Oral bisphosphonates (OBPs) are the most commonly used class of drug for the treatment of osteoporosis. Although these drugs are usually well tolerated, OBPs have been reported to have a number of more or less serious adverse effects.1 Bisphosphonate-related osteonecrosis of the jaws (BRONJ) is the most known adverse effect from the perspective of oral and maxillofacial pathology, and has been extensively described in the literature.2 However, there is little awareness regarding the risk for developing oral ulcers due to OBP treatments. Given the extensive use of OBPs in clinical practice, the limited number of case reports documenting this adverse effect suggests that there may be underdiagnoses of OBP-related oral ulcer.3 Here we report an unusual case of OBP-related oral ulcer in an elderly female patient, with the aim of bringing attention to a challenging diagnosis due to easily avoidable reasons.
A 71-year-old woman was referred to dental clinic of Marche Polytechnic University for a 9-month history painful ulcer on the dorsum of the tongue, accompanied by dysphagia. She reported a slight weight loss, due to the difficulties in chewing and swallowing.
Her medical history was significant for hypertension, unstable angina, anxiety disorders, and osteoporosis. The drug history of the patient revealed the intake of a proton pump inhibitor (Esomeprazole 20 mg), dual antiplatelet therapy (Clopidogrel 75 mg, Acetylsalicylic acid 100 mg), a combination of a benzodiazepine and a selective serotonin reuptake inhibitor (Alprazolam 0.25 mg, Sertraline 50 mg), a combination of an ACE inhibitor and a diuretic (Zofenopril 30 mg, Hydrochlorothiazide 12.5 mg), a calcium antagonist (Amplodipine 10 mg), and an OBP (Alendronate 70 mg, once weekly).
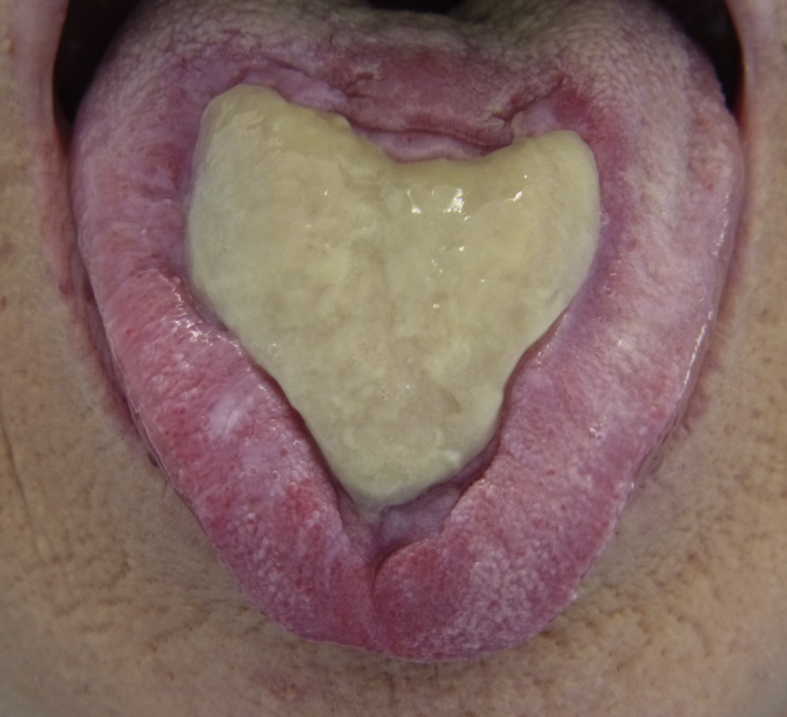
Oral physical examination showed a large heart-shaped 2 × 3 cm non-bleeding ulcer of the tongue, with regular invaginated edges and a necrotic center (Fig. 1). Other mucous membranes were not affected.
Figure 1.
A large heart-shaped non-bleeding ulcer on the dorsum of the tongue of a 71-year-old patient, showing regular invaginated edges and a necrotic center.
A contrast-enhanced CT scan of head and neck was performed 2 months earlier to investigate the nature of the oral lesion. The exam revealed the presence of a hypodense, oval shaped lesion with regular and thin margins, measuring 14 × 6 × 13 mm. Routine laboratory tests were normal.
