Abstract
Background
C14orf159, a new protein, has been identified recently. But its expression in tissues and clinicopathologic correlation is still unknown.
Patients and methods
We carried out immunohistochemistry staining in 144 gastric cancer cases in this study. Then Western blot was used to detect the expression of protein. MTT and matrigel invasion assay were used to assess the biological effects.
Results
The immunohistochemical results indicated that the expression of C14orf159 in normal gastric mucosa close to cancer tissue was remarkably higher than that in stomach carcinoma samples (63.9% and 34.7%, respectively, P<0.001). Negative C14orf159 expression was dramatically related to high TNM stages (P=0.033) and positive lymph node metastasis (P=0.008). Once C14orf159 was overexpressed, the expression levels of phosphorylated ERK and its regulated downstream molecules, such as Snail, phosphorylated P90RSK and Cyclin D1, were decreased, while the expression level of E-cadherin was increased. Finally, the invasion and proliferation capacity of gastric cancer cells was inhibited.
Conclusion
In other words, loss of C14orf159 is associated with the progression of gastric cancer. The role of C14orf159 in repression of proliferation and invasion may be due to resuming E-cadherin and abolishing Snail and Cyclin D1 expression through inactivating ERK–P90RSK pathway.
Keywords: C14orf159, gastric cancer, ERK, invasion, proliferation
Introduction
Gastric cancer is one of the most common malignant tumors in the world that ranks fourth among men and fifth among women. It is the main reason of carcinoma-related deaths globally.1 It is often found in the late clinical stages and the prognosis is not good.2 The molecular mechanisms indicating occurrence and development of gastric carcinoma have not been elaborated thoroughly so far. Therefore, it is very important to seek for new therapeutic targets for controlling gastric carcinoma progression.
C14orf159 is a newly identified protein, which comprises 661 amino acids. C14orf159 is a D-glutamate cyclase that converts D-glutamate to 5-oxo-D-proline and relates to the chemical reactions and pathways involving glutamate, the anion of 2-aminopentanedioic acid. It is localized in the matrix of mitochondria.3 At present, there is no literature on the expression pattern and clinicopathologic relevance of C14orf159 in human tissues, particularly in malignant tumors.
For the purpose of exploring the effect of C14orf159 on the progression of gastric cancer, we explored the expression of C14orf159 in both gastric carcinoma samples and cell lines and analyzed their clinicopathologic correlation. In addition, we analyzed the influences of C14orf159 on the proliferation and invasiveness of gastric cancer cell lines after C14orf159 knock-in or knock-down. Our findings revealed that C14orf159 might become a new potential therapeutic target of gastric cancer.
Patients and methods
Patients and clinical specimens
Gastric cancer specimens were obtained from 118 males and 26 females (totally 144 patients). The average age of the patients was 60 years (from 32 to 78 years). They were diagnosed with gastric adenocarcinoma and underwent major gastrectomy in the Cancer Hospital of China Medical University from 2012 to 2017. None of them adopted other treatments such as chemotherapy or radiotherapy before surgery. H&E-stained sections were made, and each section was diagnosed by two pathologists with extensive diagnostic experience in line with the WHO classification of digestive system tumors. Lymph node metastasis was found in 43 of the 144 patients. The samples were divided into two groups, stage I (n=62) and stage II–III (n=82), on the basis of the p-TNM staging system of the International Anti-Cancer Alliance (eighth edition). We have obtained written informed consent for this study from patients and ethical approval in accordance with the Declaration of Helsinki from the local trials committee of the Cancer Hospital of China Medical University.
Immunohistochemistry (IHC)
Streptavidin-peroxidase method was manipulated as described previously.4 The slices were incubated overnight at 4°C with a monoclonal mouse anti-C14orf159 antibody (1:100; Sigma-Aldrich Co., St Louis, MO, USA). After that, they were incubated with the secondary antibody at room temperature. To assess the sections, semi-quantitative scoring method was used by two pathologists with extensive diagnostic experience, but they were blinded to the clinical information. Staining intensity and percentage of stained cells were considered as described previously.5 A score ≥4 was regarded as positive C14orf159 expression.
Cell culture
The SGC-7901, BGC-823, MGC80-3 and HGC-27 cell lines were bought from the Chinese Academy of Sciences Cell Bank (Beijing, P.R. China) and were maintained in recommended growth medium.
