Abstract
Purpose:
Evidence suggests that femoroacetabular impingement (FAI) in athletes may increase the risk of anterior cruciate ligament (ACL) injury. This study correlates ACL injury with hip range of motion in a consecutive series of elite, contact athletes and tests the hypothesis that a restriction in the available hip axial rotation in a dynamic in silico model of a simulated pivot landing would increase ACL strain and the risk of ACL rupture.
Methods:
Three hundred twenty-four football athletes attending the 2012 NFL National Invitational Camp were examined. Hip range of internal rotation was measured and correlated with a history of ACL injury and surgical repair. An in silico biomechanical model was used to study the effect of FAI on the peak relative ACL strain developed during a simulated pivot landing.
Results:
The in vivo results demonstrated that a reduction in internal rotation of the left hip was associated with a statistically significant increased odds of ACL injury in the ipsilateral or contralateral knee (OR = 0.95, p = 0.0001 and p < 0.0001, respectively). A post-estimation calculation of odds ratio for ACL injury based on deficiency in hip internal rotation demonstrated that a 30-degree reduction in left hip internal rotation was associated with 4.06 and 5.29 times greater odds of ACL injury in the ipsilateral and contralateral limbs, respectively. The in silico model demonstrated that FAI systematically increased the peak ACL strain predicted during the pivot landing.
Conclusion:
FAI may be associated with ACL injury because of the increased resistance to femoral internal axial rotation during a dynamic maneuver such as a pivot landing. This insight may lead to better interventions to prevent ACL injury and improved understanding of ACL reconstruction failure.
Level of Evidence:
Cohort study, level IV.
Keywords: hip, anterior cruciate ligament, femoroacetabular impingement, football, knee, in silico model
Introduction
The spectrum of complex structural anatomic variants of the hip joint and their loading characteristics in the athlete is a growing concern [3]. Femoroacetabular impingement (FAI), femoral or acetabular retroversion, and extra-articular impingement can result in repetitive collisions and microtrauma with activity [1,16]. These morphological variants are most commonly associated with restrictions in internal rotation of the hip and regional loading of the femoral head-neck junction against the acetabular rim. These abnormal kinematics are clearly associated with labral tears, chondral delamination, and a degenerative cascade of intra-articular hip injury and secondary osteoarthrosis [16].
In addition to direct injury to the hip joint, restrictions in hip range of motion resulting in abnormal kinematics may injure joints that are proximal and distal in the kinetic chain. A hallmark of the elite athlete is the ability to recruit muscles to develop larger peak forces, joint torques, and hence body segment velocities to perform high-demand physical tasks. In this regard, compensatory increases in motion of the hemipelvis and lumbar spine may be necessary to achieve functional range of motion for sport [6]. The association of restricted hip motion with athletic pubalgia, sports hernia [13,15], adductor and hip flexor injury, lumbar and sacroiliac (SI) stress injury, increased motion across the pubic symphysis [4], and even femoral stress fractures is well known [3,15,28].
While these compensatory mechanisms of injury secondary to restricted hip motion are being increasingly recognized [3,15,28], the impact of restricted hip motion on the risk of knee injury has not been defined. Two studies have suggested a relationship between radiographic indicators of hip impingement and an increased risk for anterior cruciate ligament (ACL) injury [7,11]. However, these observational studies have not demonstrated any clinical or biomechanical support for an association between ACL rupture and FAI.[8,24]
Noncontact cutting and pivoting mechanisms are responsible for as many as 80% of anterior cruciate ligament tears in athletes [25]. A jump landing, pivot or braking maneuver that develops a large axial tibial internal rotation torque results in particularly high peak ACL strain [22,23]. Hence, restriction of internal rotation at the hip may require athletes to achieve a greater range of internal rotation of the tibia to successfully complete the athletic task [2,14]. We hypothesized that a deficiency in hip internal rotation secondary to abnormal proximal femoral or acetabular morphology may result in compensatory increases in stresses applied to the ACL with cutting and pivoting activities, thereby increasing the risk of ACL failure in athletes. The purpose of this study was to correlate ACL injury with hip range of motion in a consecutive series of elite, contact athletes. In addition, we sought to test the hypothesis that a restriction in the available hip axial rotation in a dynamic in silico model of a simulated pivot landing would increase the resulting peak ACL strain, thereby increasing the risk of ACL rupture.
Materials and Methods
This study was approved by the Safety and Injury Committee of the National Football League (NFL). History and physical examination of 324 football athletes was performed at the 2012 NFL National Invitational Camp, commonly referred to as the Scouting Combine. All players had the history and physical examination by one of three orthopaedic surgeons with fellowship training in sports medicine. Previous injuries resulting in missed games or practices were reported, including hip muscle strains (adductors, hip flexors, hamstrings), hip pointer/iliac contusion, athletic pubalgia, and osteitis pubis. Previous ACL injury was documented from an injury report as well as a complete clinical evaluation.
