Abstract
Background
Non-Hodgkin’s lymphomas (NHLs) account for 85% of lymphomas, which are characterized by high-degree malignancy, rapid progress, and even invasion into central nervous system in pediatric patients. Although the cure rate of pediatric NHL has improved, some patients have still underwent recurrence or death. This study focuses on the effects and mechanism of forskolin on the progression of NHL, aiming to find efficient therapy methods for pediatric NHL.
Methods
MTT, flow cytometry and mice tumor bearing experiments were used to evaluate the effects of forskolin on NHL cell proliferation, apoptosis and tumorigenesis. Western blotting and RT-PCR assays were used to detect protein and mRNA expression. Immunohistochemistry technology was recruited to analyze Ki-67 expression in tumor tissues.
Results
Forskolin significantly increased the expression of cleaved caspase-3/9 in both NHL Toledo and NK-92 cell lines, and inhibited cell growth. Besides, forskolin obviously reduced the expression of β-catenin protein, promoted its ubiquitination, enhanced its transportation from nuclear to cytoplasm, as well as decreased the expression of its downstream oncogenes c-myc and cyclin D1 through upregulating Axin expression and stability and inhibiting Axin ubiquitination. Moreover, forskolin enhanced the effects of SP600125, an inhibitor of c-Jun N-terminal kinase signaling on cell apoptosis promotion and tumorigenesis inhibition via Axin-induced β-catenin signaling repression.
Conclusion
The current study clarifies that forskolin can inhibit the progression of NHL through Axin-mediated inhibition of β-catenin signaling. Moreover, forskolin improves the effects of SP600125 on cell apoptosis enhancement and tumorigenesis inhibition of NHL cells. These findings provide theoretical foundation of serving forskolin as a new effective therapeutic drug for pediatric NHL.
Keywords: forskolin, SP600125, Axin, β-catenin, carcinogens
Introduction
Non-Hodgkin’s lymphomas (NHLs) account for about 85% of lymphomas with others being Hodgkin’s lymphomas. NHLs are a highly heterogeneous group with around 40 existent forms, in spite of a few common types predominating.1 NHL is the fifth most common cancer in the UK, which occurs either in lymph nodes and other lymphatic tissues, such as the tonsils, spleen, Waldeyer’s ring, and thymus, or the lymphatic cells of other organs.2 With the increasing aging population and immunodeficiency states, the incidence of it increases and seems to be more than doubled in the past 30 years.3 Although advanced progression has been made in the diagnosis and treatment, the outcome of NHL patients still remains poor. Therefore, it is urgent and essential to seek effective anticancer drugs for NHL.
Forskolin, a natural compound, is a diterpene and derived from the roots of Coleus forskohlii, a characteristic Indian plant.4 It is worth paying attention to that forskolin, which has been applied in medicine for a long time and its safeness has been confirmed by many researchers.5–7 Forskolin is reported to have the potential to directly activate the adenylate cyclase enzyme, which induces the production of cyclic adenosine monophosphate (cAMP) from ATP, leading to the increase of intracellular cAMP.8 Notably, cAMP has been identified to affect multiple cellular functions and is closely associated with tumorigenesis through protein kinase A-dependent/independent manner, including inhibition of the viability and migration and enhancement of the chemosensitivity of cancer cells.9–12 Its analog 8-CPT-cAMP [8-(4-chlorophenylthio) adenosine 3′, 5′-cyclic monophosphate] has been documented to promote apoptosis of lymphoma cells.13 Importantly, forskolin, the cAMP-elevating agent, exerts anticancer roles in many kinds of cancers, and also improves the chemosensitivity of conventional drugs.14–16 However, the effects of forskolin in NHL remain unclarified.
Many signaling transduction pathways are reported to be deregulated and strongly implicated in the occurrence and development of multiple kinds of cancers, including Hippo/Yes-associated protein (YAP),17 Wnt/β-catenin,18 phosphatidylinositol 3-hydroxy kinase/AKT,19 signal transduction and transcriptional activator (STAT),20 and P53.21 As a result, we performed the current study to explore the effects of forskolin in the progression of NHL, and determine if these signaling pathways are involved in it.
Materials and methods
Cell culture and treatment
NHL cell lines Toledo, NK-92, RL, and Farage were all purchased from American Type Culture Collection (VA, USA). Toledo, Farage, and RL cells were cultured in RPMI-1640 medium containing 10% FBS (Gibco, CA, USA); NK-92 cells were cultured in Alpha Minimum Essential Medium with ribonucleosides and deoxyribonucleosides free while with 2 mM L-glutamine and 1.5 g/L sodium bicarbonate, 0.2 mM inositol, 0.1 mM 2-mercaptoethanol, 0.02 mM folic acid, 100–200 U/mL recombinant IL-2, 12.5% horse serum (Sigma-Aldrich Co., St Louis, MO, USA), and 12.5% FBS.
Toledo or NK-92 cells were incubated with different concentrations of forskolin (0, 10, 20, 40, 80, or 160 μM; cAMP analog 8-pCPT-2′-OMe-cAMP; Sigma-Aldrich Co) for the indicated times, and 20 μM of SP600125 (Selleck Chemicals, Shanghai, China), an inhibitor of c-Jun N-terminal kinase (JNK) for 24 hours.
Cell transfection
Small interference (si) RNA targeting human Axin (No. SR305433, Origene, CA, USA) was used to downregulate the expression of Axin in Toledo and NK-92 cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
Lentivirus with puromycin resistance and green fluorescent protein label containing human β-catenin gene was purchased from GenePharma (OE-β-catenin; Shanghai, China) and was used to upregulate the expression of β-catenin in either Toledo cells or NK-92 cells.
Western blotting analysis
Total protein of cells was extracted with cold RIPA buffer (Solarbio, Beijing, China), and cytoplasmic and nucleoprotein were obtained by CelLytic™ NuCLEAR™ Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA). For Western blotting analysis, 20 μg of protein obtained from cultured cells with protein lysis buffer was separated by 10% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) porous membrane (Thermo Fisher Scientific). After incubated with 5% nonfat milk diluted with TBST to protect the antibody from nonspecific binding, the PVDF membrane was incubated with the indicated primary and the corresponding secondary antibodies successively. Finally, the membranes were incubated with ECL Chemiluminescent regent (Millipore, Darmstadt, Germany) and Meta Morph software was used to quantify the Western blotting bands after background subtraction. Primary antibodies used in this study are listed in Table S1. Histone, tubulin, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used to normalize the expression of nuclear protein cytoplasmic protein and total protein, respectively.
