Abstract
Background/purpose
Inflammatory bowel disease (IBD), comprised Crohn's disease and ulcerative colitis, is a mucosal immune response that affects gastroenterological tract. The association between IBD and periodontitis was inconclusive. In this study, we aimed to investigate the association between IBD and periodontitis by using a register-based dataset.
Materials and methods
The dataset conducting in this retrospective cohort study was obtained from the National Health Insurance Research database (NHIRD) in Taiwan. For IBD group, conditionally selected control subjects were matched in 1:4 ratio from general population. The risk of periodontitis among IBD group comparing with non-IBD group was calculated by multivariable Cox proportional hazards model.
Results
In IBD cohort, 27 IBD patients (7 Crohn's disease and 20 ulcerative colitis) with catastrophic illness registry were identified. 108 controls were selected as non-IBD cohort. The median follow-up period was 3.00 years in the IBD group and 3.15 years in the non-IBD group. The cumulative incidence of IBD was 4.32 per 100,000 persons. After adjusting for several confounding factors, IBD group had higher risk for developing periodontitis than non-IBD group (adjusted HR: 1.82; 95% CI: 1.09–3.03). To further stratification with subtype, Crohn's disease group had significantly higher risk of periodontitis (adjusted HR: 3.95; 95% CI: 1.59–9.82).
Conclusions
Taken together, this retrospective cohort study showed that patients with IBD increase risk of having periodontitis comparing with non-IBD group, especially in Crohn's disease subgroup.
Keywords: Periodontitis, Inflammatory bowel disease, Crohn's disease, Ulcerative colitis, Retrospective cohort study, National Health Insurance Research database
Introduction
Inflammatory bowel disease (IBD) is a mucosal immune response mainly located in the gastroenterological tract. IBD comprises two type of inflammatory disease: Crohn's disease and ulcerative colitis.1,2 Ulcerative colitis is characterized by recurring inflammation episodes limited to the mucosal layer of colon. It commonly involves the rectum and may extend in continuous lesion to other parts of the colon.3 Crohn's disease is characterized by transmural inflammation of the gastroenterological tract. Both types of IBD occur in the genetic susceptible individuals with antigenic effect of intestinal microbiota.1
Periodontitis is an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms or groups of specific microorganisms, resulting in progressive destruction of the periodontal ligament and alveolar bone with increased probing depth formation, recession, or both. The net result of these inflammatory changes can breakdown the fibers of the periodontal ligament, leading to clinical loss of attachment, resorption of the alveolar bone and even tooth loss.4
In 1999, an international workshop for the classification of periodontal diseases refined periodontitis as a manifestation of several hematologic and genetic disorders which have been associated with the development of periodontitis in affected individuals.5 Nowadays, the associations between periodontitis and other systemic diseases such as preterm low birth weight,6 chronic obstructive pulmonary disease,7 and diabetes8 have been established in support of the concept of perio-systemic disease connection.
The pathogenesis of both IBD and periodontitis is multi-factorial leading to a substantial defect of the mucosal barrier, deregulation of the immune response and chronic inflammation of the mucosa.9 The common oral manifestations in IBD are indurated tag-like lesions, cobblestoning, mucogingivitis, aphthous stomatitis, and pyostomatitis vegetans.10,11 In addition, severe periodontitis has been observed in individuals with IBD who suffer from secondary neutrophil impairment.12,13
Although previous studies have pointed toward an association between IBD and periodontitis,4,9,14,15 most of data were small sample size with inconclusive results. Population-based epidemiological investigations regarding the association between IBD and periodontitis are lacking. The aim of this study was to evaluate the association between IBD and periodontitis in nationwide population according to National Health Insurance Research database (NHIRD) in Taiwan.
