Abstract
Background
Alzheimer disease (AD) is the main cause of dementia in elderly people. The potential of histamine H3 receptor (H3R) antagonists as a pharmacological treatment of several neuropsychiatric diseases is well established.
Methods
The novel non-imidazole-based H3R antagonist E177 was screened for its pro-cognitive effects on the inhibitory avoidance paradigm (IAP) and novel object recognition (NOR) task in a dizocilpine (DIZ)-induced model of amnesia in male Wistar rats. Donepezil, an acetylcholine esterase inhibitor, was used as the reference drug.
Results
Acute systemic treatment with E177 (1.25, 2.5, 5, and 10 mg/kg intraperitoneally [i.p.]) significantly attenuated the cognitive impairments induced by DIZ in the IAP (all P-values <0.05, n=7), and the protective effect of the most promising dose of E177 (5 mg/kg) was abrogated when H3R agonist R-(α)-methylhistamine (RAMH; 10 mg/kg i.p.) was co-administered (P=0.281 for DIZ-amnesia group vs DIZ + E177 + RAMH group, n=7). The discrimination index calculated for E177 (5 mg/kg, i.p.) showed a significant memory-enhancing effect on DIZ-induced short-term memory impairment in the NOR task (P<0.05, n=6), with the enhancement nullified when animals were co-administered RAMH (10 mg/kg). Moreover, the results revealed that E177 (5 and 10 mg/kg, i.p.) did not alter the anxiety levels and locomotor activity of animals naïve to the open-field test (all P-values >0.05, n=8) or the elevated plus maze test (all P-values >0.05, n=6–8), which indicated that the E177-induced enhancement of memory performance in the IAP or NOR task was unrelated to changes in emotional response or in spontaneous locomotor activity.
Conclusion
The observed results suggested a possible contribution of H3Rs in the alteration of brain neurotransmitters that accompany neurodegenerative diseases, such as AD.
Keywords: Histamine H3 receptors, antagonist, dizocilpine-induced amnesia, inhibitory avoidance paradigm, novel object recognition, elevated plus maze, open field test, memory, anxiety
Introduction
Dementia is one of the most severe manifestations of cognitive impairment associated with elderly people and affectŝ50 million patients worldwide.1 The neurodegeneration caused by Alzheimer disease (AD) is the most common cause of dementia,2,3 and AD is usually considered to be one of the most serious progressive lifelong neurodegenerative brain diseases, which is characterized by memory insufficiency, confusion, and other behavioral deficits.4,5 The number of people diagnosed with AD is predicted to increase from 36 million tô100 million in 40 years.5 The pathophysiology of AD is complicated and has not been completely established, despite the existence of numerous hypotheses. AD is characterized by complex pathophysiological modifications, including insufficient or disturbed neurotransmitters such as the cholinergic, dopaminergic, adrenergic, glutamatergic, and serotonergic neurotransmitters.6 In addition, extracellular β-amyloid plaques, neurofibrillary tangles in the brain, and the involvement of oxidative stress in the development of AD have been well documented.6 In numerous studies and considering the clinically used drugs, the most tested therapeutic targets include the following: inhibition of acetylcholinesterase, antioxidant activity, inhibition of β-amyloid plaque aggregation, monoamine oxidase enzyme inhibition, and N-methyl-d-aspartic acid (NMDA) receptor antagonism.7–9 However, despite progress in current therapeutic modalities, there are 112 unique agents in the current AD treatment pipeline: 26 agents in 35 Phase III trials, 63 agents in 75 Phase II trials, and 23 agents in 25 Phase I trials, indicating that the development of novel agents with multiple pharmacological effects is a promising strategy in the current search for novel treatment options for multifactorial diseases such as AD.10–12 Histamine is a neurotransmitter of the central nervous system (CNS),13,14 and it exerts its physiological actions through interaction with four different G-protein-coupled histamine receptors (H1–H4R).15–17 H1R and H2R are extensively distributed in the CNS, gastrointestinal, reproductive, respiratory, and cardiovascular systems, whereas H4R is generally limited to immune cells.18 In contrast, histamine H3 receptors (H3Rs) are coupled to Gαi/o-proteins and are mainly expressed in the CNS presynaptically, where they function as inhibitory auto- and hetero-receptors16,17,19–25 that modulate the production and release of histamine and other neurotransmitters such as dopamine, serotonin, acetylcholine, norepinephrine, and glutamate.19–23,26 Preclinical trials have indicated the promising memory-enhancing effects of several H3R antagonists.16,17,25,27,28 Subsequently, various H3R antagonists, such as ABT-239 and A-431404, were shown to attenuate memory impairments induced by dizocilpine (DIZ) or ketamine in different animal species, and their procognitive effect was comparable with standard drugs, such as donepezil (DOZ),29 which was suggestive of the potential use for H3R antagonists in the treatment of neurodegenerative diseases such as AD.17,30–33 Consequently, H3Rs appear to be a very attractive target for the design of new H3R antagonists suitable for the treatment of many neuropsychiatric diseases, including epilepsy, schizophrenia, and AD.17,27,31,34–42 Targeting H3Rs is not commonly employed for the treatment of AD, despite the previous indications about the memory-enhancing effects in neurodegenerative disorders. Hence, the protective effect of the non-imidazole-based H3R antagonist E177, 1-(6-(naphthalen-2-yloxy)hexyl)azepane hydrogen oxalate, with high antagonist affinity (Ki=69.40 nM) and high in vitro selectivity,43 was tested in DIZ-induced memory impairments in Wistar rats subjected to the inhibitory avoidance paradigm (IAP) and novel object recognition (NOR) task (Figure 1). In addition, anxiety-like behaviors and locomotor activity of E177 on the test animals were assessed by using the elevated plus maze (EPM) and open-field test (OFT), as modulation of these parameters might confound the observations of the cognitive performances of the test rats. Moreover, abrogation experiments were conducted using the CNS-penetrant H3R agonist R-(α)-methyl-histamine dihydrochloride (RAMH) to investigate if the procognitive effects of E177 were related to central H3R antagonism.
Figure 1.
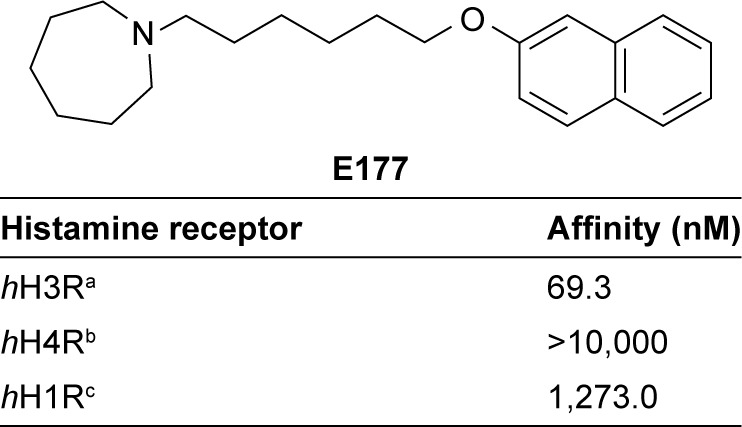
Structure and in vitro affinities of the H3R antagonist E177, 1-(6- (naphthalen-2-yloxy)hexyl)azepane, on histamine receptor subtypes.
Notes: a[3H]Nα-methylhistamine binding assay was performed using a cell membrane preparation of HEK-hH3 cells stably expressing the human H3R (n=3). b[3H]Histamine binding assay was performed using a cell membrane preparation of Sf9 cells transiently expressing the human histamine H4R and co-expressed with Gαi2 and β1γ2 subunits (n=2). c[3H]pyrilamine binding assay was performed with the cell membrane preparation of CHO-hH1R cells stably expressing the human H1R (n=2).