Immunoprecipitation (IP) assay
For IP assay, the cells were rinsed with cold PBS and lysed in IP lysis buffer (Thermo Fisher Scientific). Then the cell lysate containing 200 μg proteins was precleared with Dynabeads® protein G for 1 hour, and incubated with 2 μg of antibody against Axin or β-catenin overnight at 4°C, and then incubated with Dynabeads® protein G for an additional 1 hour of incubation. The beads were washed four times with IP lysis buffer and loaded onto gels using anti-Ub antibody.
Real time PCR (RT-PCR) analysis
Total RNA from cells and tissue samples were extracted with an RNAsimple Total RNA Kit (TIANGEN, Beijing, China). After quantification, 1 μg RNA from every sample was transcribed reversely into the first strand cDNA using SuperScript™ IV First-Strand Synthesis System (Thermo Fisher Scientific), then the RT-PCR was performed by Super-Script™ III Platinum™ One-Step qRT-PCR Kit (Thermo Fisher Scientific). GAPDH was used as an endogenous control. Axin (forward: 5′-CGACTTGCTGGACTTCTGGT-3′ and reverse: 5′-ATATGCCCTTCCCTGTCCCT-3′) and GAPDH (forward: 5′-TCTTTTGCGTCGCCAGCC-3′ and reverse: 5′-GTTCTCAGCCTTGACGGTGC-3′).
MTT assay
Toledo and NK-92 cells were seeded in a 96-well plate with 5×103 cells/well and grown in a medium containing 10% FBS for 24 hours, then they were treated with 0, 10, 20, 40, 80, and 160 μM forskolin for 1, 2, 3, 4, 5, and 6 days. After the indicated time points, cell medium was discarded and 20 μL 5 mg/mL of MTT solution was added and incubated for another 4 hours at 37°C. Then, 100 μL dimethyl sulfoxide solution (Sigma-Aldrich Co, MO, USA) was added to dissolve the insoluble formazan crystals after the medium get rid of each well. The OD values were measured at 570 nm on a plate reader (Multiskan GO, Thermo Fisher Scientific).
Flow cytometry assay
Toledo and NK-92 cells (2×105 cells/well) were seeded into 12-well plates overnight, then the cells were given 40 μM of forskolin for 48 hours or 20 μM of SP600125 for 24 hours. Next, cells were harvested and incubated with 5 μL of Alexa Fluor 488-tagged Annexin-V in the dark room for 30 minutes and incubated with 50 μg/mL of propidium iodide (PI; 5 μL) solution for 5 minutes on ice in dark with an Apoptosis Detection Kit purchased from Qcbio Science & Technologies Co., Ltd. (Shanghai, China). Cells stained with Annexin V-FITC+/PI− represent early apoptotic cells, while cells stained with Annexin V-FITC+/PI+ represent late apoptotic cells, which were analyzed by flow cytometry (BD scientific, MO, USA) and Flowjo 7.6 software.
In vivo tumorigenesis assay
Male 18–20 g BALB/c nude mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice were fed in specific pathogen-free conditions on a 12-hour light/dark cycle with standard food and water available. For tumor formation assay, Toledo and NK-92 cells were infected with OE-NC or OE-β-catenin, followed by screening with puromycin to construct stable cell lines, then 5×106 stable transfected Toledo and NK-92 cells were subcutaneously injected into nude mice, with 24 mice in each group. Then, mice infected with OE-NC-or OE-β-catenin-expressing cells were divided into four groups, respectively: control, forskolin, SP600125, forskolin + SP600125 groups or OE-β-catenin, OE-β-catenin + forskolin, OE-β-catenin + SP600125, OE-β-catenin + forskolin + SP600125 groups. Forskolin (10 mg/kg) or SP600125 (30 mg/kg) was injected to the mice every 7 days when the tumors were grown 3–4 mm in diameter and were visible. All mice were euthanized after 28 days of the implantation, then the tumors of each mouse were taken out for weighing or for immunohistochemical staining. All protocols involving animals were in accordance with the Animal Experiment Ethics Review of Ethics Committee of Henan Kaifeng Children Hospital University and this study was approved by Ethics Committee of Henan Kaifeng Children Hospital.
Immunohistochemistry
Formalin-fixed paraffin-embedded cancer tissue samples were cut into 5 μm sections. After deparaffinized, hydrated, and blocked with 5% of goat serum (AmyJet Scientific, Inc., Wuhan, China), the sections were probed with the primary antibody against Ki-67 (No. 12202, Cell Signaling Technology, CA, USA) and the corresponding second antibody (Cell Signaling Technology), followed by incubation with the chromogen 3, 30-diaminobenzidine tetrachloride (R&D Systems, Inc., Minneapolis, MN, USA). Cell nucleus was stained with Harri’s hematoxylin solution.
Data analysis
All experiments in this study were performed at least three times. Data were expressed as mean ± SD and were statistically analyzed with SPSS software (version 23.0) using Student’s t-test. A difference with a P-value <0.05 was regarded as statistically significant.
Results
Forskolin induces NHL cell apoptosis
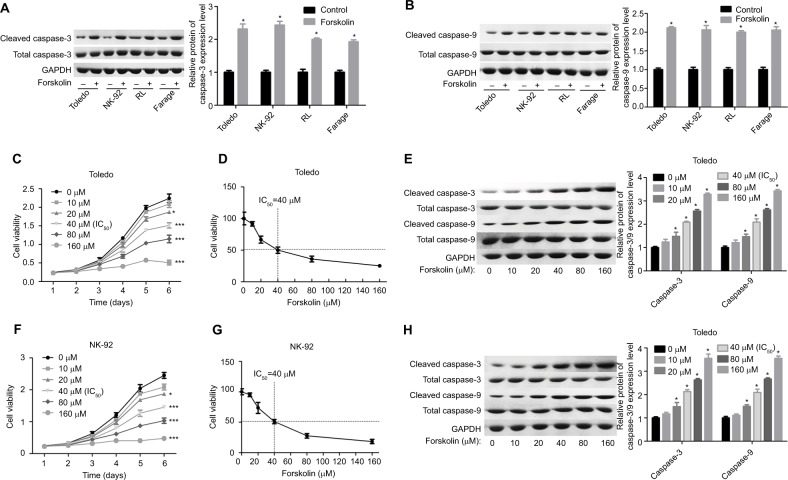
First, we explored the effects of different concentrations of forskolin on the apoptosis of diverse NHL cell lines with Western blotting analysis. Compared to the control group, forskolin (30 μM) significantly increased the expression of cleaved caspase-3/9 with total caspase-3/9 showing no obvious change, in Toledo, NK-92, RL, and Farage cells (Figure 1A, B). Then Toledo and NK-92 cells were chosen for further study. Treatment of Toledo and NK-92 cells with 20, 40, 80, and 160 μM of forskolin markedly repressed cell growth (Figure 1C, F) and promoted cell apoptosis (Figure 1E, H), with 40 μM as the IC50 (Figure 1D, G), a concentration that we used in our next study. Overall, these discoveries demonstrate that forskolin could promote the apoptosis of NHL cells.
