Abstract
Objective
We aimed to determine the safety and efficacy of irreversible electroporation (IRE) combined with chemotherapy for unresectable pancreatic carcinoma (stage III/IV).
Methods
We prospectively enrolled 54 patients (30 men; median age 61.0 years; range 41–73 years) undergoing IRE with or without chemotherapy for pancreatic cancer between July 2015 and August 2016. Kaplan–Meier estimates were used to analyze progression-free survival (PFS) and overall survival (OS). Safety was assessed based on the occurrence of adverse events.
Results
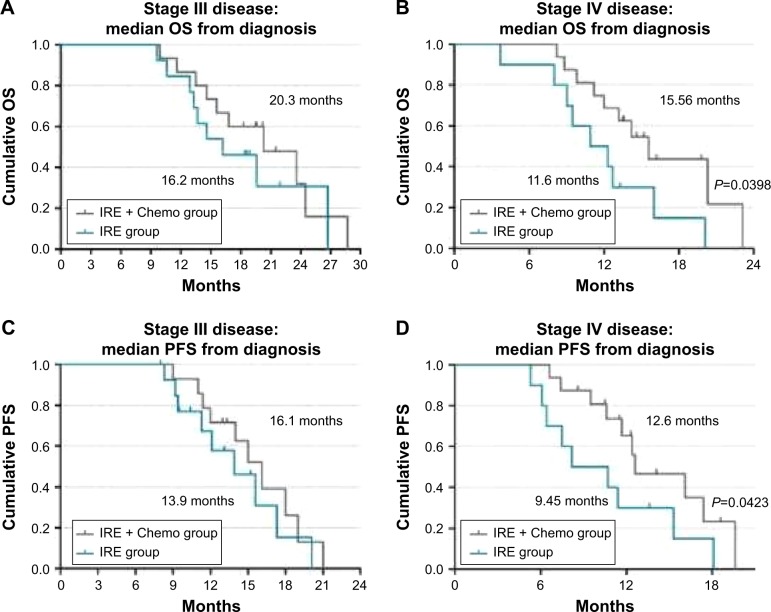
All patients successfully underwent IRE. Major IRE-related complications were observed in four patients (7.4%). Gastrointestinal hemorrhage only developed in cases undergoing IRE via the open method, and was successfully managed with interventional embolization and/or vascular ligation. Any minor complications in the cases were alleviated within 14 days after symptomatic treatment. Overall, the performance status score decreased from 1.06 to 0.89 at 3 months after IRE (P<0.05). Among those with stage III disease, after a median follow-up of 18.8 months (range 9.6–28.7 months), the median OS from diagnosis was 16.2 and 20.3 months in the IRE and IRE + Chemo groups, respectively. Among those with stage IV disease, after a median follow-up of 13.3 months (range 3.7–23.1 months), the median OS from diagnosis was 11.6 and 13.56 months in the IRE and IRE + Chemo groups, respectively. The OS was significantly poorer in the IRE group than in the IRE + Chemo group (log-rank test, P=0.0398).
Conclusion
Patients with pancreatic carcinoma could benefit from IRE, which improved the OS in certain patients who had also undergone chemotherapy. Although some severe complications were noted, IRE was generally well tolerated.
Keywords: pancreatic carcinoma, irreversible electroporation, ablation, progression-free survival, overall survival
Introduction
Pancreatic cancer is one of the most aggressive cancers, and is the fourth leading cause of cancer-related death in the USA. The condition often remains covert in the early stages and can progress rapidly; hence, most patients are diagnosed at an advanced stage. Moreover, only 10%–20% of these patients can be indicated for surgical resection.1,2 Although the resection rates have improved in recent years, the rates of postoperative complications, such as pancreatic fistula and bleeding, as well as post- operative recurrence and metastasis, have increased.3 In cases with locally advanced pancreatic cancer, tumor invasion of the surrounding blood vessels often limits the range of resection or precludes surgery altogether.4–6 At present, systemic palliative chemotherapy is the most commonly used treatment available for unresectable pancreatic cancer.7 However, pancreatic cancer is often resistant to chemotherapy, and the prognosis is poor. The median overall survival (OS) of pancreatic cancer patients reportedly ranges from 10.2 to 16.6 months. Approximately 30%–40% of patients present with locally advanced cancer at diagnosis, and the 1-year relative survival rates for all stages are poor (approximately 27%).2,8 Combined chemoradiotherapy may help to downstage locally advanced pancreatic cancer in approximately 30% of patients,9,10 and could facilitate surgery.