Materials and methods
Animals
Inbred male Wistar rats used in the current study were obtained from the central animal facility in UAE University. The animals were kept in a specific room with controlled temperature and humidity (24°C±2°C and 55%±15%, respectively), 12/12 hour light/dark sequence, and free access to food and water. All the rats were 6–8 weeks of age and weighed 180–220 g. The behavioral experiments were performed each day between 09:00 am and 01:00 pm by the same investigator in a blinded manner; all procedures were performed in accordance with the guidelines of the European Communities Council Directive of 24 November 1986 (86/609/EEC) and approval was obtained from the Institutional Animal Ethics Committee in College of Medicine and Health Sciences/United Arab Emirates University (A30-13) before the experiments were started. All efforts were made to minimize the total number of the animals used and their pain.
Drugs
E177 was synthesized by the Department of Technology and Biotechnology of Drugs (Jagiellonian University Medical College, Kraków, Poland), as previously described.43 Diazepam (DZP) was produced by Gulf Pharmaceutical Industries (Ras Al Khaimah, UAE) and was acquired from Dr Ameen Al Amaydah (Department of Emergency Medicine, Emirates International Hospital, Al Ain, UAE). The histamine H3R agonist RAMH, DIZ, and DOZ were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). All compounds were dissolved in isotonic saline and administered intraperitoneally (i.p.) at a volume of 1 mL/kg. All doses of test compounds are expressed in terms of the free base.
In vivo behavioral tests
IAP test
The IAP was examined by using inhibitory avoidance apparatus (Step-through Cage, 7550; Ugo Basile, Comerio, Italy), as described previously.11,12,44,45 The experiments were conducted in accordance with the previously published protocols.11,12,33,45–51 The test was conducted over 3 days (two consecutive training days, with the test performed on the third day). The animal was placed in the white compartment in front of the door for a 30-second habituation period at the beginning of the first training day, and then the door was routinely raised up. Once the animal passed with head and all four paws into the black compartment, a foot shock (0.4 mA, 20 Hz, 8.3 ms) was applied for a duration of 3 seconds. The step-through latency (STL) time was calculated (the cut-off time was set at 60 seconds) and the animals that did not enter the dark compartment within 60 seconds were excluded from the experiment. The intensity of the foot shock was chosen to minimize pain and reactions, such as jumping and screaming, in the examined animals. Directly after the foot shock was delivered, the respective animal was returned to its home cage and both compartments were cleaned to eliminate any olfactory signs. On the second training day (24 hours later), the experimental steps were repeated, except that the foot shock was not delivered and the cut-off latency time was increased to 300 seconds. On both training days, the rats received an injection of saline 30–45 minutes before starting the experiment, whereas on the test day (24 hours later), animals were injected with the test compound DIZ (0.1 mg/kg, i.p.) 30–45 minutes before the test. STL time was calculated on the test day with a cut-off time of 300 seconds, and no foot shock was delivered.
Treatment groups
Ten groups were used in this experiment; each group included six to eight animals matched for age and weight. All groups were treated with i.p. injections 30–45 minutes before the test. The procognitive effect of different doses of the test compound E177 was examined through the measurement of STL times for each animal in all groups. The rats were divided into the following treatment groups: Group 1, in which saline was co-injected with saline; Group 2, saline co-injected with DIZ (0.1 mg/kg); groups 3–6, DIZ co-injected with E177 (1.25, 2.5, 5, or 10 mg/kg, respectively); Group 7, DIZ co-injected with DOZ (1 mg/kg i.p.); Group 8, DIZ co-injected with the most promising dose of E177 (5 mg/kg) and the H3R agonist RAMH (10 mg/kg) for the abrogation study; Group 9, DIZ co-injected with RAMH (10 mg/kg); and Group 10, saline co-injected with RAHM (10 mg/kg). Doses of all tested compounds were selected based on previously conducted experiments for several non-imidazole-based H3R antagonists tested in adult male rats (Figures 2 and 3).33,52,53
Figure 2.
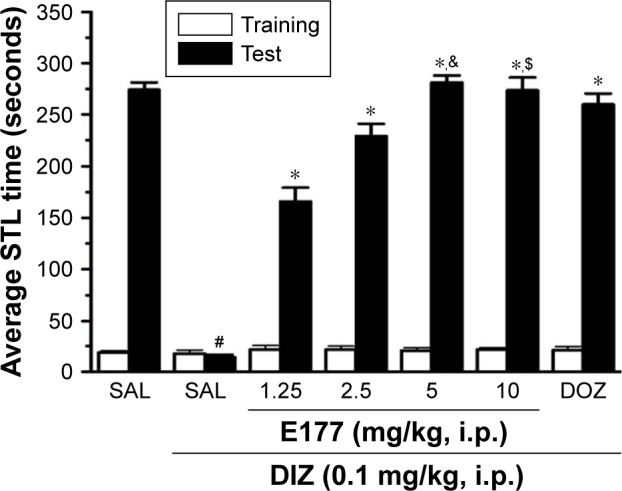
E177 attenuated DIZ-induced memory deficits in the IAP.
Notes: Average STL time measured on the first training day before the delivery of foot shock (white columns), and average STL time measured on the test day (black columns). Acute systemic administration of E177 at different doses (1.25, 2.5, 5, and 10 mg/kg i.p.) or DOZ (1 mg/kg i.p.) 30–45 minutes before the test session. #P<0.0001 for average STL time compared with that in the saline-treated group. *P<0.0001 for average STLs compared with the DIZ-treated group. &P<0.0001 compared with the DOZ-treated group. $P<0.05 compared with the DOZ-treated group. The data are expressed as the mean ± SEM (n=7).
Abbreviations: DIZ, dizocilpine; DOZ, donepezil; IAP, inhibitory avoidance paradigm; i.p., intraperitoneally; SAL, saline; SEM, standard error of the mean; STL, step-through latency.
Figure 3.
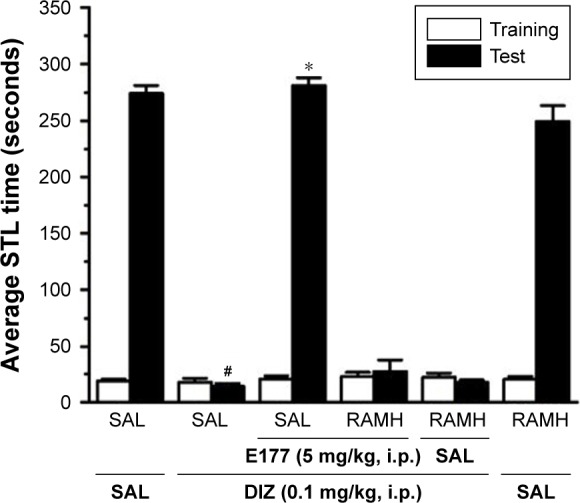
RAMH reversed E177-induced memory enhancement of the DIZ-induced deficit in the IAP.
Notes: Average of STL time measured on the first training day before the delivery of foot shock (white columns), and average of the STL time measured on the test day (black columns). Acute systemic administration of E177 (5 mg/kg, i.p.), RAMH, and E177 (5 mg/kg) + RAMH (10 mg/kg) for 15–20 minutes (for RAMH) or 30–45 minutes (for H3R antagonist E177) before the test session. #P<0.001 average STLs compared with the saline-treated group. *P<0.001 for the average STL time compared with that in the DIZ-treated group. The data are expressed as the mean ± SEM (n=7).
Abbreviations: DIZ, dizocilpine; IAP, inhibitory avoidance paradigm; i.p., intraperitoneally; RAMH, R-(α)-methyl-histamine dihydrochloride; SAL, saline; SEM, standard error of the mean; STL, step-through latency.