Materials and methods
Data collection
The dataset conducting in this cohort study was retrieved from NHIRD. The Longitudinal Health Insurance Database 2005 (LHID2005) was created and released to the public by the National Health Research Institute (NHRI), and it includes all the original claims data and registration files from 2000 to 2009 for one million individuals randomly sampled from the Registry for Beneficiaries of the National Health Institute (NHI) program on 2005 in Taiwan. Many studies have been published based on the release of claims dataset from NHIRD for population-based longitudinal studies of supporting its validity.16, 17, 18, 19, 20
This study was approved by the Chung Shan Medical University Hospital Ethics Review Board. The data retrieved from NHIRD was with proper de-identification and anonymous for patients' information. There were no specific ethical considerations in this study.
Patient identification and measurement
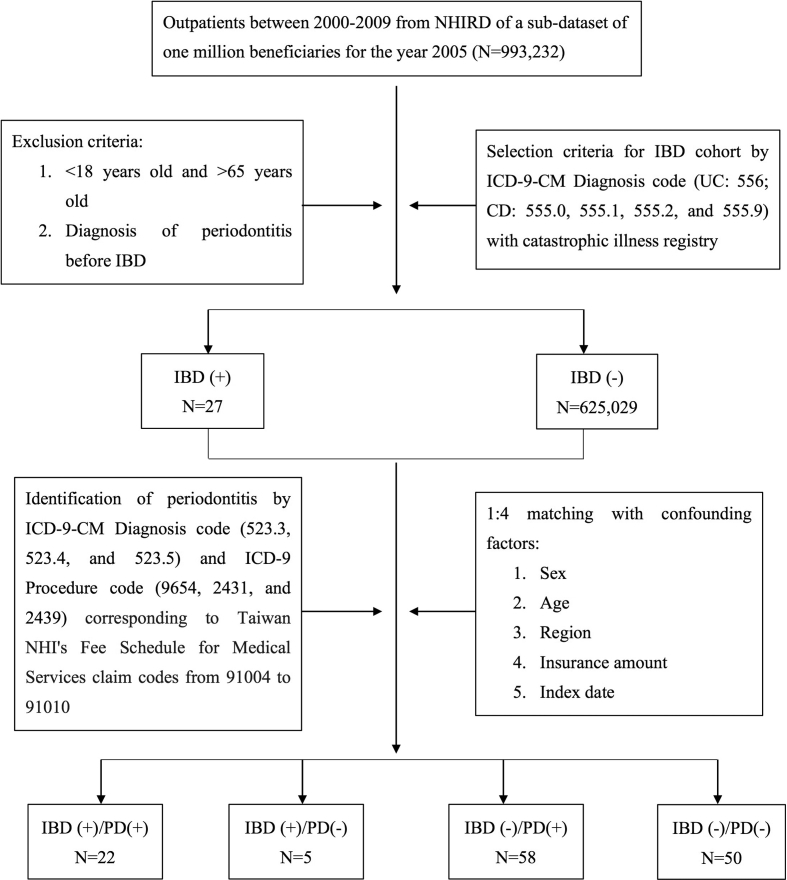
We use outpatient data between 2000 and 2009 from NHIRD of a sub-dataset of one million for the year 2005 (n = 993,232). The disease diagnoses were defined according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). We used ICD-9-CM Diagnosis codes to identify the subjects of IBD patients. Ulcerative colitis (ICD-9-CM code 556) and Crohn's disease (ICD-9-CM codes 555.0, 555.1, 555.2, and 555.9) were retrieved from the database between January 1, 2000 and December 31, 2009. To increase the validity of the IBD diagnosis, we only included subjects with identification of catastrophic illness registry for the accuracy. The patients with diagnosis of periodontitis before the diagnosis of IBD, age less than 18 years old, age older than 65 years old, withdrew from program or with missing data were excluded. Total numbers of 625,056 people were included and we identified 27 patients with IBD. The initial disease diagnosis date was set as the index date. For IBD group, we conditionally selected control subjects in 1:4 ratio by propensity score method from general population with matched sex, age, urbanization level, socioeconomic status, and index year. Those confounding variables matched between cases and controls in order to ensure that cases and controls were reasonably similar in terms of baseline demographic characteristics. We identified periodontitis based on ICD-9-CM diagnosis code (ICD-9-CM codes 523.3, 523.4, and 523.5). To ensure the criteria of indication and the accuracy of diagnosis for periodontitis, ICD-9 procedure code 9654, 2431, and 2439 were also defined. Information about the collection of radiographic and pocket depth data in the patient's record has been described for each periodontal treatment. The medical experts designated by National Health Insurance Administration (NHIA) would regularly review patients' chart for ensuring medical quality and accuracy. The designated medical experts are recommended by the Taiwan Hospital Association and the Specialist Medical Association such as Taiwan Medical Association and Taiwan Dental association.21 It is a reliable data for the conditions of the periodontitis of the selected patients in the present cohort. The flowchart of the study was showed in Fig. 1.