Figure 1.
Forskolin promoted the protein expression of cleaved caspase-3/9 and repressed cell growth in NHL cells.
Notes: (A, B) Toledo, NK-92, RL, and Farage cells were treated with 30 μM of forskolin for 48 hours, then the cells were harvested for Western blotting to assess the expression of total or cleaved caspase-3/9. (C) MTT assay was conducted to test the cell growth after Toledo cells were administered 0, 20, 40, 80, or 160 μM of forskolin for 1, 2, 3, 4, 5, or 6 days. (D) Toledo cells were administered 0, 20, 40, 80, or 160 μM of forskolin for 48 hours, then MTT assay was used to evaluate the IC50 of forskolin. (E) Toledo cells were incubated with 0, 20, 40, 80, or 160 μM of forskolin for 48 hours, then Western blotting analysis was carried out to detect the expression of total or cleaved caspase-3/9. (F) MTT assay was conducted to test the cell growth after NK-92 cells were administered 0, 20, 40, 80, or 160 μM of forskolin for 1, 2, 3, 4, 5, or 6 days. (G) NK-92 cells were administered 0, 20, 40, 80, or 160 μM of forskolin for 48 hours, then MTT assay was used to evaluate the IC50 of forskolin. (H) NK-92 cells were incubated with 0, 20, 40, 80, or 160 μM of forskolin for 48 hours, then Western blotting analysis was carried out to detect the expression of total or cleaved caspase-3/9 (n=3, *P<0.05, ***P<0.001, forskolin group vs control group).
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NHL, non-Hodgkin’s lymphoma.
Forskolin inhibits the activation of β-catenin signaling
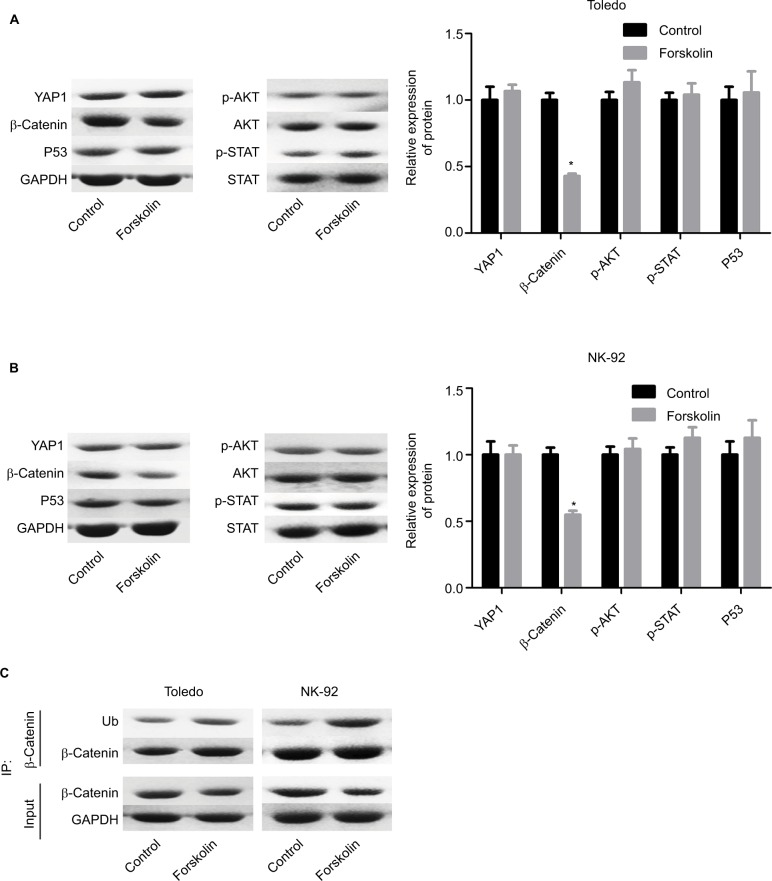
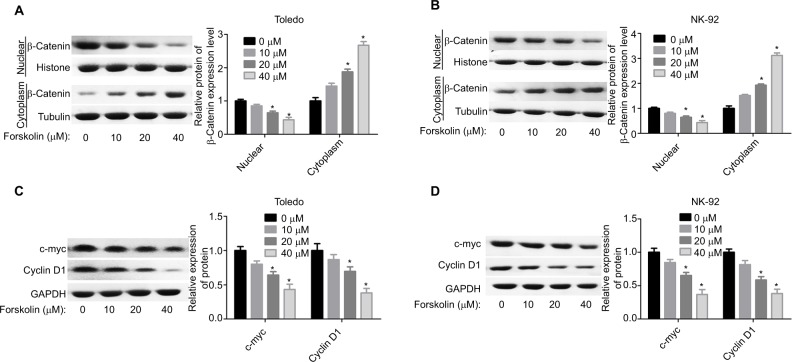
To explore the underlying mechanism of forskolin, we assessed the expression of proteins related to key signaling pathways regulating cell fate, such as Hippo/YAP, Wnt/β-catenin, AKT, JNK/STAT, and P53 in the presence of 40 μM forskolin. Results showed that the expression of β-catenin was reduced when Toledo and NK-92 cells were treated with 40 μM of forskolin for 48 hours, while the expression of other proteins p-AKT, AKT, p-STAT, STAT, and P53 showed no apparent change (Figure 2A, B). The expression of ubiquitin was also increased in the presence of forskolin as compared to the control group in both Toledo and NK-92 cells (Figure 2C). Besides, the nuclear protein of β-catenin was decreased while cytoplasmic β-catenin protein was increased after cells were treated with forskolin in a concentration-dependent manner both in Toledo (Figure 3A) and NK-92 cells (Figure 3B). Moreover, forskolin decreased the expression of oncogenic proteins cyclin D1 and c-myc, downstream proteins of β-catenin signaling (Figure 3C, D). These results suggest that forskolin represses the activation of β-catenin signaling pathway.
