Abstract
Since the blood vessel epicardial substance or Popeye domain-containing protein 1 (BVES/POPDC1) was first identified in the developing heart by two independent laboratories in 1999, an increasing number of studies have investigated the structure, function, and related diseases of BVES/POPDC1. During the first 10 years following the discovery of BVES/POPDC1, studies focused mainly on its structure, expression patterns, and functions. Based on these studies, further investigations conducted over the previous decade examined the role of BVES/POPDC1 in human diseases, such as colitis, heart diseases, and human cancers. This review provides an overview of the structure and expression of BVES/POPDC1, mainly focusing on its potential role and mechanism through which it is involved in human cancers.
Keywords: blood vessel epicardial substance, Popeye domain-containing protein 1, cancer, Rho
Introduction
Blood vessel epicardial substance (BVES), also known as Popeye domain-containing protein 1 (POPDC1), belongs to the POPDC family, which shares the same Popeye structure within the intracellular C-terminus.1,2 The POPDC family consists of BVES/ POPDC1, POPDC2, and POPDC3. These genes produce evolutionarily conserved transmembrane proteins and do not share significant structural homology with any other identified proteins.2 BVES/POPDC1 is 359 amino acids long, is principally localized to the plasma membrane, and contains an extracellular amino terminus, three transmembrane domains, and a cytoplasmic Popeye domain.3 The extracellular N-terminus of BVES/POPDC1 contains two invariant N-glycosylation sites, which are thought to be dispensable. However, they may be involved in the protection of BVES/ POPDC1 from proteolysis and localization of BVES/POPDC1 to the membrane.4,5 The intracellular C-terminus of BVES/POPDC1 includes the novel but highly conserved Popeye domain. BVES/POPDC1 protein forms a dimer or multimer in the cell within the Popeye domain (Figure 1). These BVES–BVES interactions are necessary to maintain epithelial integrity and junctional stability.6
Figure 1.
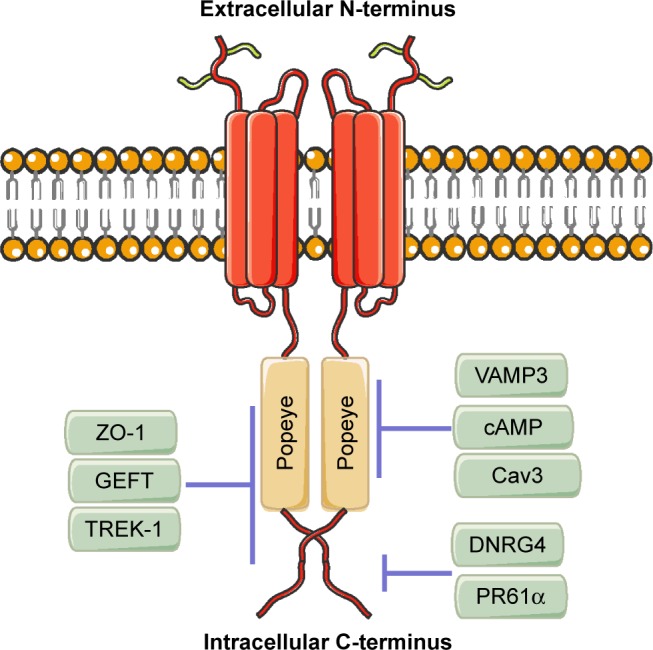
BVES/POPDC1 structure.
Notes: BVES/POPDC1 protein consists of an extracellular N-terminus, a three- pass transmembrane domain, and an intracellular C-terminus containing the highly conserved Popeye domain. BVES/POPDC1 directly binds to cAMP, VAMP3, and Cav3 in the Popeye domain, directly interacts with DNRG4 and PR61α in the intracellular C-terminus outside of Popeye domain, and directly interacts with ZO-1, GEFT, and TREK-1 in the intracellular C-terminus both inside and outside of Popeye domain.
BVES/POPDC1 transcripts have been identified in an array of eukaryotes ranging from honey bees to human beings.7 Human BVES/POPDC1 has been identified in the heart, smooth and skeletal muscle, brain, liver, gastrointestinal tract, and various epithelia.2,8–13 BVES/POPDC1 is a newly discovered cyclic 3′,5′-adenosine monophosphate (cAMP) effector14 and a caveolae-associated protein important for the tolerance of environmental stress (eg, oxygen deficiency and nutrient deprivation). Moreover, it is involved in the regulation of cell calcium and survival pathway signaling.15,16 Interestingly, BVES/POPDC1-expressing cells have a common phenotype or function: they are adherent or are at least highly interactive in nature.7 Subcellular localization studies revealed that BVES/POPDC1 is mainly localized in the cytomembrane, especially in the lateral cell membrane, cell junction, and tight junction.17 Its movement from the cytoplasm to membrane is an early event occurring concurrently with cell–cell contact.17,18 BVES/POPDC1 colocalizes in epithelial cells with Occludin and ZO-1 in an apical–lateral position within the z-axis.17 This expression pattern indicates a role of BVES/POPDC1 in cell communication or cell–cell adhesion.
The expression of BVES/POPDC1 during embryogenesis has been studied in several organisms, including chick,13 mouse,12 Xenopus laevis,19 and Drosophila.20 In these studies, BVES/POPDC1 was found in a multitude of tissue types derived from all three germ layers both in the embryo and adults. In 2008, Feng et al21 were the first to link the expression of BVES/POPDC1 to non-small-cell lung cancer (NSCLC) in human beings. In the following 10 years, accumulating evidence has revealed the role and mechanism of BVES/POPDC1 in human diseases, especially in cancers.
BVES/POPDC1 in cancers
We have summarized the articles discussing the role of BVES/POPDC1 in human cancers in Table 1.
Table 1.
The expression and function of BVES in human cancers
| Cancer | BVES expression | Detection method | Detection objects | Implied function | BVES with clinicopathological features | Publication year | Reference |
|---|---|---|---|---|---|---|---|
|
| |||||||
| NSCLC | ↓ | MethyLight assay | 49 paired NSCLC and matched normal tissues | Monitoring and detecting tumor recurrence in early-stage NSCLC after curative surgical resection | Not mentioned | 2008 | 18 |
| GC | ↓ | qPCR, MeDIP, BS-seq, pyrosequencing | 76 paired GC and normal tissues; 11 GC cell lines | Epigenetic inactivation of BVES promotes GC cell migration and invasion | None | 2010 | 20 |
| ↓ | IHC | 306 GC and 78 noncancerous gastric tissues | Potential biomarker for GC prognosis | Correlated with histological differentiation, depth of invasion, regional lymph nodes and distant metastasis, and TNM stages | 2012 | 21 | |
| UM | Not mentioned | Not mentioned | 3 UM cell lines (OM431, OMM1, and OMM2.3) | Modulating signaling pathways relevant to proliferation | Not mentioned | 2011 | 23 |
| CRC | ↓ | qPCR, Affymetrix array | 10 normal samples, 6 adenomas, and 250 CRC samples; 18 matched CRC and normal tissues | BVES prevents EMT and impairs growth and metastasis of an orthotopic xenograft | Correlated with CRC stage | 2011 | 23 |
| ↓ | Infinium HumanMethylation450 array screen, Pyrosequencing, RNAscope | 17 patients do not have UC 11 patients with UC do not have dysplasia or carcinoma, 10 patients with UC, and 10 patients with UC who have dysplasia/carcinoma | Loss of BVES promotes inflammatory tumorigenesis through dysregulation of WNT signaling | Not mentioned | 2017 | 25 | |
| HCC | ↓ | qPCR, WB, IHC | 21 HCC and corresponding paracancerous tissues; 4 HCC cell lines (Huh7, HepG2, SMMC-7721, and SK-HEP-1) | Downregulation of BVES induces EMT | None | 2014 | 31 |
| ↓ | qPCR, WB, IF | 37 HCC and matched nontumor tissues; 5 HCC cell lines (Huh7, SMMC7721, HepG2, MHCCLM3, and SK-Hep-1) | BVES inhibits migration and invasion of HCC cells and is regulated by netrin-1 via PI3K/AKT pathway | None | 2015 | 32 | |
| ↓ | MethyLight assay | 98 patients with HCC, 75 patients with LC, 90 patients with CHB, and 80 healthy individuals | Combined detection of the methylation of BVES, RASSF1A, and HOXA9 gene promoters in serum and AFP could significantly improve HBV-related HCC diagnoses | Not mentioned | 2017 | 34 | |
| BC | ↓ | IHC | 6 benign lesion, 9 DCIS, and 95 BC | BVES is suppressed in BC and can potentially be targeted to inhibit EGFR-mediated cell migration and proliferation | None | 2017 | 35 |
| ↓ | WB, ICC | BC cell lines (MDA231, SKBR3, MCF7, and MCF10A) | BVES inhibits BC cell migration and proliferation | Not mentioned | 2017 | 36 | |
Abbreviations: BC, breast cancer; BS-seq, bisulfite sequencing; CHB, chronic hepatitis B; CRC, colorectal carcinoma; DCIS, ductal carcinoma in situ; EMT, epithelial–mesenchymal transition; GC, gastric cancer; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ICC, immunocytochemical analysis; IF, immunofluorescence staining; IHC, Immunohistochemistry; LC, liver cirrhosis; MeDIP, methylated DNA immunoprecipitation; NSCLC, non-small-cell lung cancer; qPCR, quantitative PCR; UM, uveal melanoma; WB, Western blot.
