Abstract
Background/purpose
Recurrent aphthous ulceration (RAU) has an incidence of approximately 20% in general population. However, its exact cause remains unknown. Increasing evidence suggests that immunologic mechanisms may play crucial roles in the etiology of this disease.
Materials and methods
The peripheral blood samples were obtained from 85 patients with RAU during acute phase and 87 healthy controls. The serum levels of IgG, IgA, IgM, C3 and C4 were measured by immunoturbidimetry. In addition, the serum IgE levels were measured by electro-chemiluminescence immunoassay. Furthermore, the percentages of B, T, CD4+ T, CD8+ T lymphocytes and natural killer (NK) cells in peripheral blood were determined by flow cytometry.
Results
Our findings showed that the serum IgG, IgA, IgE, C3 and C4 levels of RAU patients were significantly higher than those of healthy controls. The percentages of CD4+ T cells and B cells in peripheral blood of RAU patients were significantly decreased, whereas the percentages of CD8+ T cells and NK cells of RAU patients were remarkably increased. Our results indicated that the IgG level was elevated in 18 patients (21.2%) and that the IgE level was increased in 21 patients (24.7%). Our results also showed that the frequency of abnormal IgG or IgE levels were significantly correlated with that of abnormal CD8+ T cell percentage in RAU patients.
Conclusion
The levels of both humoral and cellular immune components could be altered in RAU. The relationship between humoral and cellular immune may be potentially important immunologic aspects involved in the pathogenesis of RAU.
Keywords: stomatitis, aphthous, humoral immune, cellular immune, immunoglobulins, lymphocytes
Introduction
Recurrent aphthous ulceration (RAU) is an inflammatory disease with an incidence of approximately 20% in general population. The etiology of RAU is multifactorial, including genetic, immune, nutritional, and microbial factors and local trauma. Immune mechanisms appear to play important roles in the pathogenesis of RAU.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
In terms of humoral immunity, some studies have confirmed that immunoglobulin levels were altered in RAU patients.2, 3, 4, 5, 6 The serum IgA level was elevated in minor RAU patients compared with healthy controls.2 In addition, the levels of both salivary IgG subclasses and IgA2 were increased in RAU patients.3 Moreover, an elevated serum IgE level has been detected in RAU patients in association with disease characteristics.6 In addition, a study has confirmed that serum C3 and C4 levels were higher in RAU than that in healthy control.7
Cellular immunity was proven to play a crucial role in the pathogenesis of RAU. Diminished percentages of total T cells, CD4+ T cells and B cells have been observed in the peripheral blood of RAU patients, as well as increased percentages of CD8+ T cells and natural killer (NK) cells and a reduced CD4+/CD8+ ratio.8, 9, 10, 11, 12, 13, 14
Although previous studies have focused on the abnormality of humoral or cellular immunity in RAU, to the best of our knowledge, the relationship between humoral and cellular immunity in this disease has not yet been evaluated. In the present study, we not only assessed the alterations in humoral and cellular immunity in RAU patients, but also attempted to explore the correlations between humoral and cellular immunity of these patients.
Materials and methods
Study subjects
This study was approved by the Ethics Committee of the Nanjing Stomatological Hospital, Medical School of Nanjing University (IRB Approval Number: 2014NL-002 (ks)). A total of 85 RAU patients during the acute phase were included in this study. 87 age- and sex-matched healthy volunteers with no history of any episodes of RAU were also included in the present study. All of the subjects were recruited between 2015 and 2017, and written informed consent was obtained from each participant. The 85 RAU patients had a history of regularly recurring oral ulcers and had experienced at least three episodes in the last 6 months. For all the patients included, the clinical diagnosis was classified as minor according to the standard classification of RAU. The clinical characteristics of oral ulcer were recorded in detail. Through measuring the semi-major and semi-minor axis sizes (a and b) (cm) of one oral ulcer, we calculated the area of single ulcer according to the formula of oval area: S = πab. Then we determined the area of ulcers (cm2) in one patient with RAU by calculating total areas of all oral ulcers. Peripheral blood samples were obtained from healthy controls and all patients within 48 h after the development of aphthae. The exclusion criteria included diagnosis of an autoimmune disease (e.g., systemic lupus erythematous, rheumatoid arthritis, psoriasis, or diabetes), any type of cancer, HIV, hepatitis B or C infection, any hematologic deficiencies, other oral mucosal diseases (e.g., oral lichen planus, pemphigus), systemic disorders (e.g., Behçet's disease, Crohn disease), chronic medication use, any mental disorder, women of pregnancy or lactation. Subjects were considered eligible if they had not taken any medication for 3 months prior to examination. All the patients and healthy controls included in the present study were non-smokers.
