Abstract
Engineering approaches that mimic the process of fetal development have the potential to regenerate the zonal organization of articular cartilage. The objective of this study was to investigate the effect of sequential addition of zone-specific growth factors such as bone morphogenetic protein (BMP)-7, insulin-like growth factor (IGF)-1, and Indian hedgehog (IHH) to transforming growth factor (TGF)-β1-supplemented chondrogenic medium on zonal differentiation of human mesenchymal stem cells (hMSCs) encapsulated in an articular cartilage-derived matrix. First, fetal or adult bovine articular cartilage was decellularized, digested, and methacrylate functionalized to produce an injectable macromer (CarMa, f-CarMa for fetal, a-CarMa for adult) for encapsulation of hMSCs. Next, the optimum matrix source and initial cell density for chondrogenic differentiation of hMSCs to the superficial and calcified zone phenotypes were determined by encapsulation of the cells in CarMa hydrogel and incubated in chondrogenic medium/TGF-β1 supplemented with BMP-7 and IHH, respectively. Then, the encapsulated hMSCs were preexposed to BMP-7-supplemented chondrogenic medium/TGF-β1 and the effect of sequential addition of IGF-1 and IHH to the medium on the expression of zone-specific markers was investigated. According to the results, f-CarMa and high cell density enhanced differentiation of the encapsulated hMSCs to the superficial zone phenotype, whereas a-CarMa and low cell density enhanced differentiation to the calcified zone. The addition of IGF-1 to the chondrogenic medium/TGF-β1 stimulated differentiation of the encapsulated hMSCs, preexposed to BMP-7, to the middle zone phenotype. The addition of IHH to the chondrogenic medium/TGF-β1 stimulated maturation of the encapsulated hMSCs, preexposed to BMP-7 and IGF-1, to the calcified zone phenotype. The results are potentially useful for engineering injectable, cellular hydrogels for regeneration of full-thickness articular cartilage.
Impact Statement
The higher regenerative capacity of fetal articular cartilage compared with the adult is rooted in differences in cell density and matrix composition. We hypothesized that the zonal organization of articular cartilage can be engineered by encapsulation of mesenchymal stem cells in a single superficial zone-like matrix followed by sequential addition of zone-specific growth factors within the matrix, similar to the process of fetal cartilage development. The results demonstrate that the zonal organization of articular cartilage can potentially be regenerated using an injectable, monolayer cell-laden hydrogel with sequential release of growth factors.
Keywords: fetal articular cartilage, zonal regeneration, mesenchymal stem cells, chondrogenic differentiation, zone-specific growth factors, cell density
Introduction
The postnatal articular cartilage because of its avascularity and low biosynthetic activity lacks the ability for complete self-repair after injury.1,2 In posttraumatic osteoarthritis, the rapidly applied load focally disrupts the articular cartilage beginning with cell death at the focal point and inadequate synthesis of proteoglycans that accelerates damage to the tissue.3 The superficial zone of articular cartilage is most vulnerable to cell death after injury.4 Conventional techniques for treating full-thickness cartilage defects create fibrocartilage at the site of injury, which because of the lack of zonal organization deteriorates over time.5 Osteochondral autograft transfer suffers from an additional surgical intervention.5 Autologous chondrocyte implantation reduces fibrocartilage formation but it fails to restore the zonal organization of articular cartilage and in some cases leads to peripheral hypertrophy and calcification.4 Engineering strategies that can restore the zonal organization have the potential to regenerate full-thickness articular cartilage defects and avoid the need for total joint replacement.6
The fetal development of articular cartilage starts with condensation of mesenchymal stem cells (MSCs) in the early limb bud to form a mesenchyme characterized by high cell density.7 After condensation, a superficial zone-like tissue is formed by the expression of transforming growth factor-β (TGF-β), a master regulator of chondrogenesis.7 The expression pattern of TGF-β1 and bone morphogenetic protein-7 (BMP-7) plays a critical role during fetal development of the superficial zone by activation of chondrogenic genes through Wnt/β-catenin pathway through Smad 2/3/4 and transcription factors TCF/Lef1.8 Recently, it was shown that all chondrocytes in mouse articular cartilage originate from the superficial zone.9 The prechondrogenic cells in the superficial zone-like tissue begin to form the zonal structure of articular cartilage through the spatiotemporal gradients in matrix composition, cell density, and growth factors. Insulin-like growth factor-1 (IGF-1) is specifically expressed by the differentiating chondrocytes to form the middle zone of articular cartilage.10 Indian hedgehog (IHH) signaling accelerates hypertrophy in chondrocytes to form the calcified zone of articular cartilage through a pathway independent of parathyroid hormone-related protein (PTHrP).11
The higher regenerative capacity of fetal articular cartilage compared with the adult is rooted in differences in cell density, matrix composition, and structure.12 For example, collagen type II (Col II) accounts for 77% and 86% of the total collagen in fetal and adult articular cartilage, respectively.6 Approximately 10% of the total collagen in fetal articular cartilage is Col IX as opposed to 1% in adult cartilage.13 Similarly, 10% of the total collagen in fetal cartilage is Col XI as opposed to <3% in adult cartilage.13 The cell density in human fetal articular cartilage ranges from 350 to 150 million cells/mL depending on distance from the articular surface,14 whereas the average cell density in the adult cartilage is 30 million cells/mL.14
We recently demonstrated using a developmentally inspired approach that zone-specific chondrogenic differentiation of human MSCs (hMSCs) depended on physical properties of the matrix and zone-specific growth factors.15,16 In that study, the addition of BMP-7 to the chondrogenic medium/TGF-β1 stimulated differentiation of hMSCs to the superficial-zone phenotype, whereas the addition of IGF-1 stimulated differentiation to the middle zone phenotype. We hypothesized that the articular cartilage zones can be engineered by encapsulation of hMSCs in a single superficial zone-like matrix followed by sequential addition of zone-specific growth factors within the matrix, similar to the process of fetal development of articular cartilage. The objective of this study was to encapsulate hMSCs in a matrix derived from articular cartilage and to investigate the effect of sequential addition of BMP-7, IGF-1, and IHH growth factors to the chondrogenic medium/TGF-β1 on differentiation and maturation of the encapsulated cells to the superficial, middle, and calcified zone phenotypes, respectively. To achieve the objective, the matrix source and cell density were optimized for hMSCs encapsulated in the superficial zone matrix. Fetal and adult bovine articular cartilages were decellularized, digested, and methacrylate functionalized to produce an injectable in situ cross-linkable macromer for the encapsulation of hMSCs. Hereafter, the macromer is referred to as CarMa with f-CarMa and a-CarMa for the macromer derived from fetal and adult articular cartilage, respectively. Next, hMSCs were encapsulated in CarMa hydrogel and the effect of matrix source and initial cell density on differentiation of hMSCs to the prechondrogenic superficial zone phenotype was investigated in chondrogenic medium/TGF-β1 supplemented with BMP-7. Then, hMSCs were encapsulated in CarMa hydrogel and the effect of matrix source and initial cell density on differentiation of hMSCs to the hypertrophic calcified zone phenotype was investigated in chondrogenic medium/TGF-β1 supplemented with IHH. Next, hMSCs were encapsulated in a hydrogel matrix optimized for the superficial zone phenotype and the effect of sequential addition of growth factors (BMP-7 only, BMP-7 followed by IGF-1, BMP-7 followed by IGF-1 and IHH) on the expression of zone-specific markers was investigated with incubation time in chondrogenic medium/TGF-β1. The results indicate that the combination of different matrices, cell densities, and growth factors is required for optimum chondrogenic differentiation of hMSCs to zone-specific phenotypes of articular cartilage.
Materials and Methods
Reagents
The full-thickness adult and fetal articular cartilage harvested from the bovine femoral condyles were purchased from Animal Technologies (Tyler, TX). The photoinitiator 2-hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure-2959) was received from CIBA (Tarrytown, NY). Paraformaldehyde, formalin, paraffin, penicillin G, insulin, papain, dithiothreitol, ethylenediaminetetraacetic acid (EDTA), streptomycin, pepsin, and methacrylic anhydride were purchased from Sigma-Aldrich (St. Louis, MO). Spectro/Por dialysis tube (molecular weight cutoff 3.5 kDa) was purchased from Spectrum Laboratories (Rancho Dominquez, CA). Paper filter with 710 μm average pore size was purchased from VWR (Randor, PA). Dichloromethane (DCM) solvent was purified by distillation over calcium hydride. All other solvents were reagent grade and used as received.
hMSCs harvested and cultured from healthy human bone marrow with high expression of CD105, CD166, CD29, and CD44 and low expression of CD14, CD34, and CD45 markers were received from Lonza (Allendale, NJ). TGF-β1 and IGF-1 were purchased from Lonza. BMP-7 and bovine serum albumin (BSA) were received from Novus (Littleton, CO) and Jackson ImmunoResearch (West Grove, PA), respectively. IHH protein was purchased from Mybiosource (San Diego, CA). Iodoacetic acid was purchased from Fisher Scientific (Rockford, IL). Dulbecco's modified eagle medium (DMEM) cell culture medium, Dulbecco's phosphate-buffer saline (PBS), fetal bovine serum (FBS), trypsin-EDTA, and Quant-it PicoGreen dsDNA reagent kit were received from Invitrogen (Carlsbad, CA). The Sircol assay for determination of total collagen content was purchased from Biocolor (Carrickfergus, United Kingdom). QuantiChrom alkaline phosphatase (ALP) assay kit was purchased from Bioassay Systems (Hayward, CA). 1,9-dimethylmethylene blue (DMMB) assay kit for quantification of glycosaminoglycan (GAG) content, hematoxylin and eosin-Y (H&E) for staining cell nuclei and cytoplasm, and Alcian blue for GAG staining were purchased from Sigma-Aldrich. Masson's trichrome for collagen staining was received from Polysciences (Warrington, PA). PicoGreen assay kit for quantification of double-stranded DNA content was purchased from Molecular Probes (Thermo Fisher Scientific, Waltham, MA). All primary and secondary antibodies, luminol reagent, and Blotto blocking solution were received from Santa Cruz Biotechnology (Dallas, TX). All forward and reverse primers were synthesized by Integrated DNA Technologies (Coralville, IA).