Hip range of motion was assessed prospectively in bilateral lower extremities in all participating athletes, including a measure of hip flexion and internal rotation at 90 degrees of hip flexion while stabilizing the pelvis to engage the anterior femoral head-neck junction with the acetabular rim. Lachman, anterior drawer, and pivot-shift exams were also performed on all athletes. Previous ACL injury requiring one or more surgical reconstructions was documented based upon physical examination, presence of surgical scars, and confirmed by injury report.
Biomechanical Study
The following in vitro and in silico studies are exempted from Institutional Review Board review.
The in vitro experimental apparatus used was described by Oh et al. [22,23] and was designed to simulate a pivot landing with a restricted range of hip motion. The knee specimen was dissected leaving the knee joint ligamentous and capsular structures and then was inversely placed in the testing apparatus. Each muscle-tendon unit was pretensioned (i.e., 180 N for quadriceps and 70 N each for medial and lateral hamstrings and gastrocnemius) to maintain the initial knee flexion angle at 15 degrees. The drop-weight (‘W’ in Fig. 1B) was released onto the distal tibia of the inverted knee specimen to simulate an impulsive joint compressive force and knee flexion. The internal tibial torque was simultaneously applied to the distal tibia by using the custom designed torque converter (‘T’ in Fig. 1B). The knee abduction moment was also applied to the knee joint by abducting the knee specimen. The 3-dimensional (3D) knee joint kinematics were recorded using the Certus Optotrak motion capture camera system. The 3D forces and moments at each end of the tibia and femur were recorded (‘L’ in Fig. 1B). The ACL relative strain was recorded using a DVRT (Differential variable reluctance transducer) placed on the anteromedial (AM) bundle of the ACL, while the tensions for the five trans-knee muscle-tendon units were also monitored.
Fig. 1.
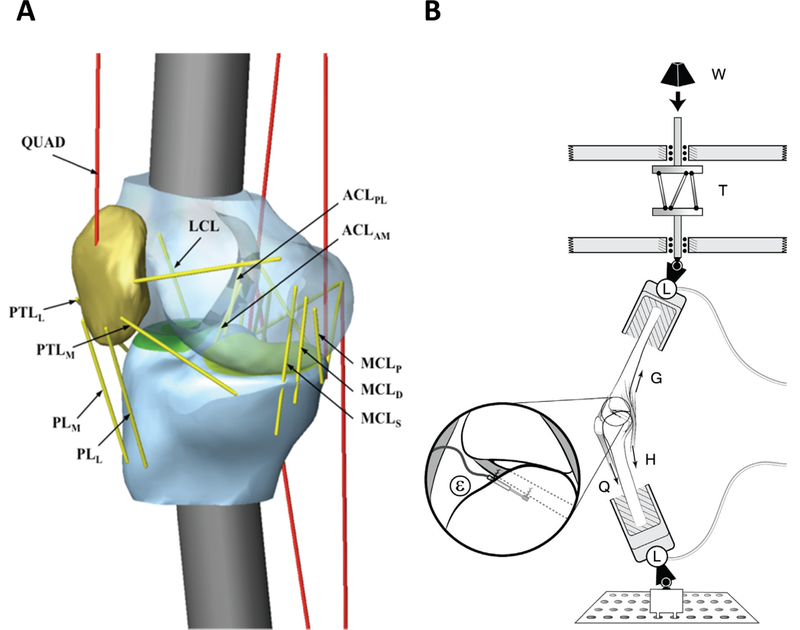
Schematic diagrams of (A) the in silico knee model and (B) the in vitro test apparatus
A 3D lower-limb in silico model (Fig. 1A) was constructed using T2-weighted sagittal magnetic resonance scans (TR/TE: 1000/35 ms, slice thickness: 0.35 mm, FOV: 160 mm) of a male cadaveric lower-limb (age: 47 yrs, weight: 778 N, height: 1.85 m). The segmented distal femur, proximal tibia, fibula and patella were imported into a dynamic motion simulation software (MD ADAMS R3, MSC Software, Inc., Santa Ana, CA) to replicate the in vitro experimental set-up (Fig. 1B). Tibia and femoral mass and moment of inertia data were calculated based on the cortical bone density obtained from the literature [18]. The knee joint ligamentous and capsular structure were modeled as viscoelastic elements (Appendix 1) [19,26,27]. As in the in vitro model, each muscle-tendon unit was pretensioned at the same values to achieve a static equilibrium at the initial knee flexion angle of 15 degrees. The impulsive compressive force, knee abduction moment, and internal tibial torque measured during the in vitro experiment were then applied to the distal tibia to drive the in silico knee model. For the in silico model validation, the quadriceps force, AM-ACL strain, knee flexion angle, knee abduction angle, and internal tibial rotation angle predicted from the simulation were quantitatively compared by Pearson cross-correlation with the corresponding values measured from the in vitro experiment. The Pearson cross-correlation coefficients were found to be r = 0.988, 0.985, 0.981, 0.976, and 0.817 for the quadriceps muscle force, AM-ACL strain, knee flexion angle, internal tibial rotation, and knee abduction angle, respectively (Appendix 2).
