Abstract
Background/purpose
Streptococcus mutans is an important pathogen in the development of dental caries. Many studies have focused on the relationship between nicotine and S. mutans in vitro. The aim of this study was to investigate the effect of nicotine on the growth of S. mutans and its cariogenic potential in vivo.
Materials and methods
Sixteen male Specific-pathogen-free Wistar rats were divided into 2 groups (nicotine-treated and nicotine-untreated group) and infected with S. mutans. The S. mutans suspension was treated with 1 mg/mL nicotine in the nicotine-treated group. The Keyes method was used to evaluate sulcal caries of rats, and dental plaque on molar teeth was observed by scanning electron microscopy (SEM).
Results
Incidence of sulcal caries was higher in nicotine-treated group compared to nicotine-untreated group (42.7 ± 1.7 vs 37.3 ± 4.9, P = 0.009). Severity of caries increased with nicotine treatment. The slightly dentinal caries scores and moderate dentinal caries scores were higher in the presence of nicotine (P < 0.001). Increased number of S. mutans cells attached to dental surface was observed under SEM in the nicotine-treated group.
Conclusion
Nicotine would promote the attachment of S. mutans to dental surface, and further increase the incidence and severity of dental caries. Therefore, nicotine might be a risk factor for smoking-induced caries.
Keywords: Dental caries, Streptococcus mutans, Nicotine, Rats
Introduction
Dental caries is a major health problem that affects 60–90% of school-age children and most adults,1 and is second only to the common cold in humans.2 Caries is a complex and multifactorial condition which results in demineralization and progressive destruction of dental hard tissue. Many factors, such as microorganisms, environment and food, are associated with dental caries.3 Dental plaque is the mainly responsible for the formation and development of caries. Streptococcus mutans is thought to be a crucial pathogen involved in the formation of dental caries and the presence of S. mutans is 70 times higher in caries-affected subjects than in caries-free subjects.4 The ability of S. mutans to synthesize extracellular polysaccharide (EPS) and produce acids leads to the establishment and development of highly cariogenic dental biofilms.5,6 And the tolerance to low pH helps S. mutans survival in oral ecosystem.7,8 Taken together, all these characteristics make S. mutans cariogenic.
That tobacco smoking is harmful to human health has been well demonstrated. It leads to cardiovascular disease, cancers and other systemic diseases.9, 10, 11 Oral cavity is inevitably affected by smoking, since it is the first part exposed to tobacco smoke. The incidence of periodontal diseases and oral cancer is much higher in smokers than in non-smokers.12,13 In recent years, more and more studies have found a close correlation between smoking and dental caries. In England, exposure tobacco products for years significantly increased coronal and root caries.14 In USA, there was a dose-dependent association between tobacco shewing and root-surface caries.15 In Japan, existence of smokers in the home and the number of smokers in the family are significantly associated with early childhood caries (ECC).16 An in vivo study demonstrated that cigarette smoke exposure and viral infections can synergistically increase the susceptibility of mice to secondary bacterial invasion.17 Another in vivo study showed that exposure to cigarette smoke expands the caries-affected area in the maxillary molars of rats.18 Consequently, the relationship between tobacco smoking and dental caries is unambiguous. Nicotine is an alkaloid and the component of cigarette smoke. Lots of studies focused on the relationship between nicotine and S. mutans in vitro. Our previous in vitro studies demonstrated that nicotine has the promotion effect on S. mutans growth, metabolic activity, cell aggregation, acids production and EPS synthesis.19, 20, 21 However, no reports ever concerns about their relationship in vivo. It would be of great interest to investigate the effect of nicotine on the growth of S. mutans and its cariogenic potential in vivo and further verify our previous in vitro studies.
Materials and methods
Ethics statement
The study was performed with the approval of the West China Hospital of Stomatology Institute Review Board (WCSHIRB) ethics committee and all experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemicals, bacterial strains and growth conditions
S. mutans UA159 (ATCC 700610, a cariogenic bacterial pathogen) was inoculated into brain heart infusion (BHI) broth with or without 1 mg/mL nicotine (Sigma–Aldrich, St Louis, MO, USA) and incubated overnight. Our previous study demonstrated that the minimum inhibitory concentration (MIC) of nicotine to S. mutans was 16 mg/mL,22 and the physiological concentration of nicotine in the saliva of a smoker ranges from 70 to 1560 μg/mL.23 In this study, 1 mg/mL nicotine was chosen as an appropriate concentration. For each treatment, the concentration of bacteria was adjusted to 1 × 109 colony-forming units (CFU)/mL. The bacteria were incubated in an atmosphere of 5% CO2 at 37 °C.