Western blotting
Total protein was obtained by a RIPA buffer (Beyotime, Shanghai, P.R. China) and quantified with bicinchoninic acid protein assay kit (Solarbio, Shanghai, P.R. China).6 The same quantity of protein (30 µg per lane) was separated by 10% SDS-PAGE. The proteins were incubated with primary antibodies overnight at 4°C against C14orf159 and GAPDH (1:200 and 1:3,000; Sigma-Aldrich Co.), p-ERK, ERK, p-P90RSK, P90RSK, p-P38, P38, p-P65, P65, p-AKT, AKT, Cyclin A2, Cyclin B1, Cyclin D1, Myc-tag, Vimentin, Snail, Slug (1:1,000; BD Transduction Laboratories, Lexington, KY, USA), E-cadherin, N-cadherin, Zo-1 and Occludin (1:1,000; Cell Signaling Technology, Danvers, MA, USA). After incubating with anti-mouse or anti-rabbit IgG (BD Transduction Laboratories) at 37°C for 2 hours, the membranes were developed with enhanced chemiluminescence reagent (Solarbio).
Plasmid transfection and siRNA
Plasmids pCMV6-ddk-myc-C14orf159 (RC223847) and pCMV6-ddk-myc were bought from Origene (Rockville, MD, USA). C14orf159-siRNA (sc-92378) and NC-siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Lipofectamine 3000 reagent (Thermo Fisher Scientific, Waltham, MA, USA) was used to transfect as we described previously.7
MTT assay
To perform the MTT assay, we plated cells of gastric cancer cell lines in 96-well plates. Cell number was about 4,000 cells per well. After 24 hours, transfection was carried out. Cells were stained after 4 days to quantitate cell viability as we performed earlier.7
Matrigel invasion assay
Transwell chamber, 24-well, 8 µm pore membranes (Corning Incorporated, Corning, NY, USA), was used to carry out cell invasion assays as we described previously.8
Statistical analysis
SPSS (v 20.0) was used for statistical analysis. P-value <0.05 was considered statistically significant.
Results
The expression of C14orf159 in gastric cancer tissues and cell lines
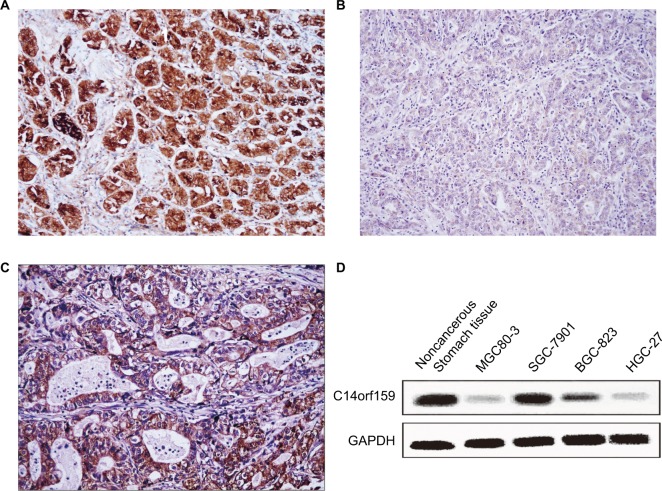
First, IHC was carried out in 144 cases of gastric carcinoma and the corresponding normal tissues to access the expression and subcellular localization of C14orf159. The results indicated that C14orf159 was strongly expressed in the cytoplasm of normal stomach tissue (Figure 1A); however, it was dimly expressed in the cytoplasm of gastric carcinoma specimens (Figure 1B). The positive expression rate of C14orf159 in gastric cancer samples was significantly lower than that in noncancerous samples (34.7%, 50/144 vs 63.9%, 92/144; P<0.001). The positive expression of C14orf159 in gastric cancer tissues is shown in Figure 1C. Subsequent statistical analysis indicated that there was no obvious relation between C14orf159 and sex, age and histological grade (P>0.05). But the expression level of C14orf159 was remarkably negatively correlated to high TNM stage (P=0.033) and positive lymph node metastasis (P=0.008; Table 1).
Figure 1.
The expression of C14orf159 in both gastric cancer specimens and cell lines.
Notes: C14orf159 revealed strong cytosolic expression in noncancerous stomach tissues (A, 200×); however, it showed dim expression in the cytoplasm of gastric carcinoma specimens (B, 200×). In some gastric cancer tissues, it was positively expressed (C, 200×). (D) C14orf159 was expressed in noncancerous stomach tissue and all four gastric cancer cells (MGC80-3, SGC-7901, BGC-823 and HGC-27), highly expressed in 7901 cells and less expressed in 803 cells. Data are mean ± SEM from three independent experiments.