Biopsy samples were taken from the ulcer borders and processed for histological analysis, immunofluorescence, and microbiological isolation. Histology was unremarkable, showing acute and chronic inflammatory infiltrates, with granulocytes near the necrotic areas. Direct immunofluorescence was negative for IgG, IgA, IgM, complement C3, and fibrinogen. Finally, the results of microbiological analyses showed the absence of pathogens.
Given the unrevealing results, further investigation of the patient's history was undertaken. At subsequent questioning, the patient reported that she allows the tablet to dissolve on the dorsum of the tongue since her general practitioner indication, as she stated.
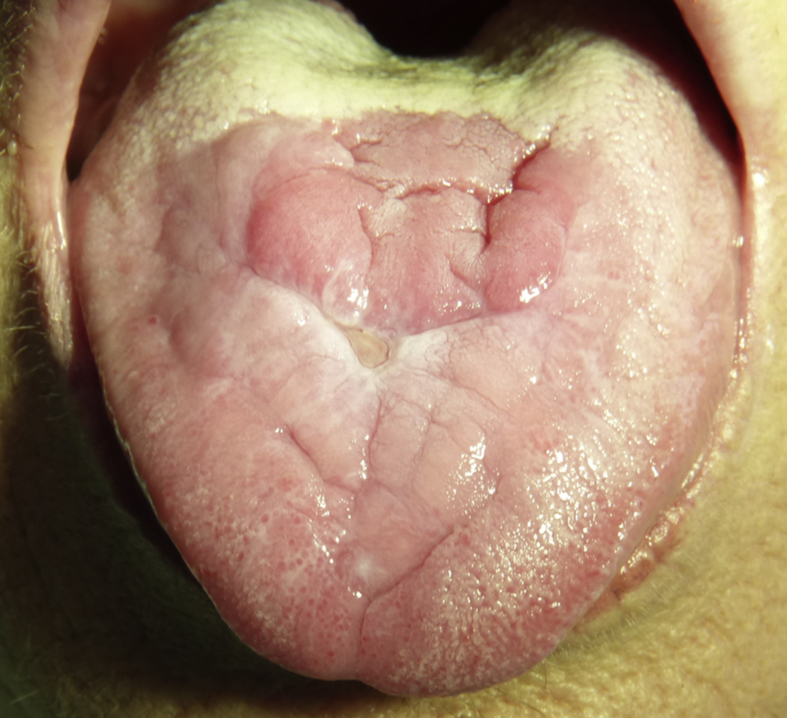
Chronic inflammation and ulceration due to alendronate was suspected. The patient was instructed to swallow the OBP with 200 ml of water in an upright position and staying upright for 30 min, without holding the tablet in her mouth. After 2 months, the ulcer had substantially improved (Fig. 2).
Figure 2.
Oral examination showed significant improvement of the lesion 2 months after the instruction about the correct intake of OBP.
There are few other cases of OBP-related oral ulcers in literature, almost always associated with an incorrect method of administration.4 The prolonged mucosal exposure to OBPs can cause damage to oral mucosa epithelial cells, probably due to the alteration of MAPK signaling pathway.3 As reported by Kharazmi et al., the period required for complete healing is related to the duration of the oral ulcer before elimination of the harmful stimulus.4 In conclusion, physicians who prescribe OBPs must be aware of this rare and subtle side effect and adequately inform patients about the proper way to use these drugs.
Conflicts of interest statement
The authors have no conflict of interest to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Solomon D.H., Rekedal L., Cadarette S.M. Osteoporosis treatments and adverse events. Curr Opin Rheumatol. 2009;21:363–368. doi: 10.1097/BOR.0b013e32832ca433. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero S.L., Dodson T.B., Fantasia J. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Lengfeld J., Buder-Bakhaya K., Goebeler M., Wobser M. Bisphosphonate-mediated oral ulcers: a rare differential diagnosis of erosive oral lesions. Dermatology. 2016;232:117–121. doi: 10.1159/000439347. [DOI] [PubMed] [Google Scholar]
- 4.Kharazmi M., Sjoqvist K., Warfvinge G. Oral ulcers, a little known adverse effect of alendronate: review of the literature. J Oral Maxillofac Surg. 2012;70:830–836. doi: 10.1016/j.joms.2011.03.046. [DOI] [PubMed] [Google Scholar]