Abstract
Background/purpose
Bacterial infection is closely associated with the failure of endodontic treatment, and use of endodontic sealer with antimicrobial activity and biological compatibility is necessary for the success of root canal treatment. The purpose of this study was to investigate and to compare the antibacterial effect of two calcium silicate-based root canal sealers (Endoseal and EndoSequence BC sealer) as recent development sealers and with three conventional root canal sealers (AH Plus, Sealapex, and Tubli-Seal), before or after setting, on Porphyromonas endodontalis, Porphyromonas gingivalis, and Enterococcus faecalis.
Materials and methods
The sealers were soaked in phosphate buffered saline to elute its compositions after and before setting, and the elutes were performed the antimicrobial assay. Also, X-ray fluorescence analysis was carried out to compare compositions of two calcium silicate-based sealers.
Results
The conventional root canal sealers have strong antibacterial activity against the Gram-negative bacteria, P. endodontalis and P. gingivalis. Endoseal sealer showed antibacterial activity against not only the Gram-negative bacteria, but also against the Gram-positive bacteria, E. faecalis. However, Endosequence BC sealer exhibited a weak antibacterial effect on all bacteria in this study. X-ray fluorescence analysis exhibited that Endoseal contained more types and more amount of the oxide compound known to have strong antimicrobial activity such as Al2O3, Fe2O3, MgO, Na2O, NiO, and SO2 than Endoseqeunce BC.
Conclusion
Endoseal, which contains various types of oxide compounds, seems to be a suitable sealer for preventing bacterial infection in both treated and untreated root canals.
Keywords: Root canal sealer, Antimicrobial activity, Oxide compound, E. faecalis
Introduction
Bacterial infection into the root canal plays an important role in the induction of pulpal and periapical inflammation and is closely associated with the failure of endodontic treatment.1 Although individual cases differ, averages of five to seven different species per canal have been detected, and the bacterial species most frequently isolated from necrotic pulps are Porphyromonas gingivalis and Porphyromonas endodontalis.2, 3, 4 P. gingivalis and P. endodontalis are associated with initial infection of the root canal, and Enterococcus faecalis has been detected in apical periodontitis lesions in root canal-treated teeth.5 Because the root canal system varies in the anatomical features including fins, isthmi, and accessory canals, complete elimination of the bacteria in the root canals is difficult. In treating the root canal, along with mechanical cleaning, various intracanal irrigants and medicaments, such as calcium hydroxide, sodium hydroxide, and chlorohexidine, are used in attempts to eradicate bacteria in the infected root canal, However, some bacteria may remain in the root canal systems.6 Therefore, a hermetic seal of the root canal space is required to entomb any residual bacteria and ultimately kill them in the filled root canal.
Root canal sealers are used to overcome the limitations of gutta-percha (GP) cones and obturation techniques by filling the space between the GP and the dentinal wall. Hence, root canals sealers that possess superior sealing ability and antibacterial activity would be clinically beneficial by preventing bacteria from re-entering the canal and by inactivating bacteria remaining in the canal after root canal obturation. Traditional root canal sealers are categorized as zinc oxide eugenol (ZOE), epoxy resin (ER), or calcium hydroxide (CH) on the basis of their composition.7, 8, 9 Recently, calcium silicate-based cement with the addition of various oxide compounds have been developed for root sealer and are called mineral trioxide aggregation (MTA).10 This cement is known to bioactive properties that have stimulation of tissue repair and induction of mineralization.11,12 For these reasons, the cement has been considered suitable for application to root canal sealer and have led to the development of root canal sealers. Antimicrobial activity is also an important factor in investigating dental materials for application to root sealer because bacterial infection is closely associated with the failure of endodontic treatment. Although the antimicrobial activity of these products against Lactobacillus acidophilus, Staphylococcus aureus, and E. faecalis has been studied,13 the evaluation has been limited to the antibacterial effect on Gram-positive bacteria notwithstanding the isolation of P. endodontalis, P. gingivalis, and E. faecalis from necrotic pulps, and the antibacterial activity has been examined only before setting of the sealer. Therefore, we investigated and compared the antibacterial activity of two calcium silicate-based root canal sealers (Endoseal and EndoSequence BC sealer) as recent development sealers and with three conventional root canal sealers (AH Plus, Sealapex, and Tubli-Seal), before or after setting, against P. endodontalis, P. gingivalis, and E. faecalis.