In vivo models of dental caries
Rats have been used as a model to establish dental caries since 1922.24 Sixteen male Specific-pathogen-free Wistar child rats aged 21 days were randomly and averagely divided into 2 groups (n = 8), one treated with nicotine and the other without nicotine. The selection of rats' age was according to the article that Bowen WH et al.25
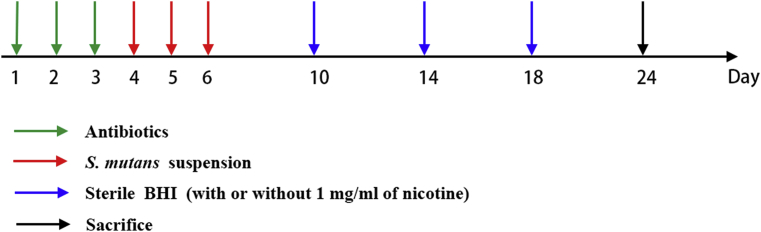
During the first three days, any indigenous oral microorganism was removed by feeding the rats a diet containing antibiotics (chloramphenicol, ampicillin, carbenicillin, 1.0 g/kg diet).26 S. mutans was incubated in culture medium (BHI) with or without 1 mg/mL of nicotine for 24 h and then inoculated to the rats' teeth. Each rat was subsequently challenged with 400 μL of 1 × 109 CFU/mL S. mutans suspension (the suspension contained 1 mg/mL of nicotine in nicotine-treated group) for the following three consecutive days (twice a day, interval of 30 min, no food or water for 1 h after inoculation) and then either sterile BHI plus 1 mg/mL nicotine (nicotine-treated group) or sterile BHI (nicotine-untreated group) every four days until the rats were sacrificed. The schedule is shown in Fig. 1. All rats were provided with the National Institutes of Health cariogenic diet 2000 and 5% sucrose water.27 The experiment lasted for 24 days and then the rats were sacrificed. The jaws were aseptically dissected and processed for Keyes caries scoring28 and scanning electron microscopy (SEM). We randomly selected 7 rats processing for Keyes caries scoring and 1 rat (4 jaws) for SEM in each group.
Figure 1.
Challenge schedule of rats. Rats were fed with antibiotics for the first three consecutive days and then were challenged with a S. mutans suspension for next three consecutive days and sterile BHI (with or without 1 mg/mL of nicotine) every four days until the rats were sacrificed.
The methods for Keyes caries scoring: Four jaws of each rat were stained in 0.4% ammonium salt solution for 16 h, protected from light. The jaws were rinsed, dried and hemisectioned, and finally observed by a stereo microscope. The caries lesions were stained in red and Keyes scoring rules were used to assess the caries of each rat. The depth and size of red ammonium salt solution disseminated into tooth represent the severity and impact area of caries lesions. According to the Keyes method, caries are divided into four grades: enamel only (E), slightly dentinal (Ds), moderate dentinal (Dm), and extensive dentinal (Dx). Ds caries involve approximately 1/4 of the dentin between the enamel and pulp chamber. Dm caries include involvement of approximately 1/4–3/4 of the dentin, while the penetration beyond 3/4 of the dentin region is labelled Dx caries. Sulcal caries lesions of every molar tooth at each level (E, Ds, Dm, and Dx) in every rat (selected rats proceeded for Keyes caries scoring) were scored. Then we added the E scores of every molar tooth in a rat up and the total number of E scores in a rat was the data we collected to statistically analyze. The same calculus was applied to collect the Ds, Dm, Dx scores data. Since dental decay starts from the enamel regions and gradually progresses to the dentin regions, The E scores represents the incidence of caries and the Ds, Dm, Dx scores represent the severity of caries.28
Scanning electron microscopy (SEM)
We randomly chose 1 rat in each group processing for SEM. After removing extra bones and flesh surrounding the jaws, the jaws were washed twice with phosphate buffer saline (PBS) and fixed overnight with 2.5% glutaraldehyde at 4 °C. The jaws were subsequently washed twice with distilled water, dehydrated by a series of ethanol rinses (30, 50, 70, 80, 85, 90, 95 and 100%), immersed for 10 min in hexamethyldisilazane and dried in a desiccator. After sputter coating with gold-palladium, samples were imaged at least three times on randomly selected positions in a scanning electron microscope at 2000×, 5000×, 10000× magnification.