NOR task
The NOR task was used to assess both the short-term memory (STM) and long-term memory (LTM) recognition in male Wistar rats by using an open square black box (50×35×50 cm), as described previously.33,45 The NOR task was conducted in the morning between 8:00 am and 12:00 pm. On the first day, two habituation sessions, separated by 1 hour, were conducted; each animal was allowed to habituate the box for 3 minutes and was then returned to its home cage and the box was cleaned. On the second day, the trial consisted of training session T1 and test session T2, which were separated by 120 minutes for the STM assessment and by 24 hours for the LTM assessment. In T1, the animals were introduced to two identical wooden objects (9×5×9 cm) placed in opposite corners of the box (30 cm apart) for 3 minutes and the time the animal spent exploring each object was calculated (exploration of an object was defined as touching or snuffing the object; other behavioral observations, such as turning around or sitting on the object, were not considered an experimental behavior). The animals which explored the objects for <10 seconds (one to three animals out of the nine animals per group) were excluded from the experiment. In T2, an identical familiar object to eliminate any olfactory clues and a novel object were introduced to the animal for a 3-minute session and the time the animal spent exploring the familiar and novel objects was measured. All objects and the test arena were cleaned by using 70% ethanol after each session. To assess the procognitive effect of our test compound E177, DIZ was used to induce amnesia. DIZ and E177 (different doses) were dissolved in saline and injected i.p. after T1; E177 was injected i.p. 30–45 minutes before T2 in the LTM assessment only, and the control groups were injected with an equal volume of saline. Doses and treatment times were chosen in accordance with previously published studies that have reported the memory-enhancing effects of H3R antagonists in the NOR task.33,45
Treatment groups
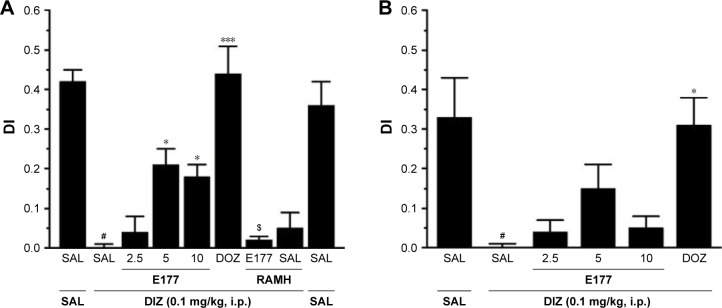
Nine groups, comprising six to eight animals matched for weight and age, were used in this experiment. The rats were divided into the following treatment groups: Group 1, in which saline was co-injected with saline; Group 2, saline co-injected with DIZ (0.1 mg/kg); groups 3–5, DIZ co-injected with E177 (2.5, 5, or 10 mg/kg); Group 6, DIZ co-injected with DOZ (1 mg/kg); Group 7, DIZ co-injected with E177 (5 mg/kg) and H3R agonist RAMH (10 mg/kg) for the abrogation study; Group 8, saline co-injected with RAMH (10 mg/kg); and Group 9, DIZ co-injected with RAMH (10 mg/kg). The systemic administration of E177 and RAMH was repeated 30–45 minutes before T2 for the LTM assessment. The discrimination index (DI) was calculated from the variables N–F/N+F (where N is the time the rat spent exploring the novel object and F is the time the rat spent exploring the familiar object in T2), as shown in Figure 4A and B.
Figure 4.
E177 mitigated DIZ-induced short-term deficits in NOR paradigm.
Notes: E177 (2.5, 5, or 10 mg/kg, i.p.) or DOZ (1 mg/kg, i.p.) was injected after T1 (training session) with DIZ (0.1 mg/kg, i.p.). The T2 (test session) was conducted 120 minutes (STM, A) or 24 hours (LTM, B) after T1 (training session). The results are expressed as DIs for the time spent exploring both objects (familiar and novel). #P<0.0001 compared with the SAL-treated group. *P<0.05 compared with the DIZ-treated group. ***P<0.0001 compared with the DIZ-treated group. $P<0.05 compared with the E177 (5 mg)-treated group. The data are expressed as the mean ± SEM (n=6).
Abbreviations: DIs, discrimination indices; DIZ, dizocilpine; DOZ, donepezil; i.p., intraperitoneally; LTM, long-term memory; NOR, novel object recognition; RAMH, R-(α)-methyl-histamine dihydrochloride; SAL, saline; SEM, standard error of the mean; STM, short-term memory.
EPM test
To estimate the anxiety-like behaviors in male Wistar rats, the EPM test was used as previously described.45,54 The EPM apparatus consisted of a central part (8×8 cm), two open arms (30×8 cm), and two closed arms (30×8 cm) bordered by non-transparent walls (height: 30 cm) and illuminated with four 60 V light-emitting diodes (LEDs). The test was conducted in the morning, between 08:00 am and 12:00 pm; each animal was placed in the center of the maze (50 cm above the floor), facing one of the open arms for a duration of 5 minutes, and the number of entries to both open and closed arms (when the rat entered with head and forepaws) as well as the time spent in the open and closed arms were measured manually. The arms and walls of the apparatus were cleaned with 70% ethanol between each animal’s use of the apparatus to remove any confounding olfactory cues. The total number of closed arm entries was considered to be an indication of locomotor activity.
Treatment groups
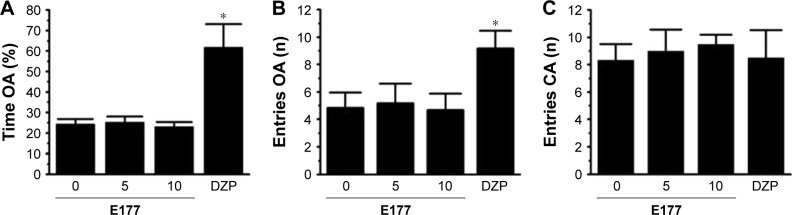
Four groups, comprising six to eight animals matched for weight and age, were used in this experiment. All the animals received i.p. injections 30–45 minutes before the test to assess the alternation effects of E177 on anxiety-like parameters and locomotor activity (Figure 5). The animals were divided into the following treatment groups: Group 1, in which saline was co-injected with saline; groups 2 and 3: E177 (5 or 10 mg/kg, respectively); and Group 4, DZP (10 mg/kg).
Figure 5.
E177 failed to alter anxiety-like behavior or locomotion in the EPM test.
Notes: Effect of E177 (5 or 10 mg/kg, i.p.) and DZP (10 mg/kg, i.p.) injections on the percentage of time spent in open arms (A), total number of open arm entries (B), and total number of closed arms entries (C). *P<0.05 for the value of the DZP-treated group compared with the saline-, E177 (5 mg)-, or E177 (10 mg)-treated groups. The data are expressed as the mean ± SEM (n=6).
Abbreviations: DZP, diazepam; EPM, elevated plus maze; i.p., intraperitoneally; SAL, saline; SEM, standard error of the mean; OA, open arms; CA, closed arms.
Open-field test
The locomotor activity was assessed by using a home-made OFT, as previously described.11,12,44 The test arena (floor, 56×56 cm of black polyvinyl chloride; walls, 56 cm high of black Formica) was open to above and illuminated with four 60 V LEDs. The floor of the box was divided into 32 equal squares, each 14 cm in diameter. The four squares in the middle were considered to be the “center” of the field. On the test day, rats were allowed to move freely in the box for 30 minutes (habituation time) and were then subsequently returned to their home cages, and the box was cleaned. Thirty minutes later, the rats received i.p. injections of the test compound, 30–45 minutes before the test was conducted. For the test session, the animals were transferred to the arena only by their tails and placed in the center where they were allowed to move freely for 3 minutes. During this time, animal activity was recorded by a digital camera placed above the arena, and video recordings were later utilized for assessment. As illustrated above, different parameters were measured, such as the total distance traveled, the time spent in the central arena as well as in the periphery, and the number of grooming incidents during the 3-minute test session, through analysis of the video recordings in accordance with previously published methods.32,44 When evaluating the results, longer time spent in the center indicated lower levels of anxiety-like behaviors (Table 2).