Figure 2.
Forskolin reduced β-catenin expression while it increased its ubiquitination.
Notes: Toledo and NK-92 cells were incubated with 0, 20, 40, 80, or 160 μM of forskolin for 48 hours, then (A, B) Western blotting was recruited to determine the expression of YAP1, β-catenin, AKT, p-AKT, STAT, and p-STAT, and (C) IP assay was used to examine the expression of ubiquitin, which binds to β-catenin protein. (n=3, *P<0.05, forskolin group vs control group).
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IP, immunoprecipitation; STAT, signal transduction and transcriptional activator; YAP, Yes-associated protein.
Figure 3.
Forskolin reduced the nuclear accumulation of β-catenin and the expression of c-myc and cyclin D1.
Notes: (A, B) The protein expression of cytoplasmic and nuclear β-catenin in Toledo and NK-92 cells in the presence of 0, 10, 20, or 40 μM forskolin was detected by Western blotting. (C, D) Western blotting analysis of the total protein expression of c-myc and cyclin D1 influenced by 0, 10, 20, or 40 μM of forskolin in Toledo and NK-92 cells (n=3, *P<0.05, forskolin group vs control group).
Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Forskolin reduces the expression of β-catenin via upregulating Axin expression in NHL cells
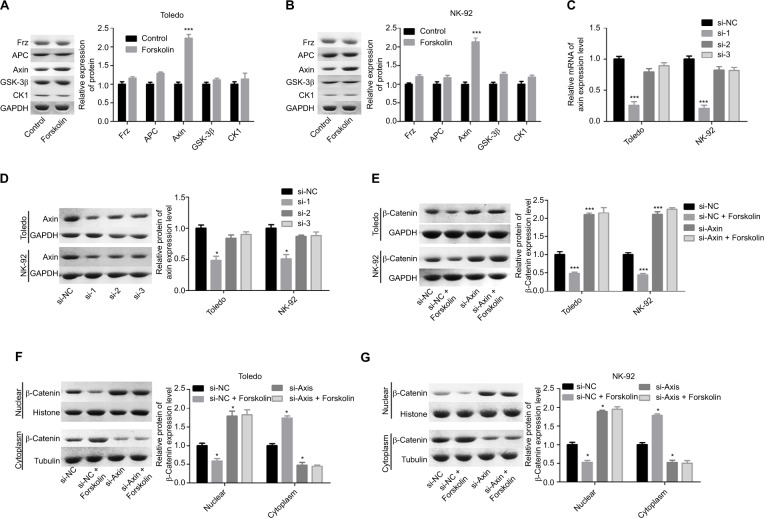
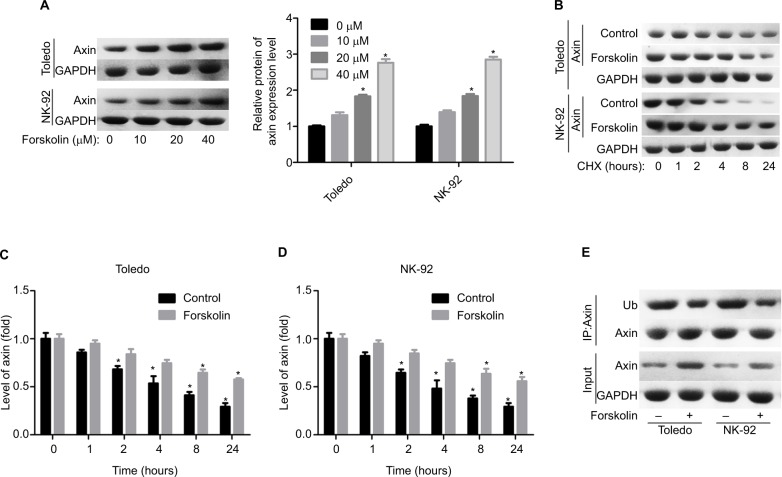
Next, we explored the expression of proteins related to β-catenin signaling, including Frz, Axin, glycogen synthase kinase 3β (GSK-3β), adenomatous polyposis coli (APC), and CK1. Results showed that only the expression of Axin was upregulated when Todelo and NK-92 cells were treated with 40 μM forskolin (Figure 4A, B), suggesting that Axin upregulation might induce the downregulation of β-catenin under forskolin treatment. Then, we specifically investigated the effects of Axin in the process where forskolin repressed the activation of β-catenin signaling through recruiting siR-NAs targeting Axin. And si-1 targeting Axin gene had the best knockdown efficiency of Axin in Toledo and NK-92 cells in both mRNA (Figure 4C) and protein levels (Figure 4D). Knockdown of Axin significantly reduced β-catenin expression regardless of forskolin presence (Figure 4E), as well as induced the reduction of nuclear β-catenin protein and cytoplasmic β-catenin increase (Figure 4F, G). Besides, forskolin promoted the expression of c-myc protein in a concentration-dependent way (Figure 5A). Furthermore, forskolin slowed down the degradation of Axin protein (Figure 5B–D) and inhibited its ubiquitination (Figure 5E). Taken these results together, we clarify that forskolin reduces the expression of β-catenin via upregulating Axin expression in NHL cells.
Figure 4.
Forskolin reduced the expression of β-catenin through upregulation of the expression of Axin.
