Abstract
Background: The fibroblast growth factor receptor (FGFR) family includes transmembrane receptors involved in a wide range of developmental and postdevelopmental biologic processes as well as a wide range of human diseases. In particular, FGFR3 has been implicated in the mechanism by which 9-cis retinoic acid (9-cisRA) induces lymphangiogenesis and improves lymphedema. The purpose of this study was to validate the efficacy of a novel small peptide FGFR3 inhibitor, peptide P3 (VSPPLTLGQLLS), and to elucidate the role of FGFR3 in 9-cisRA-induced lymphangiogenesis using this peptide.
Methods and Results: Peptide P3 effectively inhibited FGFR3 phosphorylation. In vitro, peptide P3-mediated FGFR3 inhibition did not decrease lymphatic endothelial cell (LEC) proliferation, migration, or tubule formation. However, peptide P3-mediated FGFR3 inhibition did block 9-cisRA-stimulated LEC proliferation, migration, and tubule formation. In vivo, peptide P3-mediated FGFR3 inhibition was sufficient to inhibit 9-cisRA-induced tracheal lymphangiogenesis.
Conclusion: FGFR3 does not appear to be essential to nonpromoted LEC proliferation, migration, and tubule formation. However, FGFR3 may play a key role in LEC proliferation, migration, tubule formation, and postnatal in vivo lymphangiogenesis when pharmacologically induced by 9-cisRA. P3 may have the potential to be used as a precise regulatory control element for 9-cisRA-mediated lymphangiogenesis.
Keywords: lymphangiogenesis, FGFR3, 9-cis retinoic acid, peptide inhibitor, VSPPLTLGQLLS, lymphedema, lymphatic endothelial cell
Introduction
Fibroblast growth factor receptors
The fibroblast growth factor receptor (FGFR) family involves a group of highly conserved transmembrane receptors. They vary with respect to tissue type predominance and ligand affinity. In humans, there are four identified FGFR genes, FGFR1–FGFR4.1 Due to alternative splicing events, there are seven principal FGFRs, which all act through a tyrosine kinase domain that initiates an intracellular signaling cascade.1
FGFRs are involved in a range of biologic processes during embryonic development and in adult life, including brain, limb, and vascular development, bile acid and cholesterol regulation, serum glucose regulation, vitamin D regulation, and phosphate homeostasis.2,3 Furthermore, dysfunctional FGFR signaling is implicated in a diverse array of pathologies, such as developmental disorders, skeletal deformities, and various malignant diseases.4–8
Fibroblast growth factor receptor 3
FGFR3 was discovered by Keegan et al. in 1991 when screening a cDNA library for tyrosine kinase receptors.9 Mutations in FGFR3 are responsible for a range of disorders (Table 1). In general, FGFR3 aberrations result in syndromic skeletal system disorders and various malignancies. In addition to dysfunction with FGFR3 itself, abnormalities in FGFR3 ligands can result in disease. For example, overexpression of FGF2, a high-affinity ligand for FGFR3, is present in imatinib-resistant gastrointestinal stromal tumors and esophageal squamous cell carcinoma.7,10 More recently, beyond skeletal development and cancer, FGFR3 has been shown to be important in the development and maintenance of the human vasculature.3,11
Table 1.
Fibroblast Growth Factor Receptor 3 Mutations in Human Disease
| Germline gain of function | Somatic gain of function |
|---|---|
| Achondroplasia | Ovarian and urothelial cancer |
| Crouzon syndrome with acanthosis nigricans | Glioblastoma and lung cancer |
| Hypochondroplasia | Lymphoma |
| Muenke syndrome | Multiple myeloma |
| PLSD-SD | Urothelial cancer |
| SADDAN | Gallbladder cancer |
| Thanatophoric dysplasia | Lung cancer |
| Mosaic gain of function | Loss of function |
|---|---|
| KENS | LADD syndrome47 |
As reviewed by Katoh.37
KENS, keratinocyte epidermal naevus syndrome; LADD, lacrimo-auriculo-dento-digital syndrome; PLSD-SD, platyspondylic lethal skeletal dysplasias, San Diego Types.
FGFR3 and the lymphatic system
FGFRs, in general, play a key role in the blood and lymphatic vascular systems, during both development and postnatal vascular maintenance and genesis.3,12 In fact, inhibiting FGF signaling results in loss of blood vessel integrity and ultimately vessel disintegration.12 Regarding the lymphatic system, FGFR1 and FGFR3 appear to be highly important, exemplified by the fact that Fgfr1/Fgfr3 double mutant mice have impaired lymphatic development.3 In addition, FGFR3, in particular, has been shown to be important in both growth-factor-induced and flow-activated lymphatic endothelial cell (LEC) proliferation.11,13 Beyond normal physiology, FGFR3 has been shown to play a role in the pharmacologic induction of lymphangiogenesis by 9-cis retinoic acid (9-cisRA).14
Purpose and findings
To investigate the role played by FGFR3 in modulating pharmacologically stimulated postnatal lymphatic growth, we identified a FGFR3-specific peptide inhibitor from the literature (named P3), validated its efficacy, then studied the effect of P3-mediated FGFR3 inhibition on LEC proliferation, migration, and tubule formation, 9-cisRA-promoted LEC proliferation, migration, and tubule formation, as well as tracheal lymphangiogenesis in vivo. We found that P3 effectively inhibited FGFR3 phosphorylation. In vitro, P3-mediated FGFR3 inhibition impaired 9-cisRA-stimulated LEC proliferation, migration, and tubule formation. In vivo, P3-mediated FGFR3 inhibition was sufficient to inhibit 9-cisRA-induced lymphangiogenesis. FGFR3 does not appear to be essential to nonpromoted LEC proliferation, migration, and tubule formation. However, FGFR3 does seem to play a significant role in LEC proliferation, migration, tubule formation, and postnatal in vivo lymphangiogenesis, when pharmacologically induced by 9-cisRA.
Materials and Methods
Reagents and cell culture
The sources of the antibodies used are as follows: anti-pFGFR3 (SC-33041, anti-phospho-Tyr724; Santa Cruz Biotechnology), anti-FGFR3 (SC-123; Santa Cruz Biotechnology), and anti-β-actin (AC-15; Sigma Aldrich, Inc.). Sources or other reagents used are as follows: 9-cisRA (R4643; Sigma-Aldrich, Inc.) and FGF2 (Sigma). Peptide P3 (VSPPLTLGQLLS) and scrambled peptide (LQVLSTLPSLGP) were synthesized in vitro with guaranteed TFA removal and at a purity >98% (GenScript USA, Inc.). Isolation and characterization of human LECs from human neonatal foreskins were performed as previously described.11,14–16 LECs were cultured in complete media as already reported.11,15 LECs less than eight passages were used for all experiments.