Table 2.
Effects of acute systemic administration of E177 on anxiety levels and locomotor activity in the OFT
| Groups | Time for locomotion (seconds) | Distance traveled (cm) | Time in center (seconds) | Time in periphery (seconds) | No. of rearingevents | No. of grooming events |
|---|---|---|---|---|---|---|
| Saline | 99.67±6.47 | 1,003.33±51.34 | 1.67±0.19 | 78.67±6.40 | 18.17±2.41 | 2.00±0.58 |
| E177 (5 mg/kg) | 98.33±4.78 | 1,085.67±33.95 | 1.83±0.15 | 76.67±3.93 | 16.17±1.79 | 1.50±0.39 |
| E177 (10 mg/kg) | 100.86±8.16 | 999.86±52.44 | 1.71±0.26 | 77.14±7.87 | 15.14±2.54 | 1.86±0.31 |
| DOZ (1 mg/kg) | 109.83±5.31 | 1,022.67±44.65 | 1.50±0.20 | 71.33±6.63 | 16.67±2.55 | 0.67±0.45 |
Notes: E177 (5 and 10 mg/kg, i.p.) failed to alter time spent in the center arena, time spent in the periphery, total time spent for locomotion, total distance traveled, occurrence of rearing, and incidence of grooming. The data are expressed as the mean ± SEM (n=6–7).
Abbreviations: DOZ, donepezil; i.p., intraperitoneally; OFT, open-field test; SEM, standard error of the mean.
Treatment groups
Four groups, comprising six to eight animals matched for weight and age, were used in this experiment. All the animals received i.p. injection 30–45 minutes before the test to assess the effects of E177 on anxiety-like parameters and locomotor activity. The animals were divided into the following treatment groups: Group 1, in which saline was co-injected with saline; groups 2 and 3, E177 (5 and 10 mg/kg, respectively); and Group 4, DOZ (1 mg/kg).
Statistical analyses
The data were analyzed for normality through assessment of the sample distribution or skewness (−1.3 to +1.3 was considered to be normally distributed) and the homogeneity of variance by using Levene’s test. After the results had passed the tests for normality, they were analyzed where applicable using one- or two-way ANOVA followed by Tukey’s post hoc test. For statistical comparisons, the software package SPSS 25.0 (IBM Middle East, Dubai, UAE) was used. The results are expressed as the mean and standard error of the mean (SEM). P-values <0.05 were considered to indicate statistical significance.
Results
E177 and DOZ attenuated the DIZ-induced memory impairments in the IAP
The procognitive effects of E177 (1.25, 2.5, 5, and 10 mg/kg) and the standard drug DOZ (1 mg/kg) on DIZ-induced memory deficits in IAP in adult male rats are shown in Figure 2. Following statistical analysis of STL times, acute systemic administration of E177 and DOZ (1 mg/kg) 30–45 minutes before the test conferred significant memory-enhancing effects (F(4,49)=66.81, P<0.0001; Figure 2). Post hoc analyses by the Tukey’s test revealed that DIZ (0.1 mg/kg) treatment significantly reduced STL time compared with the saline-treated group (mean difference =260.14, P<0.0001). Moreover, all four doses of E177 (1.25, 2.5, 5, and 10 mg/kg) resulted in a significant enhancement of STL times compared with the DIZ-induced amnesia group (all P-values <0.0001). Furthermore, E177 (1.25 and 2.5 mg/kg) exhibited procognitive effects on STL time, which were comparable with the reference drug DOZ (1 mg/kg), with P-values of 1.00 and 0.05, respectively (Figure 2). However, higher doses of E177 (5 and 10 mg/kg) provided significantly stronger memory-enhancing effects than DOZ (1 mg/kg), with P-values of <0.0001 and <0.05, respectively (Figure 2). Notably, no significant increase was observed between 5 and 10 mg/kg E177 (P=0.94; Figure 2).
H3R agonist RAMH reversed memory improvement provided by E177 in rats with DIZ-induced impairments in the IAP
In this experiment, animals were co-injected with the most promising dose of E177 (5 mg/kg) 30–45 minutes before the test and with RAMH (10 mg/kg) 20 minutes before the test (Figure 3). Statistical analysis and pairwise comparisons indicated that E177 (5 mg/kg) improved STL times in comparison with the DIZ-induced amnesia group (mean difference =260.14, P<0.0001; Figure 3). E177 (5 mg) treatment resulted in improved STL times, which were reversed following acute systemic co-administration with RAMH (mean difference =13.29, P=0.88, DIZ-induced amnesia group vs DIZ + E177 + RAMH group; Figure 3). Notably, acute systemic administration of DIZ (0.1 mg)-treated or saline-treated animals with RAMH (10 mg/kg) failed to significantly alter the STL times observed in each group (mean difference =3.57 [P=1.00] and 23.34 [P=0.40], respectively; Figure 3).
H3R antagonist E177 and DOZ counteracted DIZ-induced STM deficits in the NOR task
The observed DIs for E177 (5 mg/kg) and DOZ (1 mg/kg) in DIZ-induced STM deficits in the NOR task are shown in Figure 4A. The results showed that post-T1, acute systemic administration of E177 and DOZ significantly counteracted the time spent exploring the objects in the T2 session (F(8,54)=17.58, P<0.001; Figure 4A). Moreover, post hoc analyses of the DI results showed that DIZ (0.1 mg/kg i.p.) significantly impaired the novel object memory in T2 when compared with the saline-treated group (mean difference =0.42, P<0.0001). However, DOZ (1 mg/kg i.p.) and E177 (5 and 10 mg/kg i.p.) significantly counteracted the STM impairment induced by DIZ (mean differences of 0.44 [P<0.0001], 0.21 [P<0.05], and 0.20 [P<0.05], respectively; Figure 4A). In contrast, lower doses of E177 (2.5 mg/kg) failed to reverse the DIZ-induced memory impairment (mean difference =0.04, P=0.99; Figure 4A). Moreover, no significant difference in the protective effects was observed for either dose of E177, namely, 5 and 10 mg/kg (mean difference =0.005, P=1.00). Unexpectedly, and as compared with the DIZ-treated group, abrogation of the protective effect of the H3R antagonist E177 (5 mg/kg) was observed when animals were co-injected i.p. with 10 mg/kg of the H3R agonist RAMH (mean difference =0.19, P=0.075). In addition, statistical analyses revealed that RAMH (10 mg/kg i.p.) alone did not modulate STM in T2 compared with the saline- and DIZ-treated groups (mean differences of 0.067 [P=0.969] and 0.052 [P=0.994], respectively; Figure 4A). Importantly, the time spent exploring both objects through T1 and T2 was not significantly different in the DIZ-treated group and the saline-treated group (Table 1). This is essential to confirm the elimination of any confounding factors, such as the failure of DIZ post-training treatment in T1 to alter the spontaneous locomotion and/or motivations of tested animals.
Table 1.
