Abstract
Background
The 8th edition of the American Joint Committee on Cancer (AJCC) staging system for breast cancer has incorporated tumor grade, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status as staging biologic factors reflecting prognosis. The purpose of this study was to compare the 7th and 8th edition of AJCC staging system for prognostic impact.
Materials and methods
Primary breast cancer patients diagnosed from 2010 to 2014 were identified using the Surveillance, Epidemiology and End Results 18 registries research database. Breast cancer-specific survival (BCSS) and overall survival (OS) between stages were estimated using the Kaplan–Meier method and compared using the log-rank test. Multivariable analysis was performed using Cox proportional hazards regression analysis to identify factors independently associated with outcome. Akaike’s information criterion (AIC) was calculated to estimate how well the staging system fitted the data and the complexity of the model.
Results
A total of 184,221 primary breast cancer patients were identified in the 7th AJCC staging system; 16,145 (8.8%) patients could not be categorized according to 8th AJCC prognostic staging system leaving 168,076 patients included for final analyses. The 8th AJCC performed well with the BCSS and OS concordant with stage. A total of 89,494 (53.2%) of patients were restaged to a different stage group in the 8th AJCC; stage IIIA in the 7th AJCC migrated to stage IB with a worse prognosis than IIA and IIB in the 8th AJCC. Nevertheless, the 8th AJCC had a better AIC than the 7th staging system.
Conclusion
The prognostic accuracy of the 8th AJCC staging system was generally superior to the 7th AJCC, although subtle differences between the two systems should be noted in comparative studies.
Keywords: breast cancer, prognosis, AJCC
Introduction
The TNM staging system was developed and has been maintained by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control since 1959.1 It is the most commonly used staging system by medical professionals around the world, developed as a tool for doctors to discuss the prognosis of newly diagnosed cancer patients, design a treatment plan for individual patients, and standardize comparison between patients participating in clinical trials.
Breast cancer has been staged using the AJCC TNM staging system since the 1st edition cancer staging system in 19772 based on anatomic factors: the extent of the primary tumor (T), the extent of spread to the regional lymph nodes (N), and the presence of metastasis (M). Scientific developments,3,4 especially the understanding of biological markers such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and their close relationship with prognosis, selection of therapy, and response to therapy, have led to the purely anatomic TNM staging for breast cancer being challenged.5 The National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO),6 European Group on Tumor Markers (EGTM),7 and the St. Gallen International Expert Consensus8 recommend the determination of ER, PR, and HER2 status in all breast cancer patients for prognosis and for treatment planning. Another important and widely accepted prognostic factor in breast cancer is the histological grade, standardized by the Nottingham group.9–11 Recently, results from prospective clinical trials using multigene panel testing12,13 have demonstrated the value of multigene panels for selected patient management and, for one panel at least,13 have been incorporated into guidelines for treatment by ASCO6 and NCCN (Breast Cancer Version 2.2017; https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf, Accessed August 1, 2017).
Consequently, based on evidence available from the peer-reviewed literature5,14 and on findings from large but unpublished data sets,1 new “prognostic stage groups” including grade, ER, PR, HER2, and multigene testing were incorporated into the 8th edition of the AJCC breast cancer staging system to further refine prognostic information.
However, there has not yet been level I evidence to support the 8th edition of the AJCC breast cancer staging system. The primary objective of this study was to compare the two staging systems for prognostic precision using patient information extracted from the Surveillance, Epidemiology and End Results (SEER) research database.
Materials and methods
Study population and characteristics
The SEER*Stat version 8.3.4 was freely used to extract data from the SEER 18 registries research data online, including data from 1973 to 2014. We could not determine whether surgery was the first intervention, since information about neoadjuvant chemotherapy in the SEER database is lacking.
Because this database does not include detailed information on HER2 status before 2010, patients were limited to those with histologically confirmed unilateral primary breast cancer diagnosed from 2010 to 2014 with complete follow-up until December 2014 (n=383,355). Patients with a history of prior or subsequent in situ or invasive cancer of any type (n=101,432) or patients who were only initially identified by autopsy or death certificate (n=38) were excluded from the study. Patients with unknown AJCC 7 T category, N category, M category, stage group or unknown grade, ER, PR, and HER2 status (n=81,192) were excluded. The stage 0 (n=5,382) and IV (n=7,235) were also excluded because there was no change between the AJCC 7 staging system and AJCC 8 prognostic staging system. Other exclusions were of those patients with no surgery performed on the primary site (n=1,385) or surgery unknown (n=1,421) and those of unknown age (n=15) or unknown race/ethnicity (n=991). Patients were categorized according to the 7th AJCC staging system and the 8th AJCC prognostic staging system. The following patient characteristics were included: date of diagnosis, sex, breast subtype, cause of death, and survival months.