Gene and protein expression
A standard protocol was used for quantitative real-time RT-PCR (qRT-PCR) and western blotting assays as previously described.14
LEC proliferation
LEC proliferation was assayed using a Premixed WST-1 Cell Proliferation Assay kit (TaKaRa, Japan) following the manufacturer's protocol. In brief, human primary LECs were seeded at a final concentration of 2 × 104 cells per well in 24-well plates and cultured in complete media. Six hours after seeding, cells were pretreated with either P3 (10 μM) or scrambled peptide (10 μM); 15 minutes after pretreatment either ethanol or 9-cisRA (1 μM) was added. Three replicate wells were used for each treatment group. Cells were then incubated for 48 hours, after which the WST-1 reagent was added to each well and incubated for 4 hours. Absorbance at 450 nm was measured through a microplate reader (Hixex, Chameleon V, Finland).
LEC migration
LEC migration was performed as previously described with minor modification.14 In brief, human LECs were seeded (6 × 105 cells) in 60-mm dishes and pretreated with ethanol, 9-cisRA (1 μM), 9-cisRA (1 μM) and scrambled peptide (5 μM), or 9-cisRA (1 μM) and peptide P3 (5 μM) in complete media (10% fetal bovine serum [FBS]) for 24 hours. Two replicate wells were used for each treatment group. Cells were pretreated with either P3 or scrambled peptide 15 minutes before addition of ethanol or 9-cisRA. Once a confluent monolayer was established, the cells were scratched with a 1000 μL pipette tip and incubated for 24 hours. Cell migration area was captured at the same nine separate locations at 0, 24, and 48 hours for each plate and quantified in Fiji ImageJ (NIH)17 to determine the remaining migration area. The percentage of the original gap area was calculated using the gap area at 0 hours as baseline.
LEC tubule formation
LEC tubule formation was performed as previously described.14 In brief, human LECs were pretreated with ethanol or 9-cisRA (1 μM) in low-serum medium (1% FBS) for 24 hours. A 12-well plate was prepared for cell seeding by coating each well with 200 μL of growth-factor-depleted Matrigel (Corning). Two replicate wells were used for each group, and the experiment was completed twice. The Matrigel was allowed to solidify for 30 minutes at 37°C. The pretreated LECs were then seeded (7 × 104 cells) onto the Matrigel in low-serum (1% FBS) medium containing either ethanol, 9-cisRA (1 μM), 9-cisRA (1 μM) and scrambled peptide (5 μM), or 9-cisRA (1 μM) and P3 (5 μM). Tubule formation was observed as early as 12 hours after seeding. Images from each well were randomly acquired at 24 hours. Total tube length, percentage covered area, branching points, loops, and nets were measured using Wimasis Image Analysis (Onimagin Technologies SCA).
Animals
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, eighth ed., 2011) and the Animal Welfare Act. Our protocol was approved by the University of Southern California Institutional Animal Care and Use Committee. All surgery was performed while mice were anesthetized using 2.5% isofluorane, and all efforts were made to minimize pain and suffering. Animals were maintained in a temperature- and light-controlled environment with ad libitum access to a normal chow diet and water.
Mouse trachea assay
Lymphatic reporter mice, Prox1-GFP, in which lymphatic tissue expresses green fluorescent protein (GFP) were used. These mice have been previously described.18 Likewise, the mouse trachea assay was performed as previously reported.14 Either 9-cisRA (1 mM), 9-cisRA (1 mM) and scrambled peptide (1 mM), or 9-cisRA (1 mM) and P3 (1 mM) were administered intranasally to Prox1-GFP mice daily for 7 days. Five mice were used in each treatment group. Under anesthesia, 15 μL of reagent was administered to each nostril. On day 7, mice were euthanized, tracheas were harvested, fixed in paraformaldehyde (4 hours at 4°C), whole mounted, then imaged. The lymphatic reporter animals enabled the convent visualization of tracheal lymphatics in whole-mounted specimens. Low-magnification images encompassing the majority of each trachea were taken. The trachea is composed of C-shaped cartilage rings with soft tissue on the posterior aspect, connecting the points of the “C.” Normally, this soft tissue is devoid of lymphatic structures. Lymphatic outgrowth into this soft tissue was quantified in ImageJ, and recorded as total length of lymphatic vessels growing past the cartilage rings and number of sprouts.
Statistical analyses
In all graphs, mean ± standard deviation values are plotted unless otherwise noted. One-way ANOVA was used to determine differences between groups in GraphPad PRISM6 (GraphPad Software, Inc., La Jolla, CA). p-Values <0.05 were considered statistically significant. The graphs presented were generated in GraphPad PRISM6.
Results
FGFR3 is transiently upregulated in LECs incubated with 9-cisRA
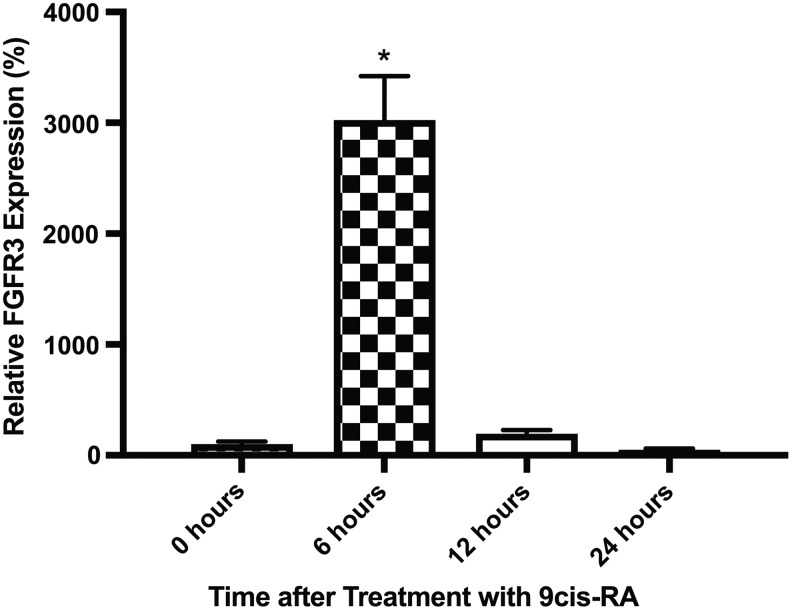
Previously, it has been shown that FGFR3 is transiently upregulated in LECs after exposure to 9-cisRA.14 To confirm and characterize this finding further, we performed quantitative RT-PCR using a different set of primers. FGFR3 expression in LECs was found to increase (28-fold) at 6 hours after incubation in 9-cisRA, and then decrease to near preincubation levels at 12 and 24 hours (Fig. 1). To better understand the significance of this spike in FGFR3 expression in the context of 9-cisRA, we searched for FGFR3 specific inhibitors. Our review of commercially available compounds that could modulate FGFR3 expression or downstream activity did not produce a suitable candidate, since all had off-target effects, including effects on the other FGFRs.
