Abstract
Purpose
This study was designed to evaluate the prognostic value of the combination of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) (neutrophil/platelet-to-lymphocyte ratio [NLR–PLR]) in patients with hepatocellular carcinoma (HCC) who receive transarterial chemoembolization (TACE) therapy.
Patients and methods
Data from 216 patients who were diagnosed with HCC after TACE therapy were retrospectively collected. R software was used to analyze the time-dependent receiver operating characteristic (ROC) curves and to compare the area under the ROC curves (AUROCs).
Results
The long-term survival rates were significantly higher for patients with lower values than those with higher values of NLR, PLR, and NLR–PLR. The mean overall survival decreased gradually with increases in the NLR–PLR score (P<0.0001). The AUROC values of the NLR–PLR score were consistently higher than those of NLR and PLR.
Conclusion
This study showed that the NLR–PLR score might be a useful predictor for patients with HCC who receive TACE therapy.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, neutrophil/platelet-to-lymphocyte ratio
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and aggressive malignancies and is the third most lethal of all human cancers.1 Curative approaches, such as hepatic resection, liver transplantation, and radio-frequency ablation, are the recommended treatment modalities for early HCC.2–4 However, the majority of patients with HCC is diagnosed at an intermediate to advanced stages. Transarterial chemo-embolization (TACE) and sorafenib are recommended for these patients, respectively, according to the Barcelona Clinic Liver Cancer (BCLC) staging classification, adopted by the American Association for the Study of Liver Disease and European Association for the Study of the Liver guidelines.5,6 Moreover, it was shown that the efficacy of sorafenib treatment was limited and costly.7 Therefore, rather than sorafenib, TACE was the most frequent first-line treatment for patients with BCLC stage C disease in real-life clinical practice for the management of HCC.8 In this study, TACE, which delivers chemotherapeutic drugs to the tumor while blocking tumor-feeding arteries, is used for patients with intermediate to advanced stages HCC.9,10
Several reports have highlighted prognostic indicators for patients with HCC, including serum α-fetoprotein (AFP), tumor size, vascular invasion, and extrahepatic spread.11 However, the heterogeneity of the study populations led to variation. What is more, these indicators may not be specific risk factors for HCC patients who receive TACE therapy, which would indicate whether a given patient could benefit from this treatment and stratify patients according to their risk levels. Thus, the search for an effective biomarker that can be used to precisely predict the disease prognosis and to inform an optimal personalized treatment strategy is necessary and urgent to improve clinical outcomes.
In 1863, the close relationship between inflammation and malignancy was first described by Virchow, who observed the presence of leukocytes in tumor tissue;12,13 since this discovery, accumulated evidence has indicated that the presence of a systemic inflammatory response and malnutrition is associated with a poor prognosis for various malignancies.14,15 Moreover, the prognostic value of the systemic inflammatory response in patients with various cancers can be reflected by the combination of hematological components. The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) have been widely used as evaluation tools for the inflammatory and immune responses and are reportedly prognostic factors in various malignancies.16–18 Furthermore, higher values of NLR and PLR are associated with unfavorable clinical features in patients with HCC.19–22 However, few reports have addressed the relationship between the combination of NLR and PLR and the prognosis of patients with HCC who received TACE therapy. In this study, we aimed to elucidate the prognostic impact of the combination of NLR and PLR for patients with HCC who received TACE therapy.
Patients and methods
Patients
Consecutive patients who were newly diagnosed with HCC after TACE therapy performed between January 2007 and July 2015 at the Department of Hepatobiliary and Pancreatic Surgery of Sun Yat-sen University Cancer Center were enrolled in this study. All patients with HCC included in this study were categorized as BCLC stage B and C in this study. The criteria for inclusion were as follows: 1) treatment with TACE as the initial therapy; 2) three courses of TACE treatment; and 3) Child-Pugh A or B liver function. Exclusion criteria included the following: 1) treatments such as hepatic resection, liver transplantation, radio-frequency treatment, or other HCC treatments before or after TACE; 2) Child-Pugh C liver function; 3) inadequate renal function (serum creatinine and serum blood urea nitrogen > the upper limit of normal); 4) obstructive jaundice; 5) diagnosis of second tumors; or 6) lost to follow-up. This study was approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Clinical data collection
All clinical and radiological data were retrieved from medical records. Hematologic examination and imaging examinations, including AFP, liver ultrasonography, contrast-enhanced dynamic computed tomography (CT), magnetic resonance imaging (MRI), and hepatic arterial angiography, were used in the clinical diagnosis. The diagnosis of HCC was established on the typical features of HCC identified by two radiological images or one radiological image combined with the elevated AFP level (≥400 ng/mL) or pathologic evidence.23