Notes: (A, B) The expressions of Axin, Frz, APC, GSK-3β, and CK1 in forskolin (40 μM)-treated or control Toledo and NK-92 cells were examined by Western blotting analysis (n=3, *P<0.05, forskolin group vs control group). (C, D) RT-PCR and Western blotting were carried out to detect the mRNA and protein levels of Axin after Toledo or NK-92 cells were transfected with si-1/2/3 targeting Axin gene for 24 or 48 hours, respectively (n=3, *P<0.05, ***P<0.001, si-1/2/3 group vs si-NC group). (E) Western blotting analysis of the expression of β-catenin in different groups of Toledo and NK-92 cells: si-NC, si-NC + forskolin, si-Axin, si-Axin + forskolin (n=3, ***P<0.001, si-NC + forskolin group or si-Axin group vs control group). (F, G) The protein expression of cytoplasmic and nuclear β-catenin in Toledo and NK-92 cells with different treatments: si-NC, si-NC + forskolin, si-Axin, si-Axin + forskolin (n=3, *P<0.05, si-NC + forskolin group or si-Axin group vs control group).
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GSK-3β, glycogen synthase kinase 3β.
Figure 5.
Forskolin increased the stability of Axin protein and reduced its ubiquitylation.
Notes: (A) Toledo and NK-92 cells were treated with 0, 10, 20, or 40 μM of forskolin for 48 hours, then cells were collected for Western blotting analysis to determine the expression of Axin. (B) Toledo and NK-92 cells were treated with 0, 10, 20, or 40 μM of forskolin for 48 hours, then the culture medium was replaced with CHX (100 mg/mL) and incubated for 0, 1, 2, 4, 8, and 24 hours, next cells were harvested for Western blotting analysis with antibody against Axin. (C, D) Bar graph of the expression of Axin related to Figure 5B. (E) IP assay was used to analyze the expression of Ub combining with Axin protein in the presence or absence of forskolin (40 μM) (n=3, *P<0.05, forskolin group vs control group).
Abbreviations: CHX, cyclohexane; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IP, immunoprecipitation; Ub, ubiquitin.
Forskolin enhances cell apoptosis induced by SP600125 in NHL cells
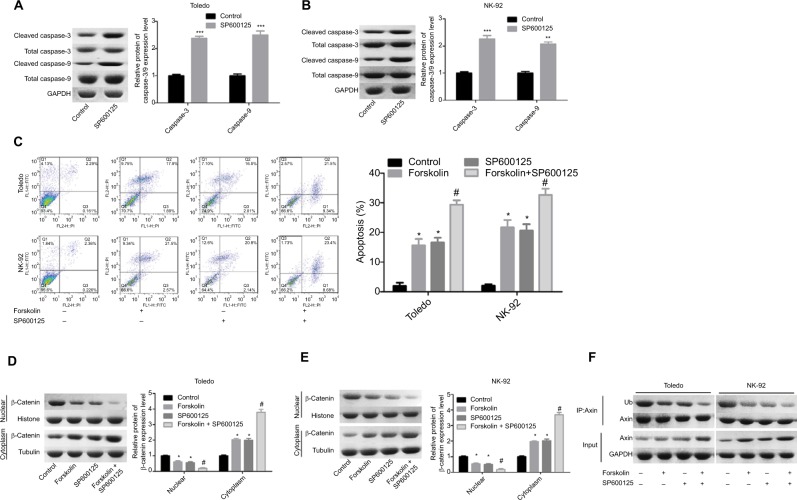
As shown in Figure 6A, SP600125 increased the expression of cleaved caspase-3/9 in Toledo and NK-92 cells, as compared to the control group (Figure 6A, B). Similarly, SP600125 and forskolin increased the apoptosis rate (total early and late apoptosis) of Toledo and NK-92 cells, and cell apoptosis was enhanced when cells were treated with SP600125 and forskolin simultaneously (Figure 6C). Besides, SP600125 enhanced the translocation of β-catenin protein from nuclear to cytoplasm (Figure 6D, E) and reduced the ubiquitination of Axin protein (Figure 6F) induced by forskolin treatment in Toledo and NK-92 cells. The results specified above suggest that SP600125 could enhance the effects induced by forskolin in the apoptosis and β-catenin location and Axin stability in NHL cells.
Figure 6.
SP600125 enhanced cell apoptosis induced by forskolin in NHL cells.
Notes: (A, B) The expression of the total or cleaved caspase-3/9 was decided by Western blotting after Toledo or NK-92 cells were treated with SP600125 (20 μM) or not (n=3, **P<0.01, ***P<0.001, SP600125 group vs control group). (C) Flow cytometry was used to determine cell apoptosis rates after Toledo or NK-92 cells were treated with SP600125 (20 μM) or not (n=3, *P<0.05, SP600125 group or forskolin group vs control group; #P<0.05, forskolin + SP600125 group vs forskolin group). (D, E) Toledo and NK-92 cells were treated with forskolin (40 μM), SP600125 (20 μM), forskolin (40 μM) + SP600125 (20 μM), or nothing, then cells were collected and the protein expression of cytoplasmic and nuclear β-catenin was examined by Western blotting (n=3, *P<0.05, SP600125 group or forskolin group vs control group; #P<0.05, forskolin + SP600125 group vs forskolin group). (F) Toledo and NK-92 cells were treated with forskolin (40 μM), SP600125 (20 μM), forskolin (40 μM) + SP600125 (20 μM) or nothing, then IP assay was conducted to measure the ubiquitination of Axin protein.
Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Forskolin/SP600125 represses tumor growth through downregulating β-catenin in vivo
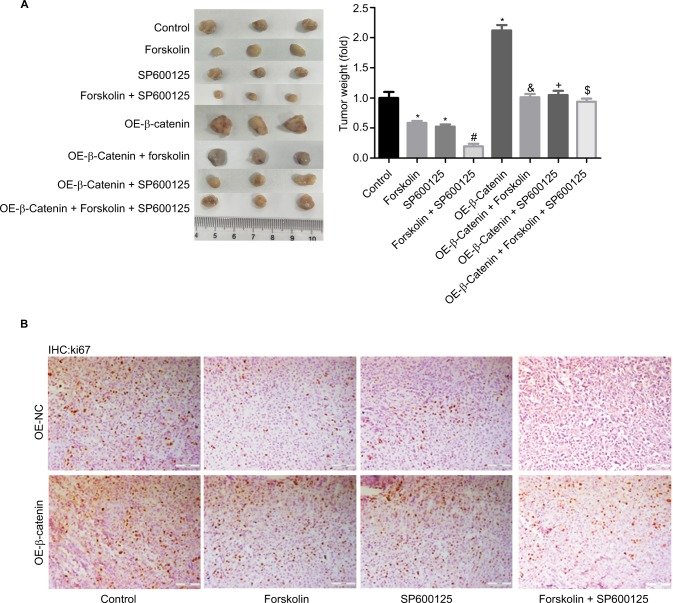
Finally, we explored the function of forskolin/SP600125 in NHL cell tumorigeneis in vivo. Results showed that tumor weights (Figure 7A) and the expression of Ki-67 (Figure 7B), a proliferative index, were all decreased when NK-92 cells were treated with SP600125, forskolin, or SP600125 + forskolin together, especially for SP600125 + forskolin when compared with the control group. Upregulation of β-catenin significantly enhanced the tumorigenesis of NK-92 cells. And the inhibition of tumorigenesis and Ki-67 expression induced by SP600125, forskolin, or SP600125 + forskolin was repressed when β-catenin was upregulated in NK-92 cells (Figure 7A, B). These results illustrate that both forskolin and SP600125 repress tumor growth through downregulating β-catenin in NHL cells.