FIG. 1.
FGFR3 RNA expression is transiently upregulated in LECs during incubation with 9-cisRA. Human LECs were grown in vitro, incubated with 9-cisRA for 0, 6, 12, and 24 hours, at which point the cells were isolated, RNA was extracted, and quantitative RT-PCR was performed. FGFR3 RNA expression was found to significantly increase at 6 hours, then sharply decrease to near pretreatment levels at 12 and 24 hours posttreatment. *Indicates a p-value < 0.05. 9-cisRA, 9-cis retinoic acid; FGFR3, fibroblast growth factor receptor 3; LECs, lymphatic endothelial cells; RT-PCR, reverse transcription-polymerase chain reaction.
Peptide P3 inhibits FGFR3 phosphorylation
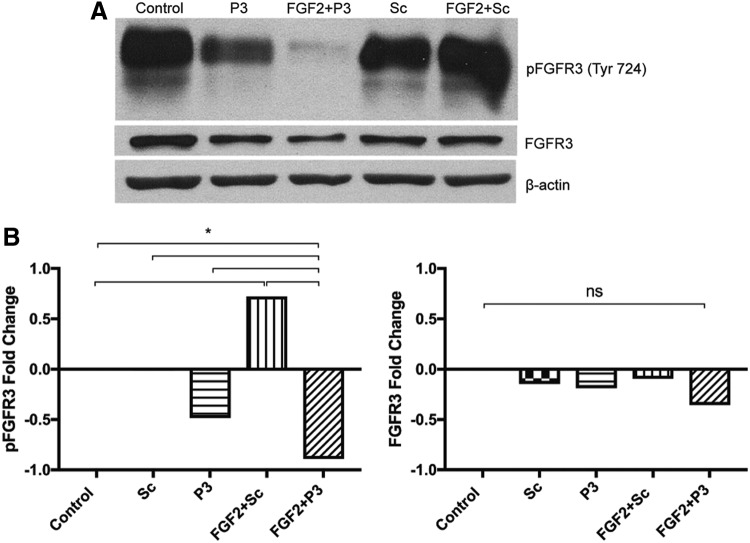
To identify alternative reagents that specifically inhibit FGFR3, we searched the published literature. In 2012, Jin et al. described a 12 amino acid peptide called P3 (VSPPLTLGQLLS) as a candidate FGFR3-specific inhibitor, and demonstrated that it was effective in ATDC5 chondrogenic cells, 293T cells, explanted metatarsal bone cultures, and an in vivo mouse model of thanatophoric dysplasia II.4 Since the effect of P3 on LECs was not described, we wanted to determine if P3 could inhibit FGFR3 in our system. Indeed, P3 was shown to effectively inhibit FGFR3 phosphorylation. Of note, total FGFR3 protein expression was not affected by treatment with peptide P3 (Fig. 2).
FIG. 2.
Peptide P3 inhibits FGFR3 phosphorylation at Tyr 724. Human LECs were grown in culture, pretreated with either the ddH2O peptide vehicle (control), peptide P3 (P3), or scrambled peptide (Sc), followed by treatment with FGF2 or FGF2 vehicle. Protein was extracted, separated through electrophoresis, and blotted. (A) Blotting for phospho-FGFR3 (pFGFR3), FGFR3, and β-actin. (B) Relative protein expression was quantified. Cells coincubated with FGF2+Sc demonstrated greater levels of pFGFR3 expression compared with control treated cells. P3 and FGF2+P3 treated cells were associated with the lowest amounts of FGFR3 expression. Whole FGFR3 expression was negligibly affected. Therefore, P3 appears to effectively inhibit FGFR3 activation even in the presence of FGF2. *Indicates a p-value < 0.05.
FGFR3 inhibition by P3 leads to decreased 9-cisRA-stimulated LEC proliferation, migration, and tubule formation
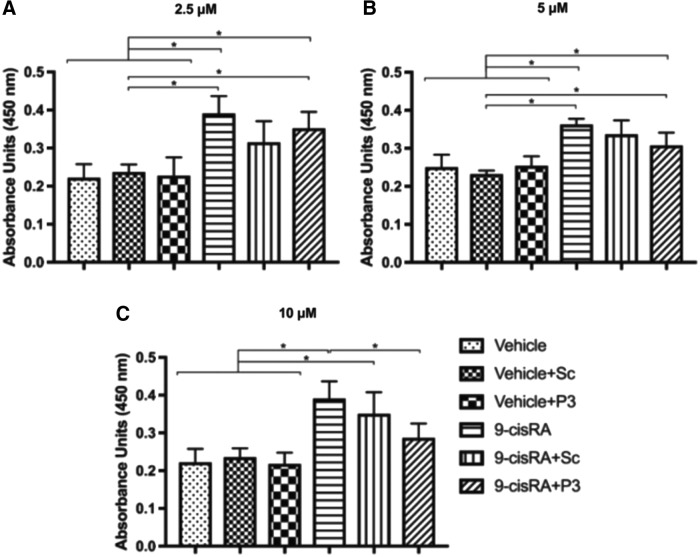
Since we demonstrated that P3 was an effective specific inhibitor of FGFR3 activation, we wanted to determine the effect of P3-mediated FGFR3 inhibition on human LEC proliferation, migration, and tubule formation. Cell proliferation was determined using a colorimetric method in which mitochondrial enzymes in metabolically active cells convert tetrazolium salt (WST-1) to formazan dye. Pretreatment of LECs with scrambled peptide or P3 did not significantly change LEC proliferation compared with the vehicle control. Therefore, at baseline, LEC proliferation is not affected by P3-mediated FGFR3 inhibition. Next, we wanted to determine if P3 had any effect on 9-cisRA-stimulated LEC proliferation. When we pretreated LECs with a control (scrambled) peptide or P3, we found that the P3 significantly inhibited 9-cisRA-induced LEC proliferation, whereas the control did not (Fig. 3).