Statistical analysis
The E, Ds, Dm, and Dx scores of each rat was examined and recorded according to the Keyes method. SPSS 21.0 software was used for data analysis. An independent t-test was performed to compare nicotine-treated and untreated groups. A significance level of p < 0.05 was adopted for statistical hypothesis testing.
Results
Sulcal caries scores after S. mutans challenge
The caries lesions on the teeth of the rats were observed by stereo microscope (Fig. 2) and scored using the Keyes method. The statistical analysis is shown in Fig. 3.
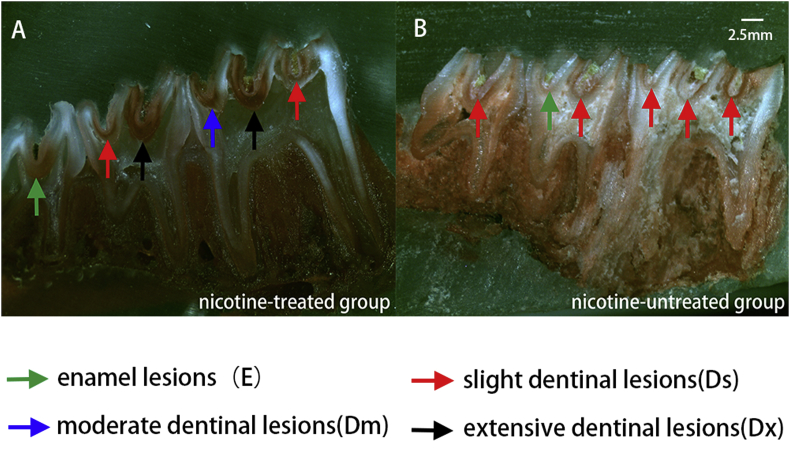
Figure 2.
Representative caries lesions were detected by stereo microscopy. Caries progressed to moderate dentine (Dm) and extensive dentine (Dx) in the nicotine-treated group. Dm & Dx caries were seldom seen in the other group.
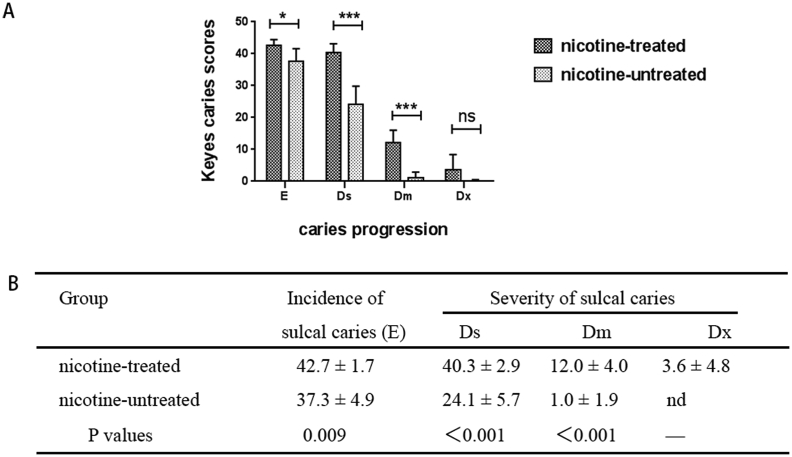
Figure 3.
Statistical analysis of Keyes scores. (A) Scores for enamel caries (E), slightly dentinal caries (Ds), and moderate dentinal caries (Dm) caries were significantly higher in nicotine-treated group (P < 0.05), while no statistical difference in extensive dentinal caries (Dx) was seen between the two groups (P > 0.05). *P < 0.05, ***P < 0.001, ns, not significant. Error bars represent SD. (B) Values denote means ± SD (n = 7), nd, not detectable. There was an obvious increase in the incidence and severity of sulcal caries.
Sulcal caries had progressed to moderate or extensive dentine (Dm, Dx) lesions in the nicotine-treated group, and only a few slight dentinal lesions (Ds) were observed (Fig. 2A). However, in nicotine-untreated group, most of the sulcal caries of the teeth had only progressed to Ds, and Dm & Dx were seldom detected (Fig. 2B).