Abbreviation: SEM, standard error of the mean.
Table 1.
Associations between C14orf159 expression and clinicopathologic features in 144 cases of gastric cancer
| Patient characteristics (N=144) | C14orf159 | P-value | |
|---|---|---|---|
| Negative | Positive | ||
| Age (years) | 0.861 | ||
| <60 | 41 | 23 | |
| ≥60 | 53 | 27 | |
| Sex | 0.656 | ||
| Male | 78 | 40 | |
| Female | 16 | 10 | |
| TNM stage | 0.033 | ||
| I | 34 | 28 | |
| II+III | 60 | 22 | |
| Lymph node metastasis | 0.008 | ||
| No | 59 | 42 | |
| Yes | 35 | 8 | |
| Histology grade | 0.484 | ||
| G1+G2 | 42 | 26 | |
| G3 | 52 | 24 |
Note: Bold indicates P<0.05.
Western blotting assay was carried out in noncancerous stomach tissue and four kinds of gastric carcinoma cell lines to examine the expression of C14orf159. The results suggested that the expression level of C14orf159 was also low in all the cell lines (Figure 1D).
C14orf159 suppressed gastric cancer cells’ invasion
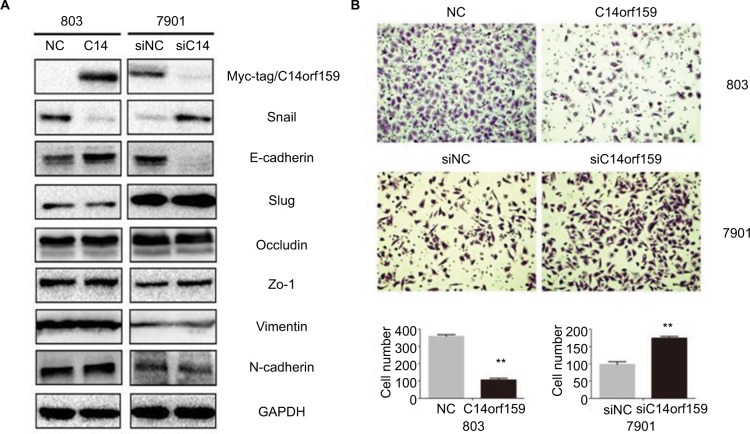
Then, we overexpressed C14orf159 in 803 cells and inhibited C14orf159 with siRNA in 7901 cells. The transfection efficiency is shown in Figure 2A. We then detected the epithelial–mesenchymal transition–related proteins and found that Snail expression level was downregulated; at the same time, E-cadherin expression level was upregulated after transfecting C14orf159 plasmid (Figure 2A). Accordingly, Snail was increased while E-cadherin was decreased following C14orf159 depletion (Figure 2A). But no significant changes were found in the expression of Slug, Occludin, Zo-1, Vimentin and N-cadherin (Figure 2A). Subsequent Transwell assay results indicated that the invasive abilities of gastric cancer cells were suppressed or enhanced following C14orf159 overexpression or inhibition, respectively (Figure 2B).
Figure 2.
Overexpression of C14orf159 inhibited gastric cancer cells’ invasion.
Notes: (A) Western blotting results suggested that the expression of Snail was downregulated and that of E-cadherin was upregulated after C14orf159 was overexpressed in 803 cells. Accordingly, the expression of Snail was increased and that of E-cadherin was decreased following silencing of C14orf159 in 7901 cells. However, the other proteins revealed no visible alteration. (B) Transwell assay results indicated the invasive abilities of gastric cancer cells were decreased or elevated after C14orf159 transfection and inhibition. Data are mean ± SEM from three independent experiments, each performed in triplicate. **P<0.01.
Abbreviations: NC, negative control; SEM, standard error of the mean.