Materials and methods
The bacteria in this study were purchased from American Type Culture Collection. E. faecalis ATCC 29221 was aerobically cultivated in brain heart infusion (BHI) broth (BD Bioscience, Sparks, MD, USA) at 37 °C, and P. endodontalis ATCC 35406, and P. gingivalis ATCC 33277 were cultured in BHI broth supplemented with hemin (1 μg/mL) and vitamin K (0.2 μg/mL) at 37 °C in an anaerobic condition (5% H2, 10% CO2, 85% N2).
Table 1 shows the composition of the root canal sealers. Sealers tested for antibacterial activity were prepared according to the manufacturers' directions. Each sealer was dispensed into each well of 12-well polystyrene microplates (SPL Life Science, Gyeonggi, South Korea), and phosphate buffer solution (PBS) was then added, for a sealer concentration of 200 mg/mL. The microplates were agitated on a shaker (50 rpm) for 4 h at room temperature. To compare the antibacterial activity between set and unset materials, eluates from each sealer were also collected after setting. The sealers were placed into the inside wells of the 12-well microplates, and PBS was added in the outside wells of the microplates to ensure stable humidity levels. The sealers were solidified for 24 h at 37 °C, and PBS was then added into the wells. Based on the initial mass, the concentration of the sealer was adjusted to 200 mg/mL by adding PBS into each well. The microplates were agitated on a shaker for 4 h at room temperature. Each eluate was transferred to a fresh 15-mL conical tube, which was then centrifuged at 5000× g for 10 min to remove any remaining insoluble particles.
Table 1.
The used root canal sealers in this study and its characterization.
| Materials | Corporation/Country | Product information |
|---|---|---|
| Sealapex | Kerr/USA | Calcium hydroxide based sealer |
| Tubli-Seal | Kerr/USA | Zinc oxide eugenol based sealer |
| AH plus | Dentsply/USA | Epoxy resin based sealer |
| EndoSequence BC | Brasseler/USA | Calcium silicate based sealer |
| Endoseal | MARUCHI/Korea | Calcium silicate based sealer |
Antimicrobial assays were performed according to the protocol of Clinical and Laboratory Standards Institute (CLSI). The incubated bacteria level was assessed using a bacterial counting chamber (Marienfeld, Lauda-Konigshofen, Germany). The concentration of E. faecalis was adjusted to a density of 1 × 106 cell/mL by adding fresh BHI broth. The BHI broth supplemented with hemin and vitamin K was added to adjust the level of P. endodontalis and P. gingivalis to 1.5 × 106 cell/mL. Subsequently, 180 μL of the specific media for each test microorganism was dispensed into each well of 96-well polystyrene plate, and 160 μL of the specific media plus 20 μL of the prepared sealer eluate were added to the first row of the plate, and serial two-fold dilution was performed using a multi-channel micropipette. Next, 20 μL of each bacterial suspension was inoculated to the wells containing the eluates from the sealers. The plates were incubated for 24 h at 37 °C, aerobically for E. faecalis, and anaerobically for P. endodontalis and P. gingivalis. Bacterial growth was monitored by measuring the absorbance at 600 nm in a microplate reader (BioTek, Winooski, VT, USA).
To investigate the difference in the antimicrobial activity between the two calcium silicate-based sealers, Endoseal and EndoSequence BC sealer, the chemical compositions of the sealers were analyzed using an X-ray fluorescence (XRF) spectrometer (ZSX primus II, Rigaku Co., Tokyo, Japan). The sealers were loaded on micro-carry paper and dried at 55 °C. The XRF spectrometer was outfitted with X-ray tubes with Rh anodes and was operated at 60 kV and 150 mA. The XRF patterns for the sealers were obtained using SC and F-PC diode detectors and analyzed using EZ Scan (Rigaku Co., Tokyo, Japan).
The data were analyzed non-parametrically by using the Kruskal–Wallis and Mann–Whitney tests. IBM SPSS Statistics Ver. 23 (IBM, Armonk, NY, USA) was used for statistical analysis. Statistical significance was defined by a P value of less than 0.05.