Clinical and radiological parameters, including age, gender, white blood cell count, platelet (PLT) count, neutrophil cell count, lymphocyte cell count, PLT count, AFP, alanine transaminase, aspartate aminotransferase (AST), total bilirubin, ALP, gamma-glutamyl transpeptidase (GGT), albumin (ALB), C-reactive protein (CRP), hepatitis B surface antigen (HBsAg), NLR, PLR, hepatitis B virus DNA load, tumor diameter, tumor number, vascular invasion, HBV infection, performance status, BCLC stage, and Cancer of the Liver Italian Program (CLIP) score were measured and analyzed. Clinical and radiological data were retrieved at the time of diagnosis before the initial TACE was initiated. Vascular invasion was defined as the presence of thrombus adjacent to the tumor in the portal system or the hepatic vein system with a vague boundary that was confirmed by at least two imaging modalities in this study.24 Apart from NLR and PLR, several inflammation-based factors have also been explored, including the combination of CRP and white cell count as the prognostic index (PI),25 a combination of serum CRP and ALB in the modified Glasgow Prognostic Score (mGPS),26 and a combination of ALB and lymphocyte count in the prognostic nutritional index (PNI).21
Treatment procedure
Three courses of uniform treatment protocols were performed for each patient. The Seldinger technique was used, as previously reported.27 Three hundred milligrams of carboplatin (Bristol-Myers Squibb, New York, NY, USA), 50 mg of epirubicin (Pharmorubicin, Pfizer, Wuxi, Jiangsu, P. R. China), and 6 mg of mitomycin (Zhejiang Hisun Pharmaceutical Co. Ltd., Taizhou, Zhejiang, P. R. China) were used in conventional chemoembolization. The delivered dose of Lipiodol (Lipiodol Ultra-Fluide; Andre Guerbet Laboratories, France) was determined in accordance with the location, size, and number of tumors, ranging from 5 to 30 mL.
Follow-up
Patients were followed up at least once every 2 months during the first years and once every 3 months thereafter. The AFP test, liver ultrasonography, CT, and MRI were selectively performed as needed. Overall survival (OS) was defined as the duration (in days) from the date of the first TACE until death or last follow-up in this study. The median follow-up period was 431.11 days.
Statistical analysis
Statistical analysis was performed using SPSS version 22 (SPSS Inc., Chicago, IL, USA). Continuous data are expressed as the means and ranges, and categorical data are shown as frequencies and proportions. Student’s t-test was used to compare continuous variables. The chi-squared test and Fisher’s exact test were used to compare the categorical variables.
Univariate and multivariate analyses were performed to assess the significance of the differences in the clinical or radiological data. The associated 95% CI was calculated. The Kaplan–Meier method was used to analyze OS. Significant differences between the groups were identified using the log-rank test. The survival curves were performed using MedCalc software version 11.4.2.0 (http://www.medcalc.be). A two tailed P-value <0.05 was considered statistically significant.
NLR or PLR was estimated by dividing an absolute neutrophil or PLT count by an absolute lymphocyte count which were obtained within 1 week before the initial TACE. Time-dependent receiver operating characteristic (ROC) curves were performed for the assessability of both NLR and PLR to predict long-term survival and to determine the optimal cutoff value for these two variables.28,29 The analyses of ROC curves and comparisons of the areas under the ROC curves (AUROCs) were performed using R software version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org) with the “survival ROC” package and the “survival ROC.C” package.
Results
Optimal cutoff values for the variables
The NLR and PLR were calculated by dividing the neutrophil or PLT counts by the lymphocyte counts. The mean values of the neutrophil, PLT, and lymphocyte counts were 4.26×109/L, 194.37×109/L, and 1.61×109/L, respectively. The analysis of the time-dependent ROC was used to decide the optimal cutoff values for the NLR and PLR, which were 1.77 and 94.62, respectively, along with the strongest Youden index for the OS prediction. The threshold for each clinical and radiological dataset was utilized as the cutoff values for these variables.
Patient characteristics
A total of 216 patients who were newly diagnosed with HCC and had received TACE as the initial therapy were included in this study. There were 200 male (92.6%) and 16 female (7.4%) patients with a median age of 53 years in the whole study cohort. Enlarged tumors and multiple tumors were common in this study. Additionally, 30.3% of the patients had vascular invasion, and 5.5% of the patients were sorted into the metastatic tumor group. Hepatitis B infection was the most common cause of HCC, and HBsAg was positive in ~97.2% of the enrolled patients. Most patients had good reserve liver function with Child-Pugh A and good performance status, with scores of 0–1 (Table 1). Elevated scores of neutrophil/platelet-to-lymphocyte ratio (NLR–PLR) were more frequently observed in HCC patients of BCLC stage C or high values of CLIP scores, while no differences in values of NLR–PLR were observed in relation to BCLC stages or CLIP scores (P>0.05).
Table 1.