Effects of E177 on dizocilpine-induced total exploratory time spent with both objects during the training and test sessions in the NOR paradigm
| Groups | n | Time exploring objects (seconds)
|
|||
|---|---|---|---|---|---|
| Training session STM | Test session STM | Training session LTM | Test session LTM | ||
| Saline | 6 | 36.50±2.69 | 40.33±2.75 | 36.67±2.93 | 38.17±3.86 |
| DIZ + saline | 6 | 37.00±2.49 | 33.75±3.09 | 37.63±2.30 | 38.14±6.47 |
| DIZ + E177 (2.5 mg/kg) | 6 | 38.50±2.37 | 40.08±1.57 | 36.83±1.99 | 36.17±1.66 |
| DIZ + E177 (5 mg/kg) | 6 | 38.67±2.52 | 38.63±2.48 | 37.67±2.55 | 33.17±2.19 |
| DIZ + E177 (10 mg/kg) | 6 | 38.17±2.17 | 36.08±0.72 | 37.50±2.63 | 37.83±3.16 |
| DIZ + DOZ (1 mg/kg) | 6 | 37.33±4.41 | 37.75±2.17 | 38.50±1.16 | 38.29±2.29 |
| DIZ + E177 (5 mg/kg) + RAMH (10 mg/kg) | 6 | 39.17±1.34 | 39.25±2.39 | 39.88±2.57 | 37.96±1.56 |
| DIZ + RAMH (10 mg/kg) | 6 | 38.00±4.22 | 37.42±1.86 | 39.33±1.56 | 37.46±1.30 |
| Saline + RAMH (10 mg/kg) | 6 | 38.25±2.88 | 37.42±2.20 | 38.75±2.14 | 37.83±4.29 |
Notes: No significant changes in total exploratory times were observed between the treatment groups. The data are expressed as the mean ± SEM (n=6).
Abbreviations: DIZ, dizocilpine; DOZ, donepezil; LTM, long-term memory; NOR, novel object recognition; RAMH, R-(α)-methyl-histamine dihydrochloride; SEM, standard error of the mean; STM, short-term memory.
E177 failed to counteract DIZ-induced LTM deficits in the NOR task
Figure 4B shows the effects observed for E177 (2.5, 5, and 10 mg/kg) and DOZ (1 mg/kg i.p.) on DIZ-induced LTM in the NOR task. The results demonstrated the significant modulatory effects of E177 and DOZ on the time spent exploring both objects in T2 (F(8,54)=5.071, P<0.0001), when injected in the post-training session T1 and 30–45 minutes before T2 on the following day (Figure 4B). Post hoc analyses of the DIs indicated that DIZ (0.1 mg/kg i.p.) significantly reduced the DI in T2 compared with the saline-treated group (mean difference =0.33, P<0.05; Figure 4B). Acute systemic pretreatment with DOZ (1 mg/kg i.p.) significantly improved the DI between the two objects (mean difference =0.07, P<0.05) compared with the DIZ-treated group. However, E177 (2.5, 5, and 10 mg/kg) failed to significantly attenuate the DI between the two objects compared with the DIZ-treated group (mean differences of 0.3 [P=1.00], 0.15 [P=0.51], and 0.09 [P=0.93], respectively; Figure 4B).
E177 did not alter animal performance in the EPM paradigm
The observed results of the anxiety-like behaviors after acute systemic administration of E177 (5 and 10 mg/kg) and DZP (10 mg/kg) are shown in Figure 5. As shown by post hoc analyses, no significant alteration was observed in the time spent in the open arms for the E177 (5 and 10 mg)-treated groups compared with the saline-treated group (F(1,10)=0.050 [P=0.828] and 0.107 [P=0.750], respectively; Figure 5A and B). In addition, the statistical analyses indicated no change in the total number of entries into the open arms for E177 (5 and 10 mg/kg), with F(1,10) values of 0.027 (P=0.872) and 0.008 (P=0.929), respectively, compared with the saline-treated group (Figure 5A and B). In contrast, both the percentage of time spent in the open arms and the total number of entries into the open arms were significantly altered following DZP (10 mg/kg) treatment compared with the saline-treated group (F(1,10)=8.605 [P<0.05] and F(1,10)=6.139 [P<0.05], respectively; Figure 5A and B). Notably, no significant change was shown in the total number of entries into the closed arms after acute systemic administration of E177 (5 and 10 mg/kg) or DZP (10 mg/kg), with F(1,10) values of 0.005 (P=0.944), 0.593 (P=0.459), and 0.004 (P=0.950), respectively (Figure 5C).
E177 did not modify anxiety-like behaviors and locomotor activity in OFT
To simultaneously exclude the possible intrinsic deficit of spontaneous locomotor activity, the effect of E177 (5 and 10 mg/kg, i.p.) was assessed in an OFT (Table 2). Locomotor activity was evaluated by the time spent in the center and periphery of the arena. In addition, the total time spent for locomotion, total distance traveled, and occurrences of rearing and grooming were assessed. One-way ANOVA showed that E177 (5 and 10 mg/kg) and DOZ (1 mg/kg) had no effect on the time spent in the center and periphery and on the total time spent for locomotion compared with the saline-treated group, with F(1,10) values of 0.385 (P=0.549), 0.059 (P=0.813), and 0.023 (P=0.883), respectively, for E177 (5 mg/kg), F(1,11) values of 0.017 (P=0.899), 0.018 (P=0.895), and 0.011 (P=0.920), respectively, for E177 (10 mg/kg), and F(1,10) values of 0.294 (P=0.599), 0.527 (P=0.484), and 1.228 (P=0.294), respectively, for DOZ (1 mg), as shown in Table 2. Statistical analyses of data describing the total distance traveled, the number of rearing events, and the number of grooming events for E177 at doses of 5 and 10 mg/kg gave essentially the same results (all P-values >0.05; Table 2). Similarly, animals that had acute systemic administration of DOZ (1 mg/kg) or E177 (5 and 10 mg/kg, i.p.) did not have a significantly different number of grooming events compared with the saline-treated group, with F(1,10) values of 2.76 (P=0.128), 0.43 (P=0.527), and 0.05 (P=0.828), respectively (Table 2).
Discussion
The results of this study clearly showed that the H3R antagonist E177 could dose-dependently (2.5, 5, and 10 mg/kg, i.p.) mitigate DIZ-induced cognitive impairments in an inhibitory paradigm in rats via the appropriate modulation of brain histaminergic neurotransmission (Figure 2). DIZ is well recognized as an NMDA receptor antagonist, and the significant involvement of NMDA receptors has been confirmed in both consolidation and retrieval processes.12,55–57 Accordingly, it is probable that E177 attenuated the memory impairment induced by DIZ, as a consequence of antagonizing the histamine H3 autoreceptors, with the resultant increase in the release of brain histamine, which is thought to interact with and activate NMDA receptors. The latter conclusions were supported by previous publications, which indicated that histamine enhanced the neurotransmission mediated by NMDA receptors in cultured hippocampal cells and signified that brain histamine and NMDA receptor interaction might help to ameliorate the memory deficits induced by DIZ in IAP. E177 also significantly and dose-dependently attenuated the memory deficits induced by DIZ, as E177 at 5 and 10 mg/kg doses significantly prolonged STL time compared to that with lower doses (1.25 and 2.5 mg/kg; Figure 2). As no additionally significant increase in the protective effect was detected with an increase in the dose of E177 from 5 to 10 mg/kg, E177 (5 mg/kg) was considered the optimum dose to confer memory-enhancing effects (Figure 2). The subsequent observations for the dose-dependent effects provided by E177 also agreed with those reported for several H3R antagonists in previous preclinical studies in animals.33,46,50,53,58,59 Notably, the protective effects of E177 (5 and 10 mg/kg) were comparable with the protective effect achieved with the standard drug DOZ, an acetylcholine esterase inhibitor clinically used for memory-enhancing effects (Figure 2). Moreover, the memory-enhancing effect of E177 (5 mg) was abrogated when rodents were co-administered with the CNS-penetrant H3R agonist RAMH, which demonstrated that H3Rs antagonism was a considerable contributor to the central neurotransmission functions linked with retrieval processes in the tested animals (Figure 3).