BVES/POPDC1 in lung cancer
Feng et al21 performed MethyLight assays to detect the DNA methylation status of 27 genes in 49 paired cancerous and noncancerous tissues obtained from patients with NSCLC who underwent surgical resection. They found that seven genes significantly more frequently methylated at high levels (percentage of methylated reference ≥4%) in cancerous vs noncancerous tissue, and among which, BVES/POPDC1 had high levels of methylation in cancerous but never noncancerous tissue.21 Feng et al’s study was the first to incorporate BVES/POPDC1 into the genes frequently methylated in NSCLC.21 In 2010, this research group studied the early events of lung cancer by analyzing lung tissue samples obtained from 151 cancer-free subjects (121 smokers and 30 nonsmokers) for hypermethylation of genes previously observed to be hypermethylated in NSCLC. They found that BVES/POPDC1 was rarely hypermethylated (<2%) in these subjects, suggesting that BVES/POPDC1 methylation is not the preneoplastic event leading to NSCLC.22 However, they speculated that the detection of BVES/POPDC1 methylation may be conducive to monitor and detect tumor recurrence in early-stage NSCLC after curative surgical resection.22
BVES/POPDC1 in gastric cancer
Kim et al23 examined the hypermethylation of POPDCs in gastric cancer and found that the expression of BVES/ POPDC1 and POPDC3 was silenced in 8/11 (73%) gastric cancer cell lines investigated. Further analysis suggested that the hypermethylation of the BVES/POPDC1 and POPDC3 promoters correlated with their decreased expression in gastric cancer cell lines. BVES/POPDC1 and POPDC3 were hypermethylated in 69% and 64% of gastric cancer tissues, respectively. Treatment with the DNA methylation inhibitor 5-aza-dC and the histone deacetylase inhibitor trichostatin A (TSA) restored the expression of BVES/POPDC1 in gastric cancer cell lines SNU-601, SNU-620, and SNU-638.23 In addition, they found that BVES/POPDC1 and POPDC3 were downregulated in EGF-induced epithelial–mesenchymal transition (EMT). Moreover, silencing of POPDC3 promoted the migration and invasion of gastric cancer cells. However, these results did not reveal a direct role of BVES/POPDC1 in gastric cancer.23 There was no significant correlation between the expression of BVES/POPDC1 or POPDC3 and clinical characteristics. The investigators speculated that the inactivation of BVES/POPDC1 and POPDC3 is an early-stage process in gastric cancer prior to the occurrence of metastasis. However, Luo et al24 examined 306 human gastric cancer and 78 noncancerous gastric tissues and found that the expression of BVES/POPDC1 was decreased in gastric cancer tissue. The low expression of BVES/POPDC1 correlated with histological differentiation, depth of invasion, regional lymph node and distant metastasis, and TNM stage. They also suggested that the reduced expression of BVES/POPDC1 is associated with the progression of gastric cancer and poor survival.24 The contradictory results obtained from these two studies may be attributed to the following: 1) Kim et al analyzed the level of BVES/POPDC1 methylation and clinicopathological characteristics, whereas Luo et al compared the protein level of BVES/POPDC1 with clinicopathological characteristics through immunohistochemistry; 2) Kim et al only analyzed a limited range of clinicopathological characteristics (ie, age, gender, histology, and TNM stage), whereas the research conducted by Luo et al included a more comprehensive clinicopathological profile (ie, age, gender, location, size, histology, histological differentiation, invasion depth, regional lymph node metastasis, distant metastasis, and TNM stage); 3) The number of experimental samples and experimental groups were different: Kim et al studied the expression of BVES/POPDC1 in 96 pairs of gastric cancer tissues and adjacent healthy tissues, whereas Luo et al performed their investigation in 306 gastric cancer tissues and 78 noncancerous gastric tissues. Further research is warranted to clarify the role of BVES/POPDC1 in gastric cancer metastasis and the molecular mechanism involved in this process.
BVES/POPDC1 in colorectal cancer
The expression of BVES/POPDC1 was found to be decreased in all stages of human colorectal carcinoma and in adenomatous polyps.25 Similar to lung cancer and gastric cancer, the low expression of BVES/POPDC1 in colorectal carcinoma and adenoma were mainly due to hypermethylation of the gene promoter on the cytosine–phosphate–guanine island.25 Overexpression of BVES/POPDC1 promoted the epithelial phenotype of colorectal cancer cells, including decreased proliferation, migration, invasion, and anchorage-independent growth as well as impaired growth and metastasis in an orthotopic xenograft.25 More recently, BVES/POPDC1 was shown to be reduced in human ulcerative colitis and colitis-associated cancer biopsy specimens.26 Furthermore, BVES/POPDC1-knockout mice (BVES/POPDC1−/−) presented with increased crypt height, elevated proliferation, decreased apoptosis, altered intestinal lineage allocation, and dysregulation of permeability and intestinal immunity.26 Furthermore, BVES/POPDC1−/− mice exhibited increased tumor multiplicity and degree of dysplasia after treatment with azoxymethane and dextran sodium sulfate.26 Molecular analysis revealed that the knockdown of BVES/POPDC1 increased c-Myc stability – an oncogene that is dysregulated in numerous malignancies – and subsequently increased the expression of downstream key target genes, such as ornithine decarboxylase and carbamoyl phosphate synthetase 2 aspartate transcarbamylase and dihydroorotase. However, the overexpression of BVES/POPDC1 reduced c-Myc stability and increased c-Myc ubiquitylation.26 In addition, BVES/POPDC1 was shown to regulate tumor proliferation in colorectal cancer via the WNT signaling pathway.25 Interestingly, Xing et al27 performed a bioinformatics analysis of colon adenocarcinoma (COAD) RNA-seq V2 exon data obtained from The Cancer Genome Atlas data portal, which included 285 tumor samples and 41 pericarcinomatous tissue samples. The results showed that BVES/POPDC1-AS1 and other three lncRNAs (MYLK-AS1, ADAMTS9-AS1, and FENDRR) were shown to have significant co-regulatory relationships or functional synergistic effects. All these lncRNAs were downregulated in COAD, suggesting their critical roles in the development and progression of COAD.27 The research also revealed another posttranscriptional modification except for hypermethylation. However, further studies are warranted to illustrate the role of BVES/POPDC1-AS1 in colorectal cancer.
BVES/POPDC1 in hepatocellular carcinoma (HCC)
The tight junction is one of the most important intercellular structures of the liver.28 The tight junction and its associated proteins are usually found to be decreased in HCC, such as ZO-1, Claudin-1, and twist.29–31 Our experimental group investigated the expression of BVES/POPDC1 in human HCC tissues and found that it was downregulated compared with that observed in corresponding paracancerous tissues.32,33 The expression of BVES/POPDC1 was also decreased in HCC cell lines. Interestingly, the decreased expression of BVES/POPDC1 was related to the invasion potential of HCC cell lines, ie, BVES/POPDC1 expression was inversely correlated with the metastatic potential of HCC cell lines.32 Knockdown of BVES/POPDC1 triggered EMT, including morphological changes in cytoskeleton rearrangement and junctional disruption, elevated expression of matrix metalloproteinase (MMP)2, MMP9, and IL-6 and decreased E-cadherin, and importantly, increased cell migration and invasion.32 Furthermore, we found that the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway may be another important upstream regulating signal pathway for BVES/POPDC1. Of note, the PI3K/Akt pathway inhibitor LY-294002 restored the decreased expression of BVES/POPDC1 inhibited by rhnetrin-1.33 DNA methylation remains an important modification leading to the decreased expression of BVES/POPDC1 in HCC.34 Dong et al35 detected the methylation status of multiple gene promoters in the serum of patients with hepatitis B virus (HBV)-related HCC, liver cirrhosis, chronic hepatitis B, and healthy individuals. The rate of hypermethylation of the BVES/POPDC1 promoter in HCC patients was 29.59%, higher than that observed in those with liver cirrhosis (4%), chronic hepatitis B (1.11%), and healthy individuals (0). The combined detection of BVES/POPDC1 methylation with that of RASSF1A, HOXA9, and AFP in HCC patients yielded a sensitivity and specificity of 83.7% and 78.9%, respectively.35 Therefore, the use of a combined methylation kit for the promoters of the BVES/POPDC1, RASSF1A, and HOXA9 genes in the serum and AFP may improve the diagnosis of HBV-related HCC in clinical practice. However, additional research is necessary to confirm the effectiveness of this approach.
