Abstract
Background/purpose
Although it has been reported that capsaicin ingestion has effects of protecting stomach mucosa and promoting energy consumption, physiological responses to oral stimulation with capsaicin has not been elucidated. Therefore, we investigated the effect of oral capsaicin stimulation on oral health and mental conditions by measuring changes in salivation, autonomic nervous activity and electroencephalogram (EEG).
Materials and methods
Eighteen healthy adults participated in this study. The stimulus concentrations of capsaicin and five basic taste solutions were determined based on the measured threshold of each stimulus in each subject. The weight of secreted saliva and the changes in concentrations of salivary secretory immunoglobulin A (SIgA) induced by capsaicin and taste stimuli were measured. Salivary α-amylase activity and heart rate variability (HRV) were measured as indicators of autonomic nervous activity. From EEG, psychological condition was analyzed by measuring the powers of theta, alpha, and beta bands.
Results
The salivary secretion rate was significantly increased by stimulation with capsaicin, NaCl, and citric acid compared with deionized water, and capsaicin demonstrated the most potent effect among tested stimuli. The secreted amount of SIgA per minute was elevated by capsaicin stimulation. Salivary α-amylase activity and HRV analysis demonstrated an elevation of sympathetic nervous activity induced by capsaicin. EEG analysis showed a significant increase in beta band power.
Conclusion
These results suggest that oral stimulation with capsaicin may be effective in improving oral conditions by increasing salivary flow and SIgA secretion, and in enhancing physical and mental conditions as indicated by sympathetic nerve and EEG changes.
Keywords: autonomic nervous activity, capsaicin, electroencephalography, pungent taste, salivary component, salivary secretion
Introduction
Pungent seasoning such as chili pepper makes foods more palatable and stimulates appetite. Capsaicin, the major pungent ingredient in chili pepper, is composed of vanillylamine and fatty acid. The vanillyl residue of capsaicin binds to the transient receptor potential cation channel subfamily V member 1 (TRPV1) and generates depolarization of the free nerve endings expressing TRPV1, which results in impulses conveying the information of irritant or pain sensation. Capsaicin applied to the mouth activates nociceptive trigeminal neurons and is recognized as pungent taste in the cerebral cortex.1 In addition to causing pungent taste, ingestion of capsaicin induces various biological responses. Ingested capsaicin was reported to have effects of protection against injury of the stomach mucosa,2, 3, 4 increase in serum adrenalin, and promotion of energy metabolism.5, 6
Regarding the effect of oral stimulation with capsaicin, Ebihara et al.7 reported that capsaicin is effective for enhancement of the swallowing reflex in dysphagia patients, however few studies have been performed on the influences of capsaicin on oral function and nervous system.8 Since saliva has important functions such as buffering, protection, lubrication, tissue repair and antibacterial actions, the approach of increasing salivary flow is quite important for promotion of oral and systemic health.9, 10 Though taste stimulation is well-recognized to increase salivary secretion, known as gustatory-salivary reflex,11, 12 the effect of pungent stimuli on salivation has never been investigated. Therefore, we investigated the effect of oral stimulation with capsaicin on salivary secretion and compared the effectiveness of capsaicin with those of the five basic tastes. In this study, we determined the stimulating concentration of each stimulus based on individual threshold rather than the constant concentration, because the effectiveness of stimulus on salivary secretion was considered to vary depending on individual sensitivity to each stimulus. In addition to salivary secretion, we measured the change in the concentration of salivary secretory immunoglobulin A (SIgA), the primary factor of mucosal immunity, induced by capsaicin.13, 14
Furthermore, it was presumed that capsaicin-induced responses were accompanied by changes in autonomic nervous activities, since modulation of sympathetic nervous activity was observed with ingestion of capsaicin.15 In order to investigate this issue, salivary α-amylase activity and variation of heart rate were measured as indices of autonomic nervous activities. Salivary α-amylase is a digestive enzyme and increased with sympathetic nervous activation.16 Power spectral analysis of fluctuation of heart rate from electrocardiogram (ECG) has been generally used for evaluation of autonomic nervous activities because the heart rate fluctuates reflecting autonomic nervous activities.17, 18, 19, 20
Since pungent seasoning causes strong sensation, psychological statuses can be changed by oral stimulation with capsaicin. In order to detect the psychological changes induced by capsaicin, we measured electroencephalogram (EEG), because power spectral analysis of EEG has been conventionally utilized to understand psychological status.21, 22, 23 Thus, we comprehensively analyzed the effects of oral stimulation with capsaicin on oral function, autonomic activity and mental condition from various indices in this study.