Statistical analysis
Breast cancer-specific survival (BCSS) was defined as the date of diagnosis to the date of death attributed to breast cancer. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause. Patients who were alive at the study cutoff data were censored.
Categorical variables were analyzed using the chi-squared test, and continuous variables were analyzed using the t-test. The Kaplan–Meier method was used to generate survival curves, and the log-rank test was performed to assess the differences in BCSS and OS between the stages. Multivariable analysis was performed using the Cox proportional hazards regression analysis to identify factors independently associated with outcome. In addition, Akaike’s information criterion (AIC) was calculated to estimate how well the staging system fitted the data and the complexity of the model, with a low AIC suggesting a more precise prognostic staging system. Age at diagnosis was included in the model. Results are expressed in HRs and 95% CIs. All tests were two-sided and P-values <0.05 considered statistically significant. Statistical analyses were performed using SAS, version 9.4, software.
Results
A total of 184,221 primary breast cancer patients were identified in the 7th AJCC staging system. However, 16,145 (8.8%) patients could not be categorized according to the 8th AJCC prognostic staging system and 58.7% of these patients (n=9,480) were T2N1M0 stage IIB (7th AJCC staging system) with grade 2 or 3, ER-positive, PR-positive, and HER2-negative subtype (Table S1). Thus, a total of 168,076 patients were included for final analyses.
The baseline characteristics of the 168,076 patients (Table 1) demonstrated a median age at diagnosis of 60 years (range, 15–108 years). A total of 107,912 (64.2%) patients were of the ER-positive, PR-positive, and HER2-negative subtype. AJCC 7 stage I (IA+IB) and the stage II (IIA+IIB) accounted for 93,552 (55.7%) and 52,686 (31.4%) patients, respectively.
Table 1.
Baseline characteristics of the included patients (n=168,076)
| Characteristics | No. | % |
|---|---|---|
| Age at diagnosis (years) | ||
| ≤39 | 8,860 | 5.3 |
| 40–49 | 29,624 | 17.6 |
| 50–59 | 42,900 | 25.5 |
| 60–69 | 44,966 | 26.8 |
| 70–79 | 27,842 | 16.6 |
| ≥80 | 13,884 | 8.3 |
| Race/ethnicity | ||
| Hispanic | 19,006 | 11.3 |
| NH Black | 17,919 | 10.7 |
| NH White | 115,520 | 68.7 |
| NH Asian or Pacific Islander | 14,662 | 8.7 |
| NH American Indian/Alaska Native | 969 | 0.6 |
| Sex | ||
| Male | 1,104 | 0.7 |
| Female | 166,972 | 99.3 |
| Grade | ||
| I | 42,252 | 25.1 |
| II | 69,759 | 41.5 |
| III–IV | 56,065 | 33.4 |
| ER status | ||
| Positive | 137,137 | 81.6 |
| Negative | 30,939 | 18.4 |
| PR status | ||
| Positive | 122,002 | 72.6 |
| Negative | 46,074 | 27.4 |
| HER2 status | ||
| Positive | 25,446 | 15.1 |
| Negative | 142,630 | 84.9 |
| ER and PR status | ||
| ER and PR positive | 120,209 | 71.5 |
| ER positive, PR negative | 16,928 | 10.1 |
| ER and PR negative | 29,146 | 17.3 |
| ER negative, PR positive | 1,793 | 1.1 |
| ER, PR and HER2 status | ||
| ER positive, PR positive, and HER2 positive | 12,297 | 7.3 |
| ER positive, PR positive, and HER2 negative | 107,912 | 64.2 |
| ER positive, PR negative, and HER2 positive | 4,707 | 2.8 |
| ER positive, PR negative, and HER2 negative | 12,221 | 7.3 |
| ER negative, PR positive, and HER2 positive | 446 | 0.3 |
| ER negative, PR positive, and HER2 negative | 1,347 | 0.8 |
| ER negative, PR negative, and HER2 positive | 7,996 | 4.8 |
| ER negative, PR negative, and HER2 negative | 21,150 | 12.6 |
| 7th AJCC TNM stage | ||
| Stage IA | 88,985 | 52.9 |
| Stage IB | 4,567 | 2.7 |
| Stage IIA | 43,124 | 25.7 |
| Stage IIB | 9,562 | 5.7 |
| Stage IIIA | 12,897 | 7.7 |
| Stage IIIB | 3,736 | 2.2 |
| Stage IIIC | 5,205 | 3.1 |
| Follow-up time (months) | ||
| Median | 20 | |
| Range | 0–59 |
Abbreviations: AJCC, American Joint Committee on Cancer; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; M, distant metastases; N, regional nodes; NH, non-Hispanic; PR, progesterone receptor; T, primary tumor.