BVES/POPDC1 in breast cancer
Contrary to the high expression of POPDC2 and POPDC3 in breast cancer, the expression of BVES/POPDC1 is decreased at all stages of ductal breast carcinoma tissues and in all molecular subtypes of breast cancer (ie, luminal A, luminal B, human EGFR2 positive, and triple negative). Moreover, it is suppressed in the more aggressive breast cancer cell lines compared with that observed in nonmalignant breast cancer cells.36 Consistently with previous studies in HCC and gastric cancer, BVES/POPDC1 is not correlated with the clinical progression of breast cancer.36 This phenomenon suggests that the inhibition of BVES/POPDC1 is a feature of all clinical stages of these cancers, and the early molecular alteration of BVES/POPDC1 may represent an initiation step in the malignant process. Indeed, the suppression of BVES/POPDC1 promotes the migration and proliferation of breast cancer cells, whereas the overexpression of BVES/ POPDC1 inhibits this malignant phenotype.37 There is a significant inverse correlation between BVES/POPDC1 and EGFR expression in both stage 2 and stage 3 breast cancer tissues. Notably, EGF protein significantly suppressed BVES/POPDC1 expression in MCF7, MDA231, and SKBR3 breast cancer cells, whereas the use of the EGFR inhibitor AG1478 (1 mM concentration) increased the level of BVES/ POPDC1.36 Further study found that the overexpression of BVES/POPDC1 attenuated EGF-mediated cell migration and proliferation in breast cancer cells.36 Previous studies also proved that the EGFR signaling pathway regulates BVES/ POPDC1 expression in certain follicle cells of Drosophila during oogenesis and in gastric cancer cells.20 The increased expression of EGF and EGF receptors has been reported to be a potent stimulator of cancer cell migration and invasion.38–40 Furthermore, EGFR-targeted therapies, including monoclonal antibodies,41,42 tyrosine kinase inhibitors,43–45 PI3K inhibitors,46,47 and antisense gene therapy,48 have been shown to be effective in cancer cells, especially those of breast cancer. Therefore, in the following 10 years, molecular drugs targeting the EGF/BVES/POPDC1 pathway may provide new strategies for cancer therapy.
BVES/POPDC1 in eye neoplasms
BVES/POPDC1 is localized to an apical–lateral position in the initial epithelial primordia of the eye.9 Later, during morphogenesis and in the adult, BVES/POPDC1 is redistributed in a cell type-specific manner in the cornea, lens, and retina.9 In an in vitro model of corneal wound healing, BVES/POPDC1 was found to be lost at the epithelial surface during cellular migration across the wound. However, it was restored at the contact area during the reinitiation of epithelial continuity.9 Morpholino knockdown of BVES/ POPDC1 expression disrupted human corneal epithelial integrity. Following injury, this treatment accelerated cell movement at the wound surface but impacted the regeneration of an intact epithelium.9 These results confirmed that BVES/POPDC1 regulates epithelial adhesion and movement during organogenesis of the eye.
Russ et al49 verified that the upregulation of BVES/ POPDC1 expression in trabecular meshwork cells leads to increased tight junction (TJ) formation with decreased activation of RhoA. Manipulation of BVES/POPDC1 expression in human corneal epithelial cell line resulted in reciprocal changes in epithelial–mesenchymal phenotypes.25 These observations indirectly identify BVES/POPDC1 as a regulator of EMT. EMT in tumor cells is similar to that observed in wound healing and organogenesis,50 suggesting that BVES/ POPDC1 may play an important role in regulating EMT processes in ocular tumor cells.
BVES/POPDC1 in other diseases
BVES/POPDC1 in heart disease
BVES/POPDC1 is strongly expressed in the heart and skeletal muscle, and this expression pattern is observed in all animal models that have been studied thus far, eg, Drosophila,20 chick,1 zebrafish,51 Xenopus,19 and mice.12 In the adult heart, BVES/POPDC1 is highly expressed in the atria vs the ventricles.14 In addition, it is elevated in the cardiac conduction which includes the sinoatrial node, the atrioventricular node, the His bundle, the bundle branches, and the Purkinje fibers.14 Functional analysis of BVES/POPDC1 suggested an overlapping role for proper electrical conduction in the heart and maintenance of structural integrity in skeletal muscle. In BVES/POPDC1−/− mice, the presence of stress-induced sinus bradycardia has been reported, following the exposure of BVES/POPDC1−/− mice to physical exercise, mental stress, or injection of isoproterenol.14 The mean heart rate of the null mouse mutants was significantly lower, and the sinoatrial node (SAN) pacemaker was pausing for different lengths of time.14 Interestingly, this pathological phenotype was not present in young mice; however, at 5–8 months of age, these mutants displayed severe stress-induced bradycardia with episodes of sinus node dysfunction.14 The age-dependent phenotype in the mutants is reminiscent of the sick sinus syndrome (SSS), which is the most frequent reason for the implantation of a pacemaker and the most prevalent condition in elderly individuals without other heart diseases. Therefore, it has been speculated that, in SSS patients, the disease may be caused by abnormal expression or function of BVES/POPDC1.
During myocardial ischemia/reperfusion (I/R), a series of damaging changes that occur in myocardial ultrastructure, energy metabolism, cardiac function, and electrophysiology are more prominent after vascular recanalization.52 This pathological phenotype is termed myocardial I/R injury. Alcalay et al16 found that both the protein and mRNA levels of BVES/POPDC1 were decreased during I/R. Induction of myocardial I/R caused a marked lower functional recovery in BVES/POPDC1 null mutants compared with the wild-type (WT) as well as a larger infarct size.16 Cardiac myocytes isolated from BVES/POPDC1 null mutants appeared impaired Ca+2 transients, increased vulnerability to oxidative stress and no pharmacologic preconditioning.16 Further research revealed the colocalization of BVES/POPDC1 with Caveolin-3 (Cav3), and BVES/POPDC1 is a caveolae-associated protein and is important for the preservation of caveolae structural and functional integrity.16 A recent study performed by this research group using myocytes treated with BVES/ POPDC1 siRNA revealed that BVES/POPDC1 is required for the survival of cardiac myocytes under serum deficiency.15 Moreover, silencing of BVES/POPDC1 in rat neonatal cardiomyocytes increases the expression of cell death regulator Bcl-2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3), while attenuating Rac1 activity and modifying the interaction of FoxO3 and NF-κB transcription factors with the Bnip3 promoter.15 These results suggest that BVES/ POPDC1 may serve as a potential target to enhance heart protection. However, at present, the evidence regarding the role of BVES/POPDC1 in human heart disease is limited.
Zhang et al53 found that BVES/POPDC1 is one of the differentially expressed genes identified in ventricular septal defect and normal human ventricular septum myocardium using suppression subtractive hybridization.53 These genes are mainly involved in energy metabolism, cell cycle and growth, cytoskeleton and cell adhesion, LIM protein, zinc finger protein, and development. Gingold-Belfer et al54 detected the expression of POPDC proteins in biopsies from non-failing and failing human hearts and found that the levels of BVES/POPDC1 and POPDC3 were decreased in failing hearts. However, inconsistent with the expression pattern observed in mice, BVES/POPDC1 was expressed in the four human heart chambers and its expression levels were higher in the ventricles than in the atria.54 These differences may be due to compartment-dependent differences. Schindler et al identified a BVES/POPDC1 missense variant (S201F) via whole-exome sequencing in a family of four individuals with cardiac arrhythmias and limb-girdle muscular dystrophy.55 Interestingly, forced expression of BVES/POPDC1S201F in murine cardiac muscle cells increased the hyperpolarization and upstroke velocity of the action potential.55 Furthermore, expressing the homologous mutation of BVES/POPDC1S191F in zebrafish resulted in heart and skeletal muscle phenotypes, as observed in patients.55 This was the first and only study to identify BVES/POPDC1 as a disease-causing gene in human heart diseases.