FIG. 3.
P3-mediated FGFR3 inhibition slows 9-cisRA-induced LEC proliferation. LEC proliferation was assayed using a Premixed WST-1 Cell Proliferation Assay kit (TaKaRa, Japan) following the manufacturer's protocol. Human LECs were grown in culture, seeded into 24-well plates, pretreated with either scrambled peptide (Sc) or peptide P3 (P3), then incubated with either vehicle control (ethanol) or 9-cisRA for 48 hours. Peptide P3 was used at three different concentrations: 2.5 (A), 5, (B), and 10 μM (C). Three replicate wells for each treatment group were used. At 48 hours, WST-1 reagent was added, and cell proliferation was quantified. 9-cisRA incubation significantly increased LEC proliferation compared with the vehicle control, vehicle+Sc, and vehicle+P3. There was no significant difference in proliferation between EtOH, EtOH+Sc, and EtOH+P3 treated cells. Coincubation of LECs with 9-cisRA+P3 was associated with significantly decreased cell proliferation compared with 9-cisRA, but not 9-cisRA+Sc. Therefore, peptide P3-mediated FGFR3 inhibition is not sufficient to slow nonpromoted LEC proliferation. However, peptide P3-mediated FGFR3 inhibition does slow 9-cisRA-promoted LEC proliferation. *Indicates a p-value < 0.05.
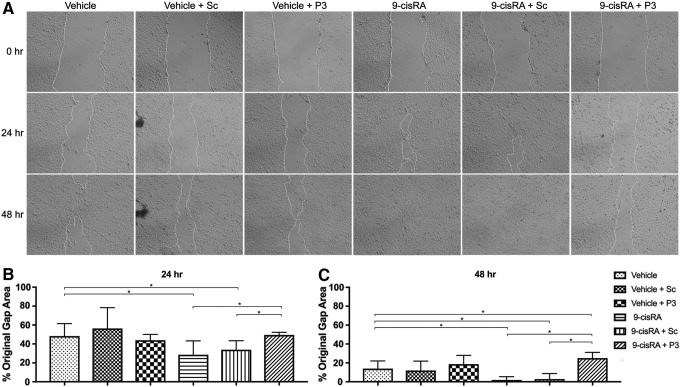
Next, we wanted to determine the effect of P3 on 9-cisRA-stimulated LEC migration using a standard scratch assay. The gap area at 0, 24, and 48 hours was measured, and the percentage of original gap area present at 24 and 48 hours after gap creation was calculated. The percentage of original gap area remaining at 24 and 48 hours was not significantly different between vehicle, vehicle+Sc, and vehicle+P3 treated cells. LECs incubated with 9-cisRA showed a significantly reduced percentage of the original gap area at 24 and 48 hours, indicating that the 9-cisRA promoted more rapid LEC migration. Migration of LECs incubated with 9-cisRA+Sc was equivalent to LECs incubated with 9-cisRA alone. Finally, LECs incubated with 9-cisRA+P3 had a significantly greater percentage of the original gap area open compared with 9-cisRA and 9-cisRA-SC treated LECs at 24 and 48 hours after gap creation. Therefore, P3-mediated FGFR3 inhibition significantly slowed 9-cisRA-directed LEC migration (Fig. 4).
FIG. 4.
P3-mediated FGFR3 inhibition does not slow nonpromoted LEC migration but does slow 9-cisRA-induced LEC migration. LEC migration was performed as previously described.14 Human LECs were grown in culture, seeded, pretreated with either scrambled peptide (Sc) or peptide P3 (P3), then incubated in either vehicle control or 9-cisRA until a confluent monolayer was established. A gap in the confluent monolayer was then created with a pipette tip, and cell migration was photographed at 0, 24, and 48 hours. Two replicate wells were used for each treatment group. (A) Representative images of the cell migration gap at 0, 24, and 48 hours. Quantified remaining migration gap area at 24 hours (B) and 48 hours (C). Total gap area was not significantly different between EtOH, EtOH+Sc, and EtOH+P3 treated cells at 24 and 48 hours after gap creation. LECs incubated with 9-cisRA and 9-cisRA+SC demonstrated significantly increased LEC migration at 24 and 48 hours. Coincubation of 9-cisRA+P3 was associated with significantly decreased LEC migration compared with 9-cisRA and 9-cisRA+Sc at 24 hours and compared with 9-cisRA, 9-cisRA+Sc, and vehicle control at 48 hours. *Indicates a p-value < 0.05.
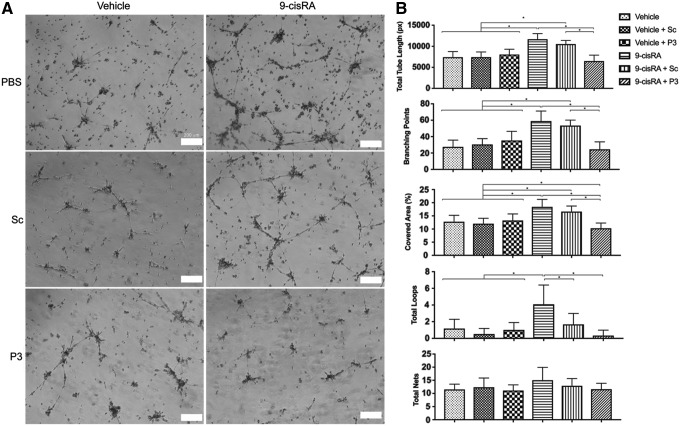
Tubule formation capacity was determined with a standard two-dimensional Matrigel assay. Total tube length, percentage covered area, number of branching points, total loops, and total nets were quantified. These metrics were compared between LECs treated with either vehicle, vehicle+Sc, vehicle+P3, 9-cisRA, 9-cisRA+Sc, or 9-cisRA+P3. Consistent with our prior studies, 9-cisRA treated cells had significantly increased total tube length, percentage covered area, branching points, and total loops compared with the vehicle control (ethanol).14 When LECs were pretreated with P3 before 9-cisRA, this condition led to significantly decreased total tube length, percentage covered area, and number of branching points compared with 9-cisRA and 9-cisRA+Sc groups. This strongly suggests that P3-mediated FGFR3 inhibition is effective in limiting 9-cisRA-mediated tube formation (Fig. 5).