Figure 1.
Flowchart indicating reasons of selection and exclusion of cases.
Statistical analysis
Statistical analyses were performed using the Student's t-test for continuous variables and the chi-squared test for categorical variables. The Kaplan–Meier analysis and log-rank test were used for calculating the difference of the cumulative incidence rates of periodontitis between IBD and non-IBD cohort group. The multivariable Cox proportional hazard model was used to estimate the hazard ratio (HR) of periodontitis with IBD compared with the people in the non-IBD cohort. Our study set with a significance level of 0.05 to detect differences in periodontitis between the IBD and non-IBD cohort. To compute achieved statistic power, post hoc analysis was performed. All analyses were conducted in SPSS version 22 (SPSS Inc., Chicago, IL, USA).
Results
In this study, 27 patients (7 Crohn's disease and 20 ulcerative colitis) were identified in IBD cohort group and 108 controls were matched in non-IBD cohort group. With sample size of total 135 individuals, post hoc analysis provided effect size 0.69 and achieved statistic power 0.99. The median follow-up period was 3.00 years in the IBD group and 3.15 years in the non-IBD group. The cumulative incidence of IBD was 4.32 per 100,000 persons. Mean age of IBD and non-IBD group were 38.0 ± 10.8 years and 36.3 ± 13.6 years (p = 0.55), respectively. As shown in Table 1, there were no statistical significant differences among sex (p = 0.23), socio-economic status (p = 0.23), and residential urbanization (p = 0.86).
Table 1.
Demographic characteristics in IBD and non-IBD group.
| IBD |
p-value | ||
|---|---|---|---|
| Yes |
No |
||
| (n = 27) | n = 108 | ||
| Age | 38.0 ± 10.8 | 36.3 ± 13.6 | 0.55 |
| Sex | 0.23 | ||
| Female | 10 (37%) | 54 (50%) | |
| Male | 17 (63%) | 54 (50%) | |
| Socioeconomic status | 0.86 | ||
| <NT $20,000 | 11 (41%) | 38 (35%) | |
| NT $20,000-NT $39,999 | 9 (33%) | 51 (47%) | |
| ≧NT $39,999 | 7 (26%) | 19 (18%) | |
| Residential urbanization | 0.12 | ||
| Urban | 18 (66%) | 60 (56%) | |
| Suburban | 8 (30%) | 34 (31%) | |
| Rural | 1 (4%) | 14 (13%) | |
| IBD type | |||
| Crohn's disease | 7 (26%) | ||
| Ulcerative colitis | 20 (74%) | ||
IBD: Inflammatory bowel disease.