Materials and methods
Subjects
Eighteen healthy adults (six males and 12 females) aged 26.6 ± 4.5 (the mean ± SD) years voluntarily participated in this study. All subjects were non-smokers and not on any medications, and had no oral problems. They refrained from eating and drinking anything except water for 2 h before the experiment.
Pungent and taste stimuli
The solutions used in the experiment are listed in Table 1. Capsaicin was dissolved in 1 mL of ethanol (99.5%) and then diluted to eight step concentrations with deionized water (DW). For the five basic tastes, sucrose, quinine hydrochloride (QHCl), monosodium glutamate (MSG), NaCl and citric acid (CA) were used for sweet, bitter, umami, salty and sour stimuli, respectively. These substances were diluted with DW to prepare eight step concentrations in two-fold serial dilution.
Table 1.
Concentration steps of each solution used for measurement of the threshold.
| Concentration steps | Pungent Capsaicin (mM) | Sweet Sucrose (M) | Bitter QHCl (mM) | Umami MSG (M) | Salty NaCl (M) | Sour CA (mM) |
|---|---|---|---|---|---|---|
| 8 | 0.3000 | 0.5000 | 0.1000 | 0.1000 | 0.3000 | 3.7500 |
| 7 | 0.1000 | 0.2500 | 0.0500 | 0.0500 | 0.1500 | 1.8750 |
| 6 | 0.0500 | 0.1250 | 0.0250 | 0.0250 | 0.0750 | 0.9375 |
| 5 | 0.0300 | 0.0625 | 0.0125 | 0.0125 | 0.0375 | 0.4688 |
| 4 | 0.0100 | 0.0313 | 0.0063 | 0.0063 | 0.0188 | 0.2344 |
| 3 | 0.0050 | 0.0156 | 0.0031 | 0.0031 | 0.0094 | 0.1172 |
| 2 | 0.0030 | 0.0078 | 0.0016 | 0.0016 | 0.0047 | 0.0586 |
| 1 | 0.0010 | 0.0039 | 0.0008 | 0.0008 | 0.0023 | 0.0283 |
CA = citric acid; MSG = monosodium glutamate; QHCl = quinine hydrochloride.
Experimental procedures
The subjects were seated on an armchair and the electrodes for recording ECG and EEG were attached on the respective parts of the body. One stimulating session in the experimental procedure involved collection of unstimulated saliva for 1 min, oral stimulation and collection of saliva for 1 min, collection of saliva for 1 min after stimulation, and an intermission of at least 4 min. This protocol of one session was repeated for stimulations of the five basic tastes, capsaicin, and DW. The order of application of stimuli was randomized among subjects except for capsaicin, which was tested last, because the effect of capsaicin tended to persist after stimulation. In the case of capsaicin, the saliva was collected until 8 min after cessation of stimulation to examine the time course of persistent effects. For EEG recording, the subjects kept their eyes closed in order to avoid large electrical waves associated with eye blink during saliva collection.
Measurement of thresholds for capsaicin and five basic tastes
Before the measurement of salivary secretion, the thresholds for capsaicin and the five basic tastes were measured in each subject. To measure the detection threshold of capsaicin, a filter paper 6 mm in diameter soaked with capsaicin solution was put on the tip of the tongue. The position of the filter paper was changed each time to avoid the influence of sensitization or desensitization.24, 25 Stimulus concentration was started with the lowest and raised until the subject felt irritation, which was taken as the detection threshold for capsaicin. The recognition thresholds for the five basic tastes were measured by the whole-mouth method. After subjects held 3 mL of taste solution in their mouth and spat it out, they chose an answer from seven options such as no taste, feeling some taste but unable to identify the taste, sweet, salty, sour, umami and bitter. Starting with the lowest concentration of solution exhibiting each taste, the concentration was raised step-by-step, and the concentration at which the subject could correctly identify the taste was taken as the recognition threshold.
Measurement of salivary secretion rate
After 5 mL of taste and capsaicin solutions and DW was poured into the subject's mouth by using a pipette, the subject held the solution in their mouth for 1 min, then spat it out together with secreted saliva. The spat solution was weighed immediately and the increased weight was taken as the amount of secreted saliva. The stimulating concentration of capsaicin solution was basically two steps higher than the detection threshold, but it was lowered by one or two steps if the subject had difficulty in keeping the solution in their mouth for 1 min due to the strong burning sensation. The stimulating concentration of each taste solution was two steps higher than the recognition threshold.