After the in silico model validation, the axial rotational stiffness of the hip joint was formulated to simulate the mechanical impingement that is associated with dynamic hip impingement (Fig. 2). Then, an internal rotation with the peak at 20 degrees along with the impulsive compressive force, knee flexion moment, and knee abduction moment were imposed on the distal tibia to investigate how the peak AM-ACL strain varies as the range of available hip internal rotation was systematically decreased to model the simulated impingement (Fig. 3). Since the in silico model was validated against representative trial data measured from a single specimen in the in vitro experiment, additional sensitivity analyses were performed. Previously, in a study in which the same in silico model was used, the effect of three morphological variables (lateral tibial slope, frontal plane limb alignment, and medial tibial concavity) on risk for ACL injury was investigated [21]. That study showed that peak AM-ACL strain was most sensitive to a change in the lateral tibial slope because the increased difference between medial and lateral tibial slopes accentuates the coupled motion between internal tibial rotation and knee abduction angle. Therefore, we varied the lateral tibial slope to determine whether our biomechanical findings from one model knee can be extrapolated to knees having other tibial slopes (Fig. 3).
Fig. 2.
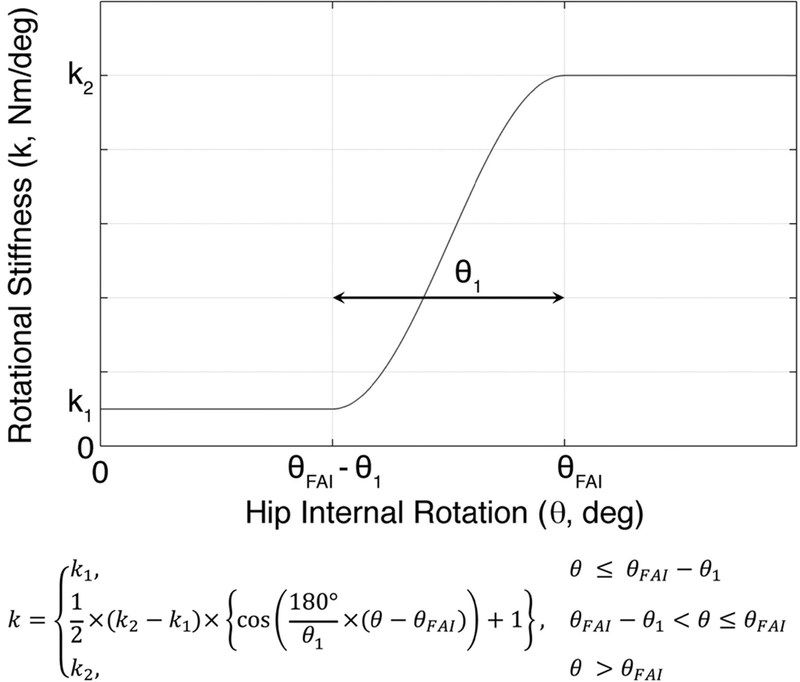
The axial hip rotational stiffness versus angular rotation relationship used to simulate femoracetabular impingement (FAI), where θ is the hip internal rotation angle; θFAI is the hip internal rotation angle at the end of the range of motion secondary to impingement; θ1 is the hip internal rotation angle where the impingement begins and is set to 5°; k1 is the stiffness coefficient when the impingement does not occur and is set to 0.5 Nm/deg; k2 is the stiffness coefficient when the hip internal rotation angle exceeds θFAI and is set to 5 Nm/deg
Fig. 3.