BVES/POPDC1 and stem cells
Stem cells are an important resource for tissue repair, regeneration, and tumorigenesis.56 A recent study investigated the role of BVES/POPDC1 in mice intestine after ionizing radiation exposure.57 In the study, BVES/POPDC1−/− mice presented with increased crypt size, and the elevated proliferation and expression of stem cell markers (eg, Lgr5, Ascl2, Olfm4, Nanog, Sox9, Lrig1, and Bmi1) and the ex vivo BVES/POPDC1−/− enteroid model exhibited increased stemness with increased plating efficiency, proportion of stem spheroids, retention of cystic structures, amplified WNT signaling, and responsiveness to WNT activation.57 BVES/POPDC1 expression was decreased in WT mice that underwent radiation.57 Moreover, after radiation, BVES/ POPDC1−/− mice showed significantly greater crypt viability compared with WT mice.57 Therefore, these data suggested that BVES/POPDC1 – apart from an intestinal epithelial adhesion molecule – is a key regulator of intestinal stem cell programming and intestinal homeostasis. However, the role of BVES/POPDC1 in cancer stem cells remains unclear.
Potential downstream signaling pathway of BVES/POPDC1
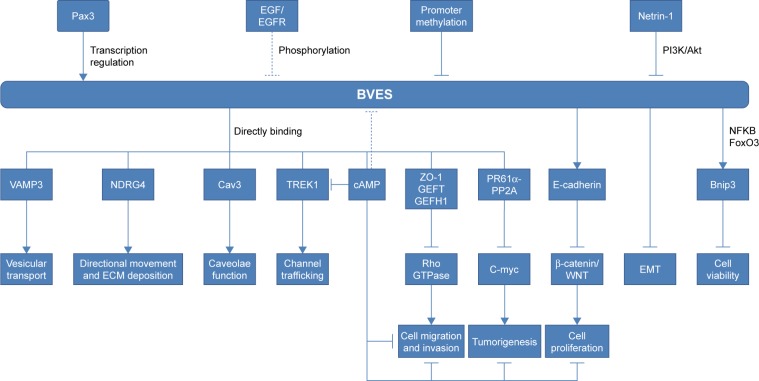
Previous studies have shown that BVES/POPDC1 is a cell adhesion molecule, inhibited in multiple types of solid tumors. However, an increasing number of recent studies have found that BVES/POPDC1 regulates EMT25,32 and modifies intestinal permeability58 and stem cell programs,57 etc. Determining the downstream signaling pathways of BVES/ POPDC1 may provide new insights into tumor research and intervention targeting BVES/POPDC1. We have shown the possible upstream and downstream mechanisms of BVES/ POPDC1 in Figure 2.
Figure 2.
The upstream effectors and downstream targets of POPDC1/BVES.
Notes: → represents promotion or elevation; ⊣ represents inhibition or decrease.
Abbreviations: ECM, extracellular matrix; EMT, epithelial–mesenchymal transition.
GEFT/Rho signaling
GEFT, also known as p63RhoGEF or ARHGEF25, belongs to the Rho guanine nucleotide exchange factor (GEF) family.59,60 GEFT modulates the active state of Rho GTPases by stimulating the exchange of guanosine diphosphate for GTP52,61,62 and further regulates cell proliferation, migration, cell–cell adhesion, and cell cycle regulation.63–66 Rac1, Cdc42, and RhoA are the main members of the Rho family of GTPases. These proteins are found to be upregulated in human tumors, such as breast, testicular, ovarian, liver, and colorectal cancers, and promote the proliferation, migration, and invasiveness of cancer cells.67–70 GEFT is the first identified protein directly interacting with BVES/POPDC1. BVES/POPDC1 is mainly localized in the plasma membrane; thus, the BVES/POPDC1–GEFT interaction results in GEFT being “detained” in the plasma membrane, leading to a decreased number and activation of GEFT in the cytoplasm.71 Exogenous expression of BVES/POPDC1 reduced Rac1 and Cdc42 activity; however, it does not affect the levels of active RhoA in NIH 3T3 cells.71 Another research group found that the increased expression of BVES/POPDC1 in trabecular meshwork cells and human corneal epithelial cells led to increased formation of the tight junction with decreased activation of RhoA.49,72 GEF-H1, also known as ARHGEF2, is another Rho GTPase.73,74 Consistent with the aforementioned study, in the research, the investigators speculated that BVES/POPDC1 is combined with ZO-1 and cingulin to form a tight junction complex, sequestering GEF-H1 in the plasma membrane and modulating RhoA signaling.72 Recently, upregulation of BVES/POPDC1 and inactivation of the RhoA/Rock pathway were observed in the mesenchymal–epithelial transition (MET) of pig fibroblasts75 and human HCC cells.76 Using an RhoA-GTPase activation assay, our group demonstrated that the suppression of BVES/ POPDC1 increased RhoA activity, thereby promoting cell migration and invasion. In contrast, the overexpression of BVES/POPDC1 decreased RhoA activity in HCC cells.33 More recently, we observed the colocalization of BVES/ POPDC1, ZO-1, and GEFT in liver tissues and cells and disappearance in HCC tissues and cells (unpublished data). Based on these findings, this colocalization may be the mechanism through which BVES/POPDC1 directly inhibits Rho activity.
WNT signaling
WNT signaling plays central roles in embryogenesis, tissue homeostasis, wound repair, and malignancy.77,78 Cellular adhesion complexes, including tight and adherens junctions, have been confirmed to be closely related to WNT signaling.79–81 Adherens junctions are modulators of canonical WNT signaling through sequestration of β-catenin at the cell membrane.82,83 Similarly, tight junctions play a fundamental role in outside-in signaling cascades for WNT signaling.84 BVES/POPDC1 is an important regulator of intercellular connection, regulating the expression of E-cadherin in multiple cell lines and tumors.25,32 In thymosin β4-treated mice, BVES/POPDC1 was increased and accompanied by elevated expression of β-catenin.85 Furthermore, BVES/POPDC1 modulates the localization of β-catenin and WNT transcriptional activity in human colorectal carcinoma cells. In addition, the overexpression of BVES/POPDC1 increased the membrane-bound localization of β-catenin, whereas it decreased cytoplasmic expression.25 These in vitro results are consistent with those observed in vivo. The cytoplasmic levels of β-catenin were excessive, and its nuclear localization in BVES/POPDC1−/− mice tumors was higher compared with that observed in WT micetumors.26 Meanwhile, WNT targets (ie, Mmp7, Wisp2, and Rspo4), were upregulated in BVES/POPDC1−/− mice tumors, further confirming that BVES/POPDC1 regulates the WNT signaling pathway.26,57
Protein phosphatase 2A (PP2A)
PP2A is one of the main serine–threonine phosphatases in mammalian cells. It is composed of a 65 kDa structural A subunit (PP2AA or PR65; α and β isoforms) and a 36-kDa catalytic C subunit (PP2AC; α and β isoforms).86 PP2A is a well-established regulator of the cell cycle and apoptosis by counteracting most of the kinase-driven intracellular signaling pathways.87 PP2A complexes inhibit mitogenic and anti-apoptotic signals by dephosphorylating and inactivating MEK1 and ERK kinases, decreasing stability, and inhibiting the function of c-MYC and STAT5.88,89 In addition, PP2A suppresses the translation of oncogenes such as MCL1 and c-MYC through direct and indirect dephosphorylation of EIF4E.90 Similarly, PP2A exhibits pro-apoptotic activity by negatively regulating the PI3K/Akt pathway through direct Akt dephosphorylation, inactivation of anti-apoptotic BCL2, and activation of pro-apoptotic factors BAD and BIM.91 PP2A has been suggested to be genetically altered or functionally inactivated in many solid cancers92–94 and leukaemias95–97 and therefore acts as a tumor suppressor. Recently, Parang et al found that BVES/POPDC1 interacted with PR61α to promote c-Myc degradation through yeast- two-hybrid, co-immunoprecipitation, and a proximity ligation assay.26 They identified a fragment (15 amino acids) of human BVES/POPDC1 required for the BVES/ POPDC1-PR61α interaction and c-Myc degradation. They also demonstrated that the regulation of WNT signaling and the oncogene c-Myc by BVES/POPDC1 was a key event in colitis-induced tumourigenesis.26 PP2A dephosphorylates a number of target proteins, many of which are implicated in tumorigenesis. Therefore, the interaction of BVES/POPDC1 and PP2A warrants further study.
Other possible targets and signaling pathways
TWIK-related K+ channel 1 (TREK-1)
TREK-1 belongs to the two-pore domain potassium channel family, which is regulated by a large number of stimulating factors, such as pH, stretch, temperature, phosphorylation, and interacting proteins.98 TREK-1 has recently been discovered to play an important role in human prostate cancer. TREK-1 is highly expressed in prostate cancer, unlike in healthy prostate or benign prostatic hyperplasia.99,100 In addition, its expression is strongly correlated with the grade and stage of prostate cancer.100 Overexpression of TREK-1 in healthy prostate epithelial cells increased their proliferation ability.100 In contrast, the knockdown of TREK-1 significantly inhibited the proliferation of prostate cancer cells.99 Recently, an investigation of ion channels and electrogenic proteins in Xenopus oocytes demonstrated that TREK-1 functionally interacts with BVES/POPDC1.101 Co-expression of BVES/ POPDC1 and TREK-1 stimulates a twofold higher current than that measured in the absence of BVES/POPDC1.101 However, the role of BVES/POPDC1–TREK-1 interaction in human cancers has not been examined.