Measurement of salivary SIgA concentration
The saliva collected before and after capsaicin stimulation was stored at −20 °C, thawed, and centrifuged at 3000 rpm (1469g) for 15 min to get the supernatant of saliva. The SIgA concentration in the supernatant was measured by using the Salivary Secretory IgA Indirect Enzyme Immunoassay Kit (Salimetrics, State College, PA, USA). Based on the measured concentration, the secreted amount of SIgA per minute was calculated by multiplying it by the salivary secretion rate.
Measurement of α-amylase activity
After impregnating a strip with saliva by holding it under the subject's tongue for 30–60 s, α-amylase activity of saliva was measured with the salivary amylase monitor (Nipro Corporation, Osaka, Japan) before and after capsaicin stimulation.
Measurement of autonomic nervous activities
ECG of the subject was derived with three electrodes attached on the thoracic skin and recorded by a portable ECG monitor (LRR-03, GMS, Tokyo, Japan). From ECG, frequency analyses of heart rate variability were performed using the analytical program (Reflex Meijin, Crosswell Co., Yokohama, Japan). The power of the high-frequency band (HF: 0.15–0.4 Hz) was taken as the parasympathetic nervous activity and the ratio of the low-frequency band (0.04–0.15 Hz) power to HF power was taken as the sympathetic nervous activity.
EEG recording
To measure EEG, Ag–AgCl plate electrodes were placed on the scalp as recording electrodes and on the right earlobe as a reference electrode. The electrical brain activity was amplified and recorded by using the EEG measuring system (ESA-16 Basic, Brain Functions Laboratory Inc., Yokohama, Japan). Power spectrum density was measured from EEG recorded at FP1, FP2, F3 and F4 points, through Fourier analysis (1k points, 50% overlap, hanning window) using LabChart Ver8 (AD Instruments, Bella Vista, Australia). The powers of theta (4–7 Hz), alpha (8–13 Hz) and beta (14–24 Hz) bands were obtained from EEG, and the change in power of each band during the first 30 s of capsaicin and DW applications was investigated.
Statistical analysis
The differences in salivary secretion rates induced by oral application of capsaicin, taste stimuli and DW were analyzed by using one-way analysis of variance (ANOVA), followed by Dunnett's test as a post hoc analysis. The changes in salivary flow after capsaicin, salivary component, autonomic nervous activities and EEG induced by capsaicin were analyzed with Wilcoxon signed-rank test. Statistical significance was set at 0.05. Statistical analyses were performed using PASW Statistics 18 (IBM Corp., Armonk, NY, USA).
Ethical consideration
All experimental procedures were approved by the ethics committee of Tokyo Medical and Dental University (Approval number 963). After explaining the purpose and the methods of the experiment by oral consultation and document, written informed consent was obtained from all subjects.
Results
The thresholds of capsaicin and five basic tastes
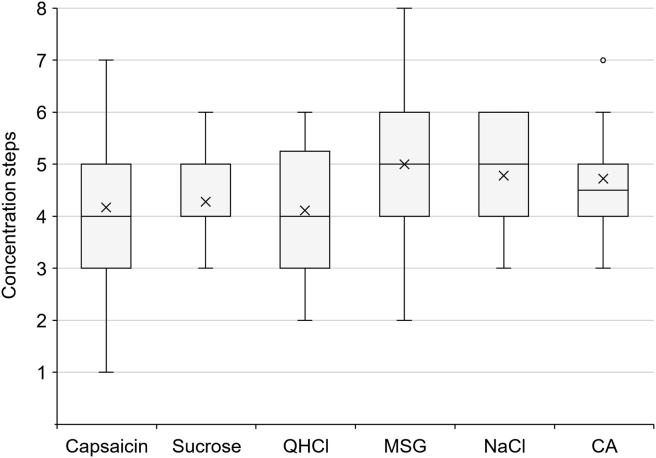
The thresholds for capsaicin and five basic tastes were measured in each subject before the measurement of salivary secretion. The mean and median threshold expressed in solution number for each stimulus are shown in Fig. 1. Based on the measured thresholds, the stimulating concentration of each solution for salivation was determined.
Figure 1.
Thresholds for each stimulus in concentration steps. The box represents the median ±25 percentile values and the vertical bar indicates the minimum and maximum values. The “x” mark indicates the mean value. CA = citric acid; MSG = monosodium glutamate; QHCl = quinine hydrochloride.