Critically, the new staging system resulted in a reallocation of 89,494 (53.2%) of the patients to a different stage group (Table 2 and Table S2). Among the downstaged groups (n=37,117) (Table S3), a total of 36,872 (99.3%) patients were ER positive and PR positive, irrespective of grade and HER2 status. As for the tumor grade, grade 1–3 accounted for 27.3%, 64.1%, and 8.6%, respectively. In the upstaged groups (n=52,377) (Table S4), 42,209 (80.6%) patients were HER2 negative while triple negative breast cancer (ER, PR, and HER2 all negative) accounted for 9,505 (18.1%) patients. The proportion of grade 3 tumors was 77.3% of patients.
Table 2.
Comparison of the 7th AJCC staging system and 8th AJCC prognostic staging system (n=168,076)
| 8th AJCC prognostic staging system | 7th AJCC staging system | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA | IB | IIA | IIB | IIIA | IIIB | IIIC | ||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| IB | 12,972 | 7.7 | 809 | 0.5 | 22,902 | 13.6 | 2,303 | 1.4 | 504 | 0.3 | ||||
| IIA | 9,842 | 5.9 | 372 | 0.2 | 6,776 | 4.0 | 954 | 0.6 | ||||||
| IIB | 4,524 | 2.7 | 90 | 0.1 | 4,785 | 2.8 | ||||||||
| IIIA | 8,922 | 5.3 | 2,721 | 1.6 | 449 | 0.3 | 192 | 0.1 | 272 | 0.2 | ||||
| IIIB | 1,459 | 0.9 | 3,784 | 2.3 | 1,173 | 0.7 | 1,819 | 1.1 | ||||||
| IIIC | 2,989 | 1.8 | 2,421 | 1.4 | 2,371 | 1.4 | 3,114 | 1.9 | ||||||
Abbreviation: AJCC, American Joint Committee on Cancer.
The greatest changes in stage for AJCC 8 were in stage IB (a rise by 20.8%), IIA (a fall by 15.0%), and IA (dropped 11.5%) compared to the 7th staging system. The stage IB changes resulted from two components: the stage IIA (13.6%), IIB (1.4%), and IIIA (0.3%) downstaged to IB and stage IA (7.7%) upstaged to IB from the 7th AJCC staging system to the 8th staging system.
Biological markers (tumor grade, ER, PR, and HER2 status) were additional significant independent predictors for survival with the 7th AJCC staging system (Table 3) in multivariable Cox proportional hazards modeling. When adjusted for grade, ER, PR, and HER2 status in the 7th AJCC staging system, stage IIIC patients (adjusted HR=18.54, 95% CI=16.62–20.69, P<0.001) had superior (and not inferior) prognosis to stage IIIB patients (adjusted HR =20.09, 95% CI=17.90–22.55, P<0.001) for BCSS. For OS, the estimates were similar (Table S5). These results demonstrated that the prognostic accuracy of the 8th AJCC prognostic staging system utilizing tumor grade, ER, PR, and HER2 status as biologic staging factors could be considered superior to the 7th staging system.
Table 3.
Cox proportional hazards model for the 7th AJCC staging system and 8th AJCC prognostic staging system for breast cancer-specific survival (n=168,076)
| Factors | 7th staging system | 8th prognostic staging system | ||||
|---|---|---|---|---|---|---|
| Univariate Cox analysis | Multivariate Cox analysis | Univariate Cox analysis | ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Stage | ||||||
| IA | Reference | Reference | Reference | |||
| IB | 1.92 (1.46, 2.53) | <0.001 | 1.97 (1.50, 2.60) | <0.001 | 2.92 (2.48, 3.42) | <0.001 |