C14orf159 decreased gastric cancer cells’ proliferation abilities
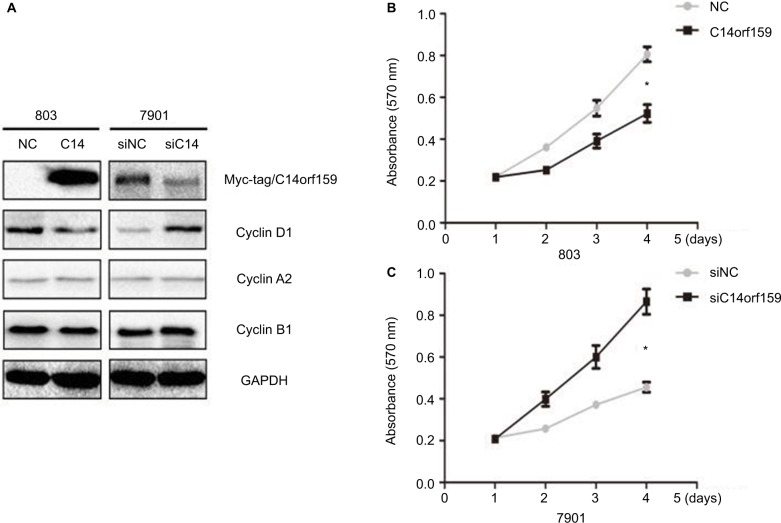
Western blotting and MTT assays were performed to detect the impact of C14orf159 on gastric carcinoma cell proliferation. Western blotting analysis showed that the expression level of Cyclin D1 was decreased after overexpressing C14orf159 in 803 cells and increased after depleting C14orf159 in 7901 cells (Figure 3A). The MTT assay results also indicated that overexpression of C14orf159 abolished gastric cancer cells’ proliferation and depletion of C14orf159 accelerated the proliferation of gastric cancer cells (Figure 3B).
Figure 3.
Transfection of C14orf159 abolished gastric cancer cells’ proliferation.
Notes: (A) The expression level of Cyclin D1 was downregulated or upregulated following C14orf159 overexpression and depletion, respectively. But there was no change in the expression of other proteins. (B) Subsequent MTT assays results revealed that the proliferation of 803 or 7901 cells was decreased or enhanced after overexpressing or silencing C14orf159, respectively. Data are mean ± SEM from three independent experiments, each performed in triplicate. *P<0.05.
Abbreviations: NC, negative control; SEM, standard error of the mean.
C14orf159 abrogated ERK-P90RSK signaling
Finally, we screened pivotal signaling pathway effector that participated in influencing gastric cancer invasion and proliferation after modulating C14orf159 expression. Western blotting results revealed that the expression levels of phosphorylated ERK and its downstream factor P90RSK were downregulated by transfecting C14orf159 in 803 cells. Correspondingly, phosphorylated ERK and P90RSK were elevated following interference of C14orf159 with siRNA in 7901 cells (Figure 4).
Figure 4.
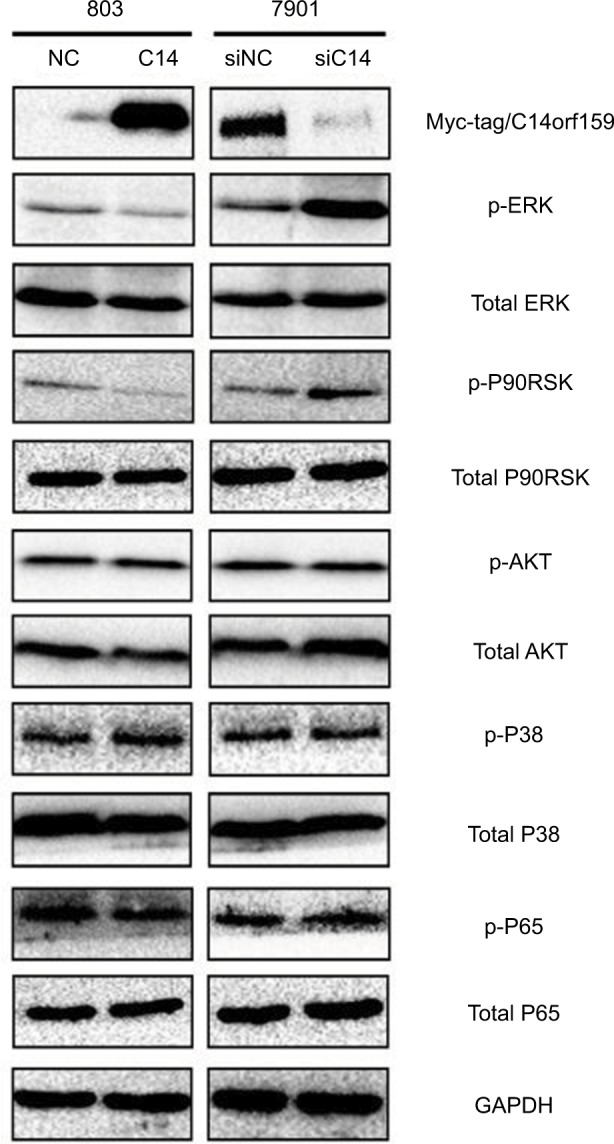
C14orf159 abrogated the phosphorylation of ERK and P90RSK.