Immunoturbidimetry
Peripheral blood samples (5 ml) were collected from the patients by venipuncture between 8 and 10 a.m. The samples were transported on ice in an upright position to the lab within 2 h. The serum samples were obtained by centrifugation and then stored at −80 °C until use. Assays for IgG, IgA, IgM, and the complements C3 and C4 were performed using immunoturbidimetry methods. In addition, the serum IgG, IgA, and IgM levels were measured using commercial kits (Sichuan Marker Biotechnology, Chengdu, China) with an automated analyzer (Roche Diagnostics, IN, Switzerland) following the manufacturer's instructions.
Electro-chemiluminescence immunoassay
Serum IgE levels were measured using electro-chemiluminescence immunoassay kits (Roche Diagnostics GmbH, Mannheim, Germany) with a Cobas e 601 system (Elecsys module) immunoassay analyzer (Roche Diagnostics, IN, Switzerland). None of the samples used for analyses of serum proteins were subjected to repetitive freeze–thaw cycles.
Flow cytometry assay
The reagents used included a BD Multitest Kit (BD Biosciences, San Jose, USA) containing BD Multitest anti-CD3 FITC/anti-CD8-PE/anti-CD45-PerCP/anti-CD4-APC, BD Multitest anti-CD3-FITC/anti-CD16+ CD56-PE/anti-CD45-PerCP/anti-CD19-APC and BD Multitest IMK Kit Lysing Solution at a 10× concentration (this solution was diluted to 1× in reagent–grade water prior to assay). Five lymphocyte subsets were analyzed, including total T cells (CD3+), TH cells (CD3+CD4+), TC cells (CD3+CD8+), B cells (CD3+CD19+) and NK cells (CD3−CD16+CD56+). The assay was performed as follows. Two tubes were prepared. Then, 20 μL of BD Multitest CD3/CD8/CD45/CD4 Reagent was added into one tube, and 20 μL of Multitest CD3/CD16+ CD56/CD45/CD19 Reagent was added into the other. Next, 50 μL EDTA–anticoagulated whole blood was added to each tube. Whereafter, the tubes were capped and mixed by gentle vortexing, followed by incubation in the dark for 15 min at room temperature (20 °C–25 °C). Subsequently, erythrocytes were lysed by adding 450 μL of 1 × BD Multitest IMK Kit Lysing Solution and the tubes were capped and mixed by gently vortexing, followed by incubation in the dark for 15 min. At last, the samples were analyzed with a flow cytometer. Samples were acquired on a BD FACS Calibur Instrument using BD Multiset software with BD Worklist Manager Software. Automatic gating was used to facilitate analysis of each subset population.
Normal reference values for immune components
According to the manufacturer's instructions of immunoturbidimetry and electro-chemiluminescence immunoassay kits, the normal ranges for serum IgG, IgA, IgM, IgE, C3 and C4 were 8.00–17.00 g/L, 0.85–4.50 g/L, 0.60–3.70 g/L, 0.0–100.0 kU/L, 0.90–1.80 g/L, and 0.10–0.40 g/L, respectively. Based on the manufacturer's instructions of flow cytometry assay, the normal ranges for lymphocyte subpopulation analysis were 6.4–22.6% for CD19+, 59.4–84.6% for CD3+, 28.5–60.5% for CD3+CD4+, 11.1–38.3% for CD3+CD8+, 5.6–30.9% for CD3−CD16+56+ and 0.9–3.6% for CD4+/CD8+, respectively.
Statistical analyses
The continuous variables were presented as mean and standard deviation (SD) and the categorical variables are presented as counts and percentages. The definitions of normal and abnormal frequencies of each variable were based on the range of reference values. Those higher or lower than reference values were defined as abnormal frequencies. The internal correlations among the frequencies of abnormal levels of immunoglobulins and complements, and the internal correlations among the frequencies of abnormal percentages/ratios of lymphocyte subsets, and the correlations between the frequencies of abnormal levels of immunoglobulins or complements and those of percentages/ratios of lymphocyte subsets were assayed using Spearman correlation analysis. To determine whether variables were normally distributed, Kolmogorov–Smirnov statistic test was applied. Normally distributed data were analyzed with unpaired t-test for differences between RAU patients and healthy control groups whereas non-normally distributed data were analyzed using Mann Whitney test. A P value <0.05 was accepted to be statistically significant. SPSS software version 18 (SPSS 18.0, SPSS Inc., Chicago, IL, USA) were performed for statistical analyses.