Caveolin-3
Caveolins are the major constructive component of the caveolae. Currently, there are three identified caveolin isoforms (Cav1, Cav2, and Cav3).102 Caveolins play an important role in the transcytosis of molecules into cells and regulation of signal transductions.103,104 Cav3 is the muscle- specific isoform, which is localized to the sarcolemma in skeletal muscle fibers and in sarcolemma and transverse tubules in cardiac myocytes.105 However, its presence has been observed in certain solid tumors. Cav3 is frequently expressed in seminoma and anaplastic carcinoma.106,107 Genetic ablation of Cav3 expression induces a lactogenic microenvironment, which is protective against mammary tumor formation.108 Cav3 has recently been identified as an interaction partner of BVES/POPDC1.16,101 This interaction is important for the preservation of caveolae structural and functional integrity.101 However, its role in the development and progression of cancer requires further investigation.
Vesicle-associated membrane protein 3 (VAMP3)
VAMP3 is a vesicular transport protein regulating the recycling of transferrins, the transferrin receptor, and integrins.109,110 It is also involved in the secretion of MMPs, degradation of the extracellular matrix (ECM), and invasiveness of human fibrosarcoma cells.111 Loss of VAMP3 function promotes cell migration and adhesion in human pancreatic cancer cells.112 BVES/POPDC1 has recently been shown to directly interact with VAMP3, and loss of BVES/POPDC1 function impairs VAMP3-mediated vesicular transport by disrupting the recycling of transferrin and β-1-integrin in MDCK epithelial cells.113 Furthermore, the expression of mutated BVES/POPDC1 disrupted integrin functions, resulting in impaired uptake during cell movement and disrupted cell spreading and adhesion.113 Based on these findings, the roles of BVES/POPDC1–VAMP3 in cancer may involve a novel BVES/POPDC1 signaling mechanism.
N-myc downstream regulated gene 4 (NDRG4)
Loss of BVES/POPDC1 or NDRG4 functions have been demonstrated to result in disrupted cell movement.37,114,115 Using SPOTs and pull-down analysis, Benesh et al116 found that NDRG4 directly binds to BVES/POPDC1 C-terminus which is outside of the conserved Popeye domain. The BVES/POPDC1/NDRG4 interaction is important for the regulation of epicardial cell directional migration, and disruption of this interaction randomizes migratory patterns.116 Furthermore, BVES/POPDC1 and NDRG4 specifically mediate the trafficking of internalized fibronectin via “autocrine ECM deposition” fibronectin recycling pathway.116 BVES/POPDC1/NDRG4 interaction also regulates fusion of cell surface-bound vesicles.116 These data suggest that BVES/POPDC1 may have multiple roles on cellular behaviors affecting development, repair, and cancer invasiveness.
Bnip3
Bnip3 is one of the Bcl-2 families of cell death regulatory factors that functions via both the activation of Bax/Bak and the opening of the mitochondria permeability transition pores.117 The expression of Bnip3 increases during stress such as hypoxia through hypoxia-inducing factor-1 dependent or independent mechanisms, such as transcriptionally regulated by RB1-E2F1, TP53, FOXO3, NF-κB, and other tumor-relevant transcription factors.117 Bnip3 is well studied in cell death, autophagy, and mitophagy.118 Recently, Kliminski et al15 found that the knockdown of BVES/POPDC1 in rat neonatal cardiomyocytes grown in serum-free medium reduced cell viability, facilitated mitochondrial injury, and upregulated Bnip3. Further, they found that BVES/ POPDC1 regulated the Bnip3 expression by modifying the competitive binding of FoxO3 and NFκB transcription factors with the Bnip3 promoter.15 In consideration of the multifaceted role of autophagy and mitophagy in cancer, the role of BVES/POPDC1-regulated Bnip3 in cancer warrants further study.119
cAMP is a second messenger molecule which is involved in many human normal physiological functions and pathological state.120 There are four effectors that directly bind to cAMP in mammalian cells as known previously, namely protein kinase A (PKA), exchange protein directly activated by cAMP (Epac), cyclic nucleotide receptor involved in sperm function (CRIS), and cyclic nucleotide-gated ion (CNG) channels.120 POPDC proteins have recently demonstrated as a novel class of cAMP effector proteins in striated muscle, the Popeye domain functioned as a high-affinity cAMP-binding site, and the binding affinity is ~10-fold higher than Epac and in the same level as that of PKA.14 Amunjela et al37 have recently demonstrated that BVES/ POPDC1 co-immunoprecipitates with cAMP in breast cancer lines. Interestingly, cAMP increases BVES/POPDC1 protein levels in these cells. In addition, cAMP was supposed to negatively modulate the interaction of TREK-1 with POPDC proteins.37 Additional studies defining the BVES/POPDC1- cAMP and its roles on cancer will be required.
Future studies
Over the previous 10 years, the expression and function of BVES/POPDC1 in cancer have been gradually recognized. All studies suggest that BVES/POPDC1 is a tumor suppressor gene.84 However, the correlation between BVES/POPDC1 and tumor prognosis requires further study to provide the possibility of BVES/POPDC1-targeted tumor intervention and follow-up. Decreased BVES/POPDC1 expression occurs in the early stage of cancer, and promoter methylation is the main mechanism of BVES/POPDC1 inhibition. Therefore, detection of the level of BVES/POPDC1 methylation may be important for the early detection of tumors and discovery of precancerous lesions.
BVES/POPDC1 is highly expressed in the developing heart and markedly downregulated during end-stage heart failure.54 Deficiency in BVES/POPDC1 leads to sinus node dysfunction in aged mice14,121 and humans born with Fallot’s tetralogy,122 suggesting its involvement in heart pathology and morphogenesis.101 Considering the differential expression of BVES/POPDC1 in the heart and tumors, we speculate that BVES/POPDC1 may play a role in tumor-related heart disease and cardiac disease caused by antineoplastic drugs. However, this potential role of BVES/POPDC1 requires further study by oncologists and cardiologists.
We recently found that BVES/POPDC1 participates in “cancer cell extrusion”, by which cancer cells leave the primary tumor and initiate local or distant metastasis.123 Mediation of this process by BVES/POPDC1 may be another important mechanism by which it inhibits tumor metastasis, especially in gastrointestinal tumors.124–126 More recently, BVES/POPDC1 was found to regulate intestinal stem cell programming after exposure to radiation,57 suggesting a potential role in the treatment of cancer using stem cells.
Overall, in the previous decade, the research on BVES/ POPDC1 has shown great promise. Further research, based on the currently available evidence, is warranted to evaluate the role of BVES/POPDC1 in human disease.