In rodents, the NOR task is a benchmark for the evaluation of recognition memory.60 Usually, there is no compensation or penalty for the animals in NOR test; the task assesses the animal’s native propensity to explore their environment as it is dependent on the animal’s innate behavior.61–64 In the current study, the most promising dose of E177 in the IAP (5 and 10 mg/kg) significantly enhanced the time spent exploring the novel object over that exploring the familiar object (Figure 4A). These results agreed with observations in earlier preclinical studies in which various imidazole-based H3R antagonists, such as thioperamide and clobenpropit,65 and non-imidazole-based H3R antagonists, such as pitolisant, GSK189254, SAR110894, ABT-239, and E159,53,66–69 attenuated the memory impairment induced by DIZ and scopolamine in the NOR task in a variety of animal species. In the current study, the H3R antagonist E177 appreciably counteracted the STM impairment associated with DIZ treatment, and the observed protective effect of E177 was completely reversed when the H3R agonist RAMH was co-injected (Figure 4A; Table 1). The previous observation followed earlier experimental results in which RAMH abolished the procognitive effect provided by ciproxifan, an imidazole-based H3R antagonist,70 and by DL77 as well as E159, the non-imidazole-based H3R antagonists, in rats in the NOR task.33,53 In contrast, acute systemic administration of E177 (2.5, 5, and 10 mg/kg) failed to counteract deficits in LTM (E177 injected in the post-training session T1 and 30–45 minutes before the test session T2), as shown in Figure 4B and Table 1. The latter results agreed with previous studies in which the non-imidazole-based H3R antagonist E159 did not improve LTM in the NOR task in rodents with DIZ-induced memory deficits.53 The obtained results in NOR indicated that the modulation of brain histamine through H3Rs is particularly involved in the neuronal circuits responsible for the E177-provided STM-enhancing effects, but not in the LTM-enhancing effects (Figure 4A and B).
The EPM paradigm is frequently used to investigate emotionally related behaviors in rodents.71 The EPM test depends on the natural tendency of the animals to prefer closed areas over opened areas (anxiety-like evaluation), and the number and/or percentage of closed arm entries usually provides information on whether the respective test compound alters the locomotion of tested animals, to exclude any confounding factors in experimental behaviors observed in EPM test.45,72–74 E177, administered at a dose of 5 mg/kg (the most promising dose in the IAP and NOR tasks), did not alter the number of open arm entries for rats in the EPM test, indicating that the anxiety-like levels of the tested rats were not altered by the effects of E177 on locomotor activity (Figure 5A–C). Moreover, E177 (5 mg/kg) altered neither the number of closed arm entries in EPM (Figure 5A–C) nor the distance traveled, the time spent in the central arena as well as in the periphery, and the number of rearing and grooming events during a 3-minute test in the OFT (Table 2). Similarly, DOZ and E177 did not modulate the number of grooming events, which indicated that neither DOZ nor E177 affected decision-making behavior, as the incidence of grooming is believed to reflect the ability of tested rodents in making decisions related to anxiety-like behaviors.75 Therefore, the observed enhanced memory performance provided by the H3R antagonist E177 in IAP, as well as NOR, was not confounded by the altered emotional responses or modified spontaneous locomotion, which signified that acute systemic administration of E177 (5 mg/kg, i.p.) provided memory-enhancing effects in the IAP and NOR paradigms owing to its capability to improve the learning tasks practiced in the training sessions of both memory test models, namely, the IAP and NOR tasks.
Conclusion
The novel non-imidazole-based H3R antagonist E177 counteracted memory impairments induced by DIZ in the IAP and NOR tasks in adult male rats without modulation of anxiety-like behaviors or locomotion, signifying the potential of H3R antagonists as drugs for the treatment of several neurodegenerative diseases, including AD. Nevertheless, more preclinical memory-related behavioral experiments in different rodents and other species are required to clarify the accurate mechanisms underlying the memory-improving effects of H3R antagonists and to understand their prospective utilization in future therapeutic treatment of neurodegenerative diseases. Moreover, consideration of the newly developed multi-targeting ligands combining pharmacophoric elements of enzymes, such as cholinesterase and/or monoamine oxidase, transporters, such as selective serotonin reuptake inhibitors, or other disease-modifying elements with H3R antagonism may yield improvements in preclinical trends in several models of neurodegenerative diseases, such as AD in rodents.76,77
Acknowledgments
BS was supported by intramural funds from the College of Medicine and Health Sciences and the Office of Graduate Studies and Research, United Arab Emirates University (UAEU Program for Advanced Research grant number 31M310). The authors acknowledge the partial support of the National Science Center granted on the basis of decision number DEC-2016/23/B/NZ7/02327 (DŁ) and the Jagiellonian University grant number K/ZDS/007121 (KKK). Support was kindly provided by the EU COST Action CA15135 (DŁ and KKK).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 2.Li KW, Ganz AB, Smit AB. Proteomics of neurodegenerative diseases: analysis of human post-mortem brain. J Neurochem. 2018 doi: 10.1111/jnc.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozaki T, Katsumata Y, Arai A. Association between changes in the use of psychotropic drugs and behavioural and psychological symptoms of dementia over 1 year among long-term care facility residents. Psychogeriatrics. 2018 Oct;:18. doi: 10.1111/psyg.12374. Epub. [DOI] [PubMed] [Google Scholar]
- 4.Shaik JB, Palaka BK, Penumala M, et al. Synthesis, pharmacological assessment, molecular modeling and in silico studies of fused tricyclic coumarin derivatives as a new family of multifunctional anti-Alzheimer agents. Eur J Med Chem. 2016;107:219–232. doi: 10.1016/j.ejmech.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 5.Khunnawutmanotham N, Chimnoi N, Saparpakorn P, Techasakul S. Synthesis and anti-acetylcholinesterase activity of scopoletin derivatives. Bioorg Chem. 2016;65:137–145. doi: 10.1016/j.bioorg.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Van Giau V, An SSA, Hulme JP. Mitochondrial therapeutic interventions in Alzheimer’s disease. J Neurol Sci. 2018;395:62–70. doi: 10.1016/j.jns.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Santos MA, Chand K, Chaves S. Recent progress in repositioning Alzheimer’s disease drugs based on a multitarget strategy. Future Med Chem. 2016;8(17):2113–2142. doi: 10.4155/fmc-2016-0103. [DOI] [PubMed] [Google Scholar]
- 8.Piemontese L. New approaches for prevention and treatment of Alzheimer’s disease: a fascinating challenge. Neural Regen Res. 2017;12(3):405–406. doi: 10.4103/1673-5374.202942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal S, Yadav A, Chaturvedi RK. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem Biophys Res Commun. 2017;483(4):1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 10.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N Y) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan N, Saad A, Nurulain SM, Darras FH, Decker M, Sadek B. The dual-acting H3 receptor antagonist and AChE inhibitor UW-MD-71 dose-dependently enhances memory retrieval and reverses dizocilpine-induced memory impairment in rats. Behav Brain Res. 2016;297:155–164. doi: 10.1016/j.bbr.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Sadek B, Khan N, Darras FH, Pockes S, Decker M. The dual-acting AChE inhibitor and H3 receptor antagonist UW-MD-72 reverses amnesia induced by scopolamine or dizocilpine in passive avoidance paradigm in rats. Physiol Behav. 2016;165:383–391. doi: 10.1016/j.physbeh.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Orr EL, Pace KR. The significance of mast cells as a source of histamine in the mouse brain. J Neurochem. 1984;42(3):727–732. doi: 10.1111/j.1471-4159.1984.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz JC, Arrang JM, Garbarg M, Korner M. Properties and roles of the three subclasses of histamine receptors in brain. J Exp Biol. 1986;124:203–224. doi: 10.1242/jeb.124.1.203. [DOI] [PubMed] [Google Scholar]