Effects of capsaicin and five basic taste stimuli on salivary secretion
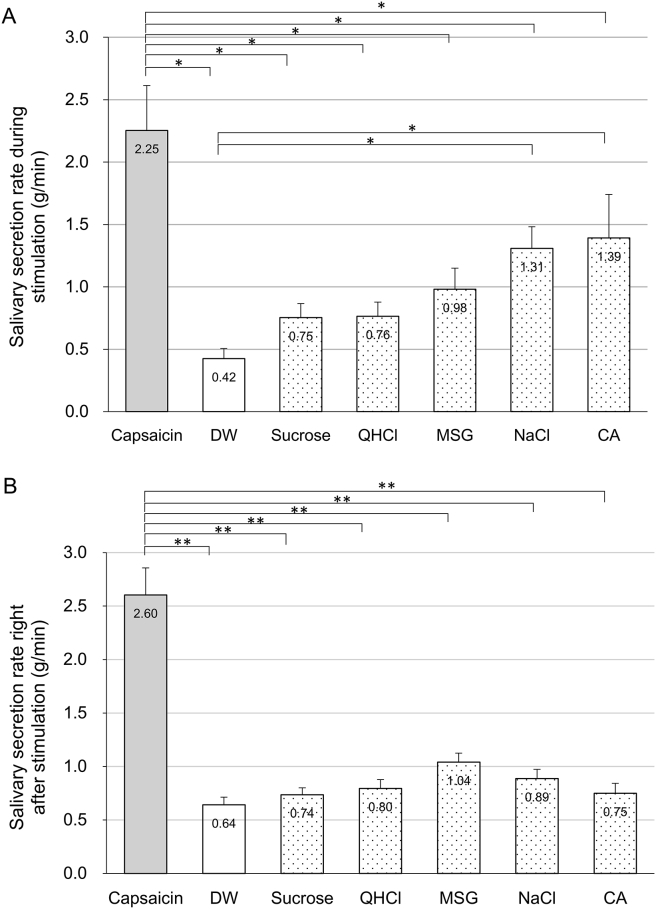
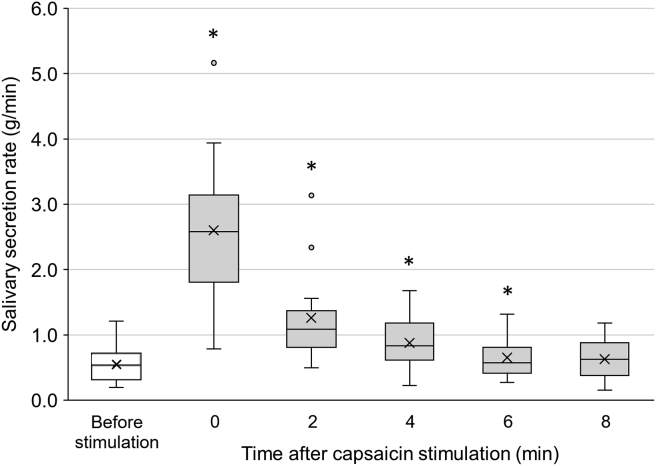
The salivary secretion rates during stimulation with each solution are illustrated in Fig. 2A. Capsaicin, NaCl and CA significantly increased salivary secretion (P < 0.05) compared with DW, and MSG showed a trend toward increase (P < 0.1). The increasing effect of capsaicin on salivation was significantly higher than those of all five basic tastes (P < 0.05). The salivary secretion rate right after cessation of stimulation (Fig. 2B) was significantly increased with capsaicin compared to DW and each of the five basic tastes (P < 0.05), and tended to be increased with MSG compared with DW (P < 0.1). The enhancement of salivary flow after capsaicin stimulation was sustained up to 6 min (P < 0.05) as indicated in Fig. 3.
Figure 2.
Changes in salivary secretion rates induced by capsaicin and five basic taste stimulations. A: Mean salivary secretion rate during each stimulation. Capsaicin, NaCl and CA significantly increased salivary secretion rates compared with DW, and the enhancing effect of capsaicin was remarkably higher than other stimuli. B: Salivary secretion rate right after each stimulation. Salivary flow after capsaicin was significantly higher than any other stimulus. Error bar indicates standard error of the mean. *P < 0.05, **P < 0.001. CA = citric acid; DW = deionized water; MSG = monosodium glutamate; QHCl = quinine hydrochloride.
Figure 3.
Persistent increase in salivary secretion after capsaicin stimulation. Increase in salivary secretion persisted up to 6 min after capsaicin stimulation. The box represents the median ±25 percentile values and the vertical bar indicates the minimum and maximum values. The “x” mark indicates the mean value. Asterisks denote significant differences compared with the baseline value before stimulation.