Acknowledgments
This review is supported by the National Natural Science Foundation of China (No 81472311, No 81572419, and No 81702396).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.de R, Zavaljevski M, Nl S, Bader D, Reese DE, Streiff NL. Bves: a novel gene expressed during coronary blood vessel development. Dev Biol. 1999;209(1):159–171. doi: 10.1006/dbio.1999.9246. [DOI] [PubMed] [Google Scholar]
- 2.Andree B, Hillemann T, Kessler-Icekson G, et al. Isolation and characterization of the novel Popeye gene family expressed in skeletal muscle and heart. Dev Biol. 2000;223(2):371–382. doi: 10.1006/dbio.2000.9751. [DOI] [PubMed] [Google Scholar]
- 3.de R, Bader DM. Cloning and expression of hbves, a novel and highly conserved mRNA expressed in the developing and adult heart and skeletal muscle in the human. Mamm Genome. 1999;10(9):913–915. doi: 10.1007/s003359901113. [DOI] [PubMed] [Google Scholar]
- 4.Knight RF, Bader DM, Backstrom JR. Membrane topology of Bves/ Pop1A, a cell adhesion molecule that displays dynamic changes in cellular distribution during development. J Biol Chem. 2003;278(35):32872–32879. doi: 10.1074/jbc.M301961200. [DOI] [PubMed] [Google Scholar]
- 5.Kukuruzinska MA, Lennon K. Protein N-glycosylation: molecular genetics and functional significance. Crit Rev Oral Biol Med. 1998;9(4):415–448. doi: 10.1177/10454411980090040301. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi M, Hager HA, Wada A, Koyama T, Chang MS, Bader DM. Identification of a novel intracellular interaction domain essential for Bves function. PLoS One. 2008;3(5):e2261. doi: 10.1371/journal.pone.0002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hager HA, Bader DM. Bves: ten years after. Histol Histopathol. 2009;24(6):777–787. doi: 10.14670/hh-24.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osler ME, Bader DM. Bves expression during avian embryogenesis. Dev Dyn. 2004;229(3):658–667. doi: 10.1002/dvdy.10490. [DOI] [PubMed] [Google Scholar]
- 9.Ripley AN, Chang MS, Bader DM. Bves is expressed in the epithelial components of the retina, lens, and cornea. Invest Ophthalmol Vis Sci. 2004;45(8):2475–2483. doi: 10.1167/iovs.04-0013. [DOI] [PubMed] [Google Scholar]
- 10.Vasavada TK, DiAngelo JR, Duncan MK. Developmental expression of Pop1/Bves. J Histochem Cytochem. 2004;52(3):371–377. doi: 10.1177/002215540405200308. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy M. Allen brain atlas maps 21,000 genes of the mouse brain. Lancet Neurol. 2006;5(11):907–908. doi: 10.1016/s1474-4422(06)70594-4. [DOI] [PubMed] [Google Scholar]
- 12.Smith TK, Bader DM. Characterization of Bves expression during mouse development using newly generated immunoreagents. Dev Dyn. 2006;235(6):1701–1708. doi: 10.1002/dvdy.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torlopp A, Breher SS, Schluter J, Brand T. Comparative analysis of mRNA and protein expression of Popdc1 (Bves) during early development in the chick embryo. Dev Dyn. 2006;235(3):691–700. doi: 10.1002/dvdy.20687. [DOI] [PubMed] [Google Scholar]
- 14.Froese A, Breher SS, Waldeyer C, et al. Popeye domain containing proteins are essential for stress-mediated modulation of cardiac pace- making in mice. J Clin Invest. 2012;122(3):1119–1130. doi: 10.1172/JCI59410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliminski V, Uziel O, Kessler-Icekson G. Popdc1/Bves functions in the preservation of cardiomyocyte viability while affecting Rac1 activity and BNIP3 expression. J Cell Biochem. 2017;118(6):1505–1517. doi: 10.1002/jcb.25810. [DOI] [PubMed] [Google Scholar]
- 16.Alcalay Y, Hochhauser E, Kliminski V, et al. Popeye domain containing 1 (Popdc1/Bves) is a caveolae-associated protein involved in ischemia tolerance. PLoS One. 2013;8(9):e71100. doi: 10.1371/journal.pone.0071100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osler ME, Chang MS, Bader DM. Bves modulates epithelial integrity through an interaction at the tight junction. J Cell Sci. 2005;118(Pt 20):4667–4678. doi: 10.1242/jcs.02588. [DOI] [PubMed] [Google Scholar]
- 18.Wada AM, Reese DE, Bader DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128(11):2085–2093. doi: 10.1242/dev.128.11.2085. [DOI] [PubMed] [Google Scholar]
- 19.Ripley AN, Osler ME, Wright CV, Bader D. Xbves is a regulator of epithelial movement during early Xenopus laevis development. Proc Natl Acad Sci U S A. 2006;103(3):614–619. doi: 10.1073/pnas.0506095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin S, Zhao D, Bownes M. Blood vessel/epicardial substance (Bves) expression, essential for embryonic development, is down regulated by Grk/EFGR signalling. Int J Dev Biol. 2007;51(1):37–44. doi: 10.1387/ijdb.052108sl. [DOI] [PubMed] [Google Scholar]
- 21.Feng Q, Se H, Stern JE, et al. DNA methylation in tumor and matched normal tissues from non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2008;17(3):645–654. doi: 10.1158/1055-9965.EPI-07-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salskov A, Hawes SE, Stern JE, et al. Hypermethylation of CCND2 may reflect a smoking-induced precancerous change in the lung. J Oncol. 2011;2011:950140. doi: 10.1155/2011/950140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M, Jang HR, Haam K, et al. Frequent silencing of Popeye domain- containing genes, Bves and POPDC3, is associated with promoter hyper- methylation in gastric cancer. Carcinogenesis. 2010;31(9):1685–1693. doi: 10.1093/carcin/bgq144. [DOI] [PubMed] [Google Scholar]
- 24.Luo D, Huang H, Lu ML, et al. Abnormal expression of adhesion protein Bves is associated with gastric cancer progression and poor survival. Pathol Oncol Res. 2012;18(2):491–497. doi: 10.1007/s12253-011-9472-x. [DOI] [PubMed] [Google Scholar]
- 25.Cs W, Zhang B, Smith JJ, et al. Bves regulates EMT in human corneal and colon cancer cells and is silenced via promoter methylation in human colorectal carcinoma. J Clin Invest. 2011;121(10):4056–4069. doi: 10.1172/JCI44228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parang B, Kaz AM, Barrett CW, et al. BVES regulates c-Myc stability via PP2A and suppresses colitis-induced tumourigenesis. Gut. 2017;66(5):852–862. doi: 10.1136/gutjnl-2015-310255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y, Zhao Z, Zhu Y, Zhao L, Zhu A, Piao D. Comprehensive analysis of differential expression profiles of mRNAs and lncRNAs and identification of a 14-lncRNA prognostic signature for patients with colon adenocarcinoma. Oncol Rep. 2018;39(5):2365–2375. doi: 10.3892/or.2018.6324. [DOI] [PubMed] [Google Scholar]
- 28.Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastro- intestinal and liver disease. Gut. 2019;65(3):547–561. doi: 10.1136/gutjnl-2018-316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagai T, Arao T, Nishio K, et al. Impact of tight junction protein ZO-1 and TWIST expression on postoperative survival of patients with hepatocellular carcinoma. Dig Dis. 2016;34(6):702–707. doi: 10.1159/000448860. [DOI] [PubMed] [Google Scholar]
- 30.Higashi Y, Suzuki S, Sakaguchi T, et al. Loss of Claudin-1 expression correlates with malignancy of hepatocellular carcinoma. J Surg Res. 2007;139(1):68–76. doi: 10.1016/j.jss.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt M, Horbach A, Kubitz R, Frilling A, Haussinger D. Disruption of hepatocellular tight junctions by vascular endothelial growth factor (VEGF): a novel mechanism for tumor invasion. J Hepatol. 2004;41(2):274–283. doi: 10.1016/j.jhep.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Han P, Fu Y, Luo M, et al. Bves inhibition triggers epithelial- mesenchymal transition in human hepatocellular carcinoma. Dig Dis Sci. 2014;59(5):992–1000. doi: 10.1007/s10620-013-2992-3. [DOI] [PubMed] [Google Scholar]
- 33.Han P, Fu Y, Liu J, et al. Netrin-1 promotes cell migration and invasion by down-regulation of Bves expression in human hepatocellular carcinoma. Am J Cancer Res. 2015;5(4):1396–1409. [PMC free article] [PubMed] [Google Scholar]
- 34.Lei Y, Han P, Lin ZY, Yan W, Tian D. Hypoxia regulates epithelial- mesenchymal transition (EMT) in hepatocellular carcinoma cells via promoting Bves methylation. Hepatology. 2018;68:1264A–1264A. [Google Scholar]
- 35.Dong X, Hou Q, Chen Y, Wang X. Diagnostic value of the methylation of multiple gene promoters in serum in hepatitis B virus-related hepatocellular carcinoma. Dis Markers. 2017;2017:2929381. doi: 10.1155/2017/2929381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amunjela JN, Tucker SJ. POPDC1 is suppressed in human breast cancer tissues and is negatively regulated by EGFR in breast cancer cell lines. Cancer Lett. 2017;406:81–92. doi: 10.1016/j.canlet.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Amunjela JN, Tucker SJ. Dysregulation of POPDC1 promotes breast cancer cell migration and proliferation. Biosci Rep. 2017;37(6):BSR20171039. doi: 10.1042/BSR20171039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng W, Gu L, Li X, et al. CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells. J Transl Med. 2016;14:32. doi: 10.1186/s12967-016-0787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claperon A, Mergey M, Nguyen Ho-Bouldoires TH, et al. EGF/EGFR axis contributes to the progression of cholangiocarcinoma through the induction of an epithelial-mesenchymal transition. J Hepatol. 2014;61(2):325–332. doi: 10.1016/j.jhep.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 40.Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29(35):4947–4958. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 41.Patel D, Bassi R, Hooper A, Prewett M, Hicklin DJ, Kang X. Anti- epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int J Oncol. 2009;34(1):25–32. [PubMed] [Google Scholar]