Results
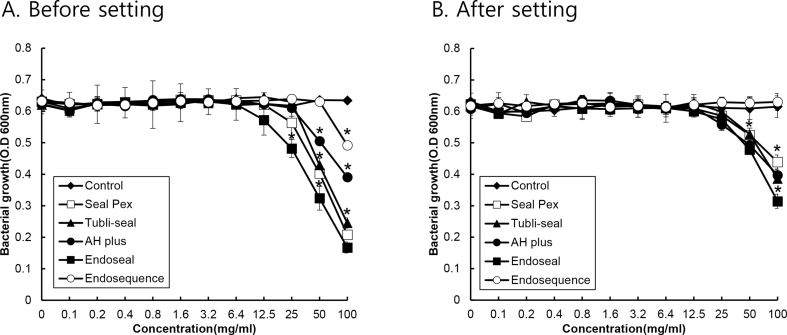
Figure 1 shows the growth of E. faecalis as a function of the sealers' concentration. The antibacterial activity against E. faecalis was the greatest in Endoseal, followed by Sealapex, Tubli-Seal, AH Plus and EndoSequence BC sealer. Endoseal exerted an inhibitory effect at 25 mg/mL, whereas Sealapex, Tubli-Seal, and AH Plus inhibited the bacterial growth at 50 mg/mL. All the sealers had less inhibitory effect against E. faecalis after the materials were set, and EndoSequence BC sealer was found to have no antibacterial activity.
Figure 1.
The antibacterial activity of traditional and calcium silicate-based root canal sealers against E. faecalis. The eluate from traditional and calcium silicate-based root canal sealers before (A) or after setting (B) was prepared using a PBS, and E. faecalis was cultivated with and without the prepared eluate of various sealers at various concentrations in a 96-well polystyrene plate. The growth of E. faecalis was measured using a microplate reader at 600 nm. The experiments were conducted three times in duplicate, and data are represented as the mean ± S.D. * Statistically significant differences compared with cultures not treated with the spent culture medium (p < 0.05).
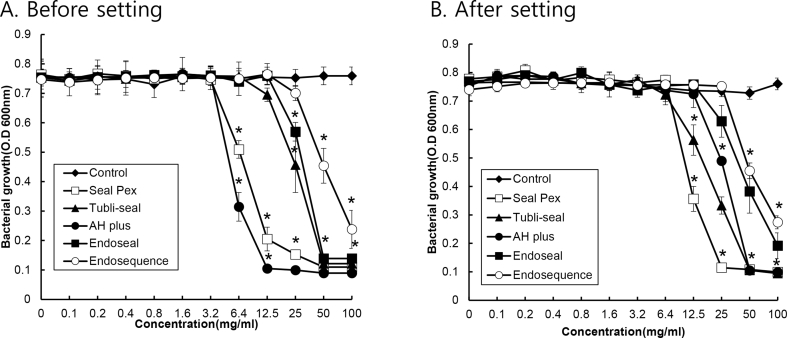
As shown Fig. 2, the growth of P. endodontalis was significantly inhibited when the concentration of AH Plus and Sealapex was greater than 6.4 mg/mL (P < 0.05). Tubli-Seal and Endoseal showed the bacterial growth at 25 mg/mL. When the materials were set, the antibacterial activity of Tubli-Seal was greater, whereas AH Plus, Sealapex, and Endoseal showed less antibacterial activity. EndoSequence BC sealer exhibited the least antibacterial activity regardless of whether or not the material was set.
Figure 2.
The antibacterial activity of traditional and calcium silicate-based root canal sealers against P. endodontalis. The eluate from traditional and calcium silicate-based root canal sealers before (A) or after setting (B) was prepared using a PBS, and P. endodontalis was cultivated with and without the prepared elute of various sealers at various concentrations in a 96-well polystyrene plate under anaerobic conditions. The growth of P. endodontalis was measured using a microplate reader at 600 nm. The experiments were conducted three times in duplicate, and data are represented as the mean ± S.D. * Statistically significant difference compared with cultures not treated with the spent culture medium (p < 0.05).