Digestion and functionalization of articular cartilage and hydrogel synthesis
The fetal or adult cartilage tissues were dissected into small pieces (5 × 5 × 2 mm). The pieces were frozen in liquid nitrogen and milled. The milled fragments were sieved using a paper filter with 710 μm average pore size. The fragments were decellularized by treatment with 10 mM Tris/1% triton for 24 h followed by sonication at 55 kHz for 2 h. The tissue fragments were kept in a nuclease solution consisting of 1 U/mL deoxyribonuclease and 1 U/mL ribonuclease in PBS for 72 h at 37°C to degrade the DNA and RNA as described.17 Next, the decellularized fetal or adult cartilage matrices were freeze-dried. The dried matrices were digested with pepsin in a 0.01 M hydrochloric acid solution at 37°C as described18 until a clear suspension was obtained (Fig. 1a). Next, pH of the digestion medium was raised to 9.0 using 1 M sodium hydroxide to inactivate the pepsin enzyme. After 1-h incubation, the pH of the digestion solution was lowered to 7.5. Next, methacrylic anhydride was added dropwise to the solution (2.5 mL/g digested cartilage) under rigorous stirring to functionalize the digested cartilage as described (Fig. 1a).19,20After functionalization, the mixture was dialyzed against distilled water for 7 days at 40°C to remove the unreacted methacrylic anhydride. According to previous studies, the denaturation of hydrated collagen commences at 58°C with a peak at 65°C corresponding to the main structural transition.21 Furthermore, according to previous reports, the peak temperature corresponding to denaturation of collagen is above 50°C regardless of collagen source.22 Therefore, the temperature of 40°C was chosen to reduce the dialysis time and minimize further denaturation of the collagenous component of the digested tissue. The methacrylate digested cartilage macromer solution was lyophilized to obtain a white foam, hereafter referred to as CarMa macromer and stored at −20°C. The degree of methacrylation of fetal and adult digested cartilage tissues was quantified using hydrogen-1 proton nuclear magnetic resonance (1H-NMR). Five milligrams of the sample was dissolved in 600 μL deuterium oxide and characterized using a Varian Mercury-300 1H-NMR (Varian, Palo Alto, CA). The chemical shifts for phenylalanine in the 6.9–7.5 ppm range were used as an internal standard to normalize the spectra as described elsewhere.23,24 The chemical shifts for amine groups of lysine (2.8–2.95 ppm) of digested cartilage, before (Car) and after methacrylation (CarMa), were integrated and the degree of methacrylation was calculated by the following equation23,24:
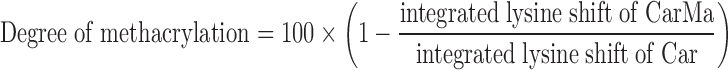 |
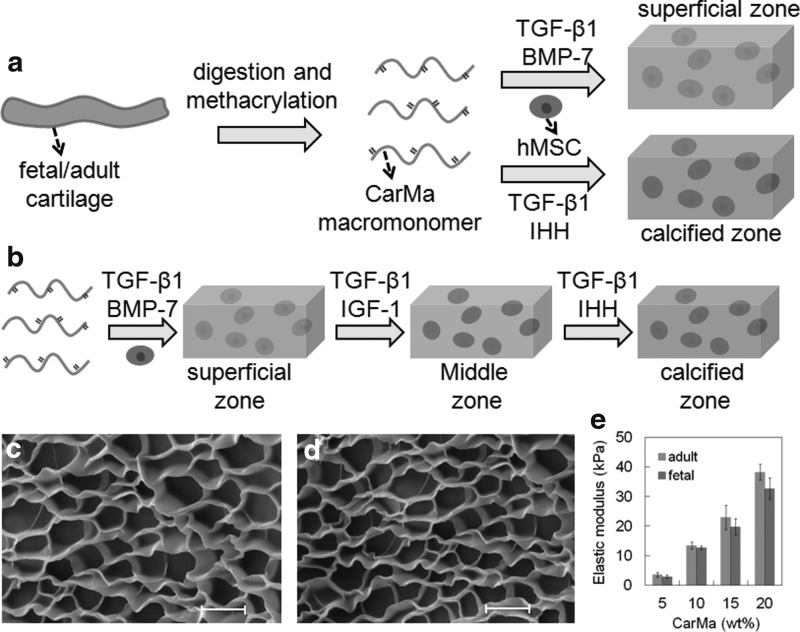
FIG. 1.
(a) Schematic representation of the fetal-mimetic approach to articular cartilage regeneration. Fetal or adult cartilage was decellularized, digested, and functionalized to produce an injectable, in situ cross-linkable, fetal (f-) or adult (a-) CarMa macromer for cell encapsulation. hMSCs were suspended in CarMa hydrogel precursor solution and cross-linked by UV irradiation. Next, the encapsulated hMSCs were cultured in chondrogenic medium/TGF-β1 supplemented with BMP-7 for differentiation to the superficial zone phenotype of chondrocytes or IHH for differentiation to the calcified zone phenotype; (b) hMSCs were encapsulated in f-CarMa hydrogel and cultured in chondrogenic medium/TGF-β1 supplemented with BMP-7 to generate a superficial zone-like cellular construct. Next, the hMSCs in the superficial zone construct were incubated in chondrogenic medium/TGF-β1 with sequential addition of IGF-1 and IHH to induce differentiation of the encapsulated cells to the middle and calcified zone phenotypes, respectively. SEM images of the lyophilized f-CarMa (c) and a-CarMa (d) hydrogels. (e) Effect of macromer concentration in the precursor solution on the elastic modulus of f-CarMa and a-CarMa hydrogels after UV cross-linking. The scale bars in (c) an (d) are 10 μm. BMP, bone morphogenetic protein; hMSC, human mesenchymal stem cell; IHH, Indian hedgehog; IGF, insulin-like growth factor; SEM, scanning electron microscope; TGF, transforming growth factor; UV, ultraviolet.
Characterization of CarMa hydrogel
The CarMa macromer was dissolved in PBS and cross-linked by ultraviolet (UV) polymerization as described previously.25 In brief, the Irgacure-2959 photoinitiator was dissolved in PBS (10 mg/mL) at 50°C by vortexing. The CarMa hydrogel precursor solution was prepared by mixing the solution of CarMa macromer in PBS with the photoinitiator solution. The precursor solution was degassed and transferred to a polytetrafluoroethylene mold (5 cm × 3 cm × 750 μm). The mold was covered with a transparent glass plate, fastened with clips, and the assembly was irradiated with an OmniCure Series 1500 UV illumination system (Excelitas, Waltham, MA) with a 200 W lamp, 8 mm diameter light guide, light intensity of 7.5 mW/cm2 with peak wavelength of 365 nm for 2 min as described.26,27 After hydrogel formation, disk-shape samples were cut from the gel using an 8 mm cork borer and loaded on the Peltier plate of an AR2000 rheometer (TA Instruments, New Castle, DE) and subjected to a uniaxial compressive force at a displacement rate of 7.5 μm/s. The slope of the linear fit to the stress–strain curve for 5–10% strain was taken as the compressive modulus of the gel as we previously described.28
Microstructure of CarMa hydrogels was imaged with a TESCAN VEGA3 SBU variable pressure scanning electron microscope (SEM; Kohoutovice, Czech Republic). The freeze-dried hydrogel samples were immersed in liquid nitrogen and cut with a surgical blade to expose a freshly cut surface. The surface was coated with gold using a Denton Desk II sputter coater (Moorestown, NJ) at 20 mA for 75 s and imaged with SEM at an accelerating voltage of 8 keV as previously described.26
Cell encapsulation in CarMa hydrogel
hMSCs (passages 3–5) were cultured in a high glucose DMEM medium supplemented with 10% FBS, 100 U/mL penicillin G, and 100 μg/mL streptomycin (basal medium, BM) at a density of 5000 cells/cm2 as we previously described.15 The CarMa hydrogel precursor solution was prepared by dissolving CarMa (100 mg/mL) and Irgacure-2959 photoinitiator (10 mg/mL) in PBS at 37°C and the solution was sterilized by filtration. The desired number of hMSCs (15, 25, 60, 100, and 200 million cells) suspended in 100 μL DMEM were mixed with 900 μL of the sterile hydrogel precursor solution. The edges of two glass slides were covered with a layer of biomedical grade adhesive tape and 50 μL of the precursor cell suspension was injected between the two glass slides and cross-linked by UV irradiation for 2 min. The disk-shaped samples, with 12 mm diameter and 450 μm thickness, were placed in standard 50 mm Petri dishes and incubated in BM.15 After incubation of the cell-laden hydrogels in BM for 24 h, the medium was changed to the medium corresponding to the superficial or calcified zone of articular cartilage (Fig. 1a and Table 1) and cultured for up to 21 days. The superficial zone medium consisted of BM supplemented with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7, whereas the calcified zone medium consisted of BM with 30 ng/mL TGF-β1 with or without IHH (Table 1).15 At each time point, the samples were evaluated by biochemical, messenger RNA (mRNA), and protein analysis for the expression of zone-specific chondrogenic markers.