| IIA | 3.95 (3.56, 4.38) | <0.001 | 2.78 (2.50, 3.08) | <0.001 | 6.51 (5.55, 7.63) | <0.001 |
| IIB | 11.43 (10.21, 12.81) | <0.001 | 5.30 (4.71, 5.97) | <0.001 | 9.15 (7.71, 10.85) | <0.001 |
| IIIA | 12.42 (11.19, 13.78) | <0.001 | 8.88 (7.98, 9.87) | <0.001 | 16.31 (14.08, 18.89) | <0.001 |
| IIIB | 32.89 (29.38, 36.83) | <0.001 | 20.09 (17.90, 22.55) | <0.001 | 24.81 (21.42, 28.74) | <0.001 |
| IIIC | 27.40 (24.61, 30.50) | <0.001 | 18.54 (16.62, 20.69) | <0.001 | 60.55 (52.96, 69.24) | <0.001 |
| Grade | ||||||
| I | Reference | Reference | _ | |||
| II | 3.00 (2.60, 3.45) | <0.001 | 1.77 (1.53, 2.04) | <0.001 | ||
| III–IV | 11.59 (10.14, 13.25) | <0.001 | 3.27 (2.84, 3.78) | <0.001 | ||
| ER status | ||||||
| Positive | Reference | Reference | _ | |||
| Negative | 4.63 (4.38, 4.90) | <0.001 | 1.55 (1.43, 1.67) | <0.001 | ||
| PR status | ||||||
| Positive | Reference | Reference | _ | |||
| Negative | 5.00 (4.72, 5.31) | <0.001 | 1.98 (1.82, 2.15) | <0.001 | ||
| HER2 status | ||||||
| Positive | Reference | Reference | _ | |||
| Negative | 0.88 (0.82, 0.95) | <0.001 | 1.84 (1.70, 1.99) | <0.001 | ||
Abbreviations: AJCC, American Joint Committee on Cancer; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
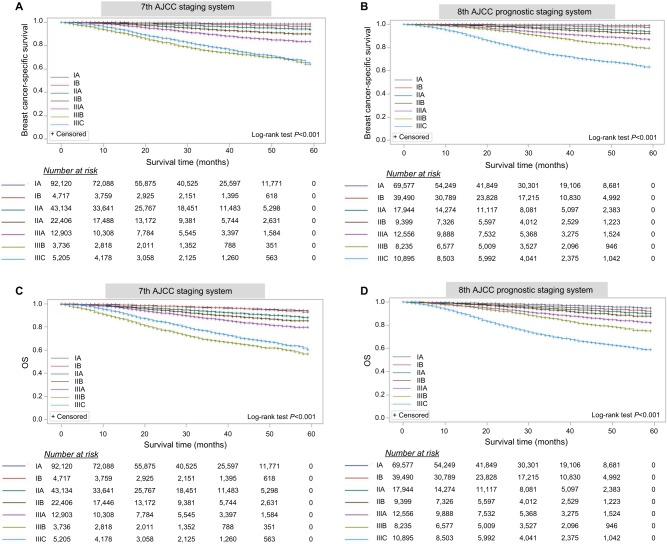
Univariate analysis demonstrated that patients with stage IIIB had a worse prognosis than those with stage IIIC within the 7th AJCC staging system (long-rank tests, P<0.001), at a median follow-up of 25 months (range, 0–59 months) (Figure 1). Three-year BCSS and OS rates by stage (Table 4) indicate that the 3-year survival for stage IIIB was inferior to stage IIIC (BCSS, 75.2% vs 78.9%; OS, 68.5% vs 75.5%) in the 7th AJCC staging system. However, this inconsistency was not reproduced in the 8th AJCC prognostic staging system (BCSS, 88.8% vs 73.7%; OS, 85.5% vs 69.9%). The higher the patients were staged, the poorer prognosis they had in the 8th AJCC.
Figure 1.
The AJCC staging systems predict survival for primary breast cancer patients and indicate the number of patients at risk.
Notes: The 7th AJCC staging system: BCSS (A) and OS (C). The 8th AJCC prognostic staging system: BCSS (B) and OS (D).
Abbreviations: AJCC, American Joint Committee on Cancer; OS, overall survival; BCSS, breast cancer-specific survival.
Table 4.
Three-year BCSS and OS according to stage (n=168,076)
| Stage | AJCC 7 | AJCC 8 | 3-year BCSS (%) | 3-year OS (%) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | AJCC 7 | AJCC 8 | AJCC 7 | AJCC 8 | |
| IA | 88,985 | 52.9 | 69,557 | 41.4 | 99.2 | 99.5 | 96.9 | 97.3 |
| IB | 4,567 | 2.7 | 39,490 | 23.5 | 98.3 | 98.7 | 96.8 | 95.7 |
| IIA | 43,124 | 25.7 | 17,944 | 10.7 | 96.6 | 96.9 | 93.2 | 94.3 |
| IIB | 9,562 | 5.7 | 9,399 | 5.6 | 90.1 | 95.6 | 87.1 | 92.8 |
| IIIA | 12,897 | 7.7 | 12,556 | 7.5 | 89.5 | 92.3 | 87.1 | 89.2 |
| IIIB | 3,736 | 2.2 | 8,235 | 4.9 | 75.2 | 88.8 | 68.5 | 85.5 |
| IIIC | 5,205 | 3.1 | 10,895 | 6.5 | 78.9 | 73.7 | 75.5 | 69.9 |
Abbreviations: AJCC, American Joint Committee on Cancer; OS, overall survival; BCSS, breast cancer-specific survival.