Tumor ablation has been used for unresectable pancreatic cancer, but damage to the surrounding structures, such as blood vessels, bile ducts, and pancreatic ducts, limits its use.11,12 Furthermore, hemorrhage and pancreatic fistulae frequently develop after conventional ablation, and peripheral tumor ablation is often incomplete.13 In contrast to conventional ablation methods, irreversible electroporation (IRE) uses high-voltage pulses to create permanent nanopores in the cell membranes, which consequently induce cell apoptosis.14 Because of its non-thermal mechanism of action, IRE can be used to target malignancies adjacent to vital structures.15 In patients with borderline resectable or unresectable locally advanced pancreatic cancer, IRE can also help to downstage the tumor, thus facilitating surgical resection.16 Furthermore, IRE can be used to treat large tumors (>5 cm) in cases where chemotherapy fails to produce a significant decrease in tumor size.17
In the present prospective study, we aimed to determine the safety and efficacy of IRE with or without chemotherapy in the treatment of unresectable pancreatic carcinoma (stage III/IV).
Materials and methods
Patient population and ethics statement
This study was registered with the US National Institutes of Health (ID: NCT02343835) and was approved by the regional ethics committee of Guangzhou Fuda Hospital. Each participant provided written informed consent for study participation, the ablation procedure, and data usage, and the study was conducted in accordance with the tenets of the Declaration of Helsinki. We prospectively enrolled patients who were scheduled to undergo IRE for pancreatic cancer between July 2015 and August 2016.
Patients were eligible for inclusion in the study if they had histologically or cytologically proven locally advanced stage III/IV pancreatic cancer (stage IV patients were eligible if they had three or fewer metastatic tumors with a maximum diameter of <3 cm); surgery was contraindicated owing to the pathological subtype, tumor stage, tumor location, or other reasons; their life expectancy was >3 months, and long-term follow-up was therefore possible; they had no serious abnormalities on blood routine examination, normal coagulation function, normal liver/lung/kidney function tests, no severe ascites, no brain metastasis, no grade 3 hypertension, no severe heart disease, and no acute or chronic infections (such as hepatitis B and HIV infection); they had a performance status score of ≤2; and they provided written informed consent.
Patients were excluded from the study if they had severe coagulation dysfunction; inability to tolerate general anesthesia; multiple metastases or multiple liver lesions (more than three); a performance status score of >2; cardiac arrhythmia and pacemakers; severe hypertension; or inducible myocardial ischemia or uncontrolled angina on a cardiac stress test.
Data collection
The following data were collected from all patients prior to IRE: age; sex; performance status score; pathological findings; and abdominal computed tomography (CT) or magnetic resonance imaging (MRI) findings, such as tumor size (maximum dimension), location, and American Joint Committee on Cancer (AJCC) tumor stage.
Preoperative preparation
All patients underwent preoperative bowel preparation and anesthesia induction with 0.3 mg/kg etomidate, 3–5 g/kg remifentanil (1.2–1.6 mg/h intravenous infusion), and 0.1 mg/kg cisatracurium besylate. Reinforced tracheal catheters, volume-controlled ventilation, and arterial catheterization for intraoperative blood pressure monitoring were used intraoperatively. Anesthesia was maintained via the intravenous infusion of cisatracurium besylate (2–6 µg/kg/min immediately after anesthesia induction), remifentanil (0.1–0.3 µg/kg/min), and propofol (60–100 µg/kg/min), with sevoflurane inhalation (0.8%–2% vol). To prevent muscle contraction and electrode displacement during IRE, patients were given muscle relaxants, and IRE was initiated after the twitch depression exceeded 90%, which indicated complete muscle relaxation. IRE was synchronized with the R-wave slope, so that the IRE device only generated pulses during the absolute refractory period of myocardial contraction to avoid inducing arrhythmia.