FIG. 5.
P3-mediated FGFR3 inhibition decreases 9-cisRA-induced LEC tubule formation. LEC tubule formation was performed as previously described.14 Human LECs were grown in culture, pretreated with either scrambled peptide (Sc) or peptide P3 (P3), incubated in either vehicle control (ethanol) or 9-cisRA for 24 hours, then seeded in Matrigel-coated wells. LEC tubule formation was then photographed at 24 hours. Two replicate wells were used for each treatment, and the experiment was completed twice. (A) Representative images of LEC tubules showing increased tubule formation with 9-cisRA incubation alone and inhibited tubule formation in 9-cisRA+P3 incubated LECs. (B) Total tube length, percentage covered area, number of branching points, total loops, and total nets were not significantly different between EtOH, EtOH+Sc, and EtOH+P3 treated cells. 9-cisRA and 9-cisRA+Sc treated cells had significantly increased total tube length, percentage covered area, and branching points compared with the vehicle control and 9-cisRA+P3 treated cells. This indicates that peptide P3-mediated FGFR3 inhibition effectively inhibited 9-cisRA-promoted tube formation. *Indicates a p-value < 0.05.
P3-mediated FGFR3 inhibition blocks 9-cisRA-induced lymphangiogenesis in vivo
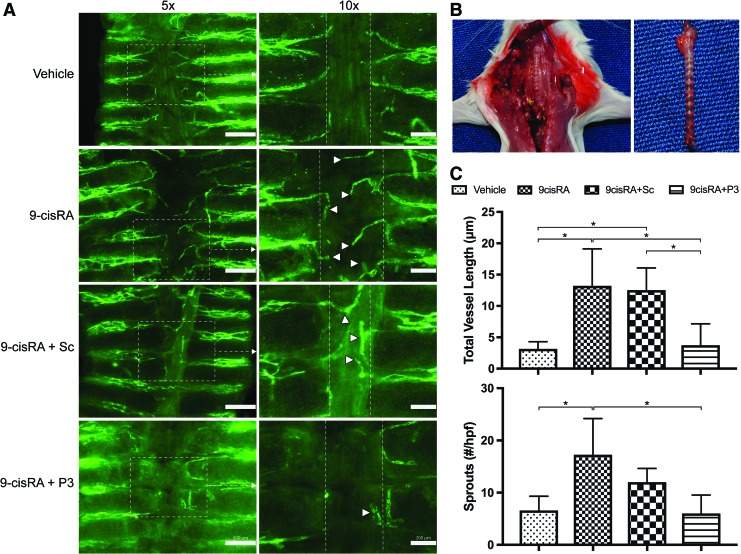
To corroborate our in vitro findings, we wanted to determine the effect of P3-mediated FGFR3 inhibition on 9-cisRA-induced tracheal lymphangiogenesis in Prox1-GFP mice.14 The lymphangiogenic effect of 9-cisRA has been demonstrated in a number of in vivo models of lymphangiogenesis and lymphedema.14,19 Of these, we chose to assess tracheal lymphangiogenesis. Prox1-GFP mice were administered either vehicle (ethanol), 9-cisRA, 9-cisRA+Sc, or 9-cisRA+P3 daily for 7 days. Whole trachea specimens were mounted, and lymphatic ingrowth into the posterior aspect of the trachea was determined. Normally, the posterior aspect of the trachea beyond the border of the cartilage rings is absent of lymphatic tissue. Lymphatic ingrowth into this area was quantified as total lymphatic vessel length and number of sprouts. 9-cisRA and 9-cisRA+Sc treated animals demonstrated significantly greater total lymphatic vessel length and number of sprouts compared with vehicle control and 9-cisRA+P3 treated animals. Thus, P3-mediated FGFR3 inhibition is sufficient to block 9-cisRA-induced lymphangiogenesis in vivo (Fig. 6).
FIG. 6.
P3-mediated FGFR3 inhibition slows 9-cisRA-induced tracheal lymphangiogenesis in Prox1-GFP mice. Prox1-GFP mice were treated daily with either vehicle control (ethanol), 9-cisRA, 9-cisRA+scrambled peptide (Sc), or 9-cisRA+peptide P3 (P3) for 7 days. Whole trachea specimens were mounted, and the lymphatics visualized under fluorescent microscopy. Five animals were used in each group. (A) Representative gross photographs of trachea in situ and a harvested trachea before whole mounting. (B) Low (5 × ) and high (10 × ) power representative images of trachea specimens. Lymphatics fluoresce green. High-magnification (10 × ) portions of each low-magnification photograph (5 × ) are shown on the right. White boxes indicate the high-magnification field of view. Vertical white lines indicate the border of tracheal cartilage, past which lymphatic vessels do not normally grow. (C) Total lymphatic vessel length and number of sprouts past the cartilage border. 9-cisRA treated animals demonstrated significantly increased total lymphatic vessel length and number of sprouts compared with vehicle control and 9-cisRA+P3 treated animals. Therefore, P3-mediated FGFR3 inhibition appears to be sufficient to block 9-cisRA-promoted lymphangiogenesis. White arrowheads indicate lymphatic vessels growing past the cartilage border. *Indicates a p-value < 0.05. GFP, green fluorescent protein. Color images are available online.
Discussion and Conclusions
FGFR3 appears to play an important but not essential role in lymphatic development. For example, FGFR3 has been closely associated with the master control gene specifying lymphatic cell fate, namely Prox1.11 Prox1 is essential for development of the lymphatic system.20 Furthermore, ectopic overexpression of Prox1 in blood vascular endothelial cells (BECs) has been shown to downregulate BEC-specific genes and upregulate LEC-specific genes.20 One of the genes upregulated by Prox1 in the reprograming of venous endothelial cells to LECs is Fgfr3.11 This highlights FGFR3 as a potential major player in lymphatic development. We propose that 9-cisRA-induced postnatal lymphangiogenesis is dependent on FGFR3 function.