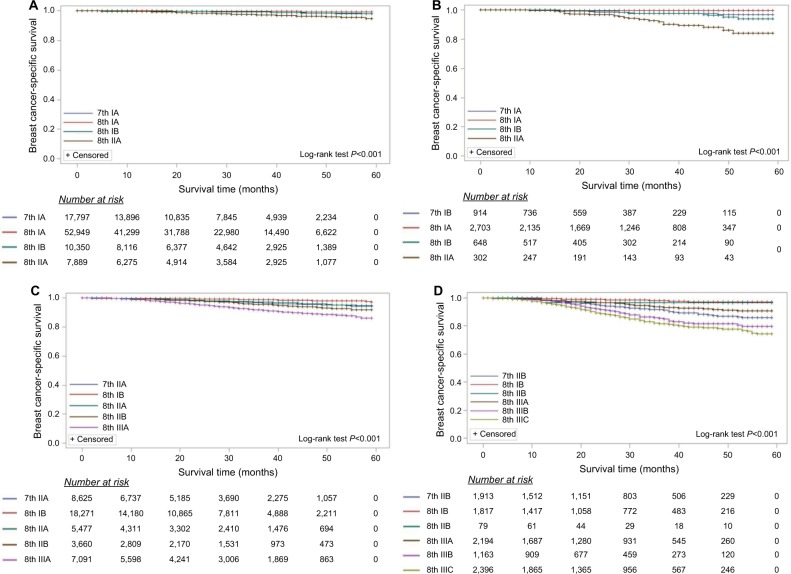
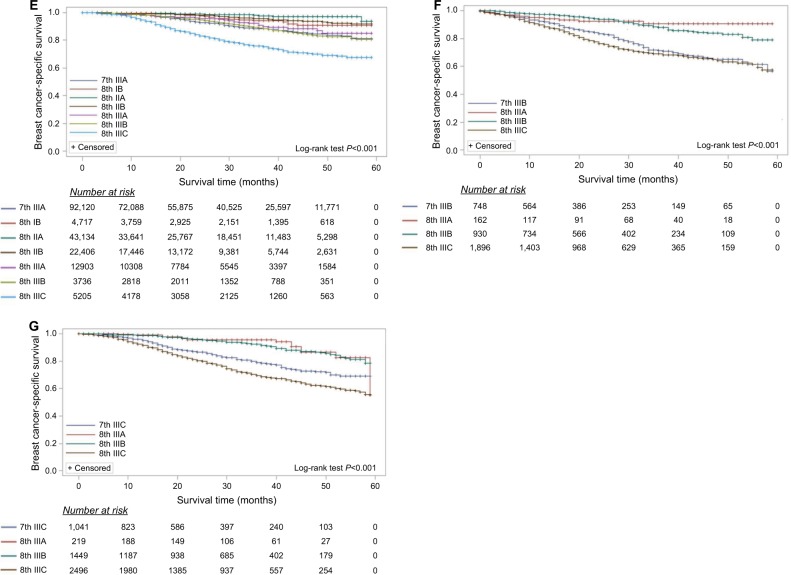
In order to further consider the prognostic accuracy of the 8th AJCC prognostic staging system, every stage in the 7th AJCC was randomly separated into two parts; 80% patients were staged according to the 8th AJCC prognostic staging system and the other 20% patients using the 7th stage as the reference.15 BCSS survival curves for stages according to the 8th AJCC prognostic staging system demonstrated that, as expected, the BCSS rates improved in the lower stages and worsened as the stage increased (Figure 2). For OS, the estimates were similar (Figure S1). The 3-year BCSS and OS rates according to stage are shown in Table 5. For example, 20% patients (n=8,625) were the 7th IIA stage as the reference, the other 80% patients (n=34,499) were staged according to the 8th AJCC prognostic staging system while 18,271 patients were downstaged to IB, 3,660 and 7,091 patients upstaged to IIB and IIIA, respectively. The 3-year BCSS rates are 98.7% (IB), 97.2% (IIA), 95.4% (IIB) and 91.6% (IIIA) in the 8th AJCC staging system.
Figure 2.
The AJCC staging systems predict breast cancer-specific survival for primary breast cancer patients and indicate the proportion of patients at risk.
Notes: In order to further estimate the prognostic accuracy of the 8th AJCC prognostic staging system, patients from every stage in the 7th AJCC were randomly separated into two parts: 80% patients were staged according to the 8th AJCC prognostic staging system and the other 20% patients were the 7th stage as the reference. (A) 7th stage IA, (B) 7th stage IB, (C) 7th stage IIA, (D) 7th stage IIB, (E) 7th stage IIIA, (F) 7th stage IIIB, and (G) 7th stage IIIC.
Abbreviation: AJCC, American Joint Committee on Cancer.
Table 5.