Table 1.
Matrix Source, Cell Density and Growth Factors Used for Chondrogenic Differentiation of hMSCs to the Superficial Zone Phenotype, Calcified Zone Phenotype, and for Sequential Differentiation of hMSCs to Superficial, Middle, and Calcified Zone Phenotypes
| Sample name | Matrix source | Cell density (million cells/mL) | Growth factors | ||
|---|---|---|---|---|---|
| Superficial zone | |||||
| a-25 | Adult cartilage | 25 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| f-25 | Fetal cartilage | 25 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| a-60 | Adult cartilage | 60 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| f-60 | Fetal cartilage | 60 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| a-100 | Adult cartilage | 100 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| f-100 | Fetal cartilage | 100 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| a-200 | Adult cartilage | 200 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| f-200 | Fetal cartilage | 200 | TGF-β1 (3 ng/mL), BMP-7 (100 ng/mL) | ||
| Calcified zone | |||||
| a-15 | Adult cartilage | 15 | TGF-β1 (30 ng/mL) | ||
| f-15 | Fetal cartilage | 15 | TGF-β1 (30 ng/mL) | ||
| a-100 | Adult cartilage | 100 | TGF-β1 (30 ng/mL) | ||
| f-100 | Fetal cartilage | 100 | TGF-β1 (30 ng/mL) | ||
| a-15-IHH | Adult cartilage | 15 | TGF-β1 (30 ng/mL), IHH (2.5 μg/mL) | ||
| f-15-IHH | Fetal cartilage | 15 | TGF-β1 (30 ng/mL), IHH (2.5 μg/mL) | ||
| a-100-IHH | Adult cartilage | 100 | TGF-β1 (30 ng/mL), IHH (2.5 μg/mL) | ||
| f-100-IHH | Fetal cartilage | 100 | TGF-β1 (30 ng/mL), IHH (2.5 μg/mL) | ||
| Sequential addition of growth factors | |||||
| Days 1–14 | Days 15–28 | Days 29–42 | |||
| BMP-7 | Fetal cartilage | 100 | TGF-β1 (3 ng/mL) | TGF-β1 (3 ng/mL) | TGF-β1 (3 ng/mL) |
| BMP-7 (100 ng/mL) | BMP-7 (100 ng/mL) | BMP-7 (100 ng/mL) | |||
| BMP-7/IGF | Fetal cartilage | 100 | TGF-β1 (3 ng/mL) | TGF-β1 (30 ng/mL) | TGF-β1 (30 ng/mL) |
| BMP-7 (100 ng/mL) | IGF-1 (100 ng/mL) | IGF-1 (100 ng/mL) | |||
| BMP-7/IGF/IHH | Fetal cartilage | 100 | TGF-β1 (3 ng/mL) | TGF-β1 (30 ng/mL) | TGF-β1 (30 ng/mL) |
| BMP-7 (100 ng/mL) | IGF-1 (100 ng/mL) | IHH (2.5 μg/mL) | |||
Biochemical analysis and staining
The cell-encapsulated hydrogel samples were homogenized in lysis buffer (10 mM Tris supplemented with 0.2% triton in PBS) and sonicated to rupture the cell membrane. Total collagen content of the homogenized samples was measured by Sircol assay according to the manufacturer's instructions as we previously described.16 ALP activity of the samples was measured with QuantiChrom ALP assay according to the manufacturer's instructions.16 GAG content of each sample was quantified using the DMMB assay, which was calibrated with reagent blanks and the standard whale chondroitin sulfate solutions, as previously described.16 The measured collagen content, ALP activity, and GAG content at each time point were normalized by dividing by the DNA content of the samples for that time point as described.16 Double-stranded DNA of the homogenized samples was measured with PicoGreen DNA assay according to manufacturer's instructions.16 For staining, the 21-day samples were fixed in formalin, embedded in paraffin, and sectioned to a thickness of 10 μm using a microtome as described.15,29 The sections were stained with H&E for visualization of cell morphology, Alcian blue for GAG, and Masson's trichrome for collagen according the manufacturer's instructions as described.16 For live/dead staining, the cell-encapsulated CarMa hydrogel samples were incubated with acetomethoxy derivative of calcein and ethidium homodimer (1 μg/mL; Life Technologies, Grand Island, NY) as we previously described.15 The stained samples were imaged using an inverted fluorescent microscope (Nikon Eclipse Ti-e; Nikon, Melville, NY). The viability of hMSCs encapsulated in CarMa hydrogels was quantified by dividing images into smaller squares and counting the number of live and dead cells as we previously described.26
mRNA analysis
Total cellular RNA of the homogenized samples was isolated using TRIzol and the genomic DNA was removed using deoxyribonuclease I (Invitrogen) as we previously described.15 The concentration of extracted RNA was measured using a Nanodrop spectrophotometer. Two hundred fifty nanograms of the extracted RNA was converted to complementary DNA (cDNA) using Promega reverse transcription system (Madison, WI) and the cDNA was amplified in SYBR green RealMasterMix (Eppendorf, Hamburg, Germany) using a Bio-Rad CXF96 real-time quantitative polymerase chain reaction system (Bio-Rad, Hercules, CA) and the appropriate gene-specific primers as described.16 Therefore, the same amount of RNA was used for all samples regardless of cell density in the hydrogels. The primer sequences15 were designed and selected by Primer3 web-based software as we previously described.30 The mRNA expressions were normalized against a housekeeping gene and fold changes were compared with those in the same group at day 0, based on the ΔΔCt method as previously described.31
Western blot analysis
The hydrogel samples were lysed in RIPA assay buffer with EDTA-free protease inhibitor cocktail (cOmplete,™ Mini; Roche Life Science, Indianapolis, IN) according to the manufacturer's instructions. The proteins (10 μg per well) in the cell lysate were separated in a 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis Mini-gel system (Bio-Rad). The pattern of separated proteins was transferred to a nitrocellulose membrane and the membranes were blocked in Blotto solution (Santa Cruz Biotechnology). The membranes were incubated in a mixture of primary antibodies (1:10,000 dilution) in PBS with 5% dry milk and 0.1% Tween-20 overnight at 4°C. The primary antibodies (Santa Cruz Biotechnology) included rabbit anti-human against superficial zone protein (SZP), Sox-9, Col II, Col X, aggrecan (AGC), and β-actin. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) at ambient conditions for 1 h. After washing, the membranes were incubated with enhanced luminol-based detection reagent (Santa Cruz Biotechnology), the luminescence was captured using a ChemiDoc MP system (Bio-Rad), and intensity of the bands was quantified with the ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
All experiments were performed in triplicate and expressed as mean ± standard deviation. Significant differences between groups were calculated using a two-way ANOVA with replication test, followed by a two-tailed Student's t-test. Values of p < 0.05 were considered statistically significant.
Results
Supplementary Figure S1a and c shows 1H-NMR spectra of fetal and adult digested cartilage before methacrylation, respectively, whereas Supplementary Figure S1b and d shows 1H-NMR spectra of fetal and adult digested cartilage after methacrylation. The chemical shifts at 5.7 ppm that appeared after methacrylation (Supplementary Fig. S1b, d), corresponded to the vinyl protons of methacrylate groups.32 Based on the analysis of NMR shifts, the calculated degree of methacrylation of fetal and adult CarMa was 70% and 68%, respectively. Supplementary Figure S2 shows the western blot bands for Col II and Col X for digested fetal cartilage before (Car) and after methacrylation (CarMa). The two major extracellular matrix (ECM) proteins of articular cartilage, namely Col II and Col X, were present before and after methacrylation of the digested samples. In addition, the relative intensity of Col II band was higher than that of Col X before and after methacrylation. SEM images of the lyophilized f-CarMa and a-CarMa hydrogels (10 wt% in PBS) are given in Figure 1c and d, respectively. The CarMa hydrogels displayed an interconnected porous microstructure with <1 μm pore wall thickness. The mean pore size of f-CarMa and a-CarMa hydrogels was 10 and 7 μm, respectively. The elastic modulus of f-CarMa hydrogel increased from 2.9 ± 0.5 kPa to 12.7 ± 0.6, 19.7 ± 2.7, and 32.6 ± 3.6 kPa with increasing macromer concentration from 5 wt% to 10 wt%, 15 wt%, and 20 wt%, respectively, whereas the modulus of a-CarMa hydrogel increased from 3.5 ± 0.8 kPa to 13.4 ± 1.2, 22.9 ± 4.1, and 38.2 ± 2.7 kPa (Fig. 1e). Elastic modulus of the native human fetal cartilage ranges from 2 to 46 kPa during development33 and the collagenous matrix accounts for 10 wt% of the articular cartilage.6 Therefore, the hydrogels with 10 wt% CarMa macromer and 13 kPa elastic modulus (13.4 ± 1.2 kPa for a-CarMa and 12.7 ± 0.6 kPa for f-CarMa) were used for cell encapsulation to simulate the matrix of fetal articular cartilage.