Changes in salivary components induced by capsaicin stimulation
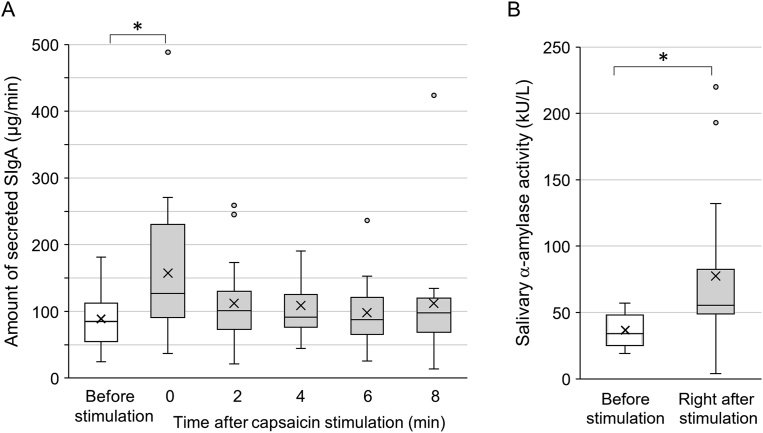
The SIgA amount was significantly elevated right after capsaicin stimulation in comparison with the baseline before stimulation (P = 0.028) (Fig. 4A). In contrast to sustained increase of salivary flow, secreted SIgA amount returned to the baseline level within 3 min after capsaicin stimulation. The salivary α-amylase activity was significantly increased by capsaicin stimulation (P = 0.004) (Fig. 4B).
Figure 4.
Changes in salivary components induced by capsaicin stimulation. A: Time course of changes in amount of SIgA secreted per minute after capsaicin stimulation. A significant elevation in SIgA amount was observed right after stimulation and returned to the baseline level by 3 min after stimulation. B: Changes in salivary α-amylase activity induced by capsaicin stimulation. Alpha-amylase activity right after capsaicin was significantly higher than the baseline. The box represents the median ±25 percentile values and the vertical bar indicates the minimum and maximum values. The “x” mark indicates the mean value. *P < 0.05. kU = kilounit.
Changes in autonomic nervous activities during stimulation
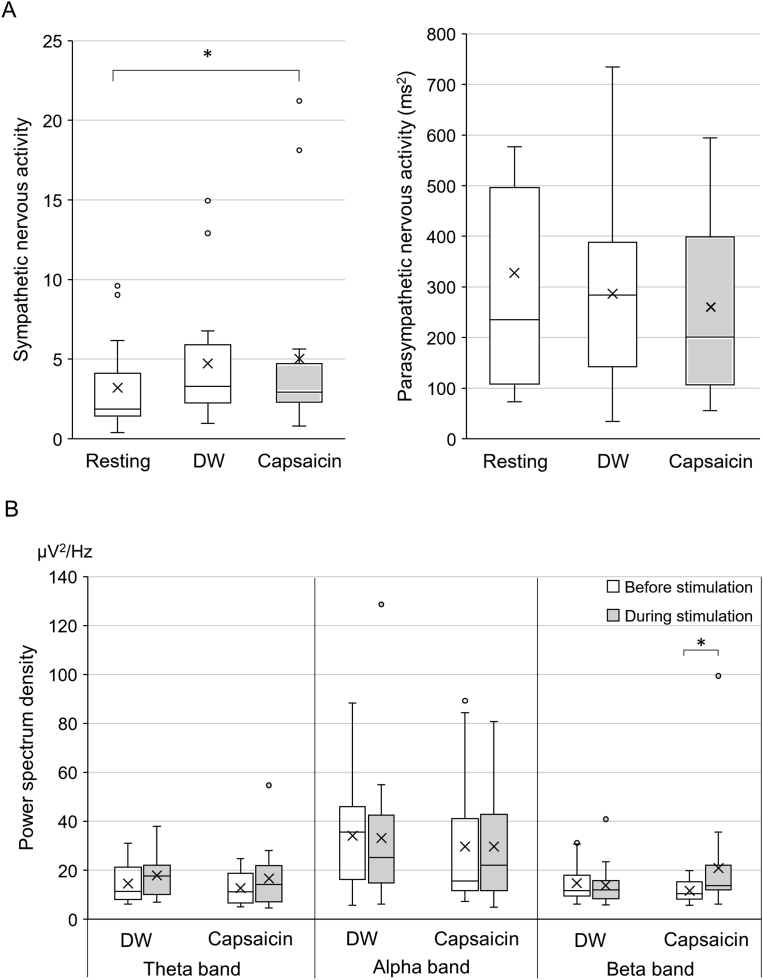
During capsaicin stimulation, the sympathetic nervous activity was significantly elevated compared to resting condition with no stimulation (P = 0.031) (Fig. 5A), which was not observed during DW application. The parasympathetic nervous activities demonstrated no significant changes during capsaicin or DW application compared to resting condition.