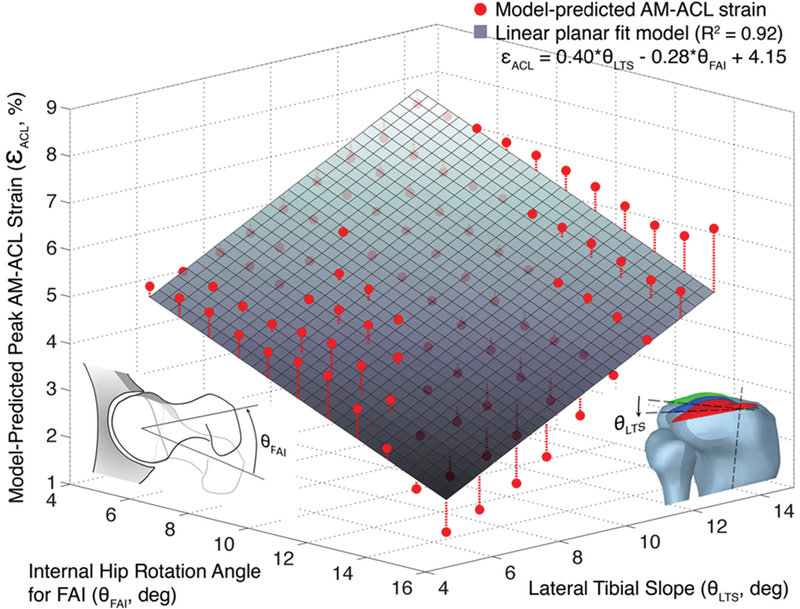
In silico model predictions for peak anteromedial bundle–anterior cruciate ligament (AM-ACL) strain during a simulated jump landing: as the available range of hip internal rotation is reduced, the peak AM-ACL strain in the knee increases. The linear planar fit model (shown as the grey plane) demonstrates how peak AM-ACL strain is predicted to be a function of both available axial hip range of motion as well as lateral tibial slope
Statistical Analysis
Clinical data from Excel (Microsoft, Redmond, Washington) spreadsheets were imported into SAS, version 9.3, statistical software (SAS Institute, Cary, North Carolina) for statistical analysis. Frequency measures were computed for all predictor and outcome variables; number and percent were calculated for each categorical variable, mean and standard deviation for each continuous variable. Chi-square tests of the categorical predictor variable “position” stratified by the outcome “ACL injury” were conducted to determine significance. For tests with cell counts of less than 5, Fisher’s Exact test was used. Generalized estimating equations (GEE) using logistic regression models were conducted to allow for adjustment for potential clustering effects by the variable “surgeon.” Univariable logistic regression was performed for each of the continuous predictor variables “IR left side” and “IR right side” on the binary outcome variables “ACL injury, left side” and “ACL injury, right side.” Coefficients resulting from these GEE logistic regression models were used to conduct post-estimation analysis to predict odds of injury for various IR values. Odds ratios were calculated from this post-estimation table to contrast estimated effects of different IR values. Odds ratios and 95% confidence intervals were produced in all analyses. An alpha level of 0.05 was considered significant for all tests.
Results
Clinical Study
A total of 34 ACL injuries (16 left and 18 right, respectively) requiring surgical reconstruction were noted in the 324 athletes, corresponding to a 10.5% prevalence of ACL injury in the study population (Table 1). While prevalence in the general population is not known, the annual incidence of primary ACL reconstructions has been reported to be 32 to 38 per 100,000 inhabitants in Scandinavian populations [12,17]. On the other hand, the population at risk—that is, the 16–39-year age group—had an incidence of between 71 to 91 primary ACL reconstructions per 100,000 Scandinavian inhabitants [12,17].
TABLE 1.
Descriptive Statistics
| N(%) | Mean(95%CI) | |
| Total | 324(100) | |
| Position | ||
| DB | 59(18.2) | |
| DL | 57(17.6) | |
| LB | 33(10.2) | |
| OL | 54(16.7) | |
| PK | 11(3.4) | |
| QB | 19(5.9) | |
| RB | 29(9.0) | |
| ST | 1(0.3) | |
| TE | 14(4.3) | |
| WO | 47(14.5) | |
| Left internal rotation | 21.2(20.1, 22.4) | |
| Right internal rotation | 21.3(20.1, 22.4) | |
| ACL injury | 34(10.5) | |
| Games missed | 2.3(1.9, 2.8) | |
| Knee injury | 158(48.8) | |
| Hip injury | 151(46.6) | |
| Foot injury | 214(66.1) | |
| Spine injury | 51(15.7) | |
| Injuries per person* | 2.6(2.4, 2.7) |
Range of injuries per person = 0–9; no data on recurrent injuries are included.
ACL = anterior cruciate ligament, DB = defensive back, DL = defensive line, LB = linebacker, OL = offensive line, PK = place kicker, QB = quarterback, RB = running back, ST = safety, TE = tight end, WO = wide out
A total of 831 injuries of the hip, knee, foot, and spine resulted in lost game or practice time.
An increased prevalence of ACL injury was noted in defensive linemen, running backs, and quarterbacks compared to other football positions, but none achieved statistical significance (Table 2).
TABLE 2.