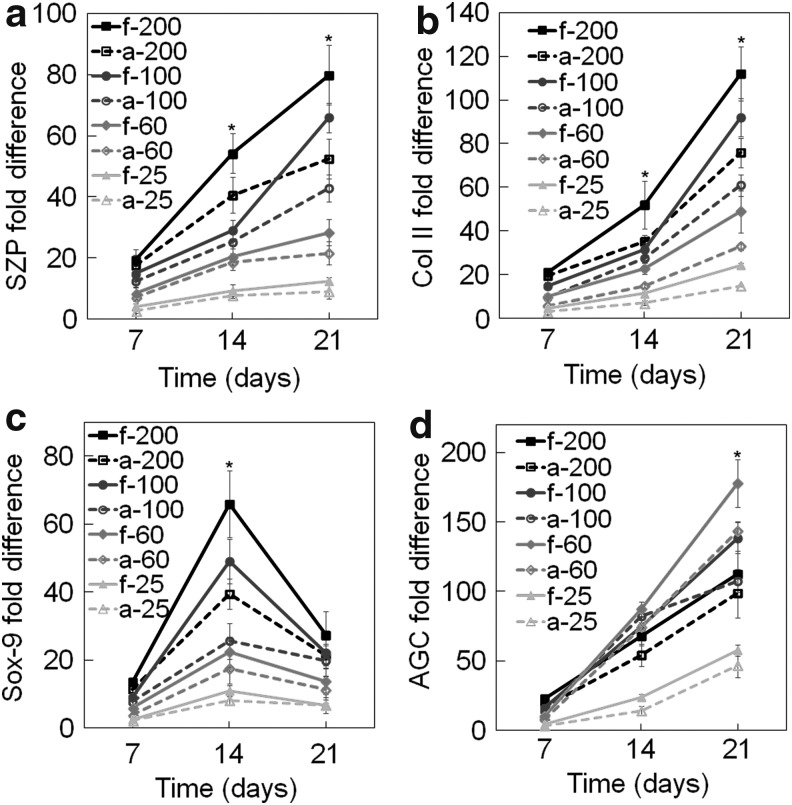
Supplementary Figure S3a–d shows live (green) and dead (red) stained images of hMSCs encapsulated in fetal CarMa matrix at cell densities of 25, 60, 100, and 200 million cells/mL 1 h after cell encapsulation. The fraction of viable hMSCs encapsulated in the CarMa matrix was >98% for all cell densities. The effect of CarMa source and cell seeding density on chondrogenic differentiation of hMSCs to the superficial zone phenotype was investigated by encapsulation of 25, 60, 100, or 200 million/mL hMSCs in CarMa and incubation in chondrogenic medium supplemented with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7 for 21 days. Figure 2a–d shows mRNA expression of SZP, Col II, Sox-9, and AGC for hMSCs encapsulated in CarMa hydrogels as a function of the initial cell density with incubation time. The SZP expression of hMSCs encapsulated in CarMa hydrogels (Fig. 2a) increased from days 7 to 21 for all cell densities. For a given cell density, the SZP expression in f-CarMa hydrogel was higher than a-CarMa. For example, the SZP expression in a-CarMa hydrogel with initial densities of 100 and 200 million cells/mL was 43.0 ± 4.5 and 52.4 ± 6.5, respectively, after 21 days compared with 66.0 ± 4.8 and 80.0 ± 9.8 for f-CarMa. The SZP expression of CarMa hydrogels increased with cell density for all time points. For example, the SZP expression in f-CarMa hydrogel for cell densities of 25, 60, 100, and 200 million/mL after 21 days increased from 12.4 ± 1.3 to 28.4 ± 4.3, 66.0 ± 4.8 and 80.0 ± 9.8, respectively.
FIG. 2.
Effect of CarMa source (fetal vs. adult) and cell density on mRNA expression of (a) SZP, (b) Col II, (c) Sox-9, and (d) AGC of the encapsulated hMSCs incubated in chondrogenic medium/TGF-β1 supplemented with BMP-7 for 21 days. Groups included 200 million cells/mL in f-CarMa (f-200, solid black) and a-CarMa (a-200, dashed black) hydrogels, 100 million cells/mL in f-CarMa (f-100, solid dark grey) and a-CarMa (a-100, dashed dark grey), 60 million cells/mL in f-CarMa (f-60, solid medium grey) and a-CarMa (a-60, dashed medium grey), and 25 million cells/mL in f-CarMa (f-25, solid light grey) and a-CarMa (a-25, dashed light grey). An asterisk (*) indicates a statistically significant difference (p < 0.05) between the test group and all other groups for a given time point. AGC, aggrecan; Col II, collagen type II; SZP, superficial zone protein.
The mRNA expression of Col II for hMSCs (Fig. 2b) encapsulated in CarMa hydrogels increased with incubation time for all cell densities. As the matrix was changed from a-CarMa to f-CarMa, the Col II expression for cell densities of 25, 60, 100, and 200 million/mL increased by 1.62, 1.49, 1.51, and 1.48 folds, respectively. The mRNA expression of Sox-9 for hMSCs (Fig. 2c) encapsulated in CarMa hydrogels increased initially from days 7 to 14 and then decreased from days 14 to 21. The Sox-9 expression in CarMa hydrogels increased significantly with cell density for all time points. The Sox-9 expression in f-CarMa hydrogel at day 14 was significantly higher than a-CarMa for all cell densities. As the matrix was changed from a-CarMa to f-CarMa with cell densities of 25, 60, 100, and 200 million/mL, the Sox-9 expression increased by 1.32, 1.27, 1.92, and 1.67 folds, respectively. The mRNA expression of AGC for hMSCs in CarMa hydrogels increased with incubation time (Fig. 2d). The AGC expression in CarMa hydrogels after 21 days increased initially with cell density from 25 to 60 million/mL and then decreased for 100 and 200 million/mL cell densities. The difference in AGC expression after 21 days was significant for cell densities of 60 and 100 million/mL, whereas the difference was not significant for cell densities of 25 or 200 million/mL.
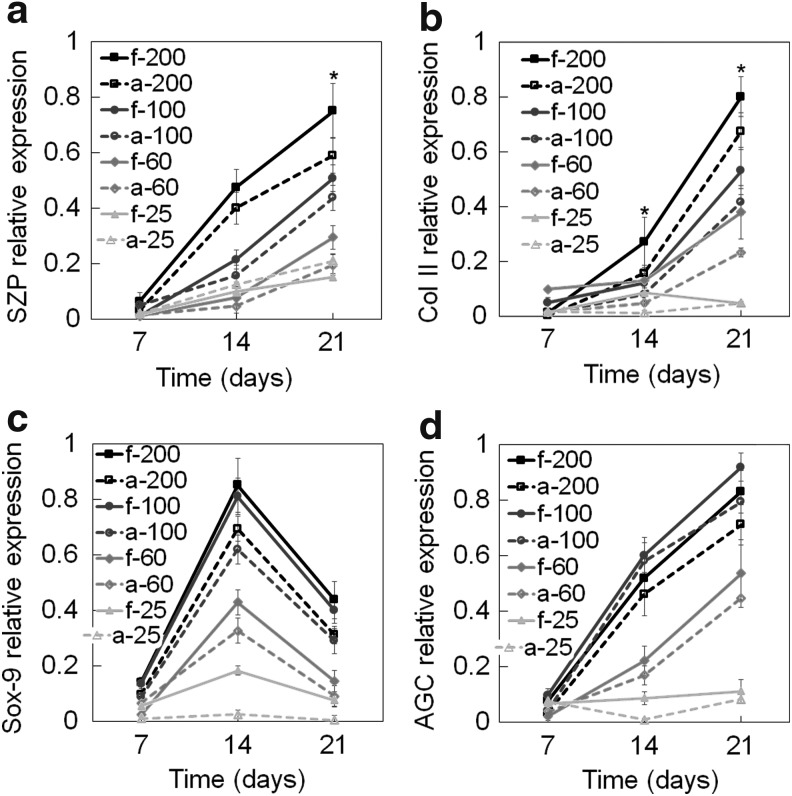
Figure 3a–d shows the protein expression of SZP, Col II, Sox-9, and AGC of hMSCs encapsulated in CarMa hydrogels with incubation time. The relative SZP protein expressions increased significantly from days 7 to 21 and for all cell densities (Fig. 3a). The protein expression of SZP in f-CarMa hydrogel after 21 days was significantly higher than a-CarMa for cell densities of 60, 100, and 200 million/mL, but the difference was not significant for 25 million cells/mL. The SZP expression in CarMa hydrogels after 21 days increased with cell density, except for a-CarMa from 25 to 60 million cells/mL. The protein expression of Col II for hMSCs in CarMa hydrogels increased with cell density for all time points, except for 25 million cells/mL (Fig. 3b). The Col II protein expressions in CarMa hydrogels at day 21 with increasing cell density (Fig. 3b) were consistent with Col II mRNA expressions in Figure 2b. The Col II protein expression in f-CarMa hydrogels at day 21 was higher than a-CarMa for all cell densities, except for 25 million cells/mL. The protein expression of Sox-9 for hMSCs in CarMa hydrogels initially increased from days 7 to 14 and then decreased from days 14 to 21 for all cell densities (Fig. 3c), consistent with the mRNA expressions in Figure 2c. The Sox-9 protein expression at day 14 increased with cell density in CarMa hydrogels and the expressions in f-CarMa were higher than a-CarMa. The protein expression of AGC (Fig. 3d) of hMSCs in CarMa hydrogels increased with incubation time for all cell densities except for 25 million/mL cell density. The AGC protein expression in f-CarMa at day 21 was higher than a-CarMa for all cell densities. The AGC protein expression in CarMa hydrogels at day 21 initially increased with cell density from 25 million/mL to 60 and 100 million/mL and then decreased slightly from 100 to 200 million cells/mL. The f-CarMa hydrogel with 100 million cells/mL and a-CarMa with 25 million cells/mL had the highest and lowest AGC protein expression, respectively.