Figure 5.
Changes in autonomic nervous and EEG activities during capsaicin stimulation. A: Changes in sympathetic and parasympathetic nervous activities. Sympathetic nervous activity was significantly elevated during capsaicin stimulation compared to the resting level, while parasympathetic nervous activity was not significantly changed. B: Changes in powers of theta, alpha and beta bands analyzed from EEG. The power of the beta band was significantly increased during capsaicin stimulation, whereas the powers of theta and alpha bands were not significantly changed. The box represents the median ±25 percentile values and the vertical bar indicates the minimum and maximum values. The “x” mark indicates the mean value. *P < 0.05. DW = deionized water.
Changes in EEG activity induced by capsaicin
The conventional frequency analysis of EEG showed that the power of the beta band was significantly increased during capsaicin stimulation compared with before stimulation (P = 0.013), which was not observed during DW application (Fig. 5B). Meanwhile, the powers of theta and alpha bands showed no significant changes during either capsaicin or DW application.
Discussion
In the present study, to investigate the oral and neural responses induced by oral stimulation with capsaicin, we measured its effects on salivation and the activities of autonomic nerves and brain neurons in humans. The measurement of salivary secretion showed that stimulation with capsaicin, NaCl, and CA significantly increased salivary flow compared with DW, and capsaicin was more effective than other stimuli during and after stimulation. The effect of capsaicin on salivary secretion persisted up to 6 min after cessation of stimulation. Some previous studies consistently reported that sour and umami stimuli were effective for enhancement of salivary secretion.11, 12, 26 In the present study, sour and salty stimuli were more effective than sweet and bitter stimuli, and umami stimulus showed a tendency toward increment of salivary secretion, which coincided with the trends of a previous study.12 Since capsaicin demonstrated larger effectiveness on salivation, it was suggested that combined stimulation with food taste and spicy seasoning can substantially increase salivary secretion and can be utilized for a means to improve oral condition of people with xerostomia.8
Besides the salivary flow, the amount of salivary SIgA secreted per minute was significantly increased by capsaicin stimulation. Schiffman et al. have reported that taste and olfactory stimulation increased the secreted amount of SIgA, and the amount was further increased with combined stimulation of taste and olfaction.27 These results suggest a possibility that combined stimulation with capsaicin and tastants can cause further increment of SIgA secretion, though the effects of taste stimulations on SIgA secretion were not investigated in this study. Salivary SIgA plays an important role in oral immune function to prevent invasion of bacteria and viruses and defend against intraoral and upper respiratory infection.13 Since salivary SIgA is recognized to decrease with aging, oral stimulation with capsaicin and tastants is suggested to improve oral health in the elderly together with salivary flow increase.28, 29
Regarding autonomic nervous responses to capsaicin, salivary α-amylase activity was elevated by oral stimulation with capsaicin. Likewise, power spectral analysis of heart rate variability showed that sympathetic nervous activities were elevated during capsaicin stimulation. It has been reported that capsaicin ingestion promoted energy consumption and suppressed body fat accumulation through sympathetic nervous activation.15, 27, 30 While these effects developed in several tens of minutes after capsaicin ingestion, the elevation of sympathetic activity in this study was observed only during capsaicin stimulation. This suggests that intraoral stimulation with capsaicin is not sufficient to cause metabolic changes, and capsaicin action on the gastrointestinal tract is required for them.