Prevalence of Injury by Position.
| N (% total) |
ACL (%) |
P-value | |
|---|---|---|---|
| Position | |||
| DB | 59(18.2) | 2(3.4) | n.s. |
| DL | 57(17.6) | 7(12.3) | n.s.* |
| LB | 33(10.2) | 3(9.1) | n.s. |
| OL | 54(16.7) | 5(9.3) | n.s. |
| PK | 11(3.4) | 1(9.1) | n.s. |
| QB | 19(5.9) | 3(15.8) | n.s. |
| RB | 29(9.0) | 4(13.8) | n.s. |
| ST | 1(0.3) | 0(0) | n.s. |
| TE | 14(4.3) | 1(7.1) | n.s. |
| WO | 47(14.5) | 4(8.5) | n.s. |
P Values from Fisher’s Exact Tests
Chi-squared Test
DB = defensive back, DL = defensive line, LB = linebacker, OL = offensive line, PK = place kicker, QB = quarterback, RB = running back, ST = safety, TE = tight end, WO = wide out n.s. = nonsignificant
The results of the GEE logistic regression analysis of ACL injury demonstrates that a reduction in internal rotation of the left hip was associated with a statistically significant increased odds of ACL injury in the ipsilateral or contralateral knee (OR = 0.95, p = 0.0001 and p < 0.0001, respectively). All models are adjusted for potential clustering effects by surgeon. A reduction in internal rotation of the right hip also trended towards an increased odds of ACL injury, but this trend was nonsignificant (Table 3).
TABLE 3.
Logistic Regression of Internal Rotation (IR) as a Predictor of Anterior Cruciate Ligament (ACL) Injury Group by Side, from Generalized Estimating Equations Adjusted for Clustering by Surgeon
| Odds Ratio | 95% Confidence Interval |
P-value | |
|---|---|---|---|
| ACL (L) | |||
| IR Left | 0.95 | 0.93, 0.98 | 0.0001 |
| IR Right | 0.95 | 0.93, 0.97 | <0.0001 |
| ACL (R) | |||
| IR Left | 0.97 | 0.92, 1.02 | n.s. |
| IR Right | 0.95 | 0.89, 1.01 | n.s. |
A post-estimation calculation of odds ratio for ACL injury based on deficiency in hip internal rotation was completed using coefficients from the logistic model predicting injury and is shown in Table 4 and Fig. 4. A 30-degree reduction in left hip internal rotation (comparing a person with IR = 0 to IR = 30 degrees) was associated with 4.06 and 5.29 times greater odds of ACL injury in the ipsilateral and contralateral limbs, respectively. A 30-degree reduction in right hip internal rotation was associated with 5.19 and 2.71 times greater odds of ACL injury in the ipsilateral and contralateral limbs, respectively.
TABLE 4.
Estimated Odds Ratios for Internal Rotation (IR) = 0, Compared to Specified IR, from Post-estimation Calculations
| Odds Ratios | ||||
|---|---|---|---|---|
| IR = 10 | IR = 20 | IR = 30 | IR = 40 | |
| ACL (L) | ||||
| IR Left | 1.59 | 2.52 | 4.06 | 6.35 |
| IR Right | 1.74 | 3.04 | 5.29 | 9.11 |
| ACL (R) | ||||
| IR Left | 1.39 | 1.95 | 2.71 | 3.83 |
| IR Right | 1.73 | 2.98 | 5.19 | 8.94 |
Fig. 4.
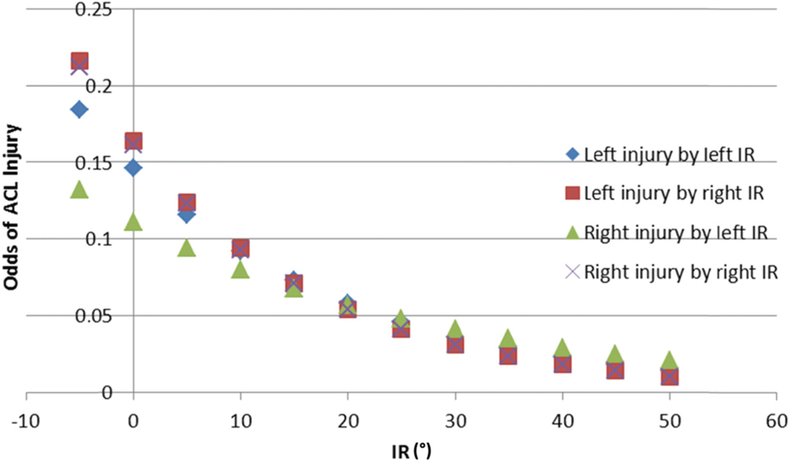
Estimated odds of anterior cruciate ligament (ACL) injury based on hip internal rotation (IR) degrees
Biomechanical Study
The in silico model simulation predicted an increase in peak AM-ACL strain as the range of hip internal rotation was decreased due to the simulated mechanical impingement at the hip (i.e., 0.28% ACL strain increase per 1.0-degree decrease in the range of hip internal rotation; Fig. 3). For example, the peak AM-ACL strain for 5-degrees of internal rotation was 22.5% greater than the corresponding value for 10-degrees of internal rotation (i.e., a peak AM-ACL strain of 5.77% vs. 4.71%, respectively). These values were obtained using the base model with a lateral tibial slope of 7.5 degrees.