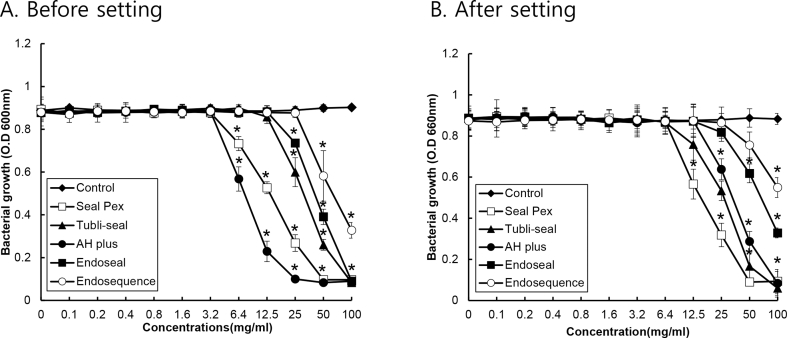
The inhibitory effects of unset sealers against P. gingivalis decreased in the order of AH Plus, Sealapex, Tubli-Seal, Endoseal, and EndoSequence BC sealer. When the materials were set, the antibacterial effect of AH Plus was significantly reduced (Fig. 3).
Figure 3.
The susceptibility of P. gingivalis for various root canal sealers. The eluate from traditional and calcium silicate-based root canal sealers before (A) or after setting (B) was prepared using a PBS, and P. gingivalis was cultivated with and without the prepared eluate of various sealers at various concentration in a 96-well polystyrene plate under anaerobic conditions. The growth of P. gingivalis was measured using a microplate reader at 600 nm. The experiments were conducted three times in duplicate, and data are represented as the mean ± S.D. * Statistically significant differences compared with cultures not treated with the spent culture medium (p < 0.05).
Endoseal sealer contained more types and larger amount of the oxide compound known to have strong antimicrobial activity such as Al2O3, Fe2O3, MgO, Na2O, NiO, and SO3 than Endoseqeunce BC sealer in XRF analysis (Table 2). The main compounds were zirconium dioxide, calcium oxide, and silicon dioxide according for approximately 97% of the total mass of EndoSequence BC sealer and 86% of Endoseal. Both EndoSeqeunce BC sealer and Endoseal are the sealer on the basis of calcium oxide and zirconium dioxide and have large amount of the two molecules. However, higher levels of metal oxide such as soidum oxide, aluminum oxide, ferric oxide, and silcon dioxide in Endoseal were detected than EndoSequence BC sealer.
Table 2.
Comparison of compositions of calcium silicate-based root canal sealers by X-ray fluorescence analysis.
| EndoSequence BC |
Endoseal |
||
|---|---|---|---|
| Components | Mass % | Components | Mass % |
| Al2O3 | 0.0035 | Na2O | 0.0706 |
| SiO2 | 5.77 | MgO | 1.20 |
| P2O5 | 1.80 | Al2O3 | 2.84 |
| K2O | 0.0358 | SiO2 | 7.56 |
| CaO | 37.8 | SO3 | 1.27 |
| MnO | 0.0157 | K2O | 0.574 |
| SrO | 0.0046 | CaO | 25.1 |
| ZrO2 | 53.3 | TiO2 | 0.141 |
| HfO2 | 1.04 | Cr2O3 | 0.105 |
| MnO | 0.0365 | ||
| Fe2O3 | 1.29 | ||
| NiO | 0.0029 | ||
| SrO | 0.0503 | ||
| Y2O3 | 0.0592 | ||
| ZrO2 | 53.0 | ||
| HfO2 | 1.05 | ||
| Bi2O3 | 5.66 | ||
Discussion
Control of the bacteria determines the success or failure of root canal treatment. Although, chemomechanical procedures, cleaning, and disinfectant treatment are performed to reduce the number of bacteria when treating the root canal, some bacteria often remain in the root canal systems.6 Therefore, the root-filling materials with antibacterial activity are required and are advantageous. Recently, calcium silicate-based root canal sealers have been developed, and their antibacterial activity against some Gram-positive bacteria, including E. faecalis, has been examined.13,14 However, other bacteria, such as P. gingivalis and P. endodontalis, are related to pulpal inflammations, and the antibacterial activity of the sealers after setting has not been evaluated. Therefore, this study investigated and compared the antibacterial activity of traditional sealers and calcium silicate-based sealers against P. gingivalis, P. endodontalis, and E. faecalis before and after setting.