- 42.Kimura H, Sakai K, Arao T, Shimoyama T, Tamura T, Nishio K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98(8):1275–1280. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1(2):85–94. [PubMed] [Google Scholar]
- 44.Feldinger K, Kong A. Profile of neratinib and its potential in the treatment of breast cancer. Breast Cancer (Dove Med Press) 2015;7:147–162. doi: 10.2147/BCTT.S54414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuler M, Awada A, Harter P, et al. A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2- negative metastatic breast cancer. Breast Cancer Res Treat. 2012;134(3):1149–1159. doi: 10.1007/s10549-012-2126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Yacoub R, Taliaferro-Smith LD, et al. Combinatorial effects of lapatinib and rapamycin in triple-negative breast cancer cells. Mol Cancer Ther. 2011;10(8):1460–1469. doi: 10.1158/1535-7163.MCT-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soulieres D, Faivre S, Mesia R, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18(3):323–335. doi: 10.1016/S1470-2045(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 48.Lai SY, Koppikar P, Sm T, et al. Intratumoral epidermal growth factor receptor antisense DNA therapy in head and neck cancer: first human application and potential antitumor mechanisms. J Clin Oncol. 2009;27(8):1235–1242. doi: 10.1200/JCO.2008.17.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russ PK, Kupperman AI, Presley SH, Haselton FR, Chang MS. Inhibition of RhoA signaling with increased Bves in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;51(1):223–230. doi: 10.1167/iovs.09-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell K. Contribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasis. Curr Opin Cell Biol. 2018;55:30–35. doi: 10.1016/j.ceb.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu YC, Liu CY, Chen YH, Chen RF, Huang CJ, Wang IJ. Blood vessel epicardial substance (Bves) regulates epidermal tight junction integrity through atypical protein kinase C. J Biol Chem. 2012;287(47):39887–39897. doi: 10.1074/jbc.M112.372078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59(4):418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Zhou L, Yang R, et al. Identification of differentially expressed genes in human heart with ventricular septal defect using suppression subtractive hybridization. Biochem Biophys Res Commun. 2006;342(1):135–144. doi: 10.1016/j.bbrc.2006.01.113. [DOI] [PubMed] [Google Scholar]
- 54.Gingold-Belfer R, Bergman M, Alcalay Y, et al. Popeye domain- containing 1 is down-regulated in failing human hearts. Int J Mol Med. 2011;27(1):25–31. doi: 10.3892/ijmm.2010.558. [DOI] [PubMed] [Google Scholar]
- 55.Schindler RF, Scotton C, Zhang J, et al. POPDC1(S201F) causes muscular dystrophy and arrhythmia by affecting protein trafficking. J Clin Invest. 2016;126(1):239–253. doi: 10.1172/JCI79562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu M, Tu J, Gingold JA, Kong CSL, Lee DF. Cancer in a dish: progress using stem cells as a platform for cancer research. Am J Cancer Res. 2018;8(6):944–954. [PMC free article] [PubMed] [Google Scholar]
- 57.Reddy VK, Short SP, Barrett CW, et al. BVES regulates intestinal stem cell programs and intestinal crypt viability after radiation. Stem Cells. 2016;34(6):1626–1636. doi: 10.1002/stem.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ya C, Reddy VK, Singh K, et al. BVES is required for maintenance of colonic epithelial integrity in experimental colitis by modifying intestinal permeability. Mucosal Immunol. 2018;11(5):1363–1374. doi: 10.1038/s41385-018-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Souchet M, Portales-Casamar E, Mazurais D, et al. Human p63RhoGEF, a novel RhoA-specific guanine nucleotide exchange factor, is localized in cardiac sarcomere. J Cell Sci. 2002;115(Pt 3):629–640. doi: 10.1242/jcs.115.3.629. [DOI] [PubMed] [Google Scholar]
- 60.Lutz S, Freichel-Blomquist A, Rumenapp U, Schmidt M, Jakobs KH, Wieland T. p63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same gene. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(5):540–546. doi: 10.1007/s00210-004-0926-5. [DOI] [PubMed] [Google Scholar]
- 61.Lutz S, Shankaranarayanan A, Coco C, et al. Structure of Galphaq- p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318(5858):1923–1927. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 62.Ki S-F, Sandquist JC, Rossol-Allison J, et al. MLK3 limits activated Galphaq signaling to Rho by binding to p63RhoGEF. Mol Cell. 2008;32(1):43–56. doi: 10.1016/j.molcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayashi A, Hiatari R, Tsuji T, Ohashi K, Mizuno K. p63RhoGEF-mediated formation of a single polarized lamellipodium is required for chemotactic migration in breast carcinoma cells. FEBS Lett. 2013;587(6):698–705. doi: 10.1016/j.febslet.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 64.Tang X, Jin R, Qu G, et al. GPR116, an adhesion G-protein-coupled receptor, promotes breast cancer metastasis via the Galphaq-p63Rho- GEF-Rho GTPase pathway. Cancer Res. 2013;73(20):6206–6218. doi: 10.1158/0008-5472.CAN-13-1049. [DOI] [PubMed] [Google Scholar]
- 65.Abraham CG, Ludwig MP, Andrysik Z, et al. DeltaNp63alpha suppresses TGFB2 expression and RhoA activity to drive cell proliferation in squamous cell carcinomas. Cell Rep. 2018;24(12):3224–3236. doi: 10.1016/j.celrep.2018.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Li C, Xiao HL, et al. CRMP4a suppresses cell motility by sequestering RhoA activity in prostate cancer cells. Cancer Biol Ther. 2018 2018 Aug 6;:1–11. doi: 10.1080/15384047.2018.1491507. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bustelo XR. RHO GTPases in cancer: known facts, open questions, and therapeutic challenges. Biochem Soc Trans. 2018;46(3):741–760. doi: 10.1042/BST20170531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narumiya S, Thumkeo D. Rho signaling research: history, current status and future directions. FEBS Lett. 2018;592(11):1763–1776. doi: 10.1002/1873-3468.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawson CD, Ridley AJ. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol. 2018;217(2):447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jansen S, Gosens R, Wieland T, Schmidt M. Paving the Rho in cancer metastasis: Rho GTPases and beyond. Pharmacol Ther. 2018;183:1–21. doi: 10.1016/j.pharmthera.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Smith TK, Hager HA, Francis R, Kilkenny DM, Lo CW, Bader DM. Bves directly interacts with GEFT, and controls cell shape and movement through regulation of Rac1/Cdc42 activity. Proc Natl Acad Sci U S A. 2008;105(24):8298–8303. doi: 10.1073/pnas.0802345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russ PK, Pino CJ, Williams CS, Bader DM, Haselton FR, Chang MS. Bves modulates tight junction associated signaling. PLoS One. 2011;6(1):e14563. doi: 10.1371/journal.pone.0014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273(52):34954–34960. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 74.Benais-Pont G, Punn A, Flores-Maldonado C, et al. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol. 2003;160(5):729–740. doi: 10.1083/jcb.200211047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi JW, Liu W, Zhang TT, et al. The enforced expression of c-myc in pig fibroblasts triggers mesenchymal-epithelial transition (MET) via F-actin reorganization and RhoA/ROCK pathway inactivation. Cell Cycle. 2013;12(7):1119–1127. doi: 10.4161/cc.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang SC, Lin XL, Li J, et al. MicroRNA-122 triggers mesenchymal- epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS One. 2014;9(7):e101330. doi: 10.1371/journal.pone.0101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 78.Murillo-Garzon V, Kypta R. WNT signalling in prostate cancer. Nat Rev Urol. 2017;14(11):683–696. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- 79.Artus C, Glacial F, Ganeshamoorthy K, et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab. 2014;34(3):433–440. doi: 10.1038/jcbfm.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah GV, Muralidharan A, Gokulgandhi M, Soan K, Thomas S. Cadherin switching and activation of beta-catenin signaling underlie proinvasive actions of calcitonin-calcitonin receptor axis in prostate cancer. J Biol Chem. 2009;284(2):1018–1030. doi: 10.1074/jbc.M807823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Preda V, Larkin SJ, Karavitaki N, Ansorge O, Grossman AB. The Wnt signalling cascade and the adherens junction complex in craniopharyn- gioma tumorigenesis. Endocr Pathol. 2015;26(1):1–8. doi: 10.1007/s12022-014-9341-8. [DOI] [PubMed] [Google Scholar]