Relationship between clinic and radiological factors and NLR–PLR score
| Characteristic | Parameter | N | NLR–PLR score | P-value | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Total | 216 | 34 | 56 | 126 | ||
| Age (years) | <60 | 158 | 26 | 40 | 92 | 0.871 |
| ≥60 | 58 | 8 | 16 | 34 | ||
| Gender | Males | 200 | 31 | 53 | 116 | 0.781 |
| Females | 16 | 3 | 3 | 10 | ||
| WBC (×109/L) | <10 | 215 | 34 | 56 | 125 | 0.699 |
| ≥10 | 1 | 0 | 0 | 1 | ||
| CRP (mg/L) | <8 | 112 | 27 | 38 | 47 | <0.001 |
| ≥8 | 104 | 7 | 18 | 79 | ||
| ALT (U/L) | <40 | 83 | 12 | 21 | 50 | 0.885 |
| ≥40 | 133 | 22 | 35 | 76 | ||
| AST (U/L) | <45 | 64 | 11 | 23 | 30 | 0.058 |
| ≥45 | 152 | 23 | 33 | 96 | ||
| ALB (g/L) | <35 | 28 | 4 | 7 | 17 | 0.958 |
| ≥35 | 188 | 30 | 49 | 109 | ||
| TBIL (mmol/L) | <20.5 | 169 | 25 | 43 | 101 | 0.675 |
| ≥20.5 | 47 | 9 | 13 | 25 | ||
| ALP (U/L) | <100 | 65 | 12 | 24 | 29 | 0.021 |
| ≥100 | 151 | 22 | 32 | 97 | ||
| GGT (U/L) | <50 | 24 | 6 | 8 | 10 | 0.189 |
| ≥50 | 192 | 28 | 48 | 116 | ||
| AFP (ng/mL) | <400 | 116 | 20 | 34 | 62 | 0.288 |
| ≥400 | 100 | 14 | 22 | 64 | ||
| HBV-DNA (IU/mL) | <100 | 54 | 8 | 13 | 33 | 0.892 |
| ≥100 | 162 | 26 | 43 | 93 | ||
| Tumor diameter (cm) | <5 | 39 | 12 | 16 | 11 | <0.001 |
| ≥5 | 177 | 22 | 40 | 115 | ||
| Tumor number | Single | 39 | 2 | 9 | 28 | 0.081 |
| Multiple | 177 | 32 | 47 | 98 | ||
| Vascular invasion | Absent | 133 | 25 | 37 | 71 | 0.136 |
| Present | 83 | 9 | 19 | 55 | ||
| HBV infection | Absent | 6 | 0 | 3 | 3 | 0.298 |
| Present | 210 | 34 | 53 | 123 | ||
| Child-Pugh classification | A | 168 | 30 | 45 | 93 | 0.172 |
| B | 48 | 4 | 11 | 33 | ||
| Performance status | 0–1 | 193 | 33 | 50 | 110 | 0.536 |
| 2 | 23 | 1 | 6 | 16 | ||
| BCLC stage | B | 36 | 6 | 12 | 18 | 0.484 |
| C | 180 | 28 | 44 | 108 | ||
| CLIP score | 1 | 56 | 10 | 13 | 33 | 0.203 |
| 2 | 60 | 12 | 11 | 37 | ||
| 3 | 75 | 11 | 26 | 38 | ||
| 4 | 25 | 1 | 6 | 18 | ||
Abbreviations: AFP, α-fetoprotein; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; CRP, C-reactive protein; GGT, gamma-glutamyl transpeptidase; HBV-DNA, hepatitis B virus DNA; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; TBIL, total bilirubin; WBC, white blood cell count.
NLR and PLR were divided into two groups: <1.77 and ≥1.77 for NLR and <94.62 and ≥94.62 for PLR. Among the 216 patients, an elevated NLR or PLR was observed in 167 (77.3%) patients or 141 (65.3%) patients, respectively. When NLR was combined with PLR, combined NLR–PLR was generated. According to NLR–PLR, patients were assigned different scores: patients with NLR <1.77 and PLR <94.62 were assigned a score of 1; patients with NLR <1.77 and PLR ≥94.62 or NLR ≥1.77 and PLR <94.62 were assigned a score of 2; and patients with NLR ≥1.77 and PLR ≥94.62 were assigned a score of 3. It was shown that a higher NLR–PLR score was associated with elevated CRP (P<0.001), elevated ALP (P=0.021), and a larger tumor size (P<0.001; Table 1). In addition, the correlations of inflammation parameters were also conducted. As shown in Table 2, there were significantly positive correlations between NLR–PLR and NLR, PLR, PI, mGPS, and CRP. In contrast, a significantly negative correlation was also observed between NLR–PLR and PNI (P<0.05, Table 2).
Table 2.