As shown in Table 2, 22 of 27 individuals in IBD group and 58 of 108 individuals in non-IBD group developed periodontitis were observed. Cumulative incidence for the development of periodontitis was 81.4% in IBD group and 53.7% in non-IBD group. The overall incident rate ratio was higher in IBD group (HR: 1.05; 95% CI: 0.64–1.72). After adjusting for confounding factors, IBD group had higher risk for developing periodontitis than non-IBD group (adjusted HR: 1.82; 95% CI: 1.09–3.03). We compared other factors including gender, age, socio-economic status, and urbanization. In female, IBD group had higher risk for developing periodontitis than non-IBD group (adjusted HR: 2.24; 95% CI: 1.04–4.83). Male group showed the similar result; however, the result did not reach statistical significant (adjusted HR: 1.58; 95% CI: 0.80–3.12). In socio-economic factor, mid-class IBD group showed the higher risk than non-IBD group (adjusted HR: 5.40; 95% CI: 2.36–12.39).
Table 2.
Incidence and hazard ratio of periodontitis between the IBD and non-IBD group.
| IBD (+) |
IBD (−) |
IRR (95% CI) | Crude HR (95%CI) | Adjust HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 27 |
n = 108 |
||||||||
| Event | Person-Year | Incidence rate | Event | Person-Year | Incidence rate | ||||
| All | 22 | 66 | 333.33 | 58 | 183 | 316.94 | 1.05 (0.64–1.72) | 1.74 (1.05–2.88) | 1.82 (1.09–3.03) |
| Age | |||||||||
| 18–44 years | 17 | 48 | 354.17 | 47 | 149 | 315.44 | 1.12 (0.64–1.96) | 1.60 (0.90–2.85) | 1.76 (0.97–3.21) |
| 45–64 years | 5 | 18 | 277.78 | 11 | 34 | 323.53 | 0.86 (0.30–2.47) | 2.23 (0.78–6.76) | 2.10 (0.66–6.70) |
| Sex | |||||||||
| Female | 10 | 40 | 250 | 30 | 105 | 285.71 | 0.88 (0.43–1.79) | 1.98 (0.93–4.21) | 2.24 (1.04–4.83) |
| Male | 12 | 26 | 461.54 | 28 | 78 | 358.97 | 1.29 (0.65–2.53) | 1.57 (0.79–3.10) | 1.58 (0.80–3.12) |
| Socialeconomic status | |||||||||
| <NT $20,000 | 6 | 18 | 333.33 | 22 | 56 | 392.86 | 0.85 (0.34–2.09) | 0.77 (0.31–1.936) | 0.73 (0.28–1.92) |
| NT $20,000-NT $39,999 | 9 | 18 | 500 | 24 | 68 | 352.94 | 1.42 (0.66–3.05) | 4.32 (1.96–9.53) | 5.40 (2.36–12.39) |
| ≧NT $39,999 | 7 | 30 | 233.33 | 12 | 59 | 203.39 | 1.14 (0.45–2.91) | 1.62 (0.60–4.36) | 1.78 (0.44–7.13) |
| Residential urbanization | |||||||||
| Urban | 13 | 27 | 481.48 | 33 | 90 | 366.67 | 1.31 (0.69–2.49) | 1.33 (0.68–2.57) | 1.34 (0.68–2.67) |
| Suburban | 8 | 39 | 205.13 | 20 | 79 | 253.16 | 0.81 (0.36–1.84) | 2.05 (0.87–4.84) | 2.18 (0.88–5.40) |
| Rural | 1 | 0 | – | 5 | 14 | 357.14 | – | 14.00 (0.88–233.87) | 9.83 (0.51–191.23) |
IBD: Inflammatory bowel disease; IRR: Incidence rate ratio; HR: Hazard ratio.
As shown in Table 3, IBD was further stratification with subtype ulcerative colitis and Crohn's disease. Crohn's disease group showed significantly higher risk for developing periodontitis (adjusted HR: 3.95; 95% CI: 1.59–9.82). However, ulcerative colitis group had borderline significance for higher risk of periodontitis (adjusted HR: 1.39; 95% CI: 0.69–2.46).
Table 3.