Notes: Expression level of phosphorylated ERK and its downstream factor P90RSK was decreased on overexpression of C14orf159 in 803 cells and was correspondingly elevated following depletion of C14orf159 with siRNA in 7901 cells. Data are mean ± SEM from three independent experiments.
Abbreviations: NC, negative control; SEM, standard error of the mean.
Discussion
C14orf159 is a recently discovered protein. Its expression site in cells, clinicopathologic correlation and its biological functions are not fully understood. Our study revealed that C14orf159 showed dim cytosolic expression in gastric carcinoma; its expression was negatively related to positive lymph node metastasis and high TNM stage. These results indicated that C14orf159 may serve as a tumor suppressor. Our study could not achieve a progression-free survival analysis because the information about the postoperative follow-up and treatment after surgery was restricted. The prognostic value and significance of C14orf159 remain to be further studied.
We also found that overexpression of C14orf159 decreased the levels of Snail and Cyclin D1 and simultaneously increased the level of E-cadherin, thus abolishing gastric cancer cells’ invasion and proliferation. Subsequently, we screened critical signaling pathway molecules that participated in modulating Snail, E-cadherin and Cyclin D1. As reported, several genes including ERK, AKT, P38, NF-κB9–14 may be related to the regulation of the above three molecules. Our study showed that overexpression of C14orf159 could inhibit the phosphorylation of ERK and its direct downstream factor P90RSK. These results were similar to those of previous studies,5,15 which suggested that C14orf159 may abrogate gastric cancer cells’ invasion and proliferation by inactivating ERK signaling. A previous study showed that ERK inhibitor U0126 could inhibit the levels of p-ERK and Snail and then restore E-cadherin expression. Correspondingly, the increased cell invasion and migration were also reversed by U0126.16 Moreover, elevated E-cadherin significantly suppressed the cell proliferation and migration and E-cadherin was inhibited by activating ERK/Snail signaling.17 To some extent, we inferred that C14orf159 repressed the proliferation and invasion via inactivating the ERK–P90RSK pathway, which needs to be validated in future studies.
How does C14orf159 abolish the phosphorylation of ERK? Floyd et al demonstrated that C14orf159 may interact with PAK7.18 As we know, PAKs is identified as the classical upstream of MEK/ERK signaling.19–21 We suspect the interplay between C14orf159 and PAK7 may interfere with MEK/ERK signaling transduction, thus suppressing the invasion and proliferation of gastric cancer cells. This hypothesis is subject to further confirmation. In addition, there is a limitation in our study. The results based on the cell lines need to be validated in vivo.
In summary, C14orf159 was expressed in the cytoplasm and its expression was lower in gastric cancer tissues than in normal gastric mucosa tissues. Negative C14orf159 expression was significantly related to positive lymph node metastasis and advanced TNM stage. By inactivating the ERK–P90RSK signaling pathway, C14orf159 inhibited Snail and Cyclin D1 and promoted E-cadherin, further decreasing the invasion and proliferation of gastric cancer cells.
Our findings indicate that C14orf159 makes an important impact on the evolution of gastric cancer and it may represent a novel target for therapeutic management of gastric cancer patients.