Lymphangiogenesis is predominantly a developmental process. In the adult, the development of new lymphatics is less common. When it does occur, it is usually associated with injury and/or inflammation, such as with traumatic wounding and chronic inflammatory disorders.21–23 Less commonly, lymphangiogenesis can also be promoted by various tumors, primarily through growth factor secretion by the tumor. FGFR3 is strongly expressed in postdevelopmental lymphatic vessels and appears to have an important role in these processes.11 In cell culture, FGFR3 has been shown to play a part in the response of LECs to proliferating signals.11 Furthermore, FGF2 is strongly associated with postnatal lymphangiogenesis. Specifically, FGF2 is one of the growth factors upregulated in lymphangiogenesis induced by herpes simplex virus type 1 infection of the cornea.24 Also, FGF2, among other factors, is expressed in tissues undergoing lymphangiogenesis from acute cutaneous hypersensitivity.25 In addition to inflammatory and healing-related lymphangiogenesis, FGFR signaling has been implicated in the formation of tumor-induced lymphatics. Tumor-induced lymphangiogenesis has now been established as a method of cancer progression and metastasis.26 With respect to FGF signaling, it has been shown that inflammatory breast cancer specimens express significantly higher levels of FGF2 mRNA compared with noninflammatory breast cancer specimens.27 The same inflammatory breast cancer specimens also had a significantly elevated amount of proliferating LECs. In summary, although the role of FGFR3 in adult lymphangiogenesis has not been extensively studied, the high expression of FGFR3 in postnatal lymphatics as well as data from studies of FGFR3 ligands and tumor-induced lymphangiogenesis do suggest that FGFR3 has a role in the adult.
Postdevelopmental lymphangiogenesis can also be induced pharmacologically. Vascular endothelial growth factor-C (VEGF-C) was one of the first recognized lymphangiogenic agents.28 VEGF-A, VEGF-D, platelet-derived growth factor-BB, and hepatocyte growth factor also promote lymphangiogenesis.29 Unfortunately, however, these factors are associated with increased lymphatic metastasis in those with a history of cancer.29 Therefore, the use of these growth factors for promoting lymphangiogenesis is limited, given that in the United States lymphedema often occurs in the postoncologic setting.30 This problem prompted the search for alternative prolymphangiogenic molecules. One promising candidate molecule our group has studied extensively is 9-cisRA.
9-cisRA is an isoform of vitamin A. A topical form of 9-cisRA has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of AIDS-related Kaposi Sarcoma. Retinoic acids have a broad range of biologic effects. Generally, they are involved in cell proliferation, metabolism, differentiation, and apoptosis.14,31,32 All-trans-retinoic acid has been shown to mediate early lymphatic vascular development.33 More recently, our group has demonstrated that 9-cisRA can affect postdevelopmental lymphatic regeneration. Specifically, 9-cisRA was shown to induce LEC proliferation, migration, and tubule formation in vitro, as well as lymphangiogenesis in an animal model.14 In addition, 9-cisRA was effective in reducing lymphedema in both a surgical tail model and a surgery+radiation hindlimb model of the disease.14,19 Importantly, the 9-cisRA treated animals had significantly increased lymphatic vessel density and functional lymph clearance.19 In a preliminary search for the molecular mechanism of 9-cisRA-mediated lymphangiogenesis, FGFR3 was found to be transiently but very significantly upregulated in LECs after incubation with 9-cisRA.14 This finding prompted us to more thoroughly explore the role of FGFR3 in 9-cisRA-induced lymphangiogenesis.
To study the role of FGFR3 in modulating pharmacologically stimulated postnatal lymphatic growth, we searched the literature for FGFR3-specific inhibitors that had been applied in vitro as well as in vivo. This search yielded peptide P3 or P3. P3 is a 12 amino acid peptide that was discovered using a phage library with the intent of identifying highly specific FGFR3 peptides.4 The initial investigators were interested in inhibiting FGFR3 as a potential therapy for thanatophoric dysplasia, which results from a gain-of-function mutation in the receptor.4 The authors verified the binding specificity of P3 to the extracellular domain of FGFR3 and its inhibition of FGFR3 tyrosine kinase. In metatarsal bone cultures, explanted from thanatophoric dysplasia mimicking mice (Fgfr3Neo–K644E/+ EIIa-Cre mice), P3 was shown to restore normal bone growth. In vivo, using the same mouse model, it was also shown that P3 rescued the lethal phenotype. In summary, Jin et al. demonstrated that P3 is an effective and specific inhibitor of FGFR3, which could translate well to an in vivo system. For these reasons, we chose to use P3 in our study of the role of FGFR3 in 9-cisRA-induced postnatal lymphangiogenesis.
LEC proliferation, migration, and tubule formation are suitable in vitro approximations of LEC function in vivo. Proliferation of LECs is necessary for in vivo lymphatic vessel formation. Subsequent to proliferation, endothelial cells typically migrate. Migration assays are a useful measure of chemokinesis and/or chemotaxis.34,35 Finally, after LECs proliferate and migrate, they must form tubules to support fluid transport. Tube formation is representative of the later stages of lymphangiogenesis and cell differentiation.34 In all, this sequence represents an in vitro surrogate for lymphangiogenesis. In this study, we found that P3-mediated FGFR3 inhibition did not affect LEC proliferation, migration, or tubule formation. This is consistent with recently published data, in which FGFR3 knockdown with siRNA did not limit human dermal LEC migration.3 Interestingly, it has been previously shown that nonspecific FGFR inhibition decreases 9-cisRA-induced LEC migration.14 This suggests that functional cooperation among FGFRs may be involved in postdevelopmental LEC proliferation. Whereas inhibition of multiple FGFRs is sufficient to inhibit LEC growth in vitro,14 inhibition of only FGFR3 is not. This concept of functional cooperation between FGFRs has been demonstrated.3,36
As mentioned, FGFR3 has been associated with the mechanism by which 9-cisRA induces lymphangiogenesis. It has been previously shown that 9-cisRA promotes LEC proliferation, migration, and tubule formation.14 Here, we show that P3-mediated FGFR3 inhibition slows the promoting effects of 9-cisRA on LEC proliferation, migration, and tubule formation in vitro. Furthermore, P3-mediated FGFR3 inhibition was shown to decrease the lymphangiogenic effects of 9-cisRA in vivo. Specifically, 9-cisRA-induced tracheal lymphangiogenesis was blocked by P3-mediated FGFR3 inhibition. Inhibition of FGFR3 alone is not sufficient to inhibit lymphatic growth in development or in other examples of postnatal lymphangiogenesis.3 However, FGFR3 appears to have a greater role in 9-cisRA-stimulated lymphangiogenesis.