In addition, the sensitivity analyses demonstrated that each 1-degree increase in the lateral tibial slope further increased the peak AM-ACL strain by 0.40%.
Discussion
The main findings in the current study suggest that restriction in hip internal rotation is associated with an increased risk of ACL rupture in the elite, pivoting athlete. While the impacts of this restricted range of motion on hip kinematics are better understood, the effect on the kinetic chain of the lower extremity has not been elucidated. Reductions in hip flexion and internal rotation of the hip appear to result in compensatory stresses on the knee that predispose to a greater risk of ACL rupture. The finding from the companion in silico modeling study is that the larger the restriction in hip internal rotation during a simulated pivot landing (which places the knee under an impulsive internal tibial torque), the greater the peak AM-ACL strain. These results are of paramount importance, as an assessment of functional internal rotation of the hip is a safe and inexpensive intervention that will help to counsel athletes on risk and prevention of ACL injury. Furthermore, a relative restriction of hip internal rotation may be an important factor in patients who have ACL reconstruction.[8,24]
The most common morphologic variations that cause dynamic hip impingement and subsequent loss of motion include femoral retroversion, cam-type morphology or loss of femoral offset, and acetabular retroversion [3]. These morphologic variations are extremely common in athletes, potentially due to developmental growth alterations that lead to nonphysiologic remodeling of the femoral head during repetitive high load and rotational activities on the physes (3). Reduced internal rotation of the hip joint secondary to these deformities leads to shearing stress in the pelvis, anteroposterior translation of one hemipelvis relative to the other during extension, and proximo-distal migration in flexion [30]. Verrall et al [29] found a significant association between osteitis pubis and the loss of both external and internal rotation at the hip in athletes. A cross-sectional study correlated limited hip joint range of motion in patients with low back pain and sacroiliac joint dysfunction [5]. Abdominal wall fascial injuries also occur during high-energy twisting activities in which restricted hip range of motion and resultant pelvic motion cause shearing across the pubic symphysis [9,10,20]. A recent series of 37 hips with symptomatic intra-articular pathology (mean patient age, 25 years) were diagnosed with symptomatic athletic pubalgia [15]. Hammoud et al [13] also recently reported a series of 38 professional athletes with a high concomitant prevalence of FAI and pubalgia symptoms. Thirty-nine percent of athletes with pubalgia symptoms noted that they resolved with FAI surgery alone [13].
The critical contributing role of a tibial internal rotation moment to ACL injury is known [22,23]. In the current study, the in silico model exposed the knee to a common form of dynamic loading during the first 100 ms of a pivot landing. The results predict that if an individual lands with the hip near its terminal range of internal rotation, the peak ACL strain will be systematically larger than if the hip is initially in a mid-range of internal rotation (Fig. 4). This is because the model tibia and femur have both been assigned mass and rotational inertia. The stimulus (or input) to the model is delivered, in distal-to-proximal fashion, as an impulsive compression, flexion moment and an axial torque to the distal tibia. As the model begins to respond, and the tibia rotates into internal rotation, the model predicts that when the femur has rotated to maximum internal rotation at the hip, the resistance of the thigh to axial rotation increases, thereby increasing the AM-ACL strain in the knee. This is the first time that the adverse effect of FAI on ACL strain has been demonstrated in any model, whether in vivo, in vitro, or in silico, and the results may have implications for education and improvement of jump and pivot-landing technique for prevention of ACL injury in sport.
This study is not without limitations. First, the study only identifies a correlation and not causation between restricted hip internal rotation and a history of ACL tears. Second, we recognize the potential for interobserver error between the surgeons evaluating hip range of motion, although this was adjusted for in our clustering model to allow each surgeon to serve as his or her own internal control. Third, it is interesting that the correlation of ACL injury with restricted internal rotation of the hip achieved significance at an alpha = 0.05 for the left but not the right side. While this may reflect inadequate power, further studies may need to define the effect of laterality; and the direction of cutting and pivoting could reflect position-specific demands on the athlete. Fourth, it should also be noted that our study likely underestimates the prevalence of ACL injury, because partial and occult injuries were not included; only those injuries that were confirmed and treated with surgical reconstruction have been included in the analysis. Fifth, the in silico models were based on many simplifying assumptions, first among them being the lack of a representation for both menisci in the knee model. Since only the initial 100 ms of the pivot landing were simulated, this may not be a major limitation, since realistic tibial plateau inclinations and concavities were simulated, and the knee joint was represented as being frictionless.