Correlations of inflammation parameters for HCC patients after TACE therapy
| Characteristic | NLR | PLR | NLR–PLR | PI | mGPS | PNI | CRP | |
|---|---|---|---|---|---|---|---|---|
| NLR | r | 1.000 | 0.394 | 0.760 | 0.279 | 0.289 | −0.368 | 0.369 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| PLR | r | 0.394 | 1.000 | 0.879 | 0.295 | 0.289 | −0.271 | 0.366 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| NLR–PLR | r | 0.760 | 0.879 | 1.000 | 0.353 | 0.353 | −0.390 | 0.449 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| PI | r | 0.279 | 0.295 | 0.353 | 1.000 | 0.972 | −0.289 | 0.867 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| mGPS | r | 0.289 | 0.289 | 0.353 | 0.972 | 1.000 | −0.324 | 0.861 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| PNI | r | −0.192 | −0.127 | −0.195 | −0.210 | −0.252 | 1.000 | −0.225 |
| P | 0.005 | 0.062 | 0.004 | 0.002 | <0.001 | 0.001 | ||
| CRP | r | 0.369 | 0.366 | 0.449 | 0.867 | 0.861 | −0.329 | 1.000 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
Abbreviations: CRP, C-reactive protein; HCC, hepatocellular carcinoma; mGPS, modified Glasgow Prognostic Score; NLR, neutrophil to lymphocyte ratio; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; TACE, transarterial chemoembolization.
OS and prognostic factors
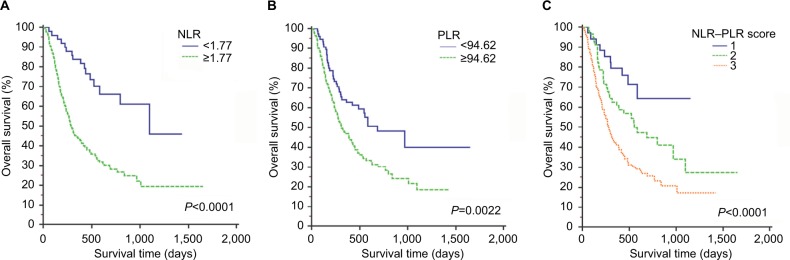
The estimated 1-, 2-, and 3-year OS rates for all patients were 61.3%, 44.2%, and 40.5%, respectively. The median OS was 17 months. The long-term survival rates were significantly higher for patients with lower values of NLR than for patients with higher values of NLR (P<0.0001, Figure 1A). Patients with PLR <94.62 also had better long-term survival than patients with PLR ≥94.62 (P=0.0022, Figure 1B). The mean OS decreased gradually along with increasing NLR–PLR. Moreover, the long-term survival rates of patients were significantly stratified by NLR–PLR scores. The 3-year OS rates of patients with NLR–PLR scores of 1, 2, and 3 were 64.3%, 27.3%, and 17.2%, respectively (P<0.0001, Figure 1C).
Figure 1.
Kaplan–Meier curves for OS in patients who were diagnosed with HCC after TACE therapy; patients were stratified according to the inflammation-based prognostic scores.
Note: (A) NLR; (B) PLR; and (C) NLR–PLR.
Abbreviations: HCC, hepatocellular carcinoma; NLR, neutrophil-to-lymphocyte ratio; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; TACE, transarterial chemoembolization.
In the univariate survival analysis, the NLR–PLR score (HR, 1.85; 95% CI, 1.41–2.42; P<0.001) was significantly associated with OS. Other significant prognostic parameters included the CRP, AST, ALB, ALP, GGT, AFP, tumor diameter, and vascular invasion (Table 3). After the multivariate Cox proportional hazards analysis, we found that the pretreatment NLR–PLR score (HR, 1.33; 95% CI, 1.00–1.77; P=0.045), the pretreatment AFP levels (HR, 1.68; 95% CI, 1.18–2.40; P=0.004), the tumor diameter (HR, 6.77; 95% CI, 2.43–18.44; P<0.001), and vascular invasion (HR, 2.03; 95% CI, 1.43–2.88; P<0.001) were independent predictors of OS (Table 3).
Table 3.