FIG. 3.
Effect of CarMa source (fetal vs. adult) and cell density on protein expression of (a) SZP, (b) Col II, (c) Sox-9, and (d) AGC of the encapsulated hMSCs incubated in chondrogenic medium/TGF-β1 supplemented with BMP-7 for 21 days. Groups included 200 million cells/mL in f-CarMa (f-200, solid black) and a-CarMa (a-200, dashed black) hydrogels, 100 million cells/mL in f-CarMa (f-100, solid dark grey) and a-CarMa (a-100, dashed dark grey), 60 million cells/mL in f-CarMa (f-60, solid medium grey) and a-CarMa (a-60, dashed medium grey), and 25 million cells/mL in f-CarMa (f-25, solid light grey) and a-CarMa (a-25, dashed light grey). An asterisk (*) indicates a statistically significant difference (p < 0.05) between the test group and all other groups for a given time point.
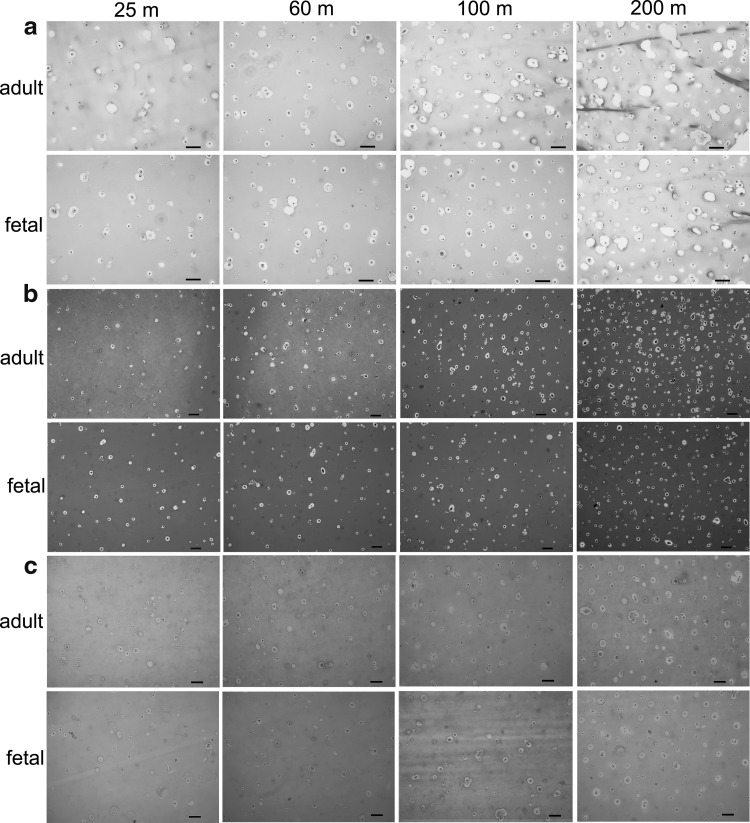
Images in Figure 4a compare H&E-stained sections of hMSCs encapsulated in CarMa hydrogels with cell densities of 25, 60, 100, and 200 million/mL after 21 days. The cells had a rounded morphology regardless of cell density and CarMa source. Images in Figure 4b compare collagen-stained sections of hMSCs in CarMa hydrogels with cell densities of 25, 60, 100, and 200 million/mL after 21 days. The cells in CarMa hydrogels with density of 200 million/mL showed highest collagen content and the staining intensity of f-CarMa was slightly higher than a-CarMa. Images in Figure 4c compare GAG-stained sections (Alcian blue) of hMSCs in CarMa hydrogels with cell densities of 25, 60, 100, and 200 million/mL after 21 days. The CarMa hydrogels with intermediate cell densities of 60 and 100 million/mL had higher GAG staining than those with low (25 million/mL) and high (200 million/mL) cell densities.
FIG. 4.
H&E- (a), collagen- (b), and GAG- (c) stained histological sections of hMSCs encapsulated in f-CarMa and a-CarMa hydrogels with cell densities of 25 (25 m), 60 (60 m), 100 (100 m), and 200 (200 m) million/mL after 21 days of incubation in chondrogenic medium/TGF-β1 supplemented with BMP-7. The scale bar in (a–c) is 20 μm. H&E, hematoxylin and eosin; GAG, glycosaminoglycan.
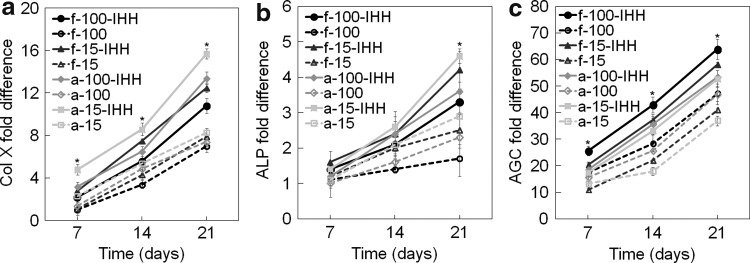
The effect of CarMa source and cell density on chondrogenic differentiation of hMSCs to the calcified zone phenotype was investigated by encapsulation of 15 or 100 million/mL hMSCs in CarMa hydrogels and incubation in chondrogenic medium supplemented with 30 ng/mL TGF-β1 with or without 2.5 μg/mL IHH. Figure 5a–c shows mRNA expression of Col X, ALP, and AGC of hMSCs encapsulated in CarMa hydrogels with incubation time. The mRNA expression of Col X increased with incubation time regardless of matrix source, cell density, or IHH addition. For example, Col X expression in f-CarMa hydrogels with 100 and 15 million cells/mL and with IHH increased by 1.53 and 1.58 folds, respectively, whereas the expression increased by 1.81 and 1.90 folds in a-CarMa. The Col X expression for those groups cultured without IHH was not significantly different. Conversely for the groups with IHH, the Col X expression in a-CarMa hydrogels was higher than f-CarMa for all cell densities. Furthermore, the Col X expression in CarMa hydrogels with IHH and cell density of 15 million/mL was higher than those hydrogels with 100 million cells/mL. The Col X expression of hMSCs in a-CarMa hydrogel with cell density of 15 million/mL cell and supplemented with IHH was highest for all time points.
FIG. 5.
Effect of CarMa source (fetal vs. adult), cell density, and exposure to IHH on mRNA expression of (a) Col X, (b) ALP, and (c) AGC of the encapsulated hMSCs incubated in chondrogenic medium/TGF-β1 with or without IHH for 21 days. Groups included 100 million cells/mL hMSCs in f-CarMa hydrogel with (f-100-IHH, solid black) or without (f-100, dashed black) IHH in the culture medium, 15 million cells/mL hMSCs in f-CarMa with (f-15-IHH, solid dark grey) or without (f-15, dashed dark grey) IHH, 100 million cells/mL hMSCs in a-CarMa with (a-100-IHH, solid medium grey) or without (a-100, dashed medium grey) IHH, 15 million cells/mL hMSCs in a-CarMa with (a-15-IHH, solid light grey) or without (a-15, dashed light grey) IHH. An asterisk (*) indicates a statistically significant difference (p < 0.05) between the test group and all other groups for a given time point. ALP, alkaline phosphatase.
ALP mRNA expression of the encapsulated hMSCs increased significantly with incubation time in CarMa hydrogels (Fig. 5b). The ALP expression in the groups incubated with IHH was significantly higher than those without IHH for all time points (Fig. 5b). The ALP expression in CarMa hydrogels increased with decreasing cell density from 100 to 15 million/mL after 21 days of incubation with or without IHH. Further for a given cell density, the ALP expression in a-CarMa hydrogels was slightly higher than f-CarMa. The hMSCs in a-CarMa hydrogel with cell density of 15 million/mL with IHH had highest ALP mRNA expression after 21 days. The AGC mRNA expression of the encapsulated hMSCs in CarMa hydrogels increased with incubation time (Fig. 5c). For a given cell density and CarMa source, the AGC expression after 21 days increased with IHH. The AGC expression in f-CarMa hydrogel after 21-day incubation increased with cell density with or without IHH. For a given cell density, the hMSCs encapsulated in f-CarMa hydrogel and incubated with IHH had higher AGC expression than those in a-CarMa. The hMSCs in f-CarMa hydrogel with 100 million/mL cell density and incubated with IHH had highest AGC mRNA expression.