The findings of this study have clinical significance for the care of any athlete engaged in cutting and pivoting activities. A correlation between the degree of restricted hip motion and risk of ACL injury is present (Fig. 3). While it is not difficult to understand how restriction in the range of motion at one joint in the lower extremity might affect the demands placed on an adjacent joint, it has not been possible to correlate the impact of this deficiency on the strain in the major passive tissues of the adjacent joint. While the clinical paradigm requires further confirmation, the present study emphasizes that having sufficient axial rotational range of motion at the hip is important and complementary to the evaluation of coronal and sagittal limb axis alignment in the surgical management of ACL tears. Identifying athletes with restricted hip internal rotation as being at risk for ACL tears will help the appropriate counseling of such athletes, and appropriate training programs may even prevent ACL injury. For example, for a particular maneuver, the athlete might be able to improve landing technique with the hip further away from its terminal range of internal rotation. Furthermore, hip rotation must be assessed and considered in the evaluation of patients who have suffered a recurrent ACL tear after reconstruction in the absence of clear technical errors or failure of biological incorporation. The results, however, do not establish causation nor advocate for prophylactic correction of restricted hip internal rotation to prevent ACL injury.
Future studies that prospectively assess hip range of motion and internal rotation deficits in athletes and follow longitudinally for ACL injury will be required to better validate this relationship. Furthermore, the impact of a corrective FAI surgery on the ACL strain and tear would be important to define the ability of our interventions to modify and prevent injury.
Conclusion
Femoroacetabular impingement may be associated with injury to the ACL because of the increased resistance to femoral internal axial rotation during dynamic maneuvers such as a pivot landing. This insight may lead to better interventions to prevent ACL injury and improved understanding of ACL reconstruction failure.
Supplementary Material
Source of Funding
This study received no external support.
Contributor Information
Asheesh Bedi, Department of Orthopaedic Surgery, MedSport, University of Michigan, 24 Frank Lloyd Wright Drive, Lobby A, Ann Arbor, MI 48106, USA, abedi@umich.edu.
Russell F. Warren, Orthopaedic Surgery, Hospital for Special Surgery, New York, NY 10021, USA
Edward M. Wojtys, Department of Orthopaedic Surgery, MedSport, University of Michigan, 24 Frank Lloyd Wright Drive, Lobby A, Ann Arbor, MI 48106, USA
You Keun Oh, Departments of Mechanical and Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109, USA.
James A. Ashton-Miller, Departments of Mechanical and Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109, USA
Hanna Oltean, Department of Orthopaedic Surgery, MedSport, University of Michigan, 24 Frank Lloyd Wright Drive, Lobby A, Ann Arbor, MI 48106, USA.
Bryan T. Kelly, Orthopaedic Surgery, Hospital for Special Surgery, New York, NY 10021, USA
References
- 1.Beck M, Kalhor M, Leunig M, Ganz R (2005) Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 87(7):1012–1018. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A, Dolan M, Hetsroni I, et al. (2011) Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med 39 Suppl:43S–49S. [DOI] [PubMed] [Google Scholar]
- 3.Bedi A, Dolan M, Leunig M, Kelly BT (2011) Static and dynamic mechanical causes of hip pain. Arthroscopy 27(2):235–251. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham PM, Kelly BT, Jacobs R, McGrady L, Wang M (2012) The effect of dynamic femoroacetabular impingement on pubic symphysis motion: a cadaveric study. Am J Sports Med 40(5):1113–1118. [DOI] [PubMed] [Google Scholar]
- 5.Cibulka MT, Sinacore DR, Cromer GS, Delitto A (1998) Unilateral hip rotation range of motion asymmetry in patients with sacroiliac joint regional pain. Spine (Phila Pa 1976) 23(9):1009–1015. [DOI] [PubMed] [Google Scholar]
- 6.Draovitch P, Edelstein J, Kelly BT (2012) The layer concept: utilization in determining the pain generators, pathology and how structure determines treatment. Curr Rev Musculoskelet Med 5(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellera Gomes JL, Palma HM, Becker R (2010) Radiographic findings in restrained hip joints associated with ACL rupture. Knee Surg Sports Traumatol Arthrosc 18(11):1562–1567. [DOI] [PubMed] [Google Scholar]