As shown in Fig. 3A, significantly more caries lesions, including enamel (E), slight dentinal (Ds) and moderate dentinal (Dm) caries lesions, were observed in the nicotine-treated group (P < 0.05). Although the total extensive dentinal (Dx) caries scores were higher in the nicotine-treated group than in the untreated group, there was no statistical difference in Dx caries lesions between the two groups (P > 0.05). As stated before, the E caries lesions represents the incidence of caries and the Ds, Dm, Dx caries lesions represent the severity of caries. Fig. 3B shows that there was an obvious increase in the incidence and severity of sulcal caries in the nicotine-treated group. Therefore, we concluded that nicotine promoted the development of dental caries in rats challenged with S. mutans.
Attachment of S. mutans to the dental surface
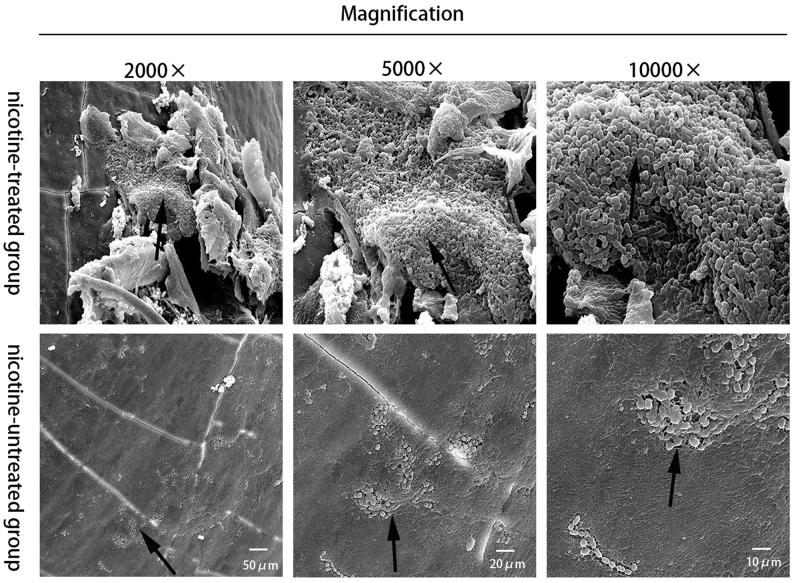
A scanning electron microscope produces the enlarging morphology images of materials on sample surface. Scanning electron microscopy images showed the bacterial plaque on the rats' molar buccal surface. Though there is no measurement method of plaque biomass or cell numbers according to a SEM image, it is intuitive that more bacterial cells were observed in the nicotine-treated group as shown in Fig. 4. Nicotine increased the number of S. mutans cells attached to the dental surface. In addition, the chain length of the cells was longer in the nicotine-treated group, with the cells also forming clumps and aggregates. However, fewer cells were observed in the control group and the cells were more randomly distributed.
Figure 4.
Representative bacterial plaques on tooth surfaces were observed by scanning electron microscopy. The two groups of nicotine treated (the upper) and nicotine untreated (the lower) samples were detected at least three times on randomly selected positions at 2000×, 5000×, 10000× magnification. More cells were observed in the nicotine-treated group, and cells formed clumps and aggregates.
Discussion
To our knowledge, this is the first study to explore the effect of nicotine on the cariogenic potential of S. mutans in vivo. Regarding the sample sizes ranged from 6 to 10 rats for each group in most caries studies,29, 30, 31, 32, 33, 34 we took 16 rats and divided them into two groups (n = 8). The results of our study provide striking evidence that nicotine promotes the attachment of S. mutans to dental surface and the development of caries in vivo, and it appears to be a promotion factor for the development of dental caries.