Acknowledgments
The current study was supported by a grant from the Key Project of Natural Science Fund Program of Liaoning Province, P.R. China (grant number 20170520046).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lin HN, Gu XY, Zhang SW, et al. Analysis on incidence and mean age at diagnosis for Global Cancer. Zhonghua Zhong Liu Za Zhi. 2018;40(7):543–549. doi: 10.3760/cma.j.issn.0253-3766.2018.07.012. Chinese. [DOI] [PubMed] [Google Scholar]
- 2.Beeharry MK, Liu WT, Yan M, et al. New blood markers detection technology: a leap in the diagnosis of gastric cancer. World J Gastroenterol. 2016;22(3):1202–1212. doi: 10.3748/wjg.v22.i3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariyoshi M, Katane M, Hamase K, et al. D-Glutamate is metabolized in the heart mitochondria. Sci Rep. 2017;7:43911. doi: 10.1038/srep43911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Zhu Y, Liu J, et al. HpSlyD inducing CDX2 and VIL1 expression mediated through TCTP protein may contribute to intestinal metaplasia in the stomach. Sci Rep. 2017;7(1):2278. doi: 10.1038/s41598-017-02642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng R, Liu Q, Wang T, et al. FAM98A promotes proliferation of non-small cell lung cancer cells via the P38-ATF2 signaling pathway. Cancer Manag Res. 2018;10:2269–2278. doi: 10.2147/CMAR.S163323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Gong Y, Li A, et al. Differential proteomic analysis reveals protein networks and pathways that may contribute to Helicobacter pylori FKBP-type PPIase-associated gastric diseases. Proteomics Clin Appl. 2018;12(3):e1700127. doi: 10.1002/prca.201700127. [DOI] [PubMed] [Google Scholar]
- 7.Jiang G, Zhang Y, Zhang X, et al. ARMc8 indicates aggressive colon cancers and promotes invasiveness and migration of colon cancer cells. Tumour Biol. 2015;36(11):9005–9013. doi: 10.1007/s13277-015-3664-z. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Chen M, Gong Y, et al. Helicobacter pylori FKBP-type PPIase promotes gastric epithelial cell proliferation and anchorage-independent growth through activation of ERK-mediated mitogenic signaling path. FEMS Microbiol Lett. 2015;362(7):1–9. doi: 10.1093/femsle/fnv023. [DOI] [PubMed] [Google Scholar]
- 9.Shen T, Yang Z, Cheng X, et al. CXCL8 induces epithelial–mesenchymal transition in colon cancer cells via the PI3K/Akt/NF-κB signaling pathway. Oncol Rep. 2017;37(4):2095–2100. doi: 10.3892/or.2017.5453. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Ou B, Han D, et al. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer. 2017;16(1):70. doi: 10.1186/s12943-017-0629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Sun JD, Yan LJ, et al. PDGF-D/PDGFRβ promotes tongue squamous carcinoma cell (TSCC) progression via activating p38/AKT/ERK/EMT signal pathway. Biochem Biophys Res Commun. 2016;478(2):845–851. doi: 10.1016/j.bbrc.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Fang R, Wang XF, et al. Stabilization of Snail through AKT/GSK-3β signaling pathway is required for TNF-α-induced epithelial– mesenchymal transition in prostate cancer PC3 cells. Eur J Pharmacol. 2013;714(1–3):48–55. doi: 10.1016/j.ejphar.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Zhou Y, Zheng X, et al. SPHK1 promotes breast epithelial cell proliferation via NF-κB-p65-mediated cyclin D1 expression. Oncotarget. 2016;7(49):80579–80585. doi: 10.18632/oncotarget.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen H, Ma JL, Zhang Y, et al. Integrin-linked kinase overexpression promotes epithelial–mesenchymal transition via nuclear factor-κB signaling in colorectal cancer cells. World J Gastroenterol. 2016;22(15):3969–3977. doi: 10.3748/wjg.v22.i15.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai S, Zhang J, Huang S, et al. HnRNPA2B1 regulates the epithelial– mesenchymal transition in pancreatic cancer cells through the ERK/snail signalling pathway. Cancer Cell Int. 2017;17(1):12. doi: 10.1186/s12935-016-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B, Xing X, Li X, et al. ZNF259 promotes breast cancer cells invasion and migration via ERK/GSK3β/snail signaling. Cancer Manag Res. 2018;10:3159–168. doi: 10.2147/CMAR.S174745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Cheng Y, Zheng Y, et al. PRKAR1A is a functional tumor suppressor inhibiting ERK/Snail/E-cadherin pathway in lung adenocarcinoma. Sci Rep. 2016;6(1):39630. doi: 10.1038/srep39630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floyd BJ, Wilkerson EM, Veling MT, et al. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function. Mol Cell. 2016;63(4):621–632. doi: 10.1016/j.molcel.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenova G, Stepanova DS, Dubyk C, et al. Targeting group I p21-activated kinases to control malignant peripheral nerve sheath tumor growth and metastasis. Oncogene. 2017;36(38):5421–5431. doi: 10.1038/onc.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steelman LS, Franklin RA, Abrams SL, et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia. 2011;25(7):1080–1094. doi: 10.1038/leu.2011.66. [DOI] [PubMed] [Google Scholar]
- 21.Pandey A, Dan I, Kristiansen TZ, et al. Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene. 2002;21(24):3939–3948. doi: 10.1038/sj.onc.1205478. [DOI] [PubMed] [Google Scholar]