In addition to providing useful information about the role of FGFR3 in 9-cisRA-induced lymphangiogenesis, P3 is a promising candidate for anti-FGFR3 therapy. Anti-FGF signaling has been used for various cancers, chondrodysplasia, and rickets; specific examples of FGF inhibitors have been clearly reviewed by Katoh.37 As a result of the similarity between FGFR1–4, receptor-specific inhibitors have only recently been developed. Several methods of interrupting FGF-FGFR signaling exist, such as siRNA knockdown, small-molecule FGFR inhibitors, FGF- and FGFR-specific antibodies, and FGF traps that sequester FGFR ligands. Each of these methods has limitations. Antibody therapy is limited by extremely high production costs.38 In addition, although several antibodies have been effective as anticancer therapy, tissue penetration and therefore solid-tumor penetration are often poor.38 Small-molecule therapy has recently seen much success in anticancer therapy39; however, to our knowledge no FGFR3-specific small-molecule inhibitors are currently available. All FGFR small-molecule inhibitors act across multiple FGFRs.37 Several FGF traps have been used in preclinical models with success.40,41 One FGF trap has reached phase I of clinical trials, namely FP-1039.42 FP-1039 is composed of an extracellular domain of FGFR1 fused with the Fc region of IgG1. Results were promising, as the therapy demonstrated minimal morbidity compared with the toxicity seen with small-molecule tyrosine kinase inhibitors. However, this therapy sequesters multiple FGFR ligands, and hence has a less specific effect than direct inhibition of the receptor. Small peptides are an emerging and promising category of therapeutics. The disadvantages of small peptide therapies include a short half-life, low membrane permeability, tendency for aggregation, and potential instability.43 However, many of these limitations can be or have been overcome.43 Peptide P3 may be a suitable alternative to current methods of FGFR3 inhibition, in that it is specific for FGFR3, theoretically limiting unintended side effects. Also, as a small peptide, it offers the general advantages of predictable metabolism, high selectivity and potency, and standard synthetic protocols.43
A number of anti-FGFR therapies have already been approved by the FDA for the treatment of various cancers.37 There is no currently FDA-approved anti-FGFR3 therapy; however, preclinical data suggest that a specific FGFR3 inhibitor would be highly useful. Activating FGFR3 mutations are frequently found in human bladder cancer.44 In preclinical models, anti-FGFR3 therapy has been shown to have antitumor effects on human bladder carcinoma cells and mouse bladder cancer.45 FGFR3 is therefore seen as a favorable target for bladder urothelial carcinoma such that clinical trials have been planned.46 Ectopic overexpression of FGFR3 is also associated with the recurrent chromosomal translocation t(4;14) that occurs in patients with multiple myeloma.47 Concurrently, FGFR3 inhibition has been shown to be efficacious in preclinical models of FGFR3-induced hematopoietic malignancies.45,47 Finally, FGFR3 has also been found to be overexpressed in oral and oropharyngeal squamous cell carcinoma.8,48 An exhaustive review of work done regarding FGFR3 as an anticancer therapy is beyond the scope of this article. However, the abundance of published work looking at FGFR3-targeted therapy speaks to FGFR3s large potential as a therapeutic target for a range of malignant diseases.
We have shown that peptide P3 effectively inhibits FGFR3 phosphorylation in human LECs. P3-mediated FGFR3 inhibition was sufficient to slow 9-cisRA-induced LEC proliferation, migration, and tubule formation. Finally, P3-mediated FGFR3 inhibition was sufficient to block 9-cisRA-induced lymphangiogenesis in vivo. This information may enable development of a more efficacious and highly specific prolymphangiogenic “therapeutic cocktail,” in which high specificity limits the side effect profile. Moving forward, P3 is a promising candidate for application in the study and treatment of disease involving FGFR3. Including the original work describing P3, this small peptide has now been validated in multiple cell types and in multiple in vivo disease models. In summary, peptide P3 has broad applicability, in that it not only effectively modulates regenerative therapy such as 9-cisRA-directed lymphangiogenesis but it may also be effective in the management of pathologic conditions associated with aberrant FGFR3 signaling.
Acknowledgment
AKW is funded by NIH 1K08HL132110-01A1.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 2005; 16:107–137 [DOI] [PubMed] [Google Scholar]
- 2. Beenken A, Mohammadi M. The FGF family: Biology, pathophysiology and therapy. Nat Rev Drug Discov 2009; 8:235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, Xie Y, Zhu J, Chen Z, De Smet F, Zhang J, Jin SW, Sun L, Sun H, Kibbey RG, Hirschi KK, Hay N, Carmeliet P, Chittenden TW, Eichmann A, Potente M, Simons M. FGF-dependent metabolic control of vascular development. Nature 2017; 545:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin M, Yu Y, Qi H, Xie Y, Su N, Wang X, Tan Q, Luo F, Zhu Y, Wang Q, Du X, Xian CJ, Liu P, Huang H, Shen Y, Deng CX, Chen D, Chen L. A novel FGFR3-binding peptide inhibits FGFR3 signaling and reverses the lethal phenotype of mice mimicking human thanatophoric dysplasia. Hum Mol Genet 2012; 21:5443–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ornitz DM, Legeai-Mallet L. Achondroplasia: Development, pathogenesis, and therapy. Dev Dyn 2017; 246:291–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung JY, Sun JM, Chang Jeong B, Il Seo S, Soo Jeon S, Moo Lee H, Yong Choi H, Young Kang S, Choi YL, Young Kwon G. FGFR3 overexpression is prognostic of adverse outcome for muscle-invasive bladder carcinoma treated with adjuvant chemotherapy. Urol Oncol 2014; 32:49..e23–e31. [DOI] [PubMed] [Google Scholar]