- 82.Klinke DJ, 2nd, Horvath N, Cuppett V, Wu Y, Deng W, Kanj R. Interlocked positive and negative feedback network motifs regulate beta-catenin activity in the adherens junction pathway. Mol Biol Cell. 2015;26(22):4135–4148. doi: 10.1091/mbc.E15-02-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Francis H, Kennedy L, Alpini G. Dual ablation of beta- and gamma- catenin: critical regulators of junctions and their functions. Hepatology. 2018;67(6):2079–2081. doi: 10.1002/hep.29761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parang B, Thompson JJ, Cs W, Williams CS. Blood vessel epicardial substance (Bves) in junctional signaling and cancer. Tissue Barriers. 2018 2018 Oct 11;:1–12. doi: 10.1080/21688370.2018.1499843. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bock-Marquette I, Shrivastava S, Pipes GC, et al. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol. 2009;46(5):728–738. doi: 10.1016/j.yjmcc.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013;14(6):e229–e238. doi: 10.1016/S1470-2045(12)70558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795(1):1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Junttila MR, Puustinen P, Niemela M, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130(1):51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 89.Ross JA, Cheng H, Zs N, Frost JA, Kirken RA. Protein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activation. J Biol Chem. 2010;285(6):3582–3591. doi: 10.1074/jbc.M109.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Yue P, Deng X, et al. Protein phosphatase 2A negatively regulates eukaryotic initiation factor 4E phosphorylation and eIF4F assembly through direct dephosphorylation of Mnk and eIF4E. Neoplasia. 2010;12(10):848–855. doi: 10.1593/neo.10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssens V, Rebollo A. The role and therapeutic potential of Ser/Thr phosphatase PP2A in apoptotic signalling networks in human cancer cells. Curr Mol Med. 2012;12(3):268–287. doi: 10.2174/156652412799218930. [DOI] [PubMed] [Google Scholar]
- 92.Bockelman C, Lassus H, Hemmes A, et al. Prognostic role of CIP2A expression in serous ovarian cancer. Br J Cancer. 2011;105(7):989–995. doi: 10.1038/bjc.2011.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tseng LM, Liu CY, Chang KC, Chu PY, Shiau CW, Chen KF. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 2012;14(2):R68. doi: 10.1186/bcr3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin YC, Chen KC, Chen CC, Cheng AL, Chen KF. CIP2A-mediated Akt activation plays a role in bortezomib-induced apoptosis in head and neck squamous cell carcinoma cells. Oral Oncol. 2012;48(7):585–593. doi: 10.1016/j.oraloncology.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Cristobal I, Garcia-Orti L, Cirauqui C, et al. Overexpression of SET is a recurrent event associated with poor outcome and contributes to protein phosphatase 2A inhibition in acute myeloid leukemia. Haematologica. 2012;97(4):543–550. doi: 10.3324/haematol.2011.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood. 2011;117(24):6660–6668. doi: 10.1182/blood-2010-08-304477. [DOI] [PubMed] [Google Scholar]
- 97.Ruvolo PP, Qui YH, Coombes KR, et al. Low expression of PP2A regulatory subunit B55alpha is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patients. Leukemia. 2011;25(11):1711–1717. doi: 10.1038/leu.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24(1):44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang GM, Wan FN, Qin XJ, et al. Prognostic significance of the TREK-1 K2P potassium channels in prostate cancer. Oncotarget. 2015;6(21):18460–18468. doi: 10.18632/oncotarget.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Voloshyna I, Besana A, Castillo M, et al. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008;68(4):1197–1203. doi: 10.1158/0008-5472.CAN-07-5163. [DOI] [PubMed] [Google Scholar]
- 101.Brand T, Simrick SL, Poon KL, Schindler RF. The cAMP-binding Popdc proteins have a redundant function in the heart. Biochem Soc Trans. 2014;42(2):295–301. doi: 10.1042/BST20130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stan RV. Structure of caveolae. Biochim Biophys Acta Mol Cell Res. 2005;1746(3):334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 103.Busija AR, Patel HH, Insel PA. Caveolins and cavins in the trafficking, maturation, and degradation of caveolae: implications for cell physiology. Am J Physiol Cell Physiol. 2017;312(4):C459–C477. doi: 10.1152/ajpcell.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yin H, Liu T, Zhang Y, Yang B. Caveolin proteins: a molecular insight into disease. Front Med. 2016;10(4):397–404. doi: 10.1007/s11684-016-0483-6. [DOI] [PubMed] [Google Scholar]
- 105.Song KS, Scherer PE, Tang Z, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271(25):15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 106.Kasahara T, Hara N, Bilim V, Tomita Y, Tsutsui T, Takahashi K. Immunohistochemical studies of caveolin-3 in germ cell tumors of the testis. Urol Int. 2002;69(1):63–68. doi: 10.1159/000064363. [DOI] [PubMed] [Google Scholar]
- 107.Kim D, Kim H, Koo JS. Expression of caveolin-1, Caveolin-2 and caveolin-3 in thyroid cancer and stroma. Pathobiology. 2012;79(1):1–10. doi: 10.1159/000329472. [DOI] [PubMed] [Google Scholar]
- 108.Sotgia F, Casimiro MC, Bonuccelli G, et al. Loss of caveolin-3 induces a lactogenic microenvironment that is protective against mammary tumor formation. Am J Pathol. 2009;174(2):613–629. doi: 10.2353/ajpath.2009.080653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu C, Hardee D, Minnear F. Membrane fusion by VAMP3 and plasma membrane t-SNAREs. Exp Cell Res. 2007;313(15):3198–3209. doi: 10.1016/j.yexcr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riggs KA, Hasan N, Humphrey D, et al. Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction. J Cell Sci. 2012;125(Pt 16):3827–3839. doi: 10.1242/jcs.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kean MJ, Williams KC, Skalski M, et al. VAMP3, syntaxin-13 and SNAP23 are involved in secretion of matrix metalloproteinases, degradation of the extracellular matrix and cell invasion. J Cell Sci. 2009;122(Pt 22):4089–4098. doi: 10.1242/jcs.052761. [DOI] [PubMed] [Google Scholar]
- 112.Luftman K, Hasan N, Day P, Hardee D, Hu C. Silencing of VAMP3 inhibits cell migration and integrin-mediated adhesion. Biochem Biophys Res Commun. 2009;380(1):65–70. doi: 10.1016/j.bbrc.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hager HA, Roberts RJ, Cross EE, Proux-Gillardeaux V, Bader DM. Identification of a novel Bves function: regulation of vesicular transport. EMBO J. 2010;29(3):532–545. doi: 10.1038/emboj.2009.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melotte V, Lentjes MH, van den Bosch SM, et al. N-Myc downstream- regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst. 2009;101(13):916–927. doi: 10.1093/jnci/djp131. [DOI] [PubMed] [Google Scholar]
- 115.Kotipatruni RP, Ferraro DJ, Ren X, et al. NDRG4, the N-Myc downstream regulated gene, is important for cell survival, tumor invasion and angiogenesis in meningiomas. Integr Biol (Camb) 2012;4(10):1185–1197. doi: 10.1039/c2ib20168b. [DOI] [PubMed] [Google Scholar]
- 116.Benesh EC, Miller PM, Pfaltzgraff ER, et al. Bves and NDRG4 regulate directional epicardial cell migration through autocrine extracellular matrix deposition. Mol Biol Cell. 2013;24(22):3496–3510. doi: 10.1091/mbc.E12-07-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vasagiri N, Kutala VK. Structure, function, and epigenetic regulation of BNIP3: a pathophysiological relevance. Mol Biol Rep. 2014;41(11):7705–7714. doi: 10.1007/s11033-014-3664-x. [DOI] [PubMed] [Google Scholar]
- 118.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16(7):939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chourasia AH, Macleod KF. Tumor suppressor functions of BNIP3 and mitophagy. Autophagy. 2015;11(10):1937–1938. doi: 10.1080/15548627.2015.1085136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brand T, Schindler R. New kids on the block: the Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscle. Cell Signal. 2017;40:156–165. doi: 10.1016/j.cellsig.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boukens BJ, Christoffels VM. Popeye proteins: muscle for the aging sinus node. J Clin Invest. 2012;122(3):810–813. doi: 10.1172/JCI62588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu M, Li Y, He X, et al. Mutational and functional analysis of the BVES gene coding region in Chinese patients with non-syndromic tetralogy of Fallot. Int J Mol Med. 2013;31(4):899–903. doi: 10.3892/ijmm.2013.1275. [DOI] [PubMed] [Google Scholar]
- 123.Han P, Fu Y, Yan W, Tian D. BVES decreases liver cancer cell extrusion in a 3D cell culture model. Gastroenterology. 2015;148(4):S1017–S1017. [Google Scholar]
- 124.Fadul J, Rosenblatt J. The forces and fates of extruding cells. Curr Opin Cell Biol. 2018;54:66–71. doi: 10.1016/j.ceb.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gu Y, Shea J, Slattum G, et al. Defective apical extrusion signaling contributes to aggressive tumor hallmarks. Elife. 2015;4:e04069. doi: 10.7554/eLife.04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gu Y, Rosenblatt J. New emerging roles for epithelial cell extrusion. Curr Opin Cell Biol. 2012;24(6):865–870. doi: 10.1016/j.ceb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]