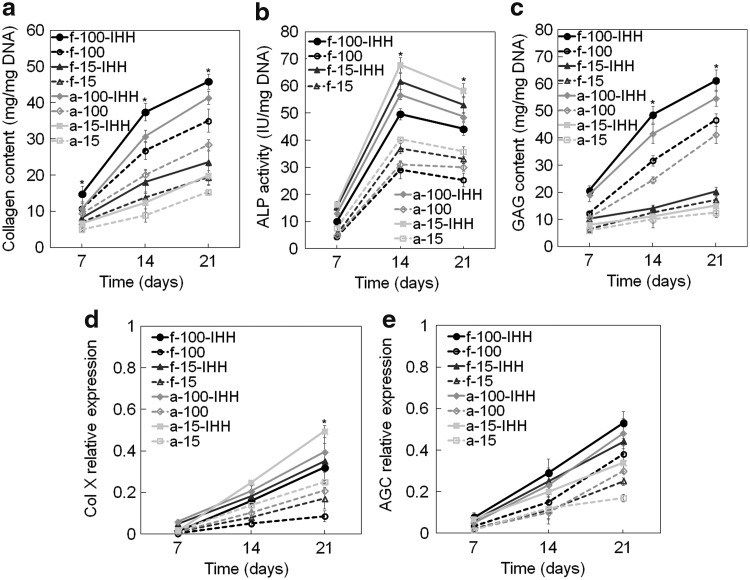
Figure 6a–c shows total collagen content, ALP activity, and GAG content of hMSCs encapsulated in CarMa hydrogels and incubated in chondrogenic medium supplemented with 30 ng/mL TGF-β1 with or without 2.5 μg/mL IHH. Total collagen content in all groups increased significantly with incubation time. Total collagen content at day 21 increased significantly with IHH regardless of cell density or CarMa source. For example, total collagen content in f-CarMa hydrogel at day 21 increased by 1.30 and 1.21 folds for cell densities of 100 and 15 million/mL, respectively, with the addition of IHH to the medium; that increase was 1.46 and 1.29 folds in a-CarMa hydrogel. For all cell densities, total collagen content in f-CarMa hydrogels with IHH was higher than a-CarMa for all time points. Furthermore, total collagen content increased significantly with increasing cell density from 15 to 100 million/mL for all time points regardless of CarMa source or IHH. The ALP activity of the encapsulated hMSCs of all groups initially increased from days 7 to 14 and then slightly decreased from days 14 to 21. For all time points, the ALP activity increased with IHH irrespective of CarMa source or cell density. For all cell densities, the peak ALP activity (day 14) in a-CarMa hydrogel was significantly higher than f-CarMa irrespective of IHH. The encapsulated hMSCs had higher peak ALP activity at the lower cell density of 15 million/mL compared with 100 million/mL, irrespective of CarMa source or IHH. The GAG content of the encapsulated hMSCs for all groups increased with incubation time (Fig. 6c). The GAG content for cell density of 100 million/mL after 21 days increased significantly with IHH. However, the addition of IHH to the medium did not significantly affect GAG content at the low cell density of 15 million/mL (after 21 days) regardless of CarMa source. The GAG content in CarMa hydrogels increased sharply with cell density from 15 to 100 million/mL irrespective of IHH. The hMSCs in f-CarMa hydrogel had higher GAG content than a-CarMa regardless of cell density or IHH.
FIG. 6.
Effect of CarMa source (fetal cartilage vs. adult), cell density and IHH on collagen content (a), ALP activity (b), GAG (c), Col X (d), and AGC protein expression (e) of the encapsulated hMSCs incubated in chondrogenic medium. Groups included 100 million cells/mL hMSCs in f-CarMa hydrogel with (f-100-IHH, solid black) or without (f-100, dashed black) IHH in the culture medium, 15 million cells/mL hMSCs in f-CarMa with (f-15-IHH, solid dark grey) or without (f-15, dashed dark grey) IHH, 100 million cells/mL hMSCs in a-CarMa with (a-100-IHH, solid medium grey) or without (a-100, dashed medium grey) IHH, and 15 million cells/mL hMSCs in a-CarMa with (a-15-IHH, solid light grey) or without (a-15, dashed light grey) IHH. An asterisk (*) indicates a statistically significant difference (p < 0.05) between the test group and all other groups for a given time point.
Figure 6d–e shows the protein expression of Col X and AGC for hMSCs encapsulated in CarMa hydrogels with incubation time. The Col X protein expression of all groups increased with incubation time (Fig. 6d), consistent with Col X mRNA expression in Figure 5a. The Col X expression in CarMa hydrogels increased with IHH for both cell densities of 15 and 100 million/mL. For all cell densities, hMSCs in a-CarMa hydrogels had higher Col X protein expression at day 21 compared with f-CarMa, with or without IHH. The Col X protein expression of hMSCs in a-CarMa hydrogels with IHH increased with decreasing cell density from 100 to 15 million/mL after 21 days. The hMSCs in a-CarMa hydrogel with cell density of 15 million/mL and with IHH had highest Col X expression after 21 days. The AGC protein expression of all groups increased with incubation time (Fig. 6e), consistent with the AGC mRNA expressions in Figure 5c. The AGC expression in CarMa hydrogels increased with IHH addition after 21 days. The AGC expression in CarMa hydrogels after 21 days increased with cell density from 15 to 100 million/mL regardless of IHH addition. For all cell densities, the hMSCs in f-CarMa hydrogel had higher AGC expression at day 21 compared with a-CarMa. The hMSCs in f-CarMa hydrogel with cell density of 100 million/mL and with IHH had highest AGC protein expression after 21 days.
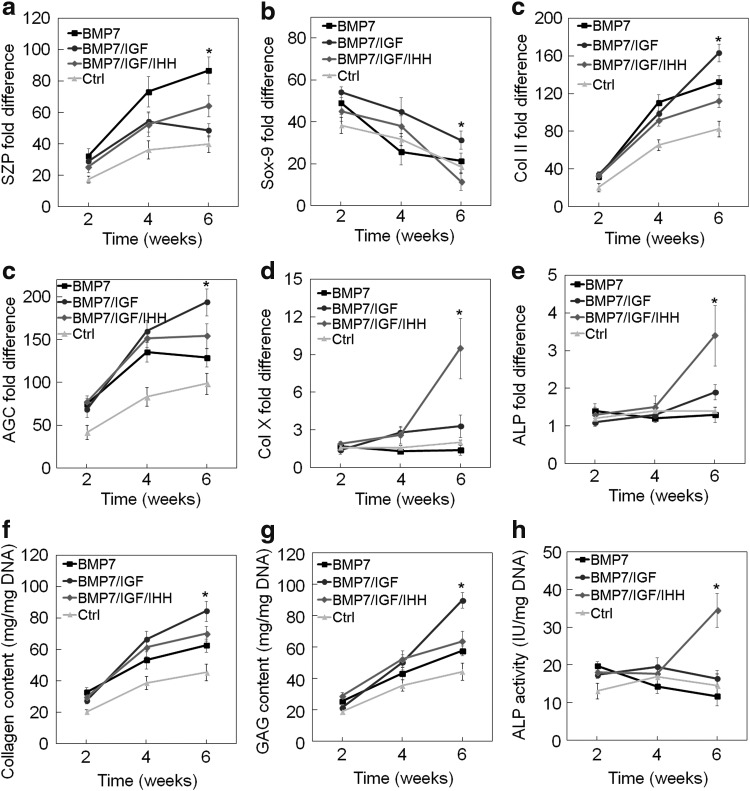
The effect of sequential addition of zone-specific growth factors on chondrogenic differentiation of hMSCs to superficial, middle and calcified zone phenotypes was investigated by encapsulation of 100 million cells/mL in f-CarMa hydrogel and incubation for 6 weeks. Experimental groups included hMSCs in f-CarMa hydrogel incubated in BM supplemented with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7 for 6 weeks (BMP-7 group); BM with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7 for 2 weeks followed by BM with 30 ng/mL TGF-β1, and 100 ng/mL IGF-1 for 4 weeks (BMP-7/IGF group); BM with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7 for 2 weeks followed by BM with 30 ng/mL TGF-β1 and 100 ng/mL IGF-1 for 2 weeks, and BM with 30 ng/mL TGF-β1 and 2.5 μg/mL IHH for 2 weeks (BMP-7/IGF/IHH group); and BM with 3 ng/mL TGF-β1 for 2 weeks followed by BM with 30 ng/mL TGF-β1 for 4 weeks (Ctrl group). Figure 7a–f shows mRNA expression of SZP, Sox-9, Col II, AGC, Col X, and ALP of hMSCs in f-CarMa hydrogel sequentially exposed to zone-specific growth factors over 6 weeks. In general, there was <10% change in dimensions of the cellular CarMa hydrogels with incubation time. The corresponding total collagen content, GAG content, and ALP activity of the hMSCs are given in Figure 7g–I, respectively. The SZP mRNA expression in BMP-7 group was significantly higher than BMP-7/IGF and BMP-7/IGF/IHH groups at weeks 4 and 6. The foregoing result indicated that the replacement of BMP-7 with IGF-1 or IHH reduced the maintenance of the superficial zone phenotype.
FIG. 7.