Since 1968 when the first reports of S. mutans inducing rampant caries in the hamsters was recorded,35 S. mutans has been considered as the major cariogenic pathogen. The ability of glucosyltransferases (Gtfs) production, synthesizing extracellular polysaccharide (EPS), generating acids and tolerance at low pH imbues S. mutans with strong cariogenicity.36 Biofilms act as an important persistence mechanism, since biofilms are capable of resisting chemotherapeutic agents, immune factors, antibiotics, as well as host-derived antibacterial agents.37 Nicotine has been reported to possess a biphasic effect on S. mutans planktonic growth, as 10−3 to 10−4 M nicotine increased growth of S. mutans, while 10−1 to 10−2 M nicotine decreased growth.19 In our study, a nicotine concentration of 1 mg/mL, i.e., 6 × 10−3 M definitely promoted the growth of S. mutans and the number of S. mutans cells attached to the dental surface. As oral bacteria mainly live as biofilms, the promotion effect of nicotine on the attachment of S. mutans to the dental surface and further increasing the biofilm formation in rats contributes to improved survival and enhanced the cariogenic ability of S. mutans.
It has been reported that the key virulence factor of S. mutans is the production of insoluble extracellular polysaccharide (EPS) through exoenzymes, such as glucosyltransferases (Gtfs).38 EPS are the prime constituents of biofilms and not only promote the colonization of S. mutans on the tooth surface, but also attract other microorganisms to form dental plaque. As a consequence, a structured community or matrix is formed.39 The EPS-rich matrix creates acidic microenvironments within the biofilms, and leads to the demineralization of dental hard tissues.40 Our previous in vitro study demonstrated that both cell numbers in biofilms as well as EPS were increased by nicotine, and Gtf protein expression was upregulated.20 The results also showed that more S. mutans cells were observed on dental surface in nicotine treated group. Since more bacterial cells secreted more EPS and more EPS developed a richer matrix, lowering the pH of the biofilm and increasing demineralization. It would be the reason why there was an obvious increase in the incidence and severity of sulcal caries in the nicotine-treated group in our study.
Some studies pointed out that the smokers had poorer oral health and were twice likely to attend the dentist compared with the non-smokers (75% vs 57%).41 Another report indicated that current smokers received significantly higher caries/endodontic treatments than non-smokers (47.1% vs 43.6%).42 And a recent report indicated that exposure to tobacco smoke at the age of 4 months old was associated with a nearly 2 fold increased risk of developing dental caries, and the risk of caries was also increased among those who were exposed to household smoking by 1–1.5 fold.43 These studies once again proved the contact with cigarette smoke and accumulation of nicotine significantly increasing the risk of caries. However, the concentration of nicotine in oral is affected by many factors, including different volumes of saliva secreted by individuals, different types of cigarettes, duration of smoking, sample locations and measuring methods. One study reported nicotine concentrations in saliva ranged from 96 ng/mL to 1.6 mg/mL.44 Another reported a nicotine range of 367 ng/mL to 2.5 mg/mL in stimulated saliva and 900 ng/mL to 4.6 mg/mL in unstimulated saliva.45 Other studies reported nicotine concentration varied from 70 ng/mL to 1.56 mg/mL.23 In our study, 1 mg/mL of nicotine promoted the development of dental caries in the molar teeth of rats. The phenomenon that there are higher caries risks in smokers might be explained.
In this in vivo study, treatment with nicotine significantly increased the number of S. mutans cells attached to dental surfaces and the development of sulcal caries (P < 0.05) in rats. Therefore, nicotine is a risk factor for dental caries.
Conflicts of interest statement
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
Acknowledgements
We are thankful to Chaoliang Zhang for the technical support. This study was supported by the National Natural Science Foundation of China (81400501 to M.L.)