- 15.Schneider EH, Seifert R, Histamine H. Histamine H(4) receptor–RGS fusion proteins expressed in Sf9 insect cells: a sensitive and reliable approach for the functional characterization of histamine H(4) receptor ligands. Biochem Pharmacol. 2009;78(6):607–616. doi: 10.1016/j.bcp.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Panula P, Chazot PL, Cowart M, et al. International Union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev. 2015;67(3):601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eissa N, Al-Houqani M, Sadeq A, Ojha SK, Sasse A, Sadek B. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front Neurosci. 2018;12:304. doi: 10.3389/fnins.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chand N, Eyre P. Classification and biological distribution of histamine receptor sub-types. Agents Actions. 1975;5(4):277–295. doi: 10.1007/BF02205232. [DOI] [PubMed] [Google Scholar]
- 19.Arrang JM, Devaux B, Chodkiewicz JP, Schwartz JC. H3-receptors control histamine release in human brain. J Neurochem. 1988;51(1):105–108. doi: 10.1111/j.1471-4159.1988.tb04841.x. [DOI] [PubMed] [Google Scholar]
- 20.Arrang JM, Garbarg M, Lancelot JC, et al. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327(6118):117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- 21.Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302(5911):832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 22.Arrang JM, Garbarg M, Schwartz JC. Autoregulation of histamine release in brain by presynaptic H3-receptors. Neuroscience. 1985;15(2):553–562. doi: 10.1016/0306-4522(85)90233-7. [DOI] [PubMed] [Google Scholar]
- 23.Arrang JM, Garbarg M, Schwartz JC. Autoinhibition of histamine synthesis mediated by presynaptic H3-receptors. Neuroscience. 1987;23(1):149–157. doi: 10.1016/0306-4522(87)90279-x. [DOI] [PubMed] [Google Scholar]
- 24.Arrang JM, Morisset S, Gbahou F. Constitutive activity of the histamine H3 receptor. Trends Pharmacol Sci. 2007;28(7):350–357. doi: 10.1016/j.tips.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Sadek B, Stark H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology. 2016;106:56–73. doi: 10.1016/j.neuropharm.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63(6):637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 27.Sadek B, Saad A, Sadeq A, Jalal F, Stark H. Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav Brain Res. 2016;312:415–430. doi: 10.1016/j.bbr.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 28.Blandina P, Efoudebe M, Cenni G, Mannaioni P, Passani MB. Acetyl-choline, histamine, and cognition: two sides of the same coin. Learn Mem. 2004;11(1):1–8. doi: 10.1101/lm.68004. [DOI] [PubMed] [Google Scholar]
- 29.Browman KE, Komater VA, Curzon P, et al. Enhancement of prepulse inhibition of startle in mice by the H3 receptor antagonists thioperamide and ciproxifan. Behav Brain Res. 2004;153(1):69–76. doi: 10.1016/j.bbr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Bardgett ME, Davis NN, Schultheis PJ, Griffith MS. Ciproxifan, an H3 receptor antagonist, alleviates hyperactivity and cognitive deficits in the APP Tg2576 mouse model of Alzheimer’s disease. Neurobiol Learn Mem. 2011;95(1):64–72. doi: 10.1016/j.nlm.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witkin JM, Nelson DL. Selective histamine H3 receptor antagonists for treatment of cognitive deficiencies and other disorders of the central nervous system. Pharmacol Ther. 2004;103(1):1–20. doi: 10.1016/j.pharmthera.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Charlier Y, Brabant C, Serrano ME, Lamberty Y, Tirelli E. The prototypical histamine H3 receptor inverse agonist thioperamide improves multiple aspects of memory processing in an inhibitory avoidance task. Behav Brain Res. 2013;253:121–127. doi: 10.1016/j.bbr.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Eissa N, Khan N, Ojha SK, Łazewska D, Kieć-Kononowicz K, Sadek B. The histamine H3 receptor antagonist DL77 ameliorates MK801-induced memory deficits in rats. Front Neurosci. 2018;12:42. doi: 10.3389/fnins.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhowmik M, Khanam R, Vohora D. Histamine H3 receptor antagonists in relation to epilepsy and neurodegeneration: a systemic consideration of recent progress and perspectives. Br J Pharmacol. 2012;167(7):1398–1414. doi: 10.1111/j.1476-5381.2012.02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada C, Hirai T, Fujii Y, Harusawa S, Kurihara T, Kamei C. Intra-cerebroventricular administration of histamine H3 receptor antagonists decreases seizures in rat models of epilepsia. Methods Find Exp Clin Pharmacol. 2004;26(4):263–270. [PubMed] [Google Scholar]
- 36.Uma Devi P, Manocha A, Khanam R, Vohora D. Beneficial interaction between clobenpropit and pyridoxine in prevention of electroshock-induced seizures in mice: lack of histaminergic mechanisms. Hum Exp Toxicol. 2011;30(1):84–88. doi: 10.1177/0960327110372398. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama H. The role of central histaminergic neuron system as an anticonvulsive mechanism in developing brain. Brain Dev. 2001;23(7):542–547. doi: 10.1016/s0387-7604(01)00261-3. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama H, Onodera K, Iinuma K, Watanabe T. Effect of thioperamide, a histamine H3 receptor antagonist, on electrically induced convulsions in mice. Eur J Pharmacol. 1993;234(1):129–133. doi: 10.1016/0014-2999(93)90717-v. [DOI] [PubMed] [Google Scholar]
- 39.Sadek B, Stark H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology. 2016;106:56–73. doi: 10.1016/j.neuropharm.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Bhowmik M, Saini N, Vohora D. Histamine H3 receptor antagonism by ABT-239 attenuates kainic acid induced excitotoxicity in mice. Brain Res. 2014;1581:129–140. doi: 10.1016/j.brainres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood D. Histamine H3 receptors and its antagonism as a novel mechanism for antipsychotic effect: a current preclinical and clinical perspective. Int J Health Sci (Qassim) 2016;10(4):543–554. [PMC free article] [PubMed] [Google Scholar]
- 42.Mahmood D, Akhtar M, Jahan K, Goswami D. Histamine H3 receptor antagonists display antischizophrenic activities in rats treated with MK-801. J Basic Clin Physiol Pharmacol. 2016;27(5):463–471. doi: 10.1515/jbcpp-2015-0045. [DOI] [PubMed] [Google Scholar]
- 43.Łażewska D, Kaleta M, Hagenow S, et al. Novel naphthyloxy derivatives – potent histamine H3 receptor ligands. Synthesis and pharmacological evaluation. Bioorg Med Chem. 2018;26(9):2573–2585. doi: 10.1016/j.bmc.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Sadek B, Saad A, Subramanian D, Shafiullah M, Łażewska D, Kieć-Kononowiczc K. Anticonvulsant and procognitive properties of the non-imidazole histamine H3 receptor antagonist DL77 in male adult rats. Neuropharmacology. 2016;106:46–55. doi: 10.1016/j.neuropharm.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Alachkar A, Łażewska D, Kieć-Kononowicz K, Sadek B. The histamine H3 receptor antagonist E159 reverses memory deficits induced by dizocilpine in passive avoidance and novel object recognition paradigm in rats. Front Pharmacol. 2017;8:709. doi: 10.3389/fphar.2017.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadek B, Saad A, Subramanian D, Shafiullah M, Lazewska D, Kiec-Kononowiczc K. Anticonvulsant and procognitive properties of the non-imidazole histamine H3 receptor antagonist DL77 in male adult rats. Neuropharmacology. 2016;106:46–55. doi: 10.1016/j.neuropharm.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Bernaerts P, Lamberty Y, Tirelli E. Histamine H3 antagonist thioperamide dose-dependently enhances memory consolidation and reverses amnesia induced by dizocilpine or scopolamine in a one-trial inhibitory avoidance task in mice. Behav Brain Res. 2004;154(1):211–219. doi: 10.1016/j.bbr.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Da Silva AL, Silva Martins B, Linck VM, et al. MK801- and scopolamine-induced amnesias are reversed by an Amazonian herbal locally used as a “brain tonic”. Psychopharmacology. 2009;202(1–3):165–172. doi: 10.1007/s00213-008-1272-y. [DOI] [PubMed] [Google Scholar]