- 8.Ellera Gomes JL, Palma HM, Ruthner R (2014) Influence of hip restriction on noncontact ACL rerupture. Knee Surg Sports Traumatol Arthrosc 22(1):188–191. [DOI] [PubMed] [Google Scholar]
- 9.Farber AJ, Wilckens JH (2007) Sports hernia: diagnosis and therapeutic approach. J Am Acad Orthop Surg 15(8):507–514. [DOI] [PubMed] [Google Scholar]
- 10.Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT (2008) Hip injuries and labral tears in the national football league. Am J Sports Med 36(11):2187–2195. [DOI] [PubMed] [Google Scholar]
- 11.Gomes JL, de Castro JV, Becker R (2008) Decreased hip range of motion and noncontact injuries of the anterior cruciate ligament. Arthroscopy 24(9):1034–1037. [DOI] [PubMed] [Google Scholar]
- 12.Granan LP, Bahr R, Steindal K, Furnes O, Engebretsen L (2008) Development of a national cruciate ligament surgery registry: the Norwegian National Knee Ligament Registry. Am J Sports Med 36(2):308–315. [DOI] [PubMed] [Google Scholar]
- 13.Hammoud S, Bedi A, Magennis E, Meyers WC, Kelly BT (2012) High incidence of athletic pubalgia symptoms in professional athletes with symptomatic femoroacetabular impingement. Arthroscopy 28(10):1388–1395. [DOI] [PubMed] [Google Scholar]
- 14.Kelly BT, Bedi A, Robertson CM, Dela Torre K, Giveans MR, Larson CM (2012) Alterations in internal rotation and alpha angles are associated with arthroscopic cam decompression in the hip. Am J Sports Med 40(5):1107–1112. [DOI] [PubMed] [Google Scholar]
- 15.Larson CM, Pierce BR, Giveans MR (2011) Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy 27(6):768–775. [DOI] [PubMed] [Google Scholar]
- 16.Leunig M, Beaule PE, Ganz R (2009) The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res 467(3):616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lind M, Menhert F, Pedersen AB (2009) The first results from the Danish ACL reconstruction registry: epidemiologic and 2 year follow-up results from 5,818 knee ligament reconstructions. Knee Surg Sports Traumatol Arthrosc 17(2):117–124. [DOI] [PubMed] [Google Scholar]
- 18.Lotz JC, Gerhart TN, Hayes WC (1991) Mechanical properties of metaphyseal bone in the proximal femur. J Biomech 24(5):317–329. [DOI] [PubMed] [Google Scholar]
- 19.Merican AM, Sanghavi S, Iranpour F, Amis AA (2009) The structural properties of the lateral retinaculum and capsular complex of the knee. J Biomech 42(14):2323–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers WC, McKechnie A, Philippon MJ, Horner MA, Zoga AC, Devon ON (2008) Experience with “sports hernia” spanning two decades. Ann Surg 248(4):656–665. [DOI] [PubMed] [Google Scholar]
- 21.Oh Y On The Mechanisms of Non-contact ACL Injury during a Simulated Jump Landing: Experimental and Theoretical Analyses [dissertation]. Ann Arbor: University of Michigan; 2011. [Google Scholar]
- 22.Oh YK, Kreinbrink JL, Wojtys EM, Ashton-Miller JA (2012) Effect of axial tibial torque direction on ACL relative strain and strain rate in an in vitro simulated pivot landing. J Orthop Res 30(4):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh YK, Lipps DB, Ashton-Miller JA, Wojtys EM (2012) What strains the anterior cruciate ligament during a pivot landing? Am J Sports Med 40(3):574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappas E, Zampeli F, Xergia SA, Georgoulis AD (2013) Lessons learned from the last 20 years of ACL-related in vivo-biomechanics research of the knee joint. Knee Surg Sports Traumatol Arthrosc 21(4):755–766. [DOI] [PubMed] [Google Scholar]
- 25.Quatman CE, Kiapour AM, Demetropoulos CK, et al. (2014) Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med 42(1):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson JR, Bull AM, Amis AA (2005) Structural properties of the medial collateral ligament complex of the human knee. J Biomech 38(5):1067–1074. [DOI] [PubMed] [Google Scholar]
- 27.Shin CS, Chaudhari AM, Andriacchi TP (2007) The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech 40(5):1145–1152. [DOI] [PubMed] [Google Scholar]
- 28.Taylor-Haas JA, Paterno MV, Shaffer MD (2011) Femoral neck stress fracture and femoroacetabular impingement. J Orthop Sports Phys Ther 41(11):905. [DOI] [PubMed] [Google Scholar]
- 29.Verrall GM, Hamilton IA, Slavotinek JP, et al. (2005) Hip joint range of motion reduction in sports-related chronic groin injury diagnosed as pubic bone stress injury. J Sci Med Sport 8(1):77–84. [DOI] [PubMed] [Google Scholar]
- 30.Williams JG (1978) Limitation of hip joint movement as a factor in traumatic osteitis pubis. Br J Sports Med 12(3):129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