Three-year BCSS and OS according to the 8th AJCC prognostic staging system
| IA | IB | IIA | IIB | IIIA | IIIB | IIIC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Rate (%) | P-value | No. (%) | Rate (%) | P-value | No. (%) | Rate (%) | P-value | No. (%) | Rate (%) | P-value | No. (%) | Rate (%) | P-value | No. (%) | Rate (%) | P-value | No. (%) | Rate (%) | P-value | |
| Ref (AJCC 7) | 17,797 (20.0) | 99.0 | 914 (20.0) | 97.4 | 8,625 (20.0) | 96.6 | 1,913 (20.0) | 91.1 | 2,580 (20.0) | 88.4 | 748 (20.0) | 71.5 | 1,041 (20.0) | 78.9 | |||||||
| IA (AJCC 8) | 52,949 (59.5) | 99.5 | <0.001 | 2,703 (59.2) | 99.6 | <0.001 | |||||||||||||||
| IB (AJCC 8) | 10,350 (11.6) | 99.1 | 0.904 | 648 (14.2) | 97.5 | 0.980 | 18,271 (42.4) | 98.7 | <0.001 | 1,817 (19.0) | 98.0 | <0.001 | 415 (3.2) | 94.2 | 0.073 | ||||||
| IIA (AJCC 8) | 7,889 (8.9) | 97.2 | <0.001 | 302 (6.6) | 91.9 | 0.002 | 5,477 (12.7) | 97.2 | 0.856 | 764 (5.9) | 97.7 | <0.001 | |||||||||
| IIB (AJCC 8) | 3,660 (8.5) | 95.4 | 0.364 | 79 (0.8) | 96.6 | 0.974 | 3,802 (29.5) | 95.6 | <0.001 | ||||||||||||
| IIIA (AJCC 8) | 7,091 (16.4) | 91.6 | <0.001 | 2,194 (22.9) | 93.8 | 0.025 | 357 (2.8) | 90.8 | 0.406 | 162 (4.3) | 90.7 | 0.010 | 219 (4.2) | 94.1 | 0.208 | ||||||
| IIIB (AJCC 8) | 1,163 (12.2) | 85.7 | 0.009 | 3,054 (23.7) | 88.7 | 1.000 | 930 (24.9) | 88.0 | <0.001 | 1,449 (27.8) | 92.2 | <0.001 | |||||||||
| III C (AJCC 8) | 2,396 (25.1) | 81.6 | <0.001 | 1,925 (14.9) | 75.8 | <0.001 | 1,896 (50.8) | 69.0 | <0.001 | 2,496 (48.0) | 69.6 | <0.001 | |||||||||
| Ref (AJCC 7) | 17,797 (20.0) | 97.0 | 914 (20.0) | 94.7 | 8,625 (20.0) | 93.6 | 1,913 (20.0) | 88.8 | 2,580 (20.0) | 86.5 | 748 (20.0) | 67.4 | 1,041 (20.0) | ||||||||
| IA (AJCC 8) | 52,949 (59.5) | 97.2 | 0.005 | 2,703 (59.2) | 98.3 | <0.001 | |||||||||||||||
| IB (AJCC 8) | 10,350 (11.6) | 96.9 | 0.996 | 648 (14.2) | 95.3 | 0.991 | 18,271 (42.4) | 95.0 | <0.001 | 1,817 (19.0) | 95.0 | <0.001 | 415 (3.2) | 92.6 | 0.224 | ||||||
| IIA (AJCC 8) | 7,889 (8.9) | 95.0 | <0.001 | 302 (6.6) | 95.8 | 0.135 | 5,477 (12.7) | 93.7 | 0.997 | 764 (5.9) | 95.8 | <0.001 | |||||||||
| IIB (AJCC 8) | 3,660 (8.5) | 91.8 | 0.196 | 79 (0.8) | 92.8 | 0.836 | 3,802 (29.5) | 93.2 | <0.001 | ||||||||||||
| IIIA (AJCC 8) | 7,091 (16.4) | 88.7 | <0.001 | 2,194 (22.9) | 90.7 | 0.238 | 357 (2.8) | 87.1 | 0.979 | 162 (4.3) | 84.5 | 0.286 | 219 (4.2) | 75.4 | 0.263 | ||||||
| IIIB (AJCC 8) | 1,163 (12.2) | 82.6 | 0.013 | 3,054 (23.7) | 86.5 | 1.000 | 930 (24.9) | 79.2 | <0.001 | 1,449 (27.8) | 91.0 | <0.001 | |||||||||
| IIIC (AJCC 8) | 2,396 (25.1) | 78.8 | <0.001 | 1,925 (14.9) | 72.7 | <0.001 | 1,896 (50.8) | 62.4 | <0.001 | 2,496 (48.0) | 89.6 | <0.001 | |||||||||
Note: The bold values emphasize the significant statistical difference.
Abbreviations: AJCC, American Joint Committee on Cancer; OS, overall survival; BCSS, breast cancer-specific survival.
When adjusted for the age at diagnosis, the 8th AJCC prognostic staging system that included grade, ER, PR, and HER2 status had a lower AIC than the 7th AJCC staging system (Table 6) further suggesting the 8th AJCC staging system yielded more precise prognostic information and performed as a superior staging system.
Table 6.