Comparisons of risk of periodontitis by subgroups of IBD.
| N | Event | Person-Year | Incidence rate | Crude HR | Adjusted HR | |
|---|---|---|---|---|---|---|
| Non-IBD cohort | 108 | 58 | 183 | 316.94 | 1 | 1 |
| IBD cohort | ||||||
| Crohn's disease | 7 | 6 | 4 | 1500 | 4.46 (1.88–10.56) | 3.95 (1.59–9.82) |
| Ulcerative colitis | 20 | 16 | 62 | 258.06 | 1.51 (0.84–2.69) | 1.39 (0.69–2.46) |
IBD: Inflammatory bowel disease; IRR: Incidence rate ratio; HR: Hazard ratio.
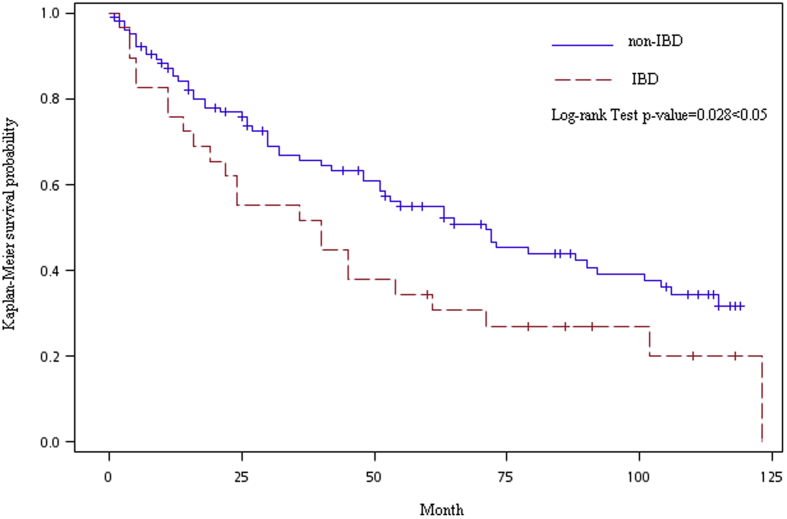
The Kaplan–Meier plot of periodontitis showed the cumulative incidence rate between the cohorts with and without IBD (Fig. 2). The gap in incidence rate of IBD and non-IBD groups has widened gradually. Log-rank test results for time-to-event analysis showed that the IBD group carries a higher risk of periodontitis than non-IBD group (p = 0.028).
Figure 2.
Adjusted Kaplan–Meier curve of periodontal disease between IBD and non-IBD group.
Discussion
In Taiwan, patients with certain serious disorders are issued a catastrophic illness card by the NHIA to reduce the financial burden of their illness. IBD diagnosis with the catastrophic illness registry may be more reliable than claim data because the application for a catastrophic illness card must be signed by a board-certificated gastroenterologist after the diagnosis verified through a number of outpatient department visits and pathological proof. This is the reason why the authors chose this criteria for the idefinition of IBD in this study.
To the best of our knowledge, this is the first population-based study to investigate the association between IBD and periodontitis. IBD patients were found to have higher risk of periodontitis. Previously, Lamster et al.12 first described the association between IBD and periodontitis due to the altered immune function in one case report. A hospital-based observational study showed that periodontitis affected more sites per subject in IBD group in US population.14 In a case control study, higher prevalence and the severity of periodontitis in IBD patients than non-IBD comparisons in Jordanian.15 Vavricka et al.11 also reported that IBD is associated with periodontitis in Swiss cohort. Taken together, the increased risk of periodontitis was observed in IBD patients. However, our current study is population-based database deviated from NHIRD with longitudinal follow-up from 2000 to 2009. This dataset with total one million participants can provide more representative result and more statistic power of evidence.
In this study, the risk of periodontitis was significantly higher in Crohn's disease as compared to ulcerative colitis group. Previously, Vavricka's et al.11 who have report that the increased risk of periodontitis was higher in Crohn's disease subgroup within IBD patients. The reason is not quite clear. The possible explanation may be the severity of disease in Crohn's disease more advanced and serious than ulcerative colitis. In addition, Crohn's disease had higher frequency for oral involvement, such as indurated tag-like lesions, cobblestoning and mucogingivitis.10 Taken together, these findings indicate that intensive regular oral check-up for periodontal status is necessary for patients with IBD, especial for Crohn's disease.