Univariate and multivariate analyses of OS in the study cohort
| Characteristic | Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | <60/60 | 0.94 (0.64–1.38) | 0.749 | NI | |
| Gender | Male/female | 1.03 (0.55–1.90) | 0.931 | NI | |
| WBC (×109/L) | <10/≥10 | 0.46 (0.15–1.44) | 0.182 | NI | |
| Neutrophil (×109/L) | <6.3/≥6.3 | 1.11 (0.667–1.85) | 0.684 | NI | |
| Lymphocyte (×109/L) | <3.2/≥3.2 | 0.05 (0.00–209.71) | 0.479 | NI | |
| PLT (×109/L) | <100/100–300/≥300 | 1.08 (0.75–1.57) | 0.669 | NI | |
| CRP (mg/L) | <8/≥8 | 2.66 (1.85–3.82) | <0.001 | 1.48 (0.98–2.21) | 0.060 |
| ALT (U/L) | <40/≥40 | 1.28 (0.90–1.83) | 0.171 | NI | |
| AST (U/L) | <45/≥45 | 2.19 (1.44–3.32) | <0.001 | 0.94 (0.60–1.48) | 0.791 |
| ALB (g/L) | <35/≥35 | 0.62 (0.40–0.97) | 0.036 | 0.74 (0.47–1.18) | 0.217 |
| TBIL (mmol/L) | <20.5/≥20.5 | 1.43 (0.97–2.12) | 0.073 | NI | |
| ALP (U/L) | <100/≥100 | 1.95 (1.30–2.90) | 0.001 | 1.20 (0.79–1.83) | 0.393 |
| GGT (U/L) | <50/≥50 | 2.84 (1.39–5.82) | 0.004 | 1.08 (0.51–2.32) | 0.836 |
| AFP (ng/mL) | <400/≥400 | 2.17 (1.54–3.05) | <0.001 | 1.68 (1.18–2.40) | 0.004 |
| HBV-DNA (IU/mL) | <100/≥100 | 0.97 (0.66–1.43) | 0.866 | NI | |
| Tumor diameter (cm) | <5/≥5 | 11.73 (4.33–31.76) | <0.001 | 6.77 (2.43–18.84) | <0.001 |
| Tumor number | Single/multiple | 1.53 (0.95–2.46) | 0.081 | NI | |
| Vascular invasion | Absent/present | 2.44 (1.74–3.43) | <0.001 | 2.03 (1.43–2.88) | <0.001 |
| HBV infection | Absent/present | 0.49 (0.197–1.20) | 0.116 | NI | |
| NLR–PLR | 1/2/3 | 1.85 (1.41–2.42) | <0.001 | 1.33 (1.00–1.77) | 0.045 |
Abbreviations: AFP, α-fetoprotein; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; CRP, C-reactive protein; GGT, gamma-glutamyl transpeptidase; HBV-DNA, hepatitis B virus DNA; NI, not included; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; PLT, platelet; OS, overall survival; TBIL, total bilirubin; WBC, white blood cell count.
Prognostic value of the NLR–PLR score
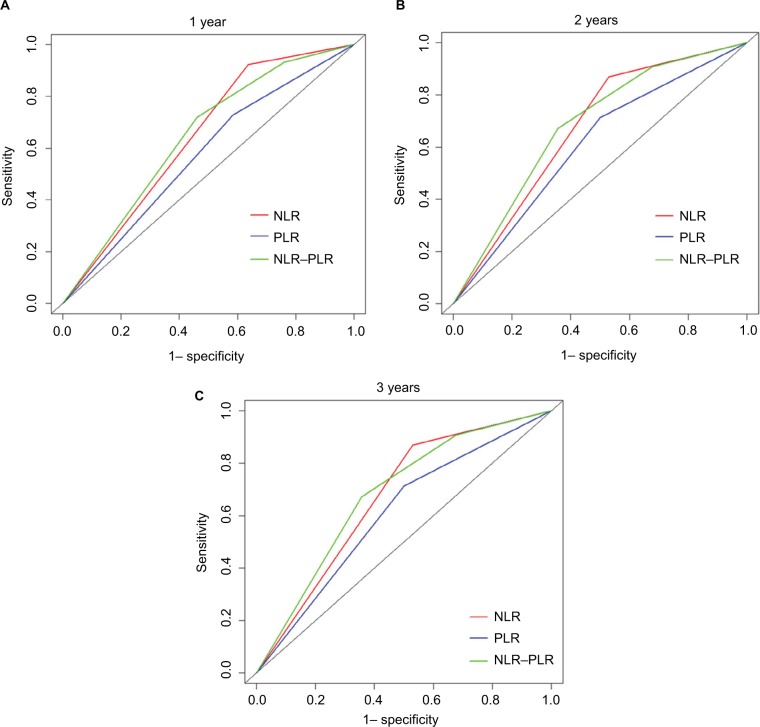
Additionally, the prognostic capacities of the NLR, PLR, and NLR–PLR scores were compared by analyzing the AUROC values to show whether the NLR–PLR score was at least equivalent or superior to the NLR or PLR scores. The ROC curves for OS prediction were calculated for the patients at 1, 2, and 3 years of follow-up (Figure 2). As shown in Table 4, the AUROC values of the NLR–PLR score were consistently higher than those of NLR and PLR. The NLR–PLR score showed a better distinguishing power for predicting the prognosis of patients with HCC who were treated with TACE compared with NLR or PLR alone. The NLR–PLR score divided patients into subgroups more precisely.
Figure 2.
Comparisons of the AUROC values for OS stratified by the inflammation-based prognostic scores at 1-year (A), 2-year (B), and 3-year (C).
Abbreviations: AUROC, area under the ROC curves; NLR, neutrophil-to-lymphocyte ratio; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; OS, overall survival.
Table 4.