Akaike’s information criterion (AIC)
| Variables | 7th AJCC staging system | 8th AJCC prognostic staging system | ||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | χ2 | P-value | AIC | HR (95% CI) | χ2 | P-value | AIC | |
| Age | 1.02 (1.02, 1.03) | 506.44 | <0.001 | 101569.38 | 1.03 (1.03, 1.03) | 729.82 | <0.001 | 99317.24 |
| Stage | ||||||||
| IA | Reference | Reference | ||||||
| IB | 2.07 (1.58, 2.73) | 27.05 | <0.001 | 3.05 (2.60, 3.58) | 185.58 | <0.001 | ||
| IIA | 4.16 (3.75, 4.61) | 728.46 | <0.001 | 6.93 (5.91, 8.13) | 566.34 | <0.001 | ||
| IIB | 12.78 (11.41, 14.33) | 1918.47 | <0.001 | 10.11 (8.52, 12.00) | 703.69 | <0.001 | ||
| IIIA | 14.19 (12.78, 15.76) | 2453.31 | <0.001 | 18.51 (15.98, 21.45) | 1510.28 | <0.001 | ||
| IIIB | 33.20 (29.65, 37.17) | 3689.94 | <0.001 | 27.85 (24.04, 32.26) | 1963.85 | <0.001 | ||
| IIIC | 29.95 (26.89, 33.35) | 3830.94 | <0.001 | 70.33 (61.49, 80.45) | 3851.01 | <0.001 | ||
Unexpectedly, stage IB patients had a worse prognosis than IIA and IIB in the 8th AJCC (Figure 2E and Table 5); stage IIIA in the 7th AJCC migrated to IB (3-year BCSS, 94.2%) and this patient group had a worse prognosis than IIA (3-year BCSS, 97.7%) or IIB (3-year BCSS, 95.6%) in the 8th prognostic staging system. This phenomenon was not found in stage IIA and IIB patients in the 7th staging system restaged to IB in the 8th staging system (Figure 2C, D and Table 5).
Discussion
The 8th AJCC prognostic staging system for breast cancer builds upon the 7th AJCC but incorporates biological factors peer-reviewed5 and unpublished data from 238,265 patients diagnosed with invasive breast cancer from 2010 to 2011 in the US National Cancer Database.16 This population-based (SEER) study examined 168,076 primary breast cancer patients, directly compared the two staging systems, and confirmed the prognostic accuracy of the 8th AJCC prognostic staging system (incorporating tumor grade, ER, PR, and HER2 status as staging biologic factors) was superior to the 7th AJCC staging system. Abdel-Rahman also reported the improvement in the discriminatory value for the 8th AJCC prognostic staging system compared to the anatomical staging system using the patient information extracted from the SEER research database.17 When we get the updated version of the 8th AJCC prognostic staging system, the uncategorized 16,145 (8.8%) patients could be restaged according to the updated version (Table S8). We analyzed all again and got the same results. 1) Three-year BCSS and OS rates by stage indicate the 3-year survival for stage IIIB was still inferior to stage IIIC in the 7th AJCC staging system. This inconsistency was not reproduced in the 8th AJCC prognostic staging system (Figure S2). 2) Stage IIIA in the 7th AJCC migrated to IB, and this patient group had a worse prognosis than IIA or IIB in the 8th prognostic staging system (Figure S3).
Prior, smaller, studies including SEER data successfully incorporated grade, ER status, and HER2 to refine patient stratification with respect to prognosis.5 Others have incorporated the triple negative phenotype (ER, PR, and HER2 all negative) to upstage the anatomic staging groups of the TNM and in doing so improved the prognostic accuracy of breast cancer staging.18 In the 7th AJCC staging system, the stage IIIB has an inferior prognosis than IIIC for BCSS and OS (Table 4 and Figure 1). As shown in Table S6, ER negative (IIIB 33.5% vs IIIC 26.4%), PR negative (IIIB 46.2% vs IIIC 24.6%), HER2 positive (IIIB 26.6% vs IIIC 23.0%), ER negative/PR negative (IIIB 31.4% vs IIIC 24.9%), and ER negative/PR negative/HER2 negative (IIIB 20.9% vs IIIC 16.1%) were mainly distributed in stage IIIB. However, these factors were independent risk factors for BCSS and OS (Table S7). Evidence from the current analyses evaluating the 7th AJCC staging system (Table 4 and Figure 1, Table S6, Table S7) demonstrated the improved prognostic staging through incorporating these biological factors into the 8th AJCC system.
It has been suggested that the new staging system results in a restaging of a substantial proportion (41%) of patients to a different stage group thus improving the grouping of patients with a similar prognosis.14 In our study, 53.2% patients were restaged to a different stage group using the 8th AJCC. However, it is unfortunate that 16,145 cases could not be restaged according to the 8th AJCC prognostic staging system (Table S1). Some 58.7% cases were T2N1M0-stage IIB in the 7th AJCC, and ER positive, PR positive and HER2 negative. Any impact of this dissonance in clinical practice and trial comparisons remains to be seen.