In order to examine psychological changes associated with oral capsaicin stimulation, power spectral analysis of EEG was conducted in this study, because the powers of alpha, beta and theta bands have been generally utilized for evaluation of psychological states. Some studies reported that a relaxing aroma induced increases in theta and alpha band powers, or a decrease in beta band power.22, 31 In the present study, the power of beta band was increased with no changes in alpha and theta bands' powers during capsaicin stimulation. This change may presumably indicate an increase in attention induced by capsaicin, because increases in the beta band were previously observed with visual stimulation under attended condition and during eye-opening.32, 33
Collectively, the present study revealed that oral stimulation with capsaicin generates pungent taste (irritating) sensation which leads to increase in beta wave in EEG suggesting attention increase, and it reflexively causes increases in salivary flow and SIgA secretion with simultaneous elevation of sympathetic activities. Since acinar cells of salivary glands express adrenergic receptors as well as cholinergic receptors, sympathetic activation by capsaicin might result in secretions of fluid, SIgA and α-amylase via α- and β-adrenergic receptors.16, 34, 35, 36 For elucidation of precise mechanisms underlying these effects and relationships between salivary changes and neuronal activities, further molecular and physiological studies will be required. In conclusion, this study provided an evidence for effectiveness of oral capsaicin stimulation in improvement of oral health conditions through increasing salivary flow and SIgA secretion, and in enhancement of physical and mental activities shown by the changes in autonomic nervous and brain activities.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement
This research was partially supported by JSPS Grant-in-Aid for Challenging Exploratory Research (Grant Number 15K12323). We thank Dr. Masako Akiyama, University Research Administrator, University Research Administrator Office, Research University Promotion Organization, Tokyo Medical and Dental University and Mr. Takashi Hashimoto, Bioresearch Co., Ltd. for their help in analyzing the data in this study.
References
- 1.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Holzer P., Pabst M.A., Lippe I.T. Intragastric capsaicin protects against aspirin-induced lesion formation and bleeding in the rat gastric mucosa. Gastroenterology. 1989;96:1425–1433. doi: 10.1016/0016-5085(89)90508-8. [DOI] [PubMed] [Google Scholar]
- 3.Gray J.L., Bunnett N.W., Orloff S.L., Mulvihill S.J., Debas H.T. A role for calcitonin gene-related peptide in protection against gastric ulceration. Ann Surg. 1994;219:58–64. doi: 10.1097/00000658-199401000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang J.Y., Teng C.H., Wee A., Chen F.C. Effect of capsaicin and chilli on ethanol induced gastric mucosal injury in the rat. Gut. 1995;36:664–669. doi: 10.1136/gut.36.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnuki K., Haramizu S., Oki K., Watanabe T., Yazawa S., Fushiki T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci Biotechnol Biochem. 2001;65:2735–2740. doi: 10.1271/bbb.65.2735. [DOI] [PubMed] [Google Scholar]
- 6.Kim K.M., Kawada T., Ishihara K., Inoue K., Fushiki T. Increase in swimming endurance capacity of mice by capsaicin-induced adrenal catecholamine secretion. Biosci Biotechnol Biochem. 1997;61:1718–1723. doi: 10.1271/bbb.61.1718. [DOI] [PubMed] [Google Scholar]
- 7.Ebihara T., Takahashi H., Ebihara S. Capsaicin troche for swallowing dysfunction in older people. J Am Geriatr Soc. 2005;53:824–828. doi: 10.1111/j.1532-5415.2005.53261.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunér-Engström M., Fredholm B.B., Larsson O., Lundberg J.M., Saria A. Autonomic mechanisms underlying capsaicin induced oral sensations and salivation in man. J Physiol. 1986;373:87–96. doi: 10.1113/jphysiol.1986.sp016036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey S.P., Williamson R.T. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 10.de Almeida Pdel V., Grégio A.M., Machado M.A., de Lima A.A., Azevedo L.R. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9:72–80. [PubMed] [Google Scholar]
- 11.Uneyama H., Kawai M., Sekine-Hayakawa Y., Torii K. Contribution of umami taste substances in human salivation during meal. J Med Invest. 2009;56(Suppl):S197–S204. doi: 10.2152/jmi.56.197. [DOI] [PubMed] [Google Scholar]
- 12.Hodson N.A., Linden R.W. The effect of monosodium glutamate on parotid salivary flow in comparison to the response to representatives of the other four basic tastes. Physiol Behav. 2006;89:711–717. doi: 10.1016/j.physbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Tamura S., Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis. 2004;57:236–247. [PubMed] [Google Scholar]