Figure 7.
Forskolin/SP600125 reduced tumor growth through downregulating β-catenin in vivo.
Notes: (A) Mouse tumor-bearing assay was used to evaluate the tumorigenesis of NK-92 cells with different treatments. (B) Immunohistochemical analysis of the expression of ki-67 in the tumor tissues from different groups in NK-92 cells (scale bar =100 μm) (n=6, *P<0.05, SP600125 group, forskolin group or OE-β-catenin group vs control group; #P<0.05, forskolin + SP600125 group vs forskolin group; δP<0.05, OE-β-catenin + forskolin group vs forskolin group; +P<0.05, OE-β-catenin + SP600125 group vs SP600125 group; $P<0.05, OE-β-catenin + forskolin + SP600125 group vs forskolin + SP600125 group).
Abbreviation: IHC, immunohistochemistry.
Discussion
Pediatric NHL is characterized by high degree of malignancy, rapid progress, and even invasion into the bone marrow and central nervous system.22 Although the cure rate of pediatric NHL has reached 70%–90%, some children still have underwent recurrence or death in the short term after chemotherapy even during chemotherapy.23 Therefore, how to optimize therapy methods to reduce the recurrence rate and additional pain to the minimum has become research hot. This study focuses on this issue and demonstrates that forskolin treatment could significantly promote cell apoptosis and inhibit cell viability through Axin-dependent β-catenin signaling inhibition in NHL. Moreover, forskolin enhances the antitumor role induced by SP600125, which provides theoretical foundation of serving forskolin as a new effective therapeutic drug for pediatric NHL.
Forskolin has been certified to be a valid medication for multiple diseases, including heart disease, diabetes, hypertension, and asthma.24,25 Besides, forskolin, an analog of cAMP, which induces cell apoptosis in multiple cancers and overcomes drug resistance,26–28 is suggested as one of the most promising molecules for potential application in cancer therapy.11 For instance, several years ago, Piontek et al29 reported that forskolin inhibited the proliferation of the gastric cancer cells through reduction of the expression of some oncogenes. Yamanaka et al30 demonstrated that forskolin inhibited cell proliferation and induced the apoptosis of rhabdomyosarcoma, neuroblastoma, and hepatoblastoma cells through inhibiting Hedgehog signaling pathway and reduced c-myc expression in a dose-dependent manner. They also revealed that forskolin could inhibit human rhabdomyosarcoma xenograft.31 Interestingly, forskolin was proved to protect the skin from UV injury and UV-induced carcinogenesis and suggested as a latent UV-protective drug in sun-sensitive or melanoma-prone individuals.32 And the current study explored the function of forskolin in NHL for the first time, and illustrated that forskolin also exerts an anticancer role in NHL, which significantly promoted the apoptosis and inhibited the tumor growth of NHL cells.
Moreover, we investigated the underlying mechanism of forskolin in NHL progression inhibition. We observed that only the expression of β-catenin was obviously decreased, while the expression levels of other signaling-related proteins such as AKT, p-AKT, STAT, p-STAT, P53, and YAP1 showed no significant change. Moreover, forskolin treatment accelerated the transportation of β-catenin protein from nuclear to cytoplasm and increased its ubiquitination. These findings suggest that β-catenin signaling pathway plays a vital role in the anticancer effects induced by forskolin. Wnt/β-catenin signaling pathway controls cell fate and tissue homeostasis, which plays a momentous role in the pathogenesis of many different kinds of disease.33,34 On the surface of cells, Wnt ligands bind to a receptor complex of two molecules consisting of a member of the Frizzled family and the low-density lipoprotein receptor-related protein family, LRP5/6, then β-catenin will be released from the destruction complex35,36 including Axin, APC, GSK-3β, casein kinase 1α, βTrCP1, and βTrCP2, which sequentially mediates a reduction of the phosphorylation and ubiquitination of β-catenin protein. Then β-catenin accumulates in cell nucleus where it forms a complex with T-cell factor/lymphoid enhancer factor family, which acts as a transcription factor for oncogenic genes such as cyclin D1 and c-myc.37 And in this study, we demonstrated that forskolin could increase the expression of Axin through slowing down the degradation of Axin protein and reducing its ubiquitination. Moreover, knockdown of Axin abolished the reduction of the expression of β-catenin, c-myc, and cyclin D1 and the cytoplasmic accumulation of β-catenin mediated by forskolin, indicating that forskolin inhibited the activation of β-catenin signaling through upregulation of Axin in NHL cells.
Furthermore, we also observed that forskolin enhanced the anticancer role of SP600125. SP600125, an inhibitor of JNK pathway, exerts an antitumor role in many kinds of cancers. Kim et al38 reported that SP600125 reduced the drug resistance of breast cancer cells and stomach cancer cells to doxorubicin in vivo and in vitro. Grassi et al39 indicated that SP600125 had a remarkable anticancer capacity against undifferentiated thyroid cancer through selective action on ROCK and p53 pathways. In this study, we revealed that SP600125 also exerted an antitumor potential in NHL partly through repressing the expression of β-catenin in an Axin-dependent manner.
Conclusion
The current study clarifies that forskolin can inhibit the progression of NHL through Axin-mediated inhibition of β-catenin signaling. Moreover, forskolin improves the effects of SP600125 on cell apoptosis enhancement and tumorigenesis inhibition of NHL cells. These findings provide theoretical foundation of serving forskolin as a new effective therapeutic drug for pediatric NHL.