Comparison of the AUROC values among the NLR, PLR, and NLR–PLR scores
| Inflammation- based score | Time | ||
|---|---|---|---|
| 1-year | 2-year | 3-year | |
| NLR | 0.643 | 0.637 | 0.669 |
| PLR | 0.572 | 0.589 | 0.606 |
| NLR–PLR | 0.644 | 0.653 | 0.681 |
Abbreviations: AUROC, area under the ROC curves; NLR, neutrophil-to-lymphocyte ratio; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Discussion
This study demonstrated that increased pretreatment NLR, PLR, and NLR–PLR scores predicted an unfavorable prognosis for OS in patients who were diagnosed with HCC and had received TACE therapy. Additionally, similar to previous studies,21,30,31 the NLR–PLR score had superior discriminative capacity for predicting prognosis compared with NLR or PLR.
Accumulated evidence shows that the systemic inflammatory response is associated with poor prognosis in multiple types of cancers.32–34 Several epidemiological studies have suggested that chronic inflammation may cause various malignancies.33 Moreover, HCC not only develops at sites of inflammation caused by the hepatitis B virus infection35,36 but also triggers regional inflammatory responses, which release inflammatory cytokines that promote the formation of an inflammatory microenvironment around the tumor.32,37,38 Multiple reports39–41 have argued that the chemicals and embolization agent in TACE can stimulate lymphocytes to become active. Furthermore, TACE treatment can sensitize p53-negative HCC cells to the cytotoxic effects of cisplatin and can result in increased HCC cell death.42 Synergistic cytotoxic effects in vitro and significant inhibition of tumor growth in vivo were observed in preclinical HCC models when TACE was administered.21,43 Peripheral inflammatory cells (ie, lymphocytes, neutrophils, and PLTs) were associated with the progression and prognosis of various types of cancer. Additionally, the NLR, PLR, and NLR–PLR scores, which represent the systematic inflammatory response, have potential value as prognostic factors for patients with HCC who received TACE therapy.
Inflammatory processes in the tumor microenvironment play important roles in tumor cell proliferation and cancer prognosis. The growth and survival factors that are released from inflammatory cells can stimulate tumor formation, progression, angiogenesis, invasion, and metastasis.44,45 The paradoxical roles of lymphocyte immune cells and circulating neutrophils in the inflammatory processes act as crucial opposing regulators in cancer occurrence.46 Neutrophils, which are a major component of the leukocyte population, produce pro-angiogenic factors, including vascular endothelial growth factors (VEGFs), to stimulate tumor development and progression.32 This phenomenon is partly explained by the upregulation of cyclooxygenase-2 or the suppression of an antitumor adaptive immune response.47–49 Similarly, an elevated PLT level can increase the production of VEGF, increasing angiogenesis and promoting tumor growth.50 However, lymphocytes are important components in cancer immune surveillance and can suppress tumor maturation.51 A decreased lymphocyte count is associated with an insufficient immunologic reaction to the tumor, which consequently enables tumor progression and metastasis.52 Additionally, several studies have suggested that adaptive immune cells, such as B-lymphocytes, CD8+cytotoxic T-lymphocytes, and CD4+ helper T-lymphocytes, play extremely important roles in the modulation of cancer development through tumor cell lysis.46,53
The NLR and PLR values are determined from the neutrophil and PLT counts, respectively, relative to the lymphocyte count, and these measurements may reflect inflammation and the immune response during tumor formation. An elevated NLR or PLR may reflect the presence of a chronic systemic inflammatory response. Patients with high values of NLR or PLR are relatively lymphocytopenic resulting in a poorer lymphocyte-mediated immune response to malignancy and therefore an increased potential for tumor recurrence and reduced survival. Numerous reports have demonstrated that NLR and PLR are useful for predicting the prognosis of various types of tumor.30 Moreover, cross-sectional studies have revealed that an elevated NLR or PLR is closely associated with poor survival,14,20 which is consistent with the findings of our study.