- 14.Gleeson M. Mucosal immunity and respiratory illness in elite athletes. Int J Sports Med. 2000;21(Suppl. 1):S33–S43. doi: 10.1055/s-2000-1450. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto T., Miyawaki C., Ue H., Yuasa T., Miyatsuji A., Moritani T. Effects of capsaicin-containing yellow curry sauce on sympathetic nervous system activity and diet-induced thermogenesis in lean and obese young women. J Nutr Sci Vitaminol. 2000;46:309–315. doi: 10.3177/jnsv.46.309. [DOI] [PubMed] [Google Scholar]
- 16.Nater U.M., Rohleder N. Salivary α-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinol. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 18.Uehara N., Takagi Y., Miwa Z., Sugimoto K. Objective assessment of internal stress in children during dental treatment by analysis of autonomic nervous activity. Int J Paediatr Dent. 2012;22:331–341. doi: 10.1111/j.1365-263X.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchihashi N., Uehara N., Takagi Y., Miwa Z., Sugimoto K. Internal stress in children and parental attitude to dental treatment with passive restraint. Pediatr Dent J. 2012;22:170–177. [Google Scholar]
- 20.Tanaka S., Uehara N., Tsuchihashi N., Sugimoto K. Emotional relationships between child patients and their mothers during dental treatments. J Dent Sci. 2016;11:287–292. doi: 10.1016/j.jds.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostyunina M.B., Kulikov M.A. Frequency characteristics of EEG spectra in the emotions. Neurosci Behav Physiol. 1996;26:340–343. doi: 10.1007/BF02359037. [DOI] [PubMed] [Google Scholar]
- 22.Sayorwan W., Siripornpanich V., Piriyapunyaporn T., Hongratanaworakit T., Kotchabhakdi N., Ruangrungsi N. The effects of lavender oil inhalation on emotional states, autonomic nervous system, and brain electrical activity. J Med Assoc Thai. 2012;85:598–606. [PubMed] [Google Scholar]
- 23.Diego M.A., Jones N.A., Field T. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int J Neurosci. 1998;96:217–224. doi: 10.3109/00207459808986469. [DOI] [PubMed] [Google Scholar]
- 24.Green B.G. Capsaicin sensitization and desensitization on the tongue produced by brief exposures to a low concentration. Neurosci Lett. 1989;107:173–178. doi: 10.1016/0304-3940(89)90812-4. [DOI] [PubMed] [Google Scholar]
- 25.Prescott J. The generalizability of capsaicin sensitization and desensitization. Physiol Behav. 1999;66:741–749. doi: 10.1016/s0031-9384(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 26.Horio T., Kawamura Y. Salivary secretion induced by umami taste. Jpn J Oral Biol. 1989;31:107–111. doi: 10.2330/joralbiosci1965.31.107. [DOI] [PubMed] [Google Scholar]
- 27.Schiffman S.S., Miletic I.D. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3:158–164. [PubMed] [Google Scholar]
- 28.Challacombe S.J., Percival R.S., Marsh P.D. Age-related changes in immunoglobulin isotypes in whole and parotid saliva and serum in healthy individuals. Oral Microbiol Immunol. 1995;10:202–207. doi: 10.1111/j.1399-302x.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 29.Miletic I.D., Schiffman S.S., Miletic V.D., Sattely-Miller E.A. Salivary IgA secretion rate in young and elderly persons. Physiol Behav. 1996;60:243–248. doi: 10.1016/0031-9384(95)02161-2. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T., Kawada T., Kurosawa M., Sato A., Iwai K. Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Am J Physiol. 1988;255:E23–E27. doi: 10.1152/ajpendo.1988.255.1.E23. [DOI] [PubMed] [Google Scholar]
- 31.Motomura N., Sakurai A., Yotsuya Y. Reduction of mental stress with lavender odorant. Percept Mot Skills. 2001;93:713–718. doi: 10.2466/pms.2001.93.3.713. [DOI] [PubMed] [Google Scholar]
- 32.Gómez C.M., Vázquez M., Vaquero E., López-Mendoza D., Cardoso M.J. Frequency analysis of the EEG during spatial selective attention. Int J Neurosci. 1998;95:17–32. doi: 10.3109/00207459809000646. [DOI] [PubMed] [Google Scholar]
- 33.Dolce G., Waldeier H. Spectral and multivariate analysis of EEG changes during mental activity in man. Electroencephalogr Clin Neurophysiol. 1974;36:577–584. doi: 10.1016/0013-4694(74)90224-7. [DOI] [PubMed] [Google Scholar]
- 34.Wallace L.J., Partlow L.M. α-Adrenergic regulation of secretion of mouse saliva rich in nerve growth factor. Proc Natl Acad Sci USA. 1976;73:4210–4214. doi: 10.1073/pnas.73.11.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bragiel A.M., Wang D., Pieczonka T.D., Shono M., Ishikawa Y. Mechanisms underlying activation of α₁-adrenergic receptor-induced trafficking of AQP5 in rat parotid acinar cells under isotonic or hypotonic conditions. Int J Mol Sci. 2016;17:1022. doi: 10.3390/ijms17071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter G.H., Garrett J.R., Hartley R.H., Proctor G.B. The influence of nerves on the secretion of immunoglobulin A into submandibular saliva in rats. J Physiol. 1998;512:567–573. doi: 10.1111/j.1469-7793.1998.567be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]