In the susceptibility test, before setting, the traditional sealers showed stronger antimicrobial activity on P. gingivalis and P. endodontalis than the calcium silicate-based. Endoseal showed the strongest antibacterial activity against E. faecalis, whereas, Endosequence BC sealer showed weak antibacterial activity against all bacteria. The comparison of antimicrobial activity among the sealers against the bacteria or the comparison of susceptibility among the bacteria for the sealer is possible by performing broth method using the elute. The used elutes were extracted after measuring same weight of the sealer at one time and then carried out the test of the antimicrobial activity. These data may be not obtained by the experiment of agar diffusion assay.
In the present study, AH plus, an epoxy resin-based sealer, showed the strongest antibacterial activity against P. gingivalis and P. endodontalis but was weaker against E. faecalis. Epoxy resin-based sealers exhibit antibacterial activity through bisphenol A diglycidyl ether and formaldehyde during polymerization.15 Therefore, Gram-negative bacteria, which have thin cell walls, are sensitive to chemicals because of easy penetration into the bacterial cytosol. Formaldehyde penetrates into the interior of bacteria and inhibits metabolism of bacteria by reacting with cytosolic proteins, RNA, and DNA.16 Endoseal is a calcium silicate-based sealer and showed an antibacterial effect against E. faecalis before or after setting. Endoseal showed the strongest antimicrobial effect against bacteria under alkaline conditions because of calcium silicate.17 However, Endosequence BC sealer, another calcium silicate-based sealer, showed weak antimicrobial activity against E. faecalis. Endosequence BC sealer and Endoseal commonly exhibit antibacterial activity because of Ca(OH)2 (calcium hydroxide) reaction, which is bactericidal against Gram-negative bacteria through damage of bacterial membrane or DNA, and denaturation proteins.18 We performed X-ray fluorescence analysis to investigate the difference between the two sealers. Endoseal contained more types and more amount of the oxide compound known to have antimicrobial activity such as Na2O, MgO, Al2O3, SO2, and Fe2O than EndoSequence BC. Among oxide compounds, these oxide compounds damage the cell wall of Gram-positive bacteria and increase the permeability of molecules into the cytoplasm through electrostatic interaction.19, 20, 21, 22 Finally, various oxide compounds with antimicrobial activity in Endoseal may damage the cell wall of bacteria and help the penetration of Ca(OH)2 into the cytosol, and then Ca(OH)2 may denature DNA and protein. Because Endosequence BC contained relatively low amount of oxide compounds with antimicrobial activity, Endosequence BC may weakly damage the cell walls of bacteria, and Ca(OH)2 may penetrate less. This indicates that calcium silicate-base sealers containing oxide compounds may show the strong antimicrobial activity against Gram-negative and Gram–positive bacteria.
P. gingivalis and P. endodontalis are related to untreated root canal infection,23 and E. faecalis is associated with re-infection of treated root canals.4,23 According to the results of this study, traditional sealers may be effective in treating primary root canal infections, whereas Endoseal, a calcium silicate-base sealer that contains oxide compounds, is more effective in preventing re-infection with E. faecalis. However, considering that traditional sealers have a cytotoxic effect on human pulp cell in vitro.17,24,25 Endoseal may be useful for preventing bacterial infection in untreated and treated root canals.
Within the limitations of the present study, all of the freshly mixed sealers exhibited higher antibacterial activity than the set sealers. The antibacterial activity of the tested sealers was found to be material- and bacteria-dependent. Endoseal continues to exhibit antibacterial activity after setting and may be the most effective in eliminating E. faecalis in the root canal. Finally, Endoseal may be the most useful sealer for preventing bacterial infection when treating the root canal.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This research was financially supported by the “the product diagnosis & improvement for rising medical device manufacturer support program” through the Gangwon Province, Wonju City and Wonju Medical Industry Techno Valley(WMIT).