Results
Clinical characteristics of RAU patients
The clinical characteristics of the RAU patients included in the present study were shown in Table 1. Of the 85 patients, 50 (58.8%) were males. The mean age ± SD was 47.54 ± 14.02 years (males, 45.86 ± 13.82 years; females, 49.94 ± 14.15 years). The most frequently affected site was tongue (46 ulcers, 54.1%), then lip (29 ulcers, 34.1%). The average duration of disease was 6.64 years (range, 1–40 years). The average number of aphthae at diagnosis was 2.67 (range, 1–20). In addition, the average ulcer area was 0.444 cm2 (range, 0.031–2.355 cm2).
Table 1.
Demographic and clinical characteristics of 85 RAU patients.
| Parameters | RAU patients |
|
|---|---|---|
| N | % | |
| Age (years) | 47.54 ± 14.02a | |
| Gender | ||
| Male | 50 | 58.8 |
| Female | 35 | 41.2 |
| Duration (years) | 6.64 ± 6.57a | |
| Number of ulcers present | 2.67 ± 3.49a | |
| Area of ulcers (cm2) | 0.444 ± 0.446a | |
| VAS | 6.46 ± 1.99a | |
| Ulcer siteb | ||
| Tongue | 46 | 54.1 |
| Lip | 29 | 34.1 |
| Buccal mucosa | 7 | 8.2 |
| Floor of the mouth | 5 | 5.9 |
| Pharyngeal mucosa | 4 | 4.7 |
RAU, recurrent aphthous ulcer; SD, standard deviation; VAS, visual analog scale.
Mean ± SD.
Some patients presented with more than one ulcer.
Increased serum IgG, IgA, IgE, C3 and C4 levels in RAU patients
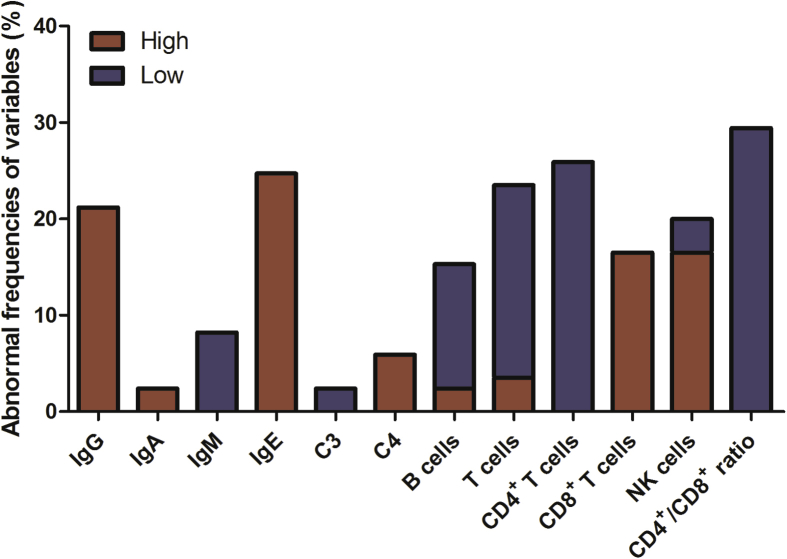
As shown in Table 2, the serum IgG, IgA, IgE, C3 and C4 levels of RAU patients were significantly higher than those of healthy controls (P < 0.05). As shown in Table 3, the serum IgG level was elevated above the normal range in 18 patients (21.2%). In addition, the serum IgE level was increased above the normal range in 21 patients (24.7%). The frequencies of abnormal levels of immunoglobulins and complements were presented in Fig. 1.
Table 2.
The comparison of immune parameter levels in peripheral blood of RAU patients and healthy control groups.
| RAU (85) | Control (87) | P-value | |
|---|---|---|---|
| IgG (g/L) | 14.57 ± 3.96 | 13.21 ± 2.42 | 0.025 |
| IgA (g/L) | 2.50 ± 0.93 | 1.83 ± 0.53 | <0.001 |
| IgM (g/L) | 1.27 ± 0.72 | 1.14 ± 0.57 | 0.279 |
| IgE (kU/L) | 37.90 (15.05–84.80) | 21.60 (15.60–82.80) | 0.005 |
| C3 (g/L) | 1.21 ± 0.16 | 1.14 ± 0.15 | 0.001 |
| C4 (g/L) | 0.29 ± 0.07 | 0.24 ± 0.05 | <0.001 |
| B cell (CD19+) (%) | 9.75 ± 3.98 | 12.15 ± 3.64 | <0.001 |
| T cell (CD3+) (%) | 68.57 ± 11.72 | 69.23 ± 7.46 | 0.544 |
| CD4+ T cell (%) | 31.51 ± 7.55 | 39.66 ± 7.22 | <0.001 |
| CD8+ T cell (%) | 30.60 ± 9.71 | 24.34 ± 7.28 | <0.001 |
| NK cell (%) | 21.31 ± 11.58 | 17.44 ± 7.38 | 0.028 |
| CD4+/CD8+ | 1.15 ± 0.51 | 1.86 ± 0.89 | <0.001 |
IgG, IgA, IgM, C3 and C4, ; IgE, median (IQR).