References
- 1.Petersen P.E., Bourgeois D., Ogawa H., Estupinan-Day S., Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–669. [PMC free article] [PubMed] [Google Scholar]
- 2.Islam B., Khan S.N., Khan A.U. Dental caries: from infection to prevention. Med Sci Monitor. 2007;13:196–203. [PubMed] [Google Scholar]
- 3.Selwitz R.H., Ismail A.I., Pitts N.B. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 4.Peterson S.N., Snesrud E., Liu J. The dental palque microbiome in health and disease. PLoS One. 2013;8:e58487. doi: 10.1371/journal.pone.0058487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowden G.H.W. Microbiology of root surface caries in humans. J Dent Res. 1990;69:1205–1210. doi: 10.1177/00220345900690051701. [DOI] [PubMed] [Google Scholar]
- 6.Marsh P.D., Bradshaw D.J. Dental plaque as a biofilm. J Ind Microbiol. 1995;15:169–175. doi: 10.1007/BF01569822. [DOI] [PubMed] [Google Scholar]
- 7.Belli W.A., Marquis R.E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svensater G., Borgstrom M., Bowden G.H.W., Edwardsson S. The acid-tolerant microbiota associated with plaque from initial caries and healthy tooth surfaces. Caries Res. 2003;37:395–403. doi: 10.1159/000073390. [DOI] [PubMed] [Google Scholar]
- 9.Mainali P., Pant S., Rodriguez A.P., Deshmukh A., Mehta J.L. Tobacco and cardiovascular health. Cardiovasc Toxicol. 2015;15:107–116. doi: 10.1007/s12012-014-9280-0. [DOI] [PubMed] [Google Scholar]
- 10.Depp C.A., Bowie C.R., Mausbach B.T. Current smoking is associated with worse cognitive and adaptive functioning in serious mental illness. Acta Psychiatr Scand. 2015;131:333–341. doi: 10.1111/acps.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizawa K., Liu C., Tang S. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int J Cancer. 2016;139:1171–1181. doi: 10.1002/ijc.30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibars A.R., Obeidat S.R., Khader Y., Mahasneh A.M., Khabour O.F. The effect of Waterpipe smoking on periodontal health. Oral Health Prev Dent. 2015;13:253–259. doi: 10.3290/j.ohpd.a32671. [DOI] [PubMed] [Google Scholar]
- 13.Maleki D., Ghojazadeh M., Mahmoudi S.S. Epidemiology of oral cancer in Iran: a systematic review. Asian Pac J Cancer Prev. 2015;16:5427–5432. doi: 10.7314/apjcp.2015.16.13.5427. [DOI] [PubMed] [Google Scholar]
- 14.Jette A.M., Feldman H.A., Tennstedt S.L. Tobacco use -A modifiable risk factor for dental disease among thee elderly. Am J Public Health. 1993;83:1271–1276. doi: 10.2105/ajph.83.9.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomar S.L., Winn D.M. Chewing tobacco use and dental caries among US men. Am J Public Health. 1993;83:1271–1276. [Google Scholar]
- 16.Nakayama Y., Mori M. Association of environmental tobacco smoke and snacking habits with the risk of early childhood caries among 3-year-old Japanese children. J Public Health Dent. 2015;75:157–162. doi: 10.1111/jphd.12085. [DOI] [PubMed] [Google Scholar]
- 17.Li M., Huang R., Zhou X., Zhang K., Zheng X., Gregory R.L. Effect of nicotine on dual-species biofilms of Streptococcus mutans and Streptococcus sanguinis. FEMS Microbiol Lett. 2014;350:125–132. doi: 10.1111/1574-6968.12317. [DOI] [PubMed] [Google Scholar]
- 18.Fujinami Y., Nakano K., Ueda O. Dental caries area of rat molar expanded by cigarette smoke exposure. Caries Res. 2011;45:561–567. doi: 10.1159/000331926. [DOI] [PubMed] [Google Scholar]
- 19.Huang R., Li M., Gregory R.L. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur J Oral Sci. 2012;120:319–325. doi: 10.1111/j.1600-0722.2012.00971.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang R., Li M., Gregory R.L. Nicotine promotes Streptococcus mutans extracellular polysaccharide synthesis, cell aggregation and overall lactate dehydrogenase activity. Arch Oral Biol. 2015;60:1083–1090. doi: 10.1016/j.archoralbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Li M., Huang R., Zhou X., Qiu W., Xu X., Gregory R.L. Effect of nicotine on cariogenic virulence of Streptococcus mutans. Folia Microbiol. 2016;61:505–512. doi: 10.1007/s12223-016-0465-8. [DOI] [PubMed] [Google Scholar]
- 22.Li M., Huang R., Zhou X., Gregory R.L. Role of sortase in Streptococcus mutans under the effect of nicotine. Int J Oral Sci. 2013;5:206–211. doi: 10.1038/ijos.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann D., Adams J.D. A study of tobacco carcinogenesis .23. carcinogenic tobacco-specific N-nitrosamines in snuff and in the saliva of snuff dippers. Cancer Res. 1981;41:4305–4308. [PubMed] [Google Scholar]