In addition, we found that an elevated NLR–PLR score was associated with increased tumor size, which showed that the inflammation-based prognostic score paralleled tumor progression. Additionally, an elevated AFP level, an enlarged tumor, and vascular invasion were also associated with poor OS by multivariate analysis in our study, which is consistent with previous studies.54 Apart from AFP, tumor size, and vascular invasion, the NLR–PLR score was identified as an independent indicator for OS. Moreover, significantly associations were shown between NLR–PLR and other inflammation-based factors, including PI, mGPS and PNI,21,25,26 which were associated with indexes, such as ALB, CRP, and ALP.55–57 It was also indicated that an elevated NLR–PLR score was also associated with elevated CRP and elevated ALP in this study. However, CRP and ALP were both excluded after the multivariate analysis, indicating that the NLR–PLR score was a more powerful predictor than CRP and ALP and was representative as an inflammation-based prognostic factor in patients with HCC, compared with other inflammation-based factors. There were some previous studies which focused on inflammation-based factors,58,59 such as NLR and PLR in HCC patients after TACE therapy, while they only discussed the prognostic effect of a single factor. Similarly, in our study, the Kaplan–Meier analysis showed that elevated NLR or PLR was associated with poor OS, these parameters were suboptimal for predicting OS. The NLR–PLR score divided the patients into subgroups more accurately in Kaplan–Meier survival analysis. Compared with values of AUROC in similar studies, a higher value for NLR–PLR scores indicated that the combined effect was greater than the individual effect of either variable alone. Another strength of our study was that it comprised a patients group whose management and follow-up were relatively homogenous. Excluding patients who received other treatments was important in removing potential confounders. Although a body of evidence existed suggesting that a high NLR or PLR was associated with adverse prognoses of patients with HCC, to date no reports focused on the combination of NLR and PLR targeting specific therapies in patients with HCC. The combined results of the AUROC and Kaplan–Meier survival curves strongly support the prognostic value of the NLR–PLR score in patients with HCC after TACE therapy for the first time. Further research is needed to evaluate the value of NLR–PLR in other patients groups with HCC and attempt independent validation of NLR–PLR for patient risk stratification.
Study limitations
This study has several limitations. First, this retrospective analysis relied on a single institutional dataset of HCC patients who were treated with TACE and there was no external validation for our study. The geographic and institutional heterogeneity that existed among these patients might affect the results of the study. Second, there might be other reasonable cutoff values for variables from other studies. Therefore, a large-scale prospective validation study is needed to confirm these results.
Conclusion
The predictive powers of NLR, PLR, and NLR–PLR were compared in patients with HCC after TACE therapy in this study. To the best of our knowledge, this study is the first to reveal that the NLR–PLR score can predict the prognosis of HCC for patients who receive TACE therapy. The NLR–PLR score is a routine parameter that is easily available and well standardized, and an elevated NLR–PLR score might correlate with a more aggressive disease phenotype. Thus, the NLR–PLR score might provide evidence for individualized treatment.
Acknowledgments
This work was supported by grants from School of Sociology and Anthropology-Sun Yat-sen University Cancer Center Joint Foundation on Medical Humanities (No 201804). We acknowledge the Medical Records Department for collecting the survival data of patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(15):4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Zhou ZG, Huang ZX, et al. Prospective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinoma. Chin J Cancer. 2016;35(1):25. doi: 10.1186/s40880-016-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Palmer DH. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(23):2498–2499. [PubMed] [Google Scholar]
- 8.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the bridge study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 13.Ferreri AJM, Illerhaus G, Zucca E, Cavalli F, International Extranodal Lymphoma Study Group Flows and flaws in primary central nervous system lymphoma. Nat Rev Clin Oncol. 2010;7(8):1–2. doi: 10.1038/nrclinonc.2010.9-c1. [DOI] [PubMed] [Google Scholar]
- 14.Templeton AJ, Knox JJ, Lin X, et al. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol. 2016;70(2):358–364. doi: 10.1016/j.eururo.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 16.Deng M, Ma X, Liang X, Zhu C, Wang M. Are pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio useful in predicting the outcomes of patients with small-cell lung cancer? Oncotarget. 2017;8(23):37200–37207. doi: 10.18632/oncotarget.16553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi S, Basso M, Strippoli A, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16(4):264–274. doi: 10.1016/j.clcc.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Song W, Tian C, Wang K, Zhang RJ, Zou SB. Preoperative platelet lymphocyte ratio as independent predictors of prognosis in pancreatic cancer: a systematic review and meta-analysis. PLoS One. 2017;12(6):e0178762. doi: 10.1371/journal.pone.0178762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(14):22854–22862. doi: 10.18632/oncotarget.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong L, Bai K, Cao Y, Huang Q, Lv L, Jiang Y. Prognostic value of pre-operative platelet to lymphocyte ratio in patients with resected primary hepatocellular carcinoma. Clin Lab. 2016;62(11):2191–2196. doi: 10.7754/Clin.Lab.2016.160414. [DOI] [PubMed] [Google Scholar]