- 49.Goshadrou F, Kermani M, Ronaghi A, Sajjadi S. The effect of ghrelin on MK-801 induced memory impairment in rats. Peptides. 2013;44:60–65. doi: 10.1016/j.peptides.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Sultan A, Yang KS, Isaev D, et al. Thujone inhibits the function of α7-nicotinic acetylcholine receptors and impairs nicotine-induced memory enhancement in one-trial passive avoidance paradigm. Toxicology. 2017;384:23–32. doi: 10.1016/j.tox.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Izquierdo I, Medina JH, Vianna MR, Izquierdo LA, Barros DM. Separate mechanisms for short- and long-term memory. Behav Brain Res. 1999;103(1):1–11. doi: 10.1016/s0166-4328(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 52.Orsetti M, Ferretti C, Gamalero R, Ghi P. Histamine H3-receptor blockade in the rat nucleus basalis magnocellularis improves place recognition memory. Psychopharmacology. 2002;159(2):133–137. doi: 10.1007/s002130100892. [DOI] [PubMed] [Google Scholar]
- 53.Alachkar A, Łażewska D, Kieć-Kononowicz K, Sadek B. The histamine H3 receptor antagonist E159 reverses memory deficits induced by dizocilpine in passive avoidance and novel object recognition paradigm in rats. Front Pharmacol. 2017;8:709. doi: 10.3389/fphar.2017.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang YM, Li XJ, Meng ZZ, et al. Effects of Xiaoyaosan on stress-induced anxiety-like behavior in rats: involvement of CRF1 receptor. Evid Based Complement Alternat Med. 2016;2016(1):1–9. doi: 10.1155/2016/1238426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brabant C, Charlier Y, Tirelli E. The histamine H3-receptor inverse agonist Pitolisant improves fear memory in mice. Behav Brain Res. 2013;243:199–204. doi: 10.1016/j.bbr.2012.12.063. [DOI] [PubMed] [Google Scholar]
- 56.Vorobjev VS, Sharonova IN, Walsh IB, Haas HL. Histamine potentiates N-methyl-d-aspartate responses in acutely isolated hippocampal neurons. Neuron. 1993;11(5):837–844. doi: 10.1016/0896-6273(93)90113-6. [DOI] [PubMed] [Google Scholar]
- 57.Xu LS, Yang LX, Hu WW, et al. Histamine ameliorates spatial memory deficits induced by MK-801 infusion into ventral hippocampus as evaluated by radial maze task in rats. Acta Pharmacol Sin. 2005;26(12):1448–1453. doi: 10.1111/j.1745-7254.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 58.Benetti F, Baldi E, Bucherelli C, Blandina P, Passani MB. Histaminergic ligands injected into the nucleus basalis magnocellularis differentially affect fear conditioning consolidation. Int J Neuropsychopharmacol. 2013;16(3):575–582. doi: 10.1017/S1461145712000181. [DOI] [PubMed] [Google Scholar]
- 59.Benetti F, Izquierdo I. Histamine infused into basolateral amygdala enhances memory consolidation of inhibitory avoidance. Int J Neuropsychopharmacol. 2013;16(7):1539–1545. doi: 10.1017/S1461145712001514. [DOI] [PubMed] [Google Scholar]
- 60.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17(1):5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown JW, Whitehead CA, Basso AM, Rueter LE, Zhang M. Preclinical evaluation of non-imidazole histamine H3 receptor antagonists in comparison to atypical antipsychotics for the treatment of cognitive deficits associated with schizophrenia. Int J Neuropsychopharmacol. 2013;16(04):889–904. doi: 10.1017/S1461145712000739. [DOI] [PubMed] [Google Scholar]
- 62.Jaaro-Peled H. Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res. 2009;179:75–86. doi: 10.1016/S0079-6123(09)17909-8. [DOI] [PubMed] [Google Scholar]
- 63.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204(2):295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callahan PM, Bertrand D, Bertrand S, Plagenhoef MR, Terry AV. Tropisetron sensitizes α7 containing nicotinic receptors to low levels of acetylcholine in vitro and improves memory-related task performance in young and aged animals. Neuropharmacology. 2017;117:422–433. doi: 10.1016/j.neuropharm.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 65.Giovannini MG, Bartolini L, Bacciottini L, Greco L, Blandina P. Effects of histamine H3 receptor agonists and antagonists on cognitive performance and scopolamine-induced amnesia. Behav Brain Res. 1999;104(1–2):147–155. doi: 10.1016/s0166-4328(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 66.Ligneau X, Perrin D, Landais L, et al. BF2.649 [1-{3-[3-(4-chlorophenyl) propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J Pharmacol Exp Ther. 2007;320(1):365–375. doi: 10.1124/jpet.106.111039. [DOI] [PubMed] [Google Scholar]
- 67.Giannoni P, Medhurst AD, Passani MB, et al. Regional differential effects of the novel histamine H3 receptor antagonist 6-[(3-cyclobutyl-2,3,4,5-tetrahydro-1H-3-benzazepin-7-yl)oxy]-N-methyl-3- pyridinecarboxamide hydrochloride (GSK189254) on histamine release in the central nervous system of freely moving rats. J Pharmacol Exp Ther. 2010;332(1):164–172. doi: 10.1124/jpet.109.158444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griebel G, Pichat P, Pruniaux M-P, et al. SAR110894, a potent histamine H3-receptor antagonist, displays procognitive effects in rodents. Pharmacol Biochem Behav. 2012;102(2):203–214. doi: 10.1016/j.pbb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Provensi G, Costa A, Passani MB, Blandina P. Donepezil, an acetylcholine esterase inhibitor, and ABT-239, a histamine H3 receptor antagonist/inverse agonist, require the integrity of brain histamine system to exert biochemical and procognitive effects in the mouse. Neuropharmacology. 2016;109:139–147. doi: 10.1016/j.neuropharm.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 70.Pascoli V, Boer-Saccomani C, Hermant JF. H3 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing retrieval. Psychopharmacology. 2009;202(1–3):141–152. doi: 10.1007/s00213-008-1171-2. [DOI] [PubMed] [Google Scholar]
- 71.Azarfarin M, Farajdokht F, Babri S, Salehpour F, Taghizadeh M, Mohaddes G. Effects of troxerutin on anxiety- and depressive-like behaviors induced by chronic mild stress in adult male rats. Iran J Basic Med Sci. 2018;21(8):781–786. doi: 10.22038/IJBMS.2018.26915.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54(1):31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- 73.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54(1):21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 74.Eissa N, Jayaprakash P, Azimullah S, et al. The histamine H3R antagonist DL77 attenuates autistic behaviors in a prenatal valproic acid-induced mouse model of autism. Sci Rep. 2018;8(1):13077. doi: 10.1038/s41598-018-31385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sestakova N, Puzserova A, Kluknavsky M, Bernatova I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol. 2013;6(3):126–135. doi: 10.2478/intox-2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Proschak E, Stark H, Merk D. Polypharmacology by design: a medicinal chemist’s perspective on multitargeting compounds. J Med Chem. 2018 doi: 10.1021/acs.jmedchem.8b00760. [DOI] [PubMed] [Google Scholar]
- 77.Khanfar MA, Affini A, Lutsenko K, Nikolic K, Butini S, Stark H. Multiple targeting approaches on histamine H3 receptor antagonists. Front Neurosci. 2016;10:201. doi: 10.3389/fnins.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]