From previous researches, a slight female predominant in Crohn's disease and male predominant in ulcerative colitis were reported in western countries. Male predominance in Asian patients with Crohn's disease is noted and equal gender distribution for ulcerative colitis.22 Our result showed that male predominant in IBD patients; however, female IBD patients had significantly higher risk for developing periodontitis than non-IBD group. Borderline significant risk was observed in male IBD group. Gender difference could be one of the risk factors for developing periodontitis, and female had higher risk than male in IBD patients in our study. Hormonal influence might play an important factor in this phenomenon.23
Our result revealed that IBD group with middle-class to high-class economic status had higher risk for developing periodontitis. Rapid socioeconomic development and exposure to environmental risk factors in childhood might be possible explanations of association between IBD and periodontitis. According to the large-scaled IBD epidemiologic study across nine countries in Asia–Pacific, the data showed geographic variability in disease incidence even within Asia.24 Incidence of IBD in Asia–Pacific region ranged from 0.50 to 3.14 per 100,000 persons. Generally, western countries had higher incidence and prevalence of IBD compared with eastern countries. Incidence of CD ranged from 3.1 to 14.6 cases per 100,000 person-years and incidence of UC ranged from 2.2 to 14.3 cases per 100,000 person-years.25 We might speculate that different degrees of urbanization/socioeconomic status could play a part in this variation. Further, lifestyle change with westernized living style might be an important factor. In previous studies,24, 25, 26, 27 westernization of lifestyle with a high dietary intake of fats, sugars and meat increased the risk for both IBD and periodontitis. Change of intestinal microbial milieu from dietary change might have a pathologic impact on this association.
With regard to environmental factors, smoking becomes a well-recognized risk factor for both diseases.28,29 We consider periodontitis in parallel with other chronic inflammatory diseases and conditions for which smoking has a detrimental effect. Previous study showed that cigarette smoking had positive association with IBD.28 Nicotine appears to increase the presence of neutrophils in gut mucosa, enhancing the chronic inflammatory condition in IBD. However, information retrieved from the NHIRD did not contain anthropometric data and health related behaviors or status such as betel nut chewing habit, smoking, and alcohol consumption. Thus, the effects of health related behaviors could not be estimated in this study. To minimize the bias, possible confounding factors, including age, gender, urbanization, and insurance cost, were carefully adjusted in our result.
In conclusion, current retrospective cohort study showed that patients with IBD increased chance of having periodontitis comparing with non-IBD group in the longitudinal investigation, especially in Crohn's disease group. Dental professionals and gastroenterologists should be more aware of the correlation of both diseases. Oral health education to patients with IBD for the potential defect on periodontal status should be encouraged.
Conflicts of interest
None declared.