Effect of sequential addition of zone-specific growth factors to the chondrogenic medium/TGF-β1 on SZP (a), Sox-9 (b), Col II (c), AGC (d), Col X (e), and ALP (f) mRNA expression, collagen content (g), GAG content (h), and ALP activity (i) of hMSCs encapsulated in f-CarMa hydrogel. Groups included hMSCs in f-CarMa hydrogel incubated in BM supplemented with 3 ng/mL TGF-β1 for 2 weeks followed by BM with 30 ng/mL TGF-β1 for 4 weeks (Ctrl, light grey), hMSCs in CarMa hydrogel incubated in chondrogenic medium supplemented with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7 for 6 weeks (BMP-7, black), BM with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7 for 2 weeks followed by BM with 30 ng/mL TGF-β1 and 100 ng/mL IGF-1 for 4 weeks (BMP-7/IGF, dark grey), BM with 3 ng/mL TGF-β1 and 100 ng/mL BMP-7 for 2 weeks followed by BM with 30 ng/mL TGF-β1 and 100 ng/mL IGF-1 for 2 weeks and BM with 30 ng/mL TGF-β1 and 2.5 μg/mL IHH for 2 weeks (BMP-7/IGF/IHH, medium grey). An asterisk (*) indicates a statistically significant difference (p < 0.05) between the test group and all other groups for a given time point. BM, basal medium.
The Sox-9 mRNA expression in all groups decreased with incubation time over 6 weeks (Fig. 7b). The Sox-9 mRNA expression in BMP-7/IGF group was higher than the BMP-7 and BMP-7/IGF/IHH groups at weeks 4 and 6, respectively. The Col II and AGC mRNA expressions (Fig. 7c, d) as well as total collagen content (Fig. 7g) and GAG content (Fig. 7h) in all groups increased over 6 weeks. The Col II and AGC mRNA expressions, collagen content, and GAG content in BMP-7/IGF group were higher than BMP-7 and BMP-7/IGF/IHH groups after 6 weeks, whereas the difference between the three groups was not significant at 4 weeks. The forgoing results indicated that IGF-1 stimulated maturation of hMSCs preexposed to BMP-7 to the middle zone phenotype with 4 weeks of IGF-1 exposure more effective than 2 weeks. The mRNA expression of hypertrophic markers Col X and ALP (Fig. 7e–f) and ALP activity (Fig. 7i) of hMSCs preexposed to BMP-7 and IGF-1 (BMP-7/IGF/IHH group) increased with IHH exposure, whereas the marker expressions did not change for BMP-7 group or slightly changed for BMP-7/IGF group. The foregoing result indicated that IHH stimulated maturation of hMSCs preexposed to BMP-7 or BMP-7/IGF-1 to the calcified zone phenotype.
Discussion
Chondrocytes during fetal development produce more ECM than in the adult articular cartilage. Chondrocytes harvested from ovine fetal articular cartilage produced higher levels of GAG and collagen than the adult chondrocytes.12 Furthermore, the harvested ovine fetal chondrocytes seeded in polyglycolic acid (PGA) scaffolds had higher Col II expression than the adult chondrocytes.12 In addition, the Col II produced by the fetal chondrocytes in PGA scaffolds resembled the Col II in the native articular cartilage tissue.12 Our results demonstrate that hMSCs encapsulated in f-CarMa hydrogel had higher expression of superficial zone (SZP, Col II, and Sox-9) and middle zone (AGC) markers than a-CarMa (Figs. 2 and 3). Conversely, the expression of calcified zone markers (Col X and ALP) in a-CarMa hydrogel was higher than f-CarMa (Figs. 5 and 6). Therefore, f-CarMa matrix is superior for regenerating the superficial zone of articular cartilage, whereas a-CarMa is superior for regenerating the calcified zone. The difference in ECM production can be attributed to the compositional and microstructural differences between the corresponding fetal and adult articular cartilage tissues. There is a difference in Col IX and Col XI contents between fetal and adult articular cartilage.34 Approximately 10% of the total collagen in fetal articular cartilage is Col IX as opposed to 1% in the adult cartilage; 10% of the total collagen in fetal cartilage is Col XI as opposed to <3% in the adult cartilage.13 It was shown that the GAG content was lower but Col X expression was higher during cartilaginous matrix development in Col IX-deficient mice 13 days after fracture.35 Therefore, a higher Col X expression and lower AGC, Col II, and Sox9 expression of hMSCs in adult CarMa may be partially because of a significantly lower Col XI content compared with fetal CarMa. In addition, microstructural differences between the fetal and adult CarMa contribute to the variation in the expression of zone-specific markers. The collagen fibrils in fetal articular cartilage are thinner and less organized than the adult cartilage that can be attributed to the differences in Col IX and Col XI content.34 Furthermore, permeability of the adult articular cartilage matrix is less than the fetal cartilage.33 In that regard, permeability of human fetal articular cartilage decreases by 20% and stiffness increases by 2.3 folds from weeks 20 to 36 of gestation.33 It was shown previously that when MSCs were seeded into poly(ɛ-caprolactone) scaffolds, the expression ratio of Col II over Col I increased with increasing scaffold permeability.36
A key factor in the initial stage of chondrogenesis in vitro and in vivo is the extent of cell–cell interaction.37 The high cell density in the mesenchyme, formed by the condensation of MSCs during articular cartilage development, leads to robust cell–cell interaction,38,39 downregulation of Yes-associated protein, and upregulation of Smad signaling.40 The cell density in the adult articular cartilage decreases from 60 million/mL in the superficial zone to 20 and 15 million/mL in the middle and calcified zones, respectively,15 which is almost an order of magnitude lower than the cell densities in fetal cartilage.14 Therefore, the higher Col II expression of the encapsulated hMSCs in CarMa hydrogels at high cell densities (100 million cells/mL) can be attributed to the higher interaction between the encapsulated cells, as observed in the fetal cartilage.12 Similarly, the upregulation of superficial zone markers at high cell densities (SZP and Col II in Figs. 2 and 3), the calcified zone markers at low densities (Col X and ALP in Figs. 5 and 6), and the middle zone markers at intermediate densities (AGC in Figs. 2 and 3) can be attributed to the zonal differences in cell density. Several studies have shown a direct correlation between cell density and the expression of superficial and middle zone markers. The Col II, AGC, and GAG expression of ovine bone marrow-derived MSCs encapsulated in a Col I matrix increased with increasing cell density from 0.5 to 50 million/mL.41 The GAG production by hMSCs encapsulated in alginate gels and cultured in chondrogenic medium peaked at 25 million cells/mL as cell density increased from 1.6 to 50 million/mL.42 The Col X expression by chondroprogenitor cells encapsulated in alginate beads and cultured in chondrogenic medium decreased as the cell density increased from 4 to 70 million/mL, whereas Col II expression increased.43 Our results show that cell density in the hydrogel significantly affected differentiation to zone-specific phenotypes of articular cartilage. The GAG expression of the encapsulated hMSCs peaked at cell density of 60 million/mL (Figs. 2 and 3), whereas the expression of calcified zone markers (Col X and ALP) decreased and total collagen increased with increasing cell density (Figs. 5 and 6). Although low-to-intermediate cell densities (<60 million cells/mL) strongly affected zone-specific differentiation of hMSCs, CarMa source had a noticeable effect at high cell densities (Figs. 2 and 3). For example, regardless of CarMa source, the encapsulated hMSCs with cell density of 60 million/mL had higher SZP and Col II expression at day 21 and Sox-9 expression at day 14 compared with 25 million cells/mL. On the contrary, SZP and Col II expressions at day 21 and Sox-9 expression at day 14 for hMSCs in f-CarMa hydrogel with 100 million cells/mL was higher than a-CarMa with 200 million cells/mL.
The results in Figures 5 and 6 demonstrate that the expression of calcified zone-specific markers (Col X and ALP) of hMSCs in CarMa hydrogels increased by supplementing the medium with IHH. It is known that IHH expression by prehypertrophic chondrocytes induces hypertrophy in mature chondrocytes39,44 through a PTHrP-independent pathway, which downregulates Sox-9 expression and activates Wnt/β-catenin and BMP pathways.11,45–47 In a previous study, concomitant transfection of hMSCs in pellet culture with IHH and TGF-β1 increased the expression of hypertrophic markers ALP and Col X as well as GAG content,48 which was consistent with our results in Figure 6. In other studies, rabbit MSCs overexpressing IHH had higher expression of hypertrophic markers ALP and Col X compared with the untreated MSCs after 21-day incubation in chondrogenic medium,49 and chondrocytes overexpressing IHH, isolated from the middle zone of articular cartilage, underwent hypertrophy and differentiated into the calcified zone chondrocytes.9,50
Several studies suggest that the superficial zone chondrocytes have the capacity to differentiate and mature to the middle and calcified zone chondrocytes.9,51,52 For example, labeled mouse superficial zone chondrocytes differentiated into mature middle and calcified zone chondrocytes in vivo.52 Therefore, the middle and calcified zones of articular cartilage can potentially be generated from hMSCs encapsulated in a superficial zone-like matrix with sequential addition of middle (IGF-1) and calcified (IHH) zone-specific growth factors. Our results show that hMSCs encapsulated in CarMa hydrogels and differentiated to the superficial zone phenotype of articular cartilage can be further differentiated and matured to the middle and calcium zone phenotypes by replacement of BMP-7 in the culture medium with IGF-1 and IHH, respectively (Fig. 7a). Furthermore, the results show that 4 weeks of IGF-1 exposure to BMP-7 preexposed hMSCs was more effective for maturation of hMSCs to the middle zone phenotype compared with 2 weeks (Fig. 7b–d, g, h).