Supplementary material
Table S1.
Antibody information
| Name | No. | Source |
|---|---|---|
| Cleaved caspase-3 | 9661 | Cell Signaling Pathway, Danvers, MA, USA |
| Caspase-3 | 9662 | Cell Signaling Pathway, Danvers, MA, USA |
| Cleaved caspase-9 | 20750 | Cell Signaling Pathway, Danvers, MA, USA |
| Caspase-9 | 9502 | Cell Signaling Pathway, Danvers, MA, USA |
| YAP1 | 4912 | Cell Signaling Pathway, Danvers, MA, USA |
| P53 | 9282 | Cell Signaling Pathway, Danvers, MA, USA |
| Phosphorylated AKT | 4060 | Cell Signaling Pathway, Danvers, MA, USA |
| AKT | 9272 | Cell Signaling Pathway, Danvers, MA, USA |
| β-catenin | 9562 | Cell Signaling Pathway, Danvers, MA, USA |
| p-STAT | 9145 | Cell Signaling Pathway, Danvers, MA, USA |
| STAT | 9139 | Cell Signaling Pathway, Danvers, MA, USA |
| Ubiquitin | 3933 | Cell Signaling Pathway, Danvers, MA, USA |
| Frz | AF1617 | R&D Systems, Minneapolis, MN, USA |
| Axin | 34-5900 | Thermo Fisher Technology, Waltham, MA, USA |
| APC | ab15270 | Abcam, Cambridge, MA, USA |
| GSK-3β | ab93926 | Abcam, Cambridge, MA, USA |
| CK1 | ab115293 | Abcam, Cambridge, MA, USA |
| Cyclin D1 | sc-450 | Santa Cruz Biotechnology, Dallas, TX, USA |
| c-myc | sc-40 | Santa Cruz Biotechnology, Dallas, TX, USA |
| Histone | ab1791 | Abcam, Cambridge, MA, USA |
| Tubulin | ab7291 | Abcam, Cambridge, MA, USA |
| Glyceraldehyde 3-phosphate dehydrogenase | 2118 | Cell Signaling Pathway, Danvers, MA, USA |
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lowry L, Linch D. Non-Hodgkin’s lymphoma. Medicine. 2013;41(5):282–289. [Google Scholar]
- 2.Newton R, Ferlay J, Beral V, Devesa SS. The epidemiology of non-Hodgkin’s lymphoma: comparison of nodal and extra-nodal sites. Int J Cancer. 1997;72(6):923–930. doi: 10.1002/(sici)1097-0215(19970917)72:6<923::aid-ijc1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y, Kristinsson SY, Goldin LR, Björkholm M, Caporaso NE, Landgren O. Increased risk for non-Hodgkin lymphoma in individuals with celiac disease and a potential familial association. Gastroenterology. 2009;136(1):91–98. doi: 10.1053/j.gastro.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanne H, Burte NP, Prasanna V, Gujjula R. Extraction and elemental analysis of Coleus forskohlii extract. Pharmacognosy Res. 2015;7(3):237–241. doi: 10.4103/0974-8490.157966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson S, Magu B, Rasmussen C, et al. Effects of coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J Int Soc Sports Nutr. 2005;2(2):54–62. doi: 10.1186/1550-2783-2-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loftus HL, Astell KJ, Mathai ML, Su XQ. Coleus forskohlii extract supplementation in conjunction with a hypocaloric diet reduces the risk factors of metabolic syndrome in overweight and obese subjects: a randomized controlled trial. Nutrients. 2015;7(11):9508–9522. doi: 10.3390/nu7115483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese C, Davinelli S, Mangano K, et al. Effects of a new combination of plant extracts plus d-mannose for the management of uncomplicated recurrent urinary tract infections. J Chemother. 2018;30(2):107–114. doi: 10.1080/1120009X.2017.1393587. [DOI] [PubMed] [Google Scholar]
- 8.Dessauer CW, Watts VJ, Ostrom RS, Conti M, Dove S, Seifert R. International Union of Basic and Clinical Pharmacology. CI. Structures and small molecule modulators of mammalian adenylyl cyclases. Pharmacol Rev. 2017;69(2):93–139. doi: 10.1124/pr.116.013078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gancedo JM. Biological roles of cAMP: variations on a theme in the different kingdoms of life. Biol Rev Camb Philos Soc. 2013;88(3):645–668. doi: 10.1111/brv.12020. [DOI] [PubMed] [Google Scholar]
- 10.Sapio L, Di Maiolo F, Illiano M, et al. Targeting protein kinase A in cancer therapy: an update. Excli J. 2014;13:843–855. [PMC free article] [PubMed] [Google Scholar]
- 11.Sapio L, Gallo M, Illiano M, et al. The natural cAMP elevating compound forskolin in cancer therapy: is it time? J Cell Physiol. 2017;232(5):922–927. doi: 10.1002/jcp.25650. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, Juhnn YS. cAMP signaling increases histone deacetylase 8 expression via the Epac2-Rap1A-Akt pathway in H1299 lung cancer cells. Exp Mol Med. 2017;49(2):e297. doi: 10.1038/emm.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem. 2004;279(20):20858–20865. doi: 10.1074/jbc.M310643200. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Hikichi M, Yukitake J, et al. Forskolin increases the effect of everolimus on aromatase inhibitor-resistant breast cancer cells. Oncotarget. 2018;9(34):23451–23461. doi: 10.18632/oncotarget.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Follin-Arbelet V, Misund K, Naderi EH, Ugland H, Sundan A, Blomhoff HK. The natural compound forskolin synergizes with dexamethasone to induce cell death in myeloma cells via Bim. Sci Rep. 2015;5:13001. doi: 10.1038/srep13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn SN, Graves SH, Dains-McGahee C, et al. Adenylyl cyclase 3/adenylyl cyclase-associated protein 1 (CAP1) complex mediates the anti-migratory effect of forskolin in pancreatic cancer cells. Mol Carcinog. 2017;56(4):1344–1360. doi: 10.1002/mc.22598. [DOI] [PubMed] [Google Scholar]