IRE procedure
IRE was performed using the NanoKnife® system (Angio- Dynamics, Queensbury, NY, USA), a high-voltage current generator (maximum power output 3 kV, 50 A), an electrocardiogram synchronization device (AccuSync 42; AccuSync Medical Research Corporation, Milford, CT, USA), and two 15 cm monopolar probes (20400103 and 20400104; NanoKnife, AngioDynamics, Latham, NY, USA).
The perioperative treatment plan was developed by an interventional radiologist (with 20 years of experience) based on the preoperative CT findings, such as tumor size and shape, which determined the number and spacing of the probes used. Since metallic stents can increase the risk of thermal injury during IRE, biliary stents, if present, were removed prior to IRE. Intraoperative ultrasonography was used to confirm tumor non-resectability and size, and to continuously monitor blood flow. Following general anesthesia induction and muscle relaxant administration, two 15-cm long, 19-G ablation probes were inserted into the pancreatic tumor in cases with stage III disease or into the pancreatic and liver tumor in cases with stage IV disease; electrode exposure ranged from 1.0 to 2.0 cm, and the electrode tips were spaced 1–2 cm apart. Probe insertion was performed percutaneously or via the open approach (Figure 1) under ultrasound (HI VISION Preirus; Hitachi, Tokyo, Japan) and CT (SOMATOM Definition 64 AS; Siemens Medical Solutions, Forchheim, Germany) guidance. Energy was applied at 1,200–1,500 V/cm for 70–90 ms/pulse, for a total of 7–9 pulses. To ensure complete coverage of the target area, we suggest the following: probes should be moved less than 1–1.5 cm at a time to ensure the integration of the ablation areas; the electrode should be kept <5 mm away from the tumor border; the current feedback should be 30–45 A; and an intratumoral gas shadow should be observed on ultrasound or enhanced CT. The procedure was completed after the removal of all probes.
Figure 1.
Flowchart for the selection of the open vs percutaneous approach for irreversible electroporation ablation.
Abbreviation: CT, computed tomography.
Owing to the use of muscle relaxants, sedation and mechanical ventilation were continued for 2 hours on average. Patients were then transferred to the observation ward for 24 hours. After their vital signs had stabilized, the patients were transferred to general wards and were provided with anti-infection treatment, drugs to protect the stomach and liver, and nutrition and other symptomatic and supportive treatment.
Follow-up
Laboratory tests (eg, serum amylase level measurement) were performed before and 1 and 7 days after IRE. All postoperative complications within 30 days were recorded prospectively and graded according to the Clavien–Dindo classification. Patients underwent triphasic abdominal CT or MRI and chest radiography 1–3 months after IRE, and every 3 months thereafter. The endpoints of interest were progression-free survival (PFS) and OS. PFS was defined as the interval between IRE and local relapse, distant metastasis (both new distant metastasis and growth of existing distant metastasis), or death, whichever occurred first. OS was calculated as the interval between the date of IRE and the date of death from any cause.
Statistical analysis
SPSS version 13.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Continuous data (tumor size, performance status score, and serum amylase and CA19-9 levels) are expressed as mean ± SD, and were compared using the Student’s t-test or Wilcoxon rank sum test. Categorical data (age, sex, tumor location, and method) are presented as frequencies, and were analyzed using the Pearson chi-squared test. PFS and OS were analyzed using the Kaplan–Meier method and compared using the log-rank test. GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) was used to plot graphs. All statistical tests were two-sided, and differences were considered significant at P<0.05.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of Guangzhou Fuda Cancer Hospital ethics committee and with the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all participants included in the study.
Results
Patient characteristics
Between July 2015 and August 2016, we evaluated 64 pancreatic cancer patients for inclusion in our study. Ten patients were ineligible owing to hypertension (four patients), duodenal tumor invasion (one patient), pacemaker implantation (one patient), and stage I/II disease (four patients). Thus, 54 consecutive patients (24 women) were included (Table 1). Their median age was 61.0 years (range 41–73 years). All patients had pathology-proven adenocarcinoma. According to the patients’ physical condition and preferences, patients received IRE alone or IRE plus chemotherapy (IRE + Chemo). Thirty-one patients received chemotherapy (gemcitabine 26 patients; FOLFIRINOX [folinic acid, 5-fluorouracil, irinotecan, oxaliplatin] five patients) for a median of four cycles (range two to six cycles). Two probes were used in each patient. The mean energy delivery time was 21.4±5.6 minutes (range 10–31 minutes), and two to 16 ablation pulses were used in each patient (mean 7.1±3.6) (Table 2; Figure 2). Stage III disease was present in 28 patients (maximum tumor diameter 2.4–6.8 cm), and stage IV disease was observed in 26 patients (maximum tumor diameter 2.2–8.9 cm).
Table 1.
Patient information
| Factors | Total (n=54) | Stage III disease group (n=28) | Stage IV disease group (n=26) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| IRE (n=13) | IRE + Chemo (n=15) | P-value | IRE (n=10) | IRE + Chemo (n=16) | P-value | ||
|
| |||||||
| Sex | 0.704 | 0.701 | |||||
| Male | 26 | 6 | 6 | 6 | 8 | ||
| Female | 28 | 7 | 9 | 4 | 8 | ||
| Age (years) | 0.445 | 1.000 | |||||
| <65 | 28 | 7 | 5 | 6 | 10 | ||
| ≥65 | 26 | 6 | 10 | 4 | 6 | ||
| Tumor size before IRE (cm)a | 4.97±1.64 | 4.90±1.28 | 5.03±1.23 | 0.764 | 4.90±2.13 | 5.01±1.44 | 0.634 |
| Tumor location | 0.435 | 0.420 | |||||
| Head and neck | 37 | 10 | 9 | 8 | 10 | ||
| Body and tail | 17 | 3 | 6 | 2 | 6 | ||
| Method | 1.000 | 0.692 | |||||
| Open operation | 16 | 3 | 4 | 4 | 5 | ||
| Percutaneous | 38 | 10 | 11 | 6 | 11 | ||
Notes:
Mean ± SD. Tumor staging was performed according to the seventh edition of the American Joint Committee on Cancer classification.
Abbreviations: Chemo, chemotherapy; IRE, irreversible electroporation.
Table 2.
Intraoperative parameters
| Parameters | Mean ± SD | Range |
|---|---|---|
|
| ||
| Number of probes | 2 | 2 |
| Cycles of ablation | 7.0±3.7 | 2–16 |
| Energy delivery time (minutes) | 21.4±5.6 | 10–31 |
| Voltage (V) | 2,456.9±624.6 | 1,725–3,000 |
| Electrode spacing (cm) | 1.6±0.4 | 1.0–2.0 |
| Pulse length (µs) | 74.1±18.6 | 70–90 |
| Number of pulses (tissue conductivity test) | 10 | 10 |
| Electrode exposure length (cm) | 1.8±0.6 | 1.5–2.0 |
Figure 2.
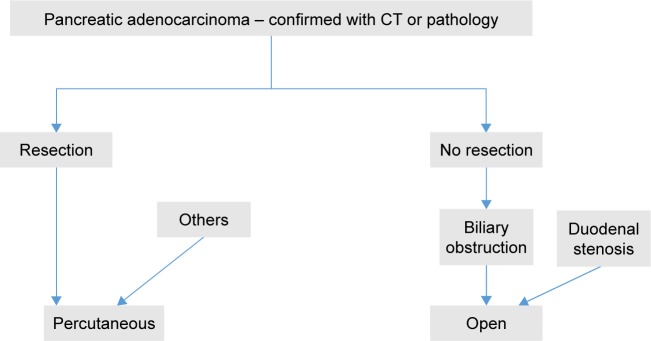
A 70-year-old woman with T4N1M0, stage III, pancreatic head and neck carcinoma.
Notes: Enhanced computed tomography shows a pancreatic tumor measuring 6.9×4.8 cm (A, B). Irreversible electroporation (IRE) is performed (C, D). At 3 months after IRE, the tumor has shrunk to 3.5×2.1 cm, and vascular retention is noted (E, F). The red arrows indicate the tumor and the blue arrows indicate the IRE probes.
Adverse events
No IRE-related deaths were noted. The most common adverse events were ascites (15 patients), pleural effusion (14 patients), fever (nine patients), and abdominal pain (six patients) (Table 3). All minor complications were resolved with or without symptomatic treatment within 2 weeks. Four major complications were noted in the cohort. Three patients developed duodenal hemorrhage, 11, 15, and 21 days after IRE. This complication was treated using surgical ligation, gastrojejunostomy, and pylorus devascularization in one patient (Figure 3), and with interventional embolization in the other two patients. One patient developed portal thrombosis (Figure 4) 9 days after IRE. A CT scan exhibited portal vein, superior mesenteric vein, and splenic vein compression, as well as a portal vein filling defect. That patient was in a hypercoagulative state before IRE, and was administered nadroparin for anticoagulation.
Table 3.
Adverse events in patients with pancreatic cancer after irreversible electroporation
| Adverse effects | Open (n=16) | Percutaneous (n=38) | ||
|---|---|---|---|---|
|
| ||||
| Cases (%) | Grade | Cases (%) | Grade | |
|
| ||||
| Major complications | ||||
| Hemorrhage | – | – | 3 | IIIb–IV |
| Portal thrombosis | 1 | IIIb | – | – |
| Minor complications | ||||
| Ascites | 5 | I–II | 10 | I–II |
| Pleural effusion | 5 | I–II | 9 | I–II |
| Fever | 3 | I | 6 | I |
| Abdominal pain | 2 | I | 4 | I–II |
| Vomiting | 1 | I | 4 | I–II |
| Hypokalemia | 1 | II | 4 | II |
| Gastroduodenal edema and retention | – | – | 3 | I–II |
| Arrhythmia | 1 | II | 1 | II |
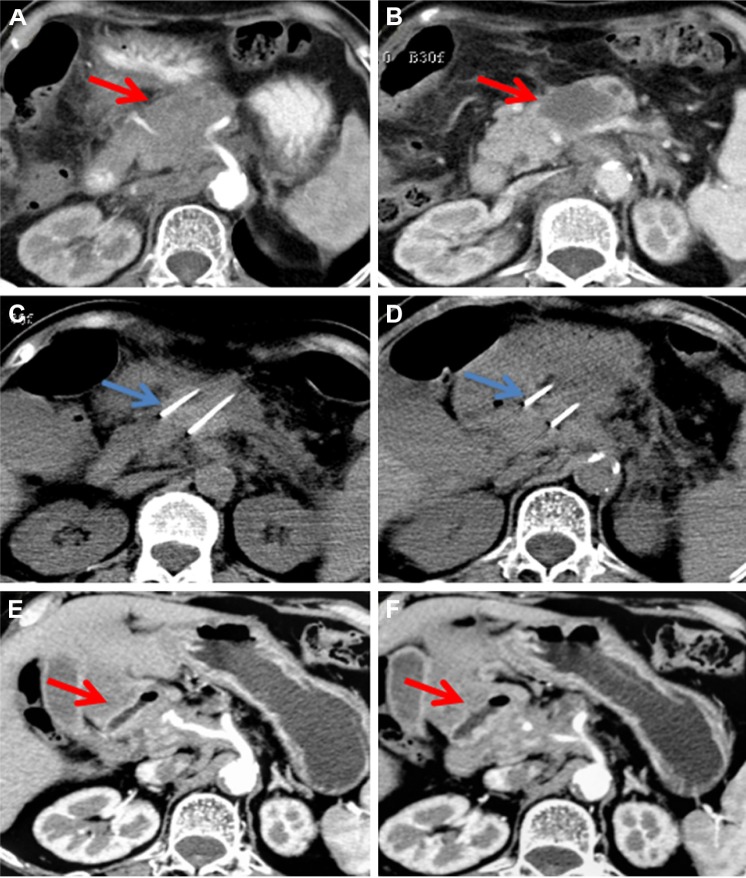
Figure 3.
A 60-year-old man with T4N1M0, stage III, pancreatic head carcinoma.
Notes: At 21 days after irreversible electroporation treatment, enhanced computed tomography shows a low-density, liquefied, necrotic area inside the tumor and a high- density shadow in the stomach and duodenum (A). Celiac artery angiography shows no obvious signs of active bleeding (B). Subsequent gastroscopy shows gastroduodenal artery rupture, and surgical ligation of the blood vessels is performed to stop the bleeding (C).
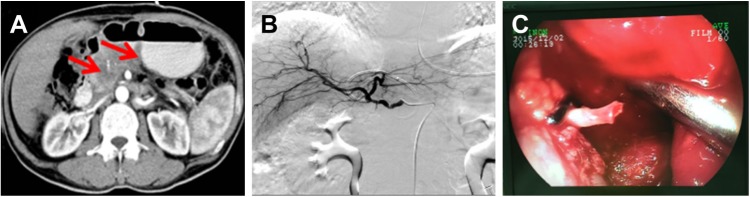
Figure 4.
A 59-year-old man with T4N1M1, stage IV, pancreatic body carcinoma.
Notes: Portal vein embolization is noted 9 days after irreversible electroporation (IRE). Computed tomography scans show the appearance of portal vein thrombosis (blue arrows) before (A) and after (B) IRE.
Performance status score
Performance status scores were calculated before and 3 and 6 months after IRE. The mean score was significantly lower 3 months after IRE (0.83±0.47) than preoperatively (1.06±0.60; P=0.038) (Figure 5). No significant differences were observed between the mean scores before and 6 months after IRE (0.91±0.52; P=0.146), and at 3 and 6 months after IRE (P=0.159).
Figure 5.
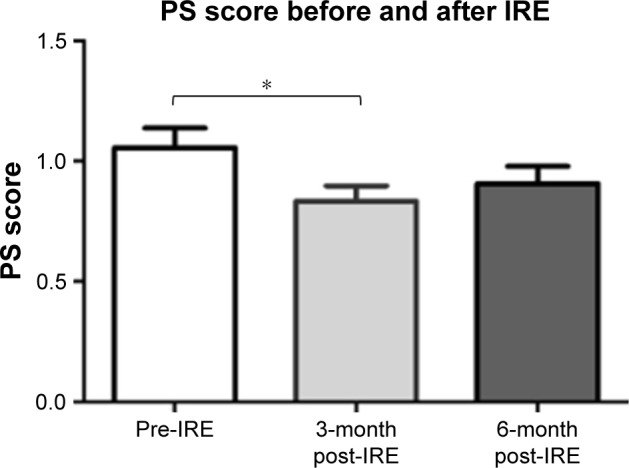
Error bar chart showing the performance status (PS) score before and after irreversible electroporation (IRE).
Note: *Significant difference (P<0.05).
PFS and OS
Among patients with stage III cancer, after a median follow-up of 18.8 months (range 9.6–28.7 months), the PFS was found to be 13.9 months (range 8.3–20.1 months) and 16.1 months (range 8–21 months) in the IRE and IRE + Chemo groups, respectively; the median OS from diagnosis was 16.2 and 20.3 months in the IRE and IRE + Chemo groups, respectively. Among patients with stage IV disease, after a median follow-up of 13.3 months (range 3.7–23.1 months), the median PFS from diagnosis was 9.45 months (range 5.3–18.1 months) and 11.7 months (range 6.6–19.6 months) in the IRE and IRE + Chemo groups, respectively; the median OS from diagnosis was 11.6 and 13.56 months in the IRE and IRE + Chemo groups, respectively (Figure 6).
Figure 6.
Kaplan–Meier survival curves showing OS from diagnosis in patients with stage III (A) and IV disease (B), as well as PFS from diagnosis in patients with stage III (C) and IV disease (D).
Abbreviations: Chemo, chemotherapy; IRE, irreversible electroporation; OS, overall survival; PFS, progression-free survival.
Discussion
Our findings show that IRE may offer local tumor control in patients with stage III and IV pancreatic cancer, including those with large tumors (>5 cm), with relatively satisfactory PFS and OS. Although certain major complications were noted in our patients, we believe that the use of appropriate measures could help to prevent these complications, and that IRE represents a potentially safe treatment for pancreatic cancer.
IRE is a soft-tissue ablation technique that uses high- voltage direct currents to produce nanopores in cell membranes and induce apoptosis. Although IRE ablation of pancreatic cancer has produced encouraging results,15–27 it has been limited to stage III pancreatic cancers thus far.19–21 Martin et al recommended that IRE should only be used in patients without metastatic disease and with tumors <4.0 cm in width after induction therapy.21
In patients with locally advanced or metastatic pancreatic cancer, gemcitabine-based chemotherapy is the main treatment; in fact, most of the patients in the present study had undergone treatment with gemcitabine alone. However, in the present study, we also used IRE to treat the patients with stage III and IV pancreatic cancer. The mean tumor diameter was >5 cm in our stage III patients. Moreover, the stage IV patients had up to three metastatic tumors each, and the maximum tumor diameter in that population was <3 cm. Martin et al recommend the use of induction gemcitabine- or FOLFIRINOX-based chemotherapy, depending on the patient’s age and performance status, for at least 3–4 months (three cycles of gemcitabine or four to six cycles of FOLFIRINOX).21–23 Although the required duration of adjuvant chemotherapy remains unclear, given the physical condition of our patients, we decided to administer two to six chemotherapy cycles after local IRE treatment.
Our findings indicate that IRE can be used to treat tumors >5 cm in diameter in patients with stage III pancreatic cancer, and can also be used in patients with stage IV pancreatic cancer with limited tumor metastasis. These findings differ from those in previously published studies. Some authors recommend the use of IRE only for tumors with maximum axial and anteroposterior dimensions of 3.5 cm.19 Scheffer et al included stage III patients with a maximum tumor diameter of 5 cm in their study of IRE treatment of pancreatic cancer, and reported PFS and OS durations of 8 and 11 months, respectively.25 Martin et al included 54 patients with locally advanced pancreatic cancer in a study comparing IRE and standard therapy, and found an improvement in the OS (20 vs 13 months, P=0.03).23 In the present study, we assigned patients to the IRE and IRE + Chemo groups based on the patients’ physical condition and preference. The mean tumor diameter was 5.03±1.29 cm in patients with stage III pancreatic cancer, and among these patients, the median OS from diagnosis (20.3 vs 16.2 months) and median PFS from diagnosis (16.1 vs 13.9 months) were better in the IRE + Chemo group than in the IRE group; these data are consistent with those from previous reports.15,23,28,29
Among metastatic pancreatic cancer patients who received gemcitabine monotherapy, the median OS ranged from 5.65 to 8.5 months and the median PFS ranged from 2.33 to 5.5 months.8,30–33 One review proposed that patients with metastatic disease do not benefit from IRE. Therefore, when setting the inclusion criteria for stage IV patients, we limited the number of metastatic tumors to three or fewer, and the maximum diameter of the metastatic tumors to <3 cm. Among stage IV patients, the median PFS from diagnosis was 9.45 and 11.7 months, and median OS from diagnosis was 11.6 and 13.56 months in the IRE and IRE + Chemo groups, respectively. The PFS and OS was significantly poorer in the IRE group than in the IRE + Chemo group (log-rank test, P=0.0423 and P=0.0398); however, further research in needed in a larger sample.
Four major complications were noted in the patients in the present study. Three patients developed duodenal bleeding, possibly due to ablation-related vascular damage, which required conversion to open IRE. This complication may be attributable to the patients’ preoperative condition, as all three patients had duodenal or gastric invasion, vascular compression or invasion, and arterial bleeding prior to IRE. Moreover, in all these patients, bleeding developed 10 days after IRE. We believe that there are three possible reasons for the bleeding: IRE of the tumor invading the duodenum may have exposed the vessels to the corrosive action of gastric juices, thus leading to arterial bleeding; excessive ablation may have caused heat damage, thus leading to vascular damage and aneurysm formation; and direct vascular puncture. To prevent such complications, we recommend the following precautions. First, if duodenal tumor invasion is detected on preoperative CT or endoscopic evaluation, open IRE should be performed prior to pylorus interruption and gastrointestinal anastomosis to prevent erosion of the duodenum by gastric juices. Second, to avoid excessive ablation, electrode exposure should be limited to 1–1.5 cm, and voltage should start from 1,200 V/cm; in cases where the current exceeds 45 A, the pulse release should be actively terminated to avoid excessive current and prevent thermal damage. Third, CT examination should be performed 1 week after IRE ablation to detect aneurysm formation, and aneurysm embolization should be promptly conducted to avoid further enlargement, rupture, and bleeding. Thus, duodenal tumor invasion may be a relative contraindication for IRE. In the present study, three cases of hemorrhage were noted during 2015–2016, and no further cases of hemorrhage were observed during 2016–2017, possibly owing to technical improvements. Moreover, the minor complications in the patient cohort had been alleviated within 14 days after symptomatic treatment. No significant increase in serum amylase levels were noted after IRE, and none of the cases developed pancreatitis. Belfiore et al15 retrospectively examined 29 patients with locally advanced pancreatic cancer treated with IRE, and