Although Abdel-Rahman reported the similar results with our analysis,17 he missed reporting the paradoxical phenomenon that stage IIIA patients in the 7th staging sys tem who migrated to stage IB had worse but not superior prognosis than IIA and IIB patients in the 8th staging system (Figure 2E and Table 5). Patients considered stage IIIA in the 7th staging system (ER positive/PR positive/HER2 positive and grade 1–2) migrated to stage IB (3-year BCSS, 94.2%) with a worse prognosis than IIA (3-year BCSS, 97.7%) and IIB (3-year BCSS, 95.6%) in the 8th staging system. Those moving from stage IIIA to IIA or IIB (n=5,739) included ER-positive/PR-positive/HER2-negative, grade 1–2 patients (86.6%) and ER-positive/PR-positive/HER2-positive and grade 3 patients (13.4%). As shown in Table S7, the ER-positive/PR-positive/HER2-negative patients have a relatively superior prognosis to the ER-positive/PR-positive/HER2-positve patients with the identical TNM stage and tumor grade. However, the impact of HER2-targeted therapy19 and consideration of the increasing use of neo-adjuvant therapy, particularly for HER2 and triple negative breast cancer, may necessitate merit further refinement of AJCC staging in future. Another needed further refinement of AJCC prognostic staging system was the stage IV group; a small retrospective study identified that triple negative breast cancer had worse OS than ER-positive and HER2-negative carcinomas among M1 patients.20 It suggested that separating stage IV patients according to biomarkers (ER, PR, and HER2) makes sense.
Recognized limitations of using SEER data include the geographical origins and missing data points, particularly in this comparison the Oncotype Dx® recurrence score. In the 8th AJCC prognostic staging system, patients with T1–2N0M0, who were ER positive and HER2 negative, and with an Oncotype Dx® recurrence score <11 should be staged as stage I, given the strong published evidence.13,21 Future comparisons using SEER data may be possible to confirm this element of the 8th AJCC.
Conclusion
This comparison of the prognostic accuracy of the 8th AJCC prognostic staging system to the 7th staging system using data from over 168,000 breast cancer patients confirms the enhanced value of the 8th AJCC. However, nuances of the modified staging, gene panel testing and influences of current therapies will require ongoing re-evaluation of the staging system.
Ethics approval and informed consent
All procedures performed in study involving human data were extracted freely from the SEER Research Data available to the public online (https://seer.cancer.gov/data/access.html).
Acknowledgments
This work was supported by National Natural Science Foundation of China (81702848) and Sun Yat-Sen University Clinical Research 5010 Program (2016007).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 2.Carr DT. The manual for the staging of cancer. Ann Intern Med. 1977;87(4):491–492. doi: 10.7326/0003-4819-87-4-491. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi M, Mittendorf EA, Cormier JN, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011;29(35):4654–4661. doi: 10.1200/JCO.2011.38.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(10):1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy MJ, Harbeck N, Nap M, et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM) Eur J Cancer. 2017;75:284–298. doi: 10.1016/j.ejca.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies – improving the management of early breast cancer: St gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26(19):3153–3158. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- 10.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 11.Elston EW, Ellis IO. Method for grading breast cancer. J Clin Pathol. 1993;46(2):189–190. doi: 10.1136/jcp.46.2.189-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 13.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez-Macgregor M, Mittendorf EA, Clarke CA, Lichtensztajn DY, Hunt KK, Giordano SH. Incorporating tumor characteristics to the American Joint Committee on Cancer Breast Cancer Staging System. Oncologist. 2017;22(11):1292–1300. doi: 10.1634/theoncologist.2017-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moshirpour M, Far B, Alhajj R. Highlighting the Importance of Big Data Management and Analysis for Various Applications. UK, London: Springer; 2017. [Google Scholar]
- 16.Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Rahman O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat. 2018;168(1):269–275. doi: 10.1007/s10549-017-4577-x. [DOI] [PubMed] [Google Scholar]
- 18.Bagaria SP, Ray PS, Sim MS, et al. Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg. 2014;149(2):125–129. doi: 10.1001/jamasurg.2013.3181. [DOI] [PubMed] [Google Scholar]
- 19.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zombori T, Lehóczky L, Cserni B, Nyári T, Cserni G. Evaluation of anatomic and prognostic stages of breast cancer according to the 8th edition of the TNM staging system – retrospective analysis based on data from deceased patients once diagnosed with breast cancer. Orv Hetil. 2017;158(35):1373–1381. doi: 10.1556/650.2017.30849. [DOI] [PubMed] [Google Scholar]
- 21.Roberts MC, Miller DP, Shak S, Petkov VI. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX recurrence score results in the SEER database. Breast Cancer Res Treat. 2017;163(2):303–310. doi: 10.1007/s10549-017-4162-3. [DOI] [PubMed] [Google Scholar]