- 17.Gujral TS, Kirschner MW. Hippo pathway mediates resistance to cytotoxic drugs. Proc Natl Acad Sci USA. 2017;114(18):E3729–E3738. doi: 10.1073/pnas.1703096114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed MK, Shao C, Wang J, et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016;3(1):11–40. doi: 10.1016/j.gendis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCubrey JA, Abrams SL, Fitzgerald TL, et al. Roles of signaling pathways in drug resistance, cancer initiating cells and cancer progression and metastasis. Adv Biol Regul. 2015;57:75–101. doi: 10.1016/j.jbior.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Kim BH, Yin CH, Guo Q, et al. A small-molecule compound identified through a cell-based screening inhibits JAK/STAT pathway signaling in human cancer cells. Mol Cancer Ther. 2008;7(9):2672–2680. doi: 10.1158/1535-7163.MCT-08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8(5):8921–8946. doi: 10.18632/oncotarget.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karadeniz C, Oguz A, Citak EC, et al. Clinical characteristics and treatment results of pediatric B-cell non-Hodgkin lymphoma patients in a single center. Pediatr Hematol Oncol. 2007;24(6):417–430. doi: 10.1080/08880010701450972. [DOI] [PubMed] [Google Scholar]
- 23.Asselin BL, Devidas M, Chen L, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group randomized trial Pediatric Oncology Group 9404. J Clin Oncol. 2016;34(8):854–862. doi: 10.1200/JCO.2015.60.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huerta M, Urzúa Z, Trujillo X, González-Sánchez R, Trujillo-Hernández B. Forskolin compared with beclomethasone for prevention of asthma attacks: a single-blind clinical trial. J Int Med Res. 2010;38(2):661–668. doi: 10.1177/147323001003800229. [DOI] [PubMed] [Google Scholar]
- 25.Ríos-Silva M, Trujillo X, Trujillo-Hernández B, et al. Effect of chronic administration of forskolin on glycemia and oxidative stress in rats with and without experimental diabetes. Int J Med Sci. 2014;11(5):448–452. doi: 10.7150/ijms.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Tang Y, Hang H, et al. cAMP induces cell apoptosis in multiple myeloma and overcomes bortezomib resistance. Am J Cancer Res. 2018;8(1):16–29. [PMC free article] [PubMed] [Google Scholar]
- 27.Perez DR, Smagley Y, Garcia M, et al. Cyclic AMP efflux inhibitors as potential therapeutic agents for leukemia. Oncotarget. 2016;7(23):33960–33982. doi: 10.18632/oncotarget.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattabiraman DR, Bierie B, Kober KI, et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability Science 20163516277)aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piontek M, Hengels KJ, Porschen R, Strohmeyer G. Protein kinase C and adenylate cyclase as targets for growth inhibition of human gastric cancer cells. J Cancer Res Clin Oncol. 1993;119(12):697–699. doi: 10.1007/BF01195338. [DOI] [PubMed] [Google Scholar]
- 30.Yamanaka H, Oue T, Uehara S, Fukuzawa M. Forskolin, a hedgehog signal inhibitor, inhibits cell proliferation and induces apoptosis in pediatric tumor cell lines. Mol Med Rep. 2010;3(1):133–139. doi: 10.3892/mmr_00000230. [DOI] [PubMed] [Google Scholar]
- 31.Yamanaka H, Oue T, Uehara S, Fukuzawa M. Hedgehog signal inhibitor forskolin suppresses cell proliferation and tumor growth of human rhabdomyosarcoma xenograft. J Pediatr Surg. 2011;46(2):320–325. doi: 10.1016/j.jpedsurg.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Amaro-Ortiz A, Yan B, D’Orazio JA. Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules. 2014;19(5):6202–6219. doi: 10.3390/molecules19056202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmeyer K, Raggioli A, Rudloff S, et al. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336(6088):1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 35.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SE, Huang H, Zhao M, et al. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340(6134):867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JH, Kim TH, Kang HS, Ro J, Kim HS, Yoon S. SP600125, an inhibitor of JNK pathway, reduces viability of relatively resistant cancer cells to doxorubicin. Biochem Biophys Res Commun. 2009;387(3):450–455. doi: 10.1016/j.bbrc.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 39.Grassi ES, Vezzoli V, Negri I, et al. SP600125 has a remarkable anticancer potential against undifferentiated thyroid cancer through selective action on rock and p53 pathways. Oncotarget. 2015;6(34):36383–36399. doi: 10.18632/oncotarget.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Antibody information
| Name | No. | Source |
|---|---|---|
| Cleaved caspase-3 | 9661 | Cell Signaling Pathway, Danvers, MA, USA |
| Caspase-3 | 9662 | Cell Signaling Pathway, Danvers, MA, USA |
| Cleaved caspase-9 | 20750 | Cell Signaling Pathway, Danvers, MA, USA |
| Caspase-9 | 9502 | Cell Signaling Pathway, Danvers, MA, USA |
| YAP1 | 4912 | Cell Signaling Pathway, Danvers, MA, USA |
| P53 | 9282 | Cell Signaling Pathway, Danvers, MA, USA |
| Phosphorylated AKT | 4060 | Cell Signaling Pathway, Danvers, MA, USA |
| AKT | 9272 | Cell Signaling Pathway, Danvers, MA, USA |
| β-catenin | 9562 | Cell Signaling Pathway, Danvers, MA, USA |
| p-STAT | 9145 | Cell Signaling Pathway, Danvers, MA, USA |
| STAT | 9139 | Cell Signaling Pathway, Danvers, MA, USA |
| Ubiquitin | 3933 | Cell Signaling Pathway, Danvers, MA, USA |
| Frz | AF1617 | R&D Systems, Minneapolis, MN, USA |
| Axin | 34-5900 | Thermo Fisher Technology, Waltham, MA, USA |
| APC | ab15270 | Abcam, Cambridge, MA, USA |
| GSK-3β | ab93926 | Abcam, Cambridge, MA, USA |
| CK1 | ab115293 | Abcam, Cambridge, MA, USA |
| Cyclin D1 | sc-450 | Santa Cruz Biotechnology, Dallas, TX, USA |
| c-myc | sc-40 | Santa Cruz Biotechnology, Dallas, TX, USA |
| Histone | ab1791 | Abcam, Cambridge, MA, USA |
| Tubulin | ab7291 | Abcam, Cambridge, MA, USA |
| Glyceraldehyde 3-phosphate dehydrogenase | 2118 | Cell Signaling Pathway, Danvers, MA, USA |