin Pharmacol Ther. 103 (2018) 758–760.. [DOI] [PubMed] [Google Scholar]
- [47].Chen L, Pawlikowski B, Schlessinger A, More SS, Stryke D, Johns SJ, Portman MA, Chen E, Ferrin TE, Sali A, Giacomini KM, Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenetics and genomics. 20(2010):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee N, Duan H, Hebert MF, Liang CJ, Rice KM, Wang J, Taste of a pill: Organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands. Journal of Biological Chemistry. 289 (2014) 27055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen EC, Liang X, Yee SW, Geier EG, Stocker SL, Chen L, Giacomini KM, Targeted disruption of organic cation transporter 3 attenuates the pharmacologic response to metformin. Molecular pharmacology. 88 (2015) 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Koepsell H, Lips K, Volk C, Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharmaceutical research. 24 (2007) 1227–51. [DOI] [PubMed] [Google Scholar]
- [51].Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslén B, Marsál K, Hansson SR, Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 25 (2004) 518–29. [DOI] [PubMed] [Google Scholar]
- [52].Grover B, Buckley D, Buckley AR, Cacini W, Reduced expression of organic cation transporters rOCT1 and rOCT2 in experimental diabetes. J. Pharmacol. Exp. Ther. 308 (2004) 949–956. [DOI] [PubMed] [Google Scholar]
- [53].Jang EH, Kim HK, Park CS, Kang JH, Increased expression of hepatic organic cation transporter 1 and hepatic distribution of metformin in high-fat diet-induced obese mice. Drug Metab Pharmacokinet. 25 (2010) 392–7.20. [DOI] [PubMed] [Google Scholar]
- [54].Chen J, Wu W, Fu Y, Yu S, Cui D, Zhao M, Du Y, Li J, Li X, Increased expression of fatty acid synthase and acetyl-CoA carboxylase in the prefrontal cortex and cerebellum in the valproic acid model of autism. Experimental and therapeutic medicine. 12(2016) 1293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Given JE, Loane M, Garne E, Addor MC, Bakker M, Bertaut-Nativel B, Gatt M, Klungsoyr K, Lelong N, Morgan M, Neville AJ, Pierini A, Rissmann A, Dolk H, Metformin exposure in first trimester of pregnancy and risk of all or specific congenital anomalies: exploratory case-control study. BMJ. 361 (2018) k2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang B, Jin Z, Sun L, Zheng Y, Jiang J, Feng C, Wang Y, Expression and correlation of sex hormone-binding globulin and insulin signal transduction and glucose transporter proteins in gestational diabetes mellitus placental tissue. Diabetes Res Clin Pract. 119 (2016) 106–17. [DOI] [PubMed] [Google Scholar]
- [57].Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, Hague WM, Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res Care. 6 (2018) e000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Van Dam JM, Garrett AJ, Schneider LA, Hodyl NA, Goldsworthy MR, Coat S, Rowan JA, Hague WM, Pitcher JB, Reduced Cortical Excitability, Neuroplasticity, and Salivary Cortisol in 11–13-Year-Old Children Born to Women with Gestational Diabetes Mellitus. EBioMedicine. 31 (2018)143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Louden ED, Luzzo KM, Jimenez PT, Chi T, Chi M, Moley KH. TallyHO obese female mice experience poor reproductive outcomes and abnormal blastocyst metabolism that is reversed by metformin. Reprod Fertil Dev. 27 (2014) 31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S, Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem. 106 (2008) 1471–82. [DOI] [PubMed] [Google Scholar]
- [61].Wultsch T, Grimberg G, Schmitt A, Painsipp E, Wetzstein H, Breitenkamp AF, Gründemann D, Schömig E, Lesch KP, Gerlach M, Reif A, Decreased anxiety in micelacking the organic cation transporter 3. J Neural Transm (Vienna). 116(2009):689–97. [DOI] [PubMed] [Google Scholar]