- 24.McCollum E.V., Simmonds N., Kinney E.M., Grieves C.J. The relation of nutrition to tooth development and tooth preservation. I. A preliminary study of gross maxillary and dental defects in two hundred and twenty rats on defective and deficient diets. Bull Johns Hopkins Hosp. 1922;33:202–215. [Google Scholar]
- 25.Bowen W.H. Rodent model in caries research. Odontology. 2013;101:9–14. doi: 10.1007/s10266-012-0091-0. [DOI] [PubMed] [Google Scholar]
- 26.Bao R., Yang J.Y., Sun Y. Flagellin-PAc fusion protein inhibits progression of established caries. J Dent Res. 2015;94:955–960. doi: 10.1177/0022034515582224. [DOI] [PubMed] [Google Scholar]
- 27.Falsetta M.L., Klein M.I., Colonne P.M. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keyes P.H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958;37:1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- 29.Garcia S.S., Blackledge M.S., Michalek S. Targeting of Streptococcus mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. J Dent Res. 2017;96:807–814. doi: 10.1177/0022034517698096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang G., Liu Y., Kim D., Li Y., Krysan D.J., Koo H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017;13:e1006407. doi: 10.1371/journal.ppat.1006407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galvao L.C.C., Rosalen P.L., Rivera-Ramos I. Inactivation of the spxA1 or spxA2 gene of Streptococcus mutans decreases virulence in the rat caries model. Mol Oral Microbiol. 2017;32:142–153. doi: 10.1111/omi.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusumaningsih T., Subijanto M.S., Indrawati R., Devijanti R.R. The level of beta defensin-2 in saliva and its expression in parotid gland epithelial cells after probiotic (Lactobacillus reuteri) induction to inhibit Streptococcus mutans in caries. Eur J Dent. 2016;10:556–560. doi: 10.4103/1305-7456.195161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Y-h, Yu F., Zeng C., Cao L-h, Zhang Z., Xu Q-a. CCL17 combined with CCL19 as a nasal adjuvant enhances the immunogenicity of an anti-caries DNA vaccine in rodents. Acta Pharmacol Sin. 2016;37:1229–1236. doi: 10.1038/aps.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachtiar E.W., Afdhal A., Meidyawati R., Soejoedono R.D., Poerwaningsih E. Effect of topical anti-Streptococcus mutans IgY gel on quantity of S. mutans on rats' tooth surface. Acta Microbiol Immunol Hung. 2016;63:159–169. doi: 10.1556/030.63.2016.2.2. [DOI] [PubMed] [Google Scholar]
- 35.Edwardsson S. Characteristics of caries-inducing human Streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968;13:637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- 36.Xu J.S., Cui Y., Liao X.M., Tan X.B., Cao X. Effect of emodin on the cariogenic properties of Streptococcus matans and development of dental caries in rats. Exp Ther Med. 2014;8:1308–1312. doi: 10.3892/etm.2014.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donlan R.M., Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo H., Bowen W.H. Candida albicans and Streptococcus mutans: a potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014;9:1295–1297. doi: 10.2217/fmb.14.92. [DOI] [PubMed] [Google Scholar]
- 39.Bowen W.H., Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao J., Klein M.I., Falsetta M.L. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csikar J., Kang J., Wyborn C., Dyer T.A., Marshman Z., Godson J. The self-reported oral health status and dental attendance of smokers and non-smokers in England. PLoS One. 2016;11:e0148700. doi: 10.1371/journal.pone.0148700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojima M., Hanioka T., Shimada K., Haresaku S., Yamamoto M., Tanaka K. The role of tobacco use on dental care and oral disease severity within community dental clinics in Japan. Tob Induc Dis. 2013;11:13. doi: 10.1186/1617-9625-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka S., Shinzawa M., Tokumasu H., Seto K., Tanaka S., Kawakami K. Secondhand smoke and incidence of dental caries in deciduous teeth among children in Japan: population based retrospective cohort study. Br Med J. 2015;351:h53978. doi: 10.1136/bmj.h5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barão V.A., Ricomini-Filho A.P., Faverani L.P. The role of nicotine, cotinine and caffeine on the electrochemical behavior and bacterial colonization to cp-Ti. Mater Sci Eng C Mater Biol Appl. 2015;56:114–124. doi: 10.1016/j.msec.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 45.Robson N., Bond A.J., Wolff K. Salivary nicotine and cotinine concentrations in unstimulated and stimulated saliva. Afr J Pharm Pharmacol. 2010;4:61–65. [Google Scholar]