Conclusion
The effect of matrix source (f-CarMa vs. a-CarMa), cell density, and zone-specific growth factors (BMP-7, IGF-1, IHH) on chondrogenic differentiation of hMSCs encapsulated in CarMa hydrogels was investigated. Encapsulation of hMSCs in f-CarMa at high cell densities and cultivation in chondrogenic medium supplemented with BMP-7 stimulated differentiation to the superficial zone phenotype of articular cartilage, whereas encapsulation in a-CarMa at low cell densities and the medium supplementation with IHH stimulated differentiation to the calcified zone. Encapsulation of hMSCs in CarMa (fetal or adult) at intermediate cell densities and medium supplementation with IGF-1 stimulated differentiation of hMSCs to the middle zone phenotype. Sequential addition of IGF-1 to the chondrogenic medium stimulated differentiation of the encapsulated hMSCs, preexposed to BMP-7, to the middle zone phenotype. Furthermore, sequential addition of IHH to the chondrogenic medium stimulated maturation of the encapsulated hMSCs, preexposed to BMP-7 and IGF-1, to the calcified zone phenotype. The results are potentially useful for engineering injectable, monolayer, cell-laden hydrogels with sequential release of growth factors for regeneration of full-thickness articular cartilage defects.
Supplementary Material
Acknowledgments
This work was supported by research grants to E. J. from the National Science Foundation under Award Numbers CBET1403545 and IIP150024 and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR063745. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Seyed Ramin Pajoum Shariati for assistance with synthesis of CarMa hydrogels.
Disclosure Statement
No competing financial interests exist.
References
- 1. Archer C., and Ralphs J. Regenerative Medicine and Biomaterials for the Repair of Connective Tissues. Boca Raton, FL: CRC Press, 2010 [Google Scholar]
- 2. Blalock D., Miller A., Tilley M., and Wang J. Joint instability and osteoarthritis. Clin Med Insights Arthritis Musculoskelet Disord 8, 15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivera J.C., Wenke J.C., Buckwalter J.A., Ficke J.R., and Johnson A.E. Posttraumatic osteoarthritis caused by battlefield injuries: the primary source of disability in warriors. J Am Acad Orthop Surg 20, S64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lotz M.K., Kraus V.B. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther 12, 211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falah M., Nierenberg G., Soudry M., Hayden M., and Volpin G. Treatment of articular cartilage lesions of the knee. Int Orthop 34, 621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahmoudifar N., and Doran P.M. Chondrogenesis and cartilage tissue engineering: the longer road to technology development. Trends Biotechnol 30, 166, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Lefebvre V., and Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol 90, 291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu L., Bluguermann C., Kyupelyan L., et al. Human developmental chondrogenesis as a basis for engineering chondrocytes from pluripotent stem cells. Stem Cell Rep 1, 575, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L., Newton P.T., Bouderlique T., et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J 31, 1067, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verschure P.J., Marle J.V., Joosten L.A., Helsen M.M., Lafeber F.P., and Berg W.B. Localization of insulin-like growth factor-1 receptor in human normal and osteoarthritic cartilage in relation to proteoglycan synthesis and content. Br J Rheumatol 35, 1044, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Mak K.K., Kronenberg H.M., Chuang P.T., Mackem S., and Yang Y.Z. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 135, 1947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuchs J.R., Terada S., Hannouche D., Ochoa E.R., Vacanti J.P., and Fauza D.O. Engineered fetal cartilage: structural and functional analysis in vitro. J Pediatr Surg 37, 1720, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Eyre D. Collagen of articular cartilage. Arthritis Res 4, 30, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilmore R.S., and Palfrey A.J. Chondrocyte distribution in the articular-cartilage of human femoral condyles. J Anat 157, 23, 1988 [PMC free article] [PubMed] [Google Scholar]
- 15. Karimi T., Barati D., Karaman O., Moeinzadeh S., and Jabbari E. A developmentally inspired combined mechanical and biochemical signaling approach on zonal lineage commitment of mesenchymal stem cells in articular cartilage regeneration. Integr Biol 7, 112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moeinzadeh S., Pajoum Shariati S.R.P., and Jabbari E. Comparative effect of physicomechanical and biomolecular cues on zone-specific chondrogenic differentiation of mesenchymal stem cells. Biomaterials 92, 57, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoshiba T., Kawazoe N., and Chen G.P. The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development. Biomaterials 33, 2025, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Visser J., Levett P.A., te Moller N.C.R., et al. Crosslinkable hydrogels derived from cartilage, meniscus, and tendon tissue. Tissue Eng A 21, 1195, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barati D., Shariati S.R.P., Moeinzadeh S., Melero-Martin J.M., Khademhosseini A., and Jabbari E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J Contr Rel 223, 126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., and Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 1, 31, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Bozec L., and Odlyha M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys J 101, 228, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClain P.E., and Wiley E.R. Differential scanning calorimeter studies of the thermal transitions of collagen. implications on structure and stability. J Biol Chem 247, 692, 1972 [PubMed] [Google Scholar]
- 23. Hoch E., Schuh C., Hirth T., Tovar G.E.M., and Borchers K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J Mater Sci Mater Med 23, 2607, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Wang Z.J., Tian Z.L., Menard F., and Kim K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 9, 044101, 2017 [DOI] [PubMed] [Google Scholar]
- 25. Moeinzadeh S., Barati D., He X., and Jabbari E. Gelation characteristics and osteogenic differentiation of stromal cells in inert hydrolytically degradable micellar polyethylene glycol hydrogels. Biomacromolecules 13, 2073, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Barati D., Kader S., Pajoum Shariati S.R.P., Moeinzadeh S., Sawyer R.H., and Jabbari E. Synthesis and characterization of photo-cross-linkable keratin hydrogels for stem cell encapsulation. Biomacromolecules 18, 398, 2017 [DOI] [PubMed] [Google Scholar]
- 27. Zuo Y.C., Liu X.L., Wei D., et al. Photo-cross-linkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Appl Mater Interfaces 7, 10386, 2015 [DOI] [PubMed] [Google Scholar]
- 28. Barati D., Moeinzadeh S., Karaman O., and Jabbari E. Time dependence of material properties of polyethylene glycol hydrogels chain extended with short hydroxy acid segments. Polymer 55, 3894, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Correia C.R., Moreira-Teixeira L.S., Moroni L., et al. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng C Methods 17, 717, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Henderson J.A., He X., and Jabbari E. Concurrent differentiation of marrow stromal cells to osteogenic and vasculogenic lineages. Macromol Biosci 8, 499, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Livak K.J., and Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Shin H., Olsen B.D., and Khademhosseini A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 33, 3143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahmoodian R., Leasure J., Philip P., Pleshko N., Capaldi F., and Siegler S. Changes in mechanics and composition of human talar cartilage anlagen during fetal development. Osteoarthritis Cartilage 19, 1199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hagg R., Bruckner P., and Hedbom E. Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX. J Cell Biol 142, 285, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Opolka A., Ratzinger S., Schubert T., et al. Collagen IX is indispensable for timely maturation of cartilage during fracture repair in mice. Matrix Biol 26, 85, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Kemppainen J.M., and Hollister S.J. Differential effects of designed scaffold permeability on chondrogenesis by chondrocytes and bone marrow stromal cells. Biomaterials 31, 279, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Tew S.R., Murdoch A.D., Rauchenberg R.P., and Hardingham T.E. Cellular methods in cartilage research: primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods 45, 2, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Las Heras F., Gahunia H.K., and Pritzker K.P. Articular cartilage development: a molecular perspective. Orthop Clin North Am 43, 155, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Gadjanski I., Spiller K., and Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Rev 8, 863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karystinou A., Roelofs A.J., Neve A., Cantatore F.P., Wackerhage H., and De Bari C. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res Ther 17, 147, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bornes T.D., Jomha N.M., Mulet-Sierra A., and Adesida A.B. Optimal seeding densities for in vitro chondrogenesis of two- and three-dimensional isolated and expanded bone marrow-derived mesenchymal stromal stem cells within a porous collagen scaffold. Tissue Eng C Methods 22, 208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kavalkovich K.W., Boynton R.E., Murphy J.M., and Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim 38, 457, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Bernstein P., Dong M., Graupner S., et al. Sox9 expression of alginate-encapsulated chondrocytes is stimulated by low cell density. J Biomed Mater Res A 91, 910, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Mackie E.J., Tatarczuch L., and Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol 211, 109, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Akiyama H., Lyons J.P., Mori-Akiyama Y., et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 18, 1072, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karimi T., Moeinzadeh S., and Jabbari E. Growth factors for musculoskeletal tissue engineering. In: Nukavarapu S., Freeman J., and Laurencin C., eds. Regenerative engineering of musculoskeletal tissues and interfaces. Cambridge, UK: Woodhead Publishing, 2015, pp. 43–76. Chap. 3 [Google Scholar]
- 47. Shimoyama A., Wada M., Ikeda F., et al. Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol Biol Cell 18, 2411, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steinert A.F., Weissenberger M., Kunz M., et al. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther 14, R168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu P.C., Liu K., Liu J.F., Xia K., Chen L.Y., and Wu X. Transfection of the IHH gene into rabbit BMSCs in a simulated microgravity environment promotes chondrogenic differentiation and inhibits cartilage aging. Oncotarget 7, 62873, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amano K., Densmore M., Nishimura R., and Lanske B. Indian hedgehog signaling regulates transcription and expression of collagen type x via runx2/smads interactions. J Biol Chem 289, 24898, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dowthwaite G.P., Bishop J.C., Redman S.N., et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci 117, 889, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Kozhemyakina E., Zhang M.J., Ionescu A., et al. Identification of a PRG4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol 67, 1261, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.