References
- 1.Abraham C., Cho J.H. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrokhyar F., Swarbrick E.T., Irvine E.J. A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:2–15. doi: 10.1080/00365520150218002. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart D.C., Sandborn W.J. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim J., Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 6.D'Aiuto F., Graziani F., Tete S., Gabriele M., Tonetti M.S. Periodontitis: from local infection to systemic diseases. Int J Immunopathol Pharmacol. 2005;18:1–11. [PubMed] [Google Scholar]
- 7.Chung J.H., Hwang H.J., Kim S.H., Kim T.H. Associations between periodontitis and chronic obstructive pulmonary disease: the 2010 to 2012 Korean national health and nutrition examination survey. J Periodontol. 2016;87:864–871. doi: 10.1902/jop.2016.150682. [DOI] [PubMed] [Google Scholar]
- 8.Preshaw P.M., Alba A.L., Herrera D. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. Inflammatory bowel disease: clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odontol Scand. 2001;59:235–243. doi: 10.1080/00016350152509265. [DOI] [PubMed] [Google Scholar]
- 10.Lankarani K.B., Sivandzadeh G.R., Hassanpour S. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol. 2013;19:8571–8579. doi: 10.3748/wjg.v19.i46.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vavricka S.R., Brun L., Ballabeni P. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- 12.Lamster I.B., Rodrick M.L., Sonis S.T., Falchuk Z.M. An analysis of peripheral blood and salivary polymorphonuclear leukocyte function, circulating immune complex levels and oral status in patients with inflammatory bowel disease. J Periodontol. 1982;53:231–238. doi: 10.1902/jop.1982.53.4.231. [DOI] [PubMed] [Google Scholar]
- 13.Lavine W.S., Maderazo E.G., Stolman J. Impaired neutrophil chemotaxis in patients with juvenile and rapidly progressing periodontitis. J Periodontal Res. 1979;14:10–19. doi: 10.1111/j.1600-0765.1979.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 14.Flemmig T.F., Shanahan F., Miyasaki K.T. Prevalence and severity of periodontal disease in patients with inflammatory bowel disease. J Clin Periodontol. 1991;18:690–697. doi: 10.1111/j.1600-051x.1991.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 15.Habashneh R., Khader Y., Alhumouz M., Jadallah K., Ajlouni Y. The association between inflammatory bowel disease and periodontitis among Jordanians: a case–control study. J Periodontal Res. 2012;47:293–298. doi: 10.1111/j.1600-0765.2011.01431.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang R.Y., Lin Y.F., Kao S.Y., Shieh Y.S., Chen J.S. Dental restorative treatment expenditure and resource utilization in patients with chronic kidney disease: a nationwide population-based study. J Dent Sci. 2017;12:275–282. doi: 10.1016/j.jds.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C.K., Wu Y.T., Chang Y.C. Periodontal inflammatory disease is associated with the risk of Parkinson's disease: a population-based retrospective matched-cohort study. Peer J. 2017;5:3647. doi: 10.7717/peerj.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang P.Y., Su N.Y., Lu M.Y., Wei C., Yu H.C., Chang Y.C. Trends in the prevalence of diagnosed temporomandibular disorder from 2004 to 2013 using a Nationwide Health Insurance Database in Taiwan. J Dent Sci. 2017;12:249–252. doi: 10.1016/j.jds.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H.C., Su N.Y., Huang J.Y., Lee S.S., Chang Y.C. Trends in the prevalence of periodontitis in Taiwan from 1997 to 2013: a nationwide population-based retrospective study. Medicine. 2017;96:e8585. doi: 10.1097/MD.0000000000008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S.F., Wang Y.H., Su N.Y. Changes in prevalence of pre-cancerous oral submucous fibrosis from 1996-2013 in Taiwan: a nationwide population-based retrospective study. J Formos Med Assoc. 2018;117:147–152. doi: 10.1016/j.jfma.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 21.National Health Insurance Administration Ministry of Health and Welfare, Taiwan. https://www.nhi.gov.tw/Content_List.aspx
- 22.Prideaux L., Kamm M.A., De Cruz P.P., Chan F.K., Ng S.C. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266–1280. doi: 10.1111/j.1440-1746.2012.07150.x. [DOI] [PubMed] [Google Scholar]
- 23.Ng S.C. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363–372. doi: 10.1016/j.bpg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Ng S.C., Tang W., Ching J.Y. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158–165. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Loftus E.V., Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 26.Ng S.C., Bernstein C.N., Vatn M.H. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 27.Hou J.K., Abraham B., El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 28.Mahid S.S., Minor K.S., Soto R.E., Hornung C.A., Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 29.Haber J., Wattles J., Crowley M., Mandell R., Joshipura K., Kent R.L. Evidence for cigarette smoking as a major risk factor for periodontitis. J Periodontol. 1993;64:16–23. doi: 10.1902/jop.1993.64.1.16. [DOI] [PubMed] [Google Scholar]