- 21.He CB, Lin XJ. Inflammation scores predict the survival of patients with hepatocellular carcinoma who were treated with transarterial chemoembolization and recombinant human type-5 adenovirus H101. PLoS One. 2017;12(3):e0174769. doi: 10.1371/journal.pone.0174769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T, Zhu J, Zhao L, et al. Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J Surg Oncol. 2017;115(6):718–728. doi: 10.1002/jso.24549. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Hsu CY, Huang YH, et al. Vascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impact. J Clin Gastroenterol. 2014;48(8):734–741. doi: 10.1097/MCG.0b013e3182a8a254. [DOI] [PubMed] [Google Scholar]
- 25.Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29(5):3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 26.Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome study. Br J Cancer. 2011;104(4):726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18(2):413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 28.Galun D, Bogdanovic A, Djokic Kovac J, Bulajic P, Loncar Z, Zuvela M. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative-intent surgery for hepatocellular carcinoma: experience from a developing country. Cancer Manag Res. 2018;10:977–988. doi: 10.2147/CMAR.S161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 30.Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci Rep. 2017;7(1):1494. doi: 10.1038/s41598-017-01652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitano Y, Yamashita YI, Yamamura K, et al. Effects of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios on survival in patients with extrahepatic cholangiocarcinoma. Anticancer Res. 2017;37(6):3229–3237. doi: 10.21873/anticanres.11685. [DOI] [PubMed] [Google Scholar]
- 32.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bard-Chapeau EA, Nguyen AT, Rust AG, et al. Transposon mutagenesis identifies genes driving hepatocellular carcinoma in a chronic hepatitis B mouse model. Nat Genet. 2014;46(1):24–32. doi: 10.1038/ng.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang DK, Sun J, Cao G, et al. Genetic variants in Stat4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat Genet. 2013;45(1):72–75. doi: 10.1038/ng.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endig J, Buitrago-Molina LE, Marhenke S, et al. Dual role of the adaptive immune system in liver injury and hepatocellular carcinoma development. Cancer Cell. 2016;30(2):308–323. doi: 10.1016/j.ccell.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Mohs A, Kuttkat N, Reißing J, et al. Functional role of CCL5/RAN-TES for HCC progression during chronic liver disease. J Hepatol. 2017;66(4):743–753. doi: 10.1016/j.jhep.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Zheng C, Liang B, et al. Hepatocellular necrosis, apoptosis, and proliferation after transcatheter arterial embolization or chemo-embolization in a standardized rabbit model. J Vasc Interv Radiol. 2011;22(11):1606–1612. doi: 10.1016/j.jvir.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Chao Y, Wu CY, Kuo CY, et al. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol Int. 2013;7(3):883–892. doi: 10.1007/s12072-012-9409-9. [DOI] [PubMed] [Google Scholar]
- 41.McNally ME, Martinez A, Khabiri H, et al. Inflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Surg Oncol. 2013;20(3):923–928. doi: 10.1245/s10434-012-2639-1. [DOI] [PubMed] [Google Scholar]
- 42.Sieben M, Herzer K, Zeidler M, et al. Killing of p53-deficient hepatoma cells by parvovirus H-1 and chemotherapeutics requires promyelocytic leukemia protein. World J Gastroenterol. 2008;14(24):3819–3828. doi: 10.3748/wjg.14.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Yu DC, Chen Y, et al. A hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res. 2001;61(17):6428–6436. [PubMed] [Google Scholar]
- 44.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal M, Larusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60(1):184–190. [PubMed] [Google Scholar]
- 46.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577–583. [PubMed] [Google Scholar]
- 47.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4(6):431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 48.Schoppmann SF, Birner P, Stöckl J, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161(3):947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276(21):18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 50.Wiesner T, Bugl S, Mayer F, Hartmann JT, Kopp HG. Differential changes in platelet VEGF, Tsp, CXCL12, and CXCL4 in patients with metastatic cancer. Clin Exp Metastasis. 2010;27(3):141–149. doi: 10.1007/s10585-010-9311-6. [DOI] [PubMed] [Google Scholar]
- 51.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 54.Zhang N, Gu J, Yin L, et al. Incorporation of alpha-fetoprotein (AFP) into subclassification of BCLC C stage hepatocellular carcinoma according to a 5-year survival analysis based on the SEER database. Oncotarget. 2016;7(49):81389–81401. doi: 10.18632/oncotarget.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Xue TQ, Chen XY. Predictive values of serum VEGF and CRP levels combined with contrast enhanced MRI in hepatocellular carcinoma patients after TACE. Am J Cancer Res. 2016;6(10):2375–2385. [PMC free article] [PubMed] [Google Scholar]
- 56.Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36(Pt A):143–151. doi: 10.1016/j.ijsu.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 57.Rekik S, Guyot E, Bhais M, et al. The CRP level and state score predict survival in cirrhotic patients with hepatocellular carcinoma treated by transarterial embolization. Dig Liver Dis. 2016;48(9):1088–1092. doi: 10.1016/j.dld.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Liu C, Jia BS, Zou BW, et al. Neutrophil-to-lymphocyte and aspartate-to-alanine aminotransferase ratios predict hepatocellular carcinoma prognosis after transarterial embolization. Medicine. 2017;96(45):e8512. doi: 10.1097/MD.0000000000008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian XC, Liu XL, Zeng FR, Chen Z, Wu DH. Platelet-to-lymphocyte ratio acts as an independent risk factor for patients with hepatitis B virus-related hepatocellular carcinoma who received transarterial chemoembolization. Eur Rev Med Pharmacol Sci. 2016;20(11):2302–2309. [PubMed] [Google Scholar]