- 7. Javidi-Sharifi N, Traer E, Martinez J, Gupta A, Taguchi T, Dunlap J, Heinrich MC, Corless CL, Rubin BP, Druker BJ, Tyner JW. Crosstalk between KIT and FGFR3 promotes gastrointestinal stromal tumor cell growth and drug resistance. Cancer Res 2015; 75:880–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koole K, van Kempen PM, Swartz JE, Peeters T, van Diest PJ, Koole R, van Es RJ, Willems SM. Fibroblast growth factor receptor 3 protein is overexpressed in oral and oropharyngeal squamous cell carcinoma. Cancer Med 2016; 5:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keegan K, Johnson DE, Williams LT, Hayman MJ. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc Natl Acad Sci U S A 1991; 88:1095–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol 2015; 4:215–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: A role for FGF signaling in lymphangiogenesis. Mol Biol Cell 2006; 17:576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest 2008; 118:3355–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi D, Park E, Jung E, Seong YJ, Hong M, Lee S, Burford J, Gyarmati G, Peti-Peterdi J, Srikanth S, Gwack Y, Koh CJ, Boriushkin E, Hamik A, Wong AK, Hong YK. ORAI1 activates proliferation of lymphatic endothelial cells in response to laminar flow through Kruppel-like factors 2 and 4. Circ Res 2017; 120:1426–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi I, Lee S, Kyoung Chung H, Suk Lee Y, Eui Kim K, Choi D, Park EK, Yang D, Ecoiffier T, Monahan J, Chen W, Aguilar B, Lee HN, Yoo J, Koh CJ, Chen L, Wong AK, Hong YK. 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: Therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation 2012; 125:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong YK. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood 2009; 113:1856–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol 2003; 162:575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: An open-source platform for biological-image analysis. Nat Methods 2012; 9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi I, Chung HK, Ramu S, Lee HN, Kim KE, Lee S, Yoo J, Choi D, Lee YS, Aguilar B, Hong YK. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood 2011; 117:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bramos A, Perrault D, Yang S, Jung E, Hong YK, Wong AK. Prevention of postsurgical lymphedema by 9-cis retinoic acid. Ann Surg 2016; 264:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 2002; 225:351–357 [DOI] [PubMed] [Google Scholar]
- 21. Paupert J, Sounni NE, Noel A. Lymphangiogenesis in post-natal tissue remodeling: Lymphatic endothelial cell connection with its environment. Mol Aspects Med 2011; 32:146–158 [DOI] [PubMed] [Google Scholar]
- 22. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005; 438:946–953 [DOI] [PubMed] [Google Scholar]
- 23. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol 2008; 6:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gurung HR, Carr MM, Bryant K, Chucair-Elliott AJ, Carr DJ. Fibroblast growth factor-2 drives and maintains progressive corneal neovascularization following HSV-1 infection. Mucosal Immunol 2018; 11:172–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lachance PA, Hazen A, Sevick-Muraca EM. Lymphatic vascular response to acute inflammation. PLoS One 2013; 8:e76078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Da MX, Wu Z, Tian HW. Tumor lymphangiogenesis and lymphangiogenic growth factors. Arch Med Res 2008; 39:365–372 [DOI] [PubMed] [Google Scholar]
- 27. Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, Vermeulen PB, Dirix LY. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res 2004; 10:7965–7971 [DOI] [PubMed] [Google Scholar]
- 28. Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997; 276:1423–1425 [DOI] [PubMed] [Google Scholar]
- 29. Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci 2008; 1131:225–234 [DOI] [PubMed] [Google Scholar]
- 30. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377 [DOI] [PubMed] [Google Scholar]
- 31. Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008; 134:921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ 2004; 11(Suppl 2):S126–S143 [DOI] [PubMed] [Google Scholar]
- 33. Marino D, Dabouras V, Brandli AW, Detmar M. A role for all-trans-retinoic acid in the early steps of lymphatic vasculature development. J Vasc Res 2011; 48:236–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adair TH, Monani J-P. Angiogenesis. San Rafeal, CA: Morgan & Claypool Life Sciences; 2010 [PubMed] [Google Scholar]
- 35. Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol 2006; 4:83–100 [DOI] [PubMed] [Google Scholar]
- 36. Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 1998; 125:3615–3623 [DOI] [PubMed] [Google Scholar]
- 37. Katoh M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol Sci 2016; 37:1081–1096 [DOI] [PubMed] [Google Scholar]
- 38. Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br J Pharmacol 2009; 157:220–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoelder S, Clarke PA, Workman P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol Oncol 2012; 6:155–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morita J, Nakamura M, Kobayashi Y, Deng CX, Funato N, Moriyama K. Soluble form of FGFR2 with S252W partially prevents craniosynostosis of the apert mouse model. Dev Dyn 2014; 243:560–567 [DOI] [PubMed] [Google Scholar]
- 41. Ronca R, Giacomini A, Di Salle E, Coltrini D, Pagano K, Ragona L, Matarazzo S, Rezzola S, Maiolo D, Torrella R, Moroni E, Mazzieri R, Escobar G, Mor M, Colombo G, Presta M. Long-pentraxin 3 derivative as a small-molecule FGF trap for cancer therapy. Cancer Cell 2015; 28:225–239 [DOI] [PubMed] [Google Scholar]
- 42. Tolcher AW, Papadopoulos KP, Patnaik A, Wilson K, Thayer S, Zanghi J, Gemo AT, Kavanaugh WM, Keer HN, LoRusso PM. A phase I, first in human study of FP-1039 (GSK3052230), a novel FGF ligand trap, in patients with advanced solid tumors. Ann Oncol 2016; 27:526–532 [DOI] [PubMed] [Google Scholar]
- 43. Fosgerau K, Hoffmann T. Peptide therapeutics: Current status and future directions. Drug Discov Today 2015; 20:122–128 [DOI] [PubMed] [Google Scholar]
- 44. Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet 1999; 23:18–20 [DOI] [PubMed] [Google Scholar]
- 45. Hadari Y, Schlessinger J. FGFR3-targeted mAb therapy for bladder cancer and multiple myeloma. J Clin Invest 2009; 119:1077–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turo R, Harnden P, Thygesen H, Fleischmann A, Thalmann GN, Seiler R, Cross WR, Knowles MA. FGFR3 expression in primary invasive bladder cancers and matched lymph node metastases. J Urol 2015; 193:325–330 [DOI] [PubMed] [Google Scholar]
- 47. Chen J, Lee BH, Williams IR, Kutok JL, Mitsiades CS, Duclos N, Cohen S, Adelsperger J, Okabe R, Coburn A, Moore S, Huntly BJ, Fabbro D, Anderson KC, Griffin JD, Gilliland DG. FGFR3 as a therapeutic target of the small molecule inhibitor PKC412 in hematopoietic malignancies. Oncogene 2005; 24:8259–8267 [DOI] [PubMed] [Google Scholar]
- 48. Nayak S, Goel MM, Makker A, Bhatia V, Chandra S, Kumar S, Agarwal SP. Fibroblast growth factor (FGF-2) and its receptors FGFR-2 and FGFR-3 may be putative biomarkers of malignant transformation of potentially malignant oral lesions into oral squamous cell carcinoma. PLoS One 2015; 10:e0138801. [DOI] [PMC free article] [PubMed] [Google Scholar]