- [62].Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN, Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 171 (2010) 1197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Duan H, Wang J, Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. Journal of Pharmacology and Experimental Therapeutics. 335(2010):743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Song IS, Lee DY, Shin MH, Kim H, Ahn YG, Park I, Kim KH, Kind T, Shin JG, Fiehn O, Liu KH, Pharmacogenetics meets metabolomics: discovery of tryptophan as a new endogenous OCT2 substrate related to metformin disposition. PLoS One. 7(2012):e36637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Daws LC, Gould GG, Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther. 131(2011):61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bonnin A, Levitt P, Placental source for 5-HT that tunes fetal brain development. Neuropsychopharmacology. 37(2012):299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gaspar P, Cases O, Maroteaux L, The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 4(2003):1002–12. [DOI] [PubMed] [Google Scholar]
- [68].Côté F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G, (2007). Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 104(2007):329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P, A transient placental source of serotonin for the fetal forebrain. Nature. 472(2011):347- [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Velasquez JC, Goeden N, Bonnin A, Placental serotonin: implications for the developmental effects of SSRIs and maternal depression. Front Cell Neurosci. 7(2013):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ligam P, Manuelpillai U, Wallace EM, Walker D, Localisation of indoleamine 2, 3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: implications for role of the kynurenine pathway in pregnancy. Placenta. 26(2005):498–504. [DOI] [PubMed] [Google Scholar]
- [72].Nishizawa H, Hasegawa K, Suzuki M, Achiwa Y, Kato T, Saito K, Kurahashi H, Udagawa Y, Mouse model for allogeneic immune reaction against fetus recapitulates human pre-eclampsia. J Obstet Gynaecol Res. 34(2008):1–6. [DOI] [PubMed] [Google Scholar]
- [73].Vanky E, Zahlsen K, Spigset O, Carlsen SM, Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 83(2005):1575–8. [DOI] [PubMed] [Google Scholar]
- [74].Wu HH, Choi S, Levitt P, Differential patterning of genes involved in serotonin metabolism and transport in extra-embryonic tissues of the mouse. Placenta, 42:(2016) 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP, Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 inOrct3/Slc22a3-deficient mice. Molecular and Cellular Biology. 21(2001). 4188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Saito J, Hirota T, Kikunaga N, Otsubo K, Ieiri I, Interindividual differences in placental expression of the SLC22A2 (OCT2) gene: relationship to epigenetic variations in the 5'-upstream regulatory region. J Pharm Sci. 100(2011):3875–83. [DOI] [PubMed] [Google Scholar]
- [77].Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GDV, Hebert MF,. Pharmacokinetics of Metformin during Pregnancy. Drug Metabolism and Disposition, 38(2010): 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lamhonwah AM, Mai L, Chung C, Lamhonwah D, Ackerley C, Tein I I, Upregulation of mammary gland OCTNs maintains carnitine homeostasis in suckling infants. Biochem Biophys Res Commun. 404(2011):1010–5. [DOI] [PubMed] [Google Scholar]
- [79].Futatsugi A, Masuo Y, Kawabata S, Nakamichi N, Kato Y, L503F variant of carnitine/organic cation transporter 1 efficiently transports metformin and other biguanides. J Pharm Pharmacol. 68(2016):1160–9. [DOI] [PubMed] [Google Scholar]
- [80].Nigam SK, What do drug transporters really do? Nat Rev Drug Discov. 14(2015):29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ito N, Ito K, Ikebuchi Y, Kito T, Miyata H, Toyoda Y, Takada T, Hisaka A, Honma M, Oka A, Kusuhara H, Suzuki H, Organic cation transporter/solute carrier family 22a is involved in drug transfer into milk in mice. J Pharm Sci. 103(2014):3342–8. [DOI] [PubMed] [Google Scholar]
- [82].Forcato S, Novi DRBDS, Costa NO, Borges LI, Góes MLM, Ceravolo GS, Gerardin DCC, In utero and lactational exposure to metformin induces reproductive alterations in male rat offspring. Reprod Toxicol. 2017. December;74:48–58. [DOI] [PubMed] [Google Scholar]
- [83].Niemuth NJ, Klaper RD, Low-dose metformin exposure causes changes in expression of endocrine disruption-associated genes. Aquat Toxicol. 195 (2018):33–40. [DOI] [PubMed] [Google Scholar]
- [84].Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD MD, Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 21(2001):6348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]