References
- 1.Kakehashi S., Stanley H.R., Fitzgerald R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Gomes B.P., Pinheiro E.T., Gade-Neto C.R. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19:71–76. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 3.Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427–430. doi: 10.1016/S0099-2399(06)80842-3. [DOI] [PubMed] [Google Scholar]
- 4.Sundqvist G. Taxonomy, ecology, and pathogenicity of the root canal flora. Oral Surg Oral Med Oral Pathol. 1994;78:522–530. doi: 10.1016/0030-4220(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 5.Molander A., Reit C., Dahlen G., Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 6.Ozcan E., Eldeniz A.U., Ari H. Bacterial killing by several root filling materials and methods in an ex vivo infected root canal model. Int Endod J. 2011;44:1102–1109. doi: 10.1111/j.1365-2591.2011.01928.x. [DOI] [PubMed] [Google Scholar]
- 7.Zmener O. Evaluation of the apical seal obtained with two calcium hydroxide based endodontic sealers. Int Endod J. 1987;20:87–90. doi: 10.1111/j.1365-2591.1987.tb00594.x. [DOI] [PubMed] [Google Scholar]
- 8.von Fraunhofer J.A., Branstetter J. The physical properties of four endodontic sealer cements. J Endod. 1982;8:126–130. doi: 10.1016/S0099-2399(82)80248-3. [DOI] [PubMed] [Google Scholar]
- 9.Mutoh N., Satoh T., Watabe H., Tani-Ishii N. Evaluation of the biocompatibility of resin-based root canal sealers in rat periapical tissue. Dent Mater J. 2013;32:413–419. doi: 10.4012/dmj.2012-218. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri J., Montesin F.E., Brady K., Sweeney R., Curtis R.V., Ford T.R. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Maroto M., Barberia E., Planells P., Garcia Godoy F. Dentin bridge formation after mineral trioxide aggregate (MTA) pulpotomies in primary teeth. Am J Dent. 2005;18:151–154. [PubMed] [Google Scholar]
- 12.Aminozarbian M.G., Barati M., Salehi I., Mousavi S.B. Biocompatibility of mineral trioxide aggregate and three new endodontic cements: an animal study. Dent Res J. 2012;9:54–59. doi: 10.4103/1735-3327.92944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jafari F., Samadi Kafil H., Jafari S., Aghazadeh M., Momeni T. Antibacterial activity of MTA fillapex and AH 26 root canal sealers at different time intervals. Iran Endod J. 2016;11:192–197. doi: 10.7508/iej.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh G., Gupta I., Elshamy F.M., Boreak N., Homeida H.E. In vitro comparison of antibacterial properties of bioceramic-based sealer, resin-based sealer and zinc oxide eugenol based sealer and two mineral trioxide aggregates. Eur J Dermatol. 2016;10:366–369. doi: 10.4103/1305-7456.184145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonardo M.R., Bezerra da Silva L.A., Filho M.T., Santana da Silva R. Release of formaldehyde by 4 endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:221–225. doi: 10.1016/s1079-2104(99)70119-8. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim E.S., Park Y.B., Kwon Y.S., Shon W.J., Lee K.W., Min K.S. Physical properties and biocompatibility of an injectable calcium-silicate-based root canal sealer: in vitro and in vivo study. BMC Oral Health. 2015;15:129. doi: 10.1186/s12903-015-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siqueira J.F., Jr., Lopes H.P. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32:361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 19.Hajipour M.J., Fromm K.M., Ashkarran A.A. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Catauro M., Raucci M.G., De Gaetano F., Marotta A. Antibacterial and bioactive silver-containing Na2O x CaO x 2SiO2 glass prepared by sol-gel method. J Mater Sci Mater Med. 2004;15:831–837. doi: 10.1023/b:jmsm.0000032825.51052.00. [DOI] [PubMed] [Google Scholar]
- 21.Fang T.J., Dalmasso J.P. Antimicrobial activity of sulfur dioxide to certain lactic acid bacteria isolated from wines. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1993;26(3):116–131. [PubMed] [Google Scholar]
- 22.Azam A., Ahmed A.S., Oves M., Khan M.S., Habib S.S., Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed. 2012;7:6003–6009. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gajan E.B., Aghazadeh M., Abashov R., Salem Milani A., Moosavi Z. Microbial flora of root canals of pulpally-infected teeth: Enterococcus faecalis a prevalent species. J Dent Res Dent Clin Dent Prospects. 2009;3:24–27. doi: 10.5681/joddd.2009.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camargo C.H., Camargo S.E., Valera M.C., Hiller K.A., Schmalz G., Schweikl H. The induction of cytotoxicity, oxidative stress, and genotoxicity by root canal sealers in mammalian cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:952–960. doi: 10.1016/j.tripleo.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Pires C.W., Botton G., Cadona F.C. Induction of cytotoxicity, oxidative stress and genotoxicity by root filling pastes used in primary teeth. Int Endod J. 2016;49:737–745. doi: 10.1111/iej.12502. [DOI] [PubMed] [Google Scholar]