CD19+, CD3+, CD4+ T, CD8+ T, CD4+/CD8+ and NK, .
Table 3.
Frequencies of abnormal levels of immunoglobulins and complements and abnormal percentages/ratios of lymphocyte subsets in the RAU patients.
| Parameters | Normal |
Abnormal |
Abnormal |
|
|---|---|---|---|---|
| N (%) | N (%) | High |
Low |
|
| N (%) | N (%) | |||
| IgG | 67 (78.8) | 18 (21.2) | 18 (21.2) | 0 (0.0) |
| IgA | 83 (97.6) | 2 (2.4) | 2 (2.4) | 0 (0.0) |
| IgM | 78 (91.8) | 7 (8.2) | 0 (0.0) | 7 (8.2) |
| IgE | 64 (75.3) | 21 (24.7) | 21 (24.7) | 0 (0.0) |
| C3 | 83 (97.6) | 2 (2.4) | 0 (0.0) | 2 (2.4) |
| C4 | 80 (94.1) | 5 (5.9) | 5 (5.9) | 0 (0.0) |
| Percentage of B cells (CD19+) | 72 (84.7) | 13 (15.3) | 2 (2.4) | 11 (12.9) |
| Percentage of T cells (CD3+) | 65 (76.5) | 20 (23.5) | 3 (3.5) | 17 (20.0) |
| Percentage of CD4+ T cells | 63 (74.1) | 22 (25.9) | 0 (0.0) | 22 (25.9) |
| Percentage of CD8+ T cells | 71 (83.5) | 14 (16.5) | 14 (16.5) | 0 (0.0) |
| Percentage of NK cells | 68 (80.0) | 17 (20.0) | 14 (16.5) | 3 (3.5) |
| Ratio of CD4+/CD8+ T cells | 60 (70.6) | 25 (29.4) | 0 (0.0) | 25 (29.4) |
Figure 1.
Frequencies of abnormal levels of immunoglobulins and complements and abnormal percentages/ratios of lymphocyte subsets in the RAU patients. The red columns indicate the frequencies higher than the upper limit of reference range, while the blue columns indicate the frequencies lower than the lower limit of reference range.
Altered percentages of lymphocyte subsets in RAU patients
As shown in Table 2, the percentage of CD4+ T cells and B cells of RAU patients were significantly lower than those of healthy controls (P < 0.001). Whereas the percentage of CD8+ T cells and NK cells of RAU patients were significantly higher than those of healthy controls (P < 0.05). And the ratio of CD4+/CD8+ T cells of RAU patients was significantly lower than that of healthy control group (P < 0.001). As shown in Table 3, the percentage of total T cells was decreased in 17 patients (20.0%). The percentage of CD4+ T cells was decreased below the normal range in 22 patients (25.9%), whereas the percentage of CD8+ T cells was elevated above the normal range in 14 patients (16.5%). The CD4+/CD8+ ratio was decreased below the normal range in 25 patients (29.4%). Additionally, the percentage of B cells was diminished in 11 patients (12.9%). The percentage of NK cells was augmented in 14 patients (16.5%). The frequencies of abnormal percentages of B, T and NK cells were presented in Fig. 1.
Correlations of the frequencies of abnormal IgG and IgE levels with that of abnormal CD8+ T cell percentage in RAU patients
No significant correlations were detected between the frequencies of abnormal serum levels of immunoglobulins or complements in the patients. However, the frequency of abnormal total T cell percentage was significantly correlated with that of abnormal B cell percentage (r = 0.227, P = 0.037). The frequency of abnormal CD4+ T cell percentage was significantly correlated with that of abnormal total T cell percentage (r = 0.286, P = 0.008). Besides, the frequency of abnormal NK cell percentage was significantly correlated with that of abnormal total T cell percentage (r = 0.624, P < 0.001), as well as with that of abnormal CD4+ T cell percentage (r = 0.357, P = 0.001). Interestingly, the frequency of abnormal serum IgG level was significantly correlated with that of abnormal CD8+ T cell percentage in the patients (r = 0.313, P = 0.004). Moreover, the frequency of abnormal serum IgE level was significantly correlated with that of abnormal CD8+ T cell percentage (r = 0.260, P = 0.016). In addition, the frequency of abnormal serum IgM level was significantly correlated with that of abnormal B cell percentage in the patients (r = 0.229, P = 0.035) (Table 4).
Table 4.
Correlations between the frequencies of abnormal levels of immunoglobulins or complements and abnormal percentages/ratios of lymphocyte subsets in the individuals analyzed.
| IgG | IgA | IgM | IgE | C3 | C4 | |
|---|---|---|---|---|---|---|
| CD19+ | r = 0.020 P = 0.858 |
r = −0.066 P = 0.549 |
r = 0.229 P = 0.035* |
r = −0.092 P = 0.403 |
r = −0.066 P = 0.549 |
r = −0.106 P = 0.333 |
| CD3+ | r = −0.016 P = 0.885 |
r = −0.086 P = 0.433 |
r = 0.136 P = 0.213 |
r = 0.132 P = 0.227 |
r = −0.086 P = 0.433 |
r = −0.021 P = 0.850 |
| CD4+ | r = −0.121 P = 0.269 |
r = −0.095 P = 0.389 |
r = 0.107 P = 0.332 |
r = 0.142 P = 0.194 |
r = −0.095 P = 0.389 |
r = −1.152 P = 0.164 |
| CD8+ | r = 0.313 P = 0.004* |
r = −0.069 P = 0.531 |
r = −0.133 P = 0.225 |
r = 0.260 P = 0.016* |
r = −0.069 P = 0.531 |
r = −0.111 P = 0.312 |
| NK | r = −0.043 P = 0.695 |
r = −0.078 P = 0.480 |
r = 0.171 P = 0.117 |
r = 0.055 P = 0.620 |
r = −0.078 P = 0.480 |
r = 0.000 P = 1.000 |
| CD4+/CD8+ | r = 0.093 P = 0.395 |
r = −0.103 P = 0.348 |
r = −0.013 P = 0.905 |
r = 0.093 P = 0.396 |
r = −0.103 P = 0.348 |
r = −0.057 P = 0.602 |
Statistical significance: *P < 0.05.
Discussion
RAU is a common disease that affects a large number of individuals. However, its exact cause remains unclear. Recently, increasing evidence indicates that immunologic mechanisms play crucial roles in the etiology of RAU.13 The results of the present study showed that 65 of the 85 RAU patients had one or more frequencies of abnormal immune parameter levels in peripheral blood.
Humoral immunity plays a critical role in defending microbial infection, and the associated defense mechanisms may involve immunoglobulins, complements and their subclass responses. A previous study has demonstrated the increased serum and salivary IgA levels in minor RAU patients compared with controls.2 Nevertheless, the same study reported no difference of serum IgG or IgM level between RAU patients and controls.2 Another study has demonstrated that the salivary levels of all IgG subclasses, as well as that of IgA2, are increased in RAU patients compared with controls.3 Marked increase of salivary IgA has been reported in minor RAU in comparison with the levels in healthy controls.4, 5 Furthermore, an elevated serum IgE level has been detected in RAU in association with disease characteristics.6 In addition, a study has confirmed that the levels of C3c, C4 and hemolytic activity of the complement system were higher in RAU than in healthy people.7 The results of our study indicated that the serum IgG, IgA, IgE, C3 and C4 levels were elevated in RAU patients compared to healthy controls. However, no significant alteration of the serum IgM level was observed. Our data also suggested that the percentages of CD4+ T cells and B cells in peripheral blood of RAU patients were significantly decreased, whereas the percentages of CD8+ T cells and NK cells of RAU patients were remarkably increased. Our findings were in accordance with most results in the published literature.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The discrepancies between our findings and those of previous studies may be due to the difference of research methods, distinct periods of RAU (acute or remission phases) and diversified genetic background of RAU patients.
The serum and salivary levels of immunoglobulins may be elevated in some infectious, inflammatory and autoimmune diseases. IgG is the main isotype of human immunoglobulins which has the longest serum half-life. IgG antibodies take part in neutralizing viruses and toxins.15 Notably, elevated levels of IgG antibodies to Helicobacter pylori have been detected in the serum and saliva of RAU patients.16, 17 With regard to the etiopathogenesis of RAU, microbial infection is considered as a potential trigger factor. Some bacteria or viruses may act as causative pathogens to modify the immunologic response and invoke RAU.13 Some recent studies have revealed that RAU is associated with dysbiosis of mucosal and salivary microbiota.18, 19, 20, 21 Furthermore, a previous study have shown that H. pylori may play an etiological role in RAU.22 Moreover, the associations between RAU and several viral infections have been reported.23 IgE is not only central to the pathogenesis of many allergic diseases but also important in the defense against parasitic infections. Recently, an elevated serum IgE level has been identified as a possible biomarker for the pathogenesis of different diseases, and it may be considered as part of the workup of RAU patients.6, 24 Accordingly, food allergies have also been regarded as potential etiological factors for RAU.12, 25, 26, 27
Serum IgA is in monomeric form and next to IgG at level. A majority of IgA, however, is in dimeric form and called secretary IgA. IgA is also the principal immunoglobulin isotype found in the external secretions including saliva. Secretary IgA has an important role in protection of aerodigestive tract mucosa and acts as a barrier against pathogens, antigens and even allergens. Thus, any defect in the defense system could be an important risk factor for increasing the susceptibility to infection, autoimmune disorders and allergy.28 In comparison with serum IgG and IgE, the frequency of elevated serum IgA level in RAU patients was relatively low in the present study. The possible explanation is mainly that the alteration extent of serum IgA level in RAU patients could be milder than that of serum IgG or IgE level. Additionally, serum IgA may play a less pivotal part in the pathogenesis of RAU compared to salivary IgA. Several previous studies have confirmed marked increase of salivary IgA in RAU, which suggested an important role for salivary IgA in pathogenesis of RAU.2, 3, 4, 5
Cellular immunity plays a crucial role in RAU pathogenesis. RAU development has been proposed to be dependent on the hyper-responsiveness of effector Th1 cells and keratinocyte cytolysis on account of cytotoxic CD8+ T cells.12 A decreased percentage of CD4+ T cells, elevated percentages of CD8+ T and NK cells, and a diminished CD4+/CD8+ ratio have been reported in RAU patients.12, 13 In accordance with these previous results, our data supported that an abnormal cellular immunological response played an important role in the pathogenesis of RAU. CD8+ T cell response is modulated by inflammatory cytokines. Some studies have demonstrated increased production of Type 1 cytokines IL-2, IFN-γ and TNF-α as well as IL-5, IL-6 and IL-8 by peripheral blood mononuclear cells in RAU. In contrast, IL-10 and TGF-β anti-inflammatory cytokine (Type 2) production was decreased in RAU.29 Moreover, some authors have found that T regulatory cell (Treg) proportion and function were decreased in peripheral blood of RAU patients.29, 30 Furthermore, other researchers have reported that serum IL-17 level was increased in RAU.31, 32 It is well known that Treg cells are anti-inflammatory and Th17 cells are pro-inflammatory in function. In brief, activated Th1, Th17 cells and inhibited Th2, Treg cells could contribute to imbalance in pro- and anti-inflammatory cytokines network in RAU, which promote excessively cytotoxic immune response by stimulating CD8+ T cells towards harmless colonized microorganisms or self-antigens of oral mucosa.29, 31, 32
The results of the present study revealed associations of the frequencies of abnormal serum IgG or IgE levels with that of an abnormal CD8+ T cell percentage in RAU, indicating a potential link between humoral and cellular immunity. Many studies have suggested that Th1-type immunologic response plays a crucial role in the development of RAU.14, 29, 33, 34, 35 Th1-type cytokines are associated with enhanced autoimmunity, induction of the cellular immune response and stimulation of IgG secretion.16 In turn, the cellular immune response and IgG release are considered as the main processes that confer protection from microbial infections. CD8+ cytotoxic T cells (CTLs) are critical in the control of viral and bacterial infections, and they function by directly killing infected cells. Moreover, a previous study has revealed an important immunoregulatory role of IgE in the nonallergic inflammatory response, and has reported that IgE deficiency may decrease the activation of CD8+ T cells and promote normalization of the CD4+/CD8+ T cell ratio in lupus-prone mice.36
Collectively, the levels of both humoral and cellular immune components may be altered in the peripheral blood of RAU patients. The close correlation between the abnormal humoral and cellular immune responses implies that they may both be synergistically involved in the pathogenesis of this disease. Further studies including larger sample sizes and investigation of the fundamental underlying mechanisms are necessary to elucidate this correlation.
Disclosure of conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgment
This work was supported by the Key Project of Science and Technology Department of Jiangsu Province (No. BL2014018), the Nonprofit Industry Research Specific Fund of National Health and Family Planning Commission of China (No. 201502018), the National Natural Scientific Foundation of China (No. 81570978 & No. 81400521) and the Project of Jiangsu Provincial Medical Youth Talent (No. QNRC2016118).
Contributor Information
Xiang Wang, Email: yuwx999@sina.com.
Wen-Mei Wang, Email: wenmei-wang@hotmail.com.
References
- 1.Li X.Y., Zhang Z.C. Assessment of serum malondialdehyde, uric acid, and vitamins C and E levels in patients with recurrent aphthous stomatitis. J Dent Sci. 2016;11:401–404. doi: 10.1016/j.jds.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brozović S., Vucićević-Boras V., Buković D. Serum IgA, IgG, IgM and salivary IgA in recurrent aphthous ulceration. Coll Antropol. 2001;25:633–637. [PubMed] [Google Scholar]
- 3.Sistig S., Vucićević-Boras V., Lukac J., Kusić Z. Salivary IgA and IgG subclasses in oral mucosal diseases. Oral Dis. 2002;8:282–286. doi: 10.1034/j.1601-0825.2002.20844.x. [DOI] [PubMed] [Google Scholar]
- 4.Saluja R., Kale A., Hallikerimath S. Determination of levels of salivary IgA subclasses in patients with minor recurrent aphthous ulcer. J Oral Maxillofac Pathol. 2012;16:49–53. doi: 10.4103/0973-029X.92973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammad R., Halboub E., Mashlah A., Abou-Hamed H. Levels of salivary IgA in patients with minor recurrent aphthous stomatitis: a matched case-control study. Clin Oral Invest. 2013;17:975–980. doi: 10.1007/s00784-012-0785-2. [DOI] [PubMed] [Google Scholar]
- 6.Almoznino G., Zini A., Mizrahi Y., Aframian D.J. Elevated serum IgE in recurrent aphthous stomatitis and associations with disease characteristics. Oral Dis. 2014;20:386–394. doi: 10.1111/odi.12131. [DOI] [PubMed] [Google Scholar]
- 7.Lewkowicz N., Lewkowicz P., Kurnatowska A. Innate immune system is implicated in recurrent aphthous ulcer pathogenesis. J Oral Pathol Med. 2003;32:475–481. doi: 10.1034/j.1600-0714.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- 8.Sistig S., Cekic-Arambasin A., Rabatic S., Vucicevic-Boras V., Kleinheinz V., Piffko J. Natural immunity in recurrent aphthous ulceration. J Oral Pathol Med. 2001;30:275–280. doi: 10.1034/j.1600-0714.2001.300504.x. [DOI] [PubMed] [Google Scholar]
- 9.Savage N.W., Mahanonda R., Seymour G.J., Bryson G.J., Collins R.J. The proportion of suppressor-inducer T-lymphocytes is reduced in recurrent aphthous stomatitis. J Oral Pathol. 1988;17:293–297. doi: 10.1111/j.1600-0714.1988.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun A., Chiang C.P., Chiou P.S., Wang J.T., Liu B.Y., Wu Y.C. Immunomodulation by levamisole in patients with recurrent aphthous ulcers or oral lichen planus. J Oral Pathol Med. 1994;23:172–177. doi: 10.1111/j.1600-0714.1994.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun A., Chu C.T., Liu B.Y., Wang J.T., Leu J.S., Chiang C.P. Expression of interleukin-2 receptor by activated peripheral blood lymphocytes upregulated by the plasma level of interleukin-2 in patients with recurrent aphthous ulcers. Proc Natl Sci Counc Repub China B. 2000;24:116–122. [PubMed] [Google Scholar]
- 12.Wardhana, Datau E.A. Recurrent aphthous stomatitis caused by food allergy. Acta Med Indones. 2010;42:236–240. [PubMed] [Google Scholar]
- 13.Slebioda Z., Szponar E., Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz) 2014;62:205–215. doi: 10.1007/s00005-013-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freysdottir J., Lau S., Fortune F. Gammadelta T cells in Behçet's disease (BD) and recurrent aphthous stomatitis (RAS) Clin Exp Immunol. 1999;118:451–457. doi: 10.1046/j.1365-2249.1999.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder H.W., Jr., Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansour-Ghanaei F., Asmar M., Bagherzadeh A.H., Ekbataninezhad S. Helicobacter pylori infection in oral lesions of patients with recurrent aphthous stomatitis. Med Sci Mon. 2005;11:CR576–C579. [PubMed] [Google Scholar]
- 17.Albanidou-Farmaki E., Giannoulis L., Markopoulos A. Outcome following treatment for Helicobacter pylori in patients with recurrent aphthous stomatitis. Oral Dis. 2005;11:22–26. doi: 10.1111/j.1601-0825.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.J., Choi Y.S., Baek K.J., Yoon S.H., Park H.K., Choi Y. Mucosal and salivary microbiota associated with recurrent aphthous stomatitis. BMC Microbiol. 2016;16(Suppl 1):57. doi: 10.1186/s12866-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seoudi N., Bergmeier L.A., Drobniewski F., Paster B., Fortune F. The oral mucosal and salivary microbial community of Behcet's syndrome and recurrent aphthous stomatitis. J Oral Microbiol. 2015;7:27150. doi: 10.3402/jom.v7.27150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hijazi K., Lowe T., Meharg C., Berry S.H., Foley J., Hold G.L. Mucosal microbiome in patients with recurrent aphthous stomatitis. J Dent Res. 2015;94:87S–94S. doi: 10.1177/0022034514565458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bankvall M., Sjöberg F., Gale G., Wold A., Jontell M., Östman S. The oral microbiota of patients with recurrent aphthous stomatitis. J Oral Microbiol. 2014;6:25739. doi: 10.3402/jom.v6.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gülseren D., Karaduman A., Kutsal D., Nohutcu R.M. The relationship between recurrent aphthous stomatitis, and periodontal disease and Helicobacter Pylori infection. Clin Oral Invest. 2016;20:2055–2060. doi: 10.1007/s00784-015-1704-0. [DOI] [PubMed] [Google Scholar]
- 23.Seoudi N., Bergmeier L.A., Hagi-Pavli E., Bibby D., Fortune F. The seroprevalence and salivary shedding of herpesviruses in Behcet's syndrome and recurrent aphthous stomatitis. J Oral Microbiol. 2015;7:27156. doi: 10.3402/jom.v7.27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand P., Singh B., Jaggi A.S., Singh N. Mast cells: an expanding pathophysiological role from allergy to other disorders. Naunyn Schmiedeb Arch Pharmacol. 2012;385:657–670. doi: 10.1007/s00210-012-0757-8. [DOI] [PubMed] [Google Scholar]
- 25.Besu I., Jankovic L., Konic-Ristic A., Damjanovic A., Besu V., Juranic Z. Good tolerance to goat's milk in patients with recurrent aphthous ulcers with increased immunoreactivity to cow's milk proteins. J Oral Pathol Med. 2013;42:523–527. doi: 10.1111/jop.12052. [DOI] [PubMed] [Google Scholar]
- 26.Besu I., Jankovic L., Magdu I.U., Konic-Ristic A., Raskovic S., Juranic Z. Humoral immunity to cow's milk proteins and gliadin within the etiology of recurrent aphthous ulcers? Oral Dis. 2009;15:560–564. doi: 10.1111/j.1601-0825.2009.01595.x. [DOI] [PubMed] [Google Scholar]
- 27.Calderón P.E., Valenzuela F.A., Carreño L.E., Madrid A.M. A possible link between cow milk and recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. 2008;22:898–899. doi: 10.1111/j.1468-3083.2007.02542.x. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadiafshar A., Mohsenifard M.R., Mazloomzadeh S. Evaluation of serum & salivary IgA in patients with type 1 diabetes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewkowicz N., Lewkowicz P., Banasik M., Kurnatowska A., Tchórzewski H. Predominance of Type 1 cytokines and decreased number of CD4(+) CD25(+high) T regulatory cells in peripheral blood of patients with recurrent aphthous ulcerations. Immunol Lett. 2005;99:57–62. doi: 10.1016/j.imlet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Lewkowicz N., Lewkowicz P., Dzitko K. Dysfunction of CD4+CD25 high T regulatory cells in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2008;37:454–461. doi: 10.1111/j.1600-0714.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 31.Lewkowicz N., Kur B., Kurnatowska A., Tchorzewski H., Lewkowicz P. Expression of Th1/Th2/Th3/Th17-related genes in recurrent aphthous ulcers. Arch Immunol Ther Exp (Warsz) 2011;59:399–406. doi: 10.1007/s00005-011-0134-1. [DOI] [PubMed] [Google Scholar]
- 32.Ozyurt K., Celik A., Sayarlıoglu M. Serum Th1, Th2 and Th17 cytokine profiles and alpha-enolase levels in recurrent aphthous stomatitis. J Oral Pathol Med. 2014;43:691–695. doi: 10.1111/jop.12182. [DOI] [PubMed] [Google Scholar]
- 33.Borra R.C., Andrade P.M., Silva I.D. The Th1/Th2 immune-type response of the recurrent aphthous ulceration analyzed by cDNA microarray. J Oral Pathol Med. 2004;33:140–146. doi: 10.1111/j.0904-2512.2004.00089.x. [DOI] [PubMed] [Google Scholar]
- 34.Dalghous A.M., Freysdottir J., Fortune F. Expression of cytokines, chemokines, and chemokine receptors in oral ulcers of patients with Behcet's disease (BD) and recurrent aphthous stomatitis is Th1-associated, although Th2-association is also observed in patients with BD. Scand J Rheumatol. 2006;35:472–475. doi: 10.1080/03009740600905380. [DOI] [PubMed] [Google Scholar]
- 35.Albanidou-Farmaki E., Markopoulos A.K., Kalogerakou F., Antoniades D.Z. Detection, enumeration and characterization of T helper cells secreting type 1 and type 2 cytokines in patients with recurrent aphthous stomatitis. Tohoku J Exp Med. 2007;212:101–105. doi: 10.1620/tjem.212.101. [DOI] [PubMed] [Google Scholar]
- 36.Dema B., Charles N., Pellefigues C. Immunoglobulin E plays an immunoregulatory role in lupus. J Exp Med. 2014;211:2159–2168. doi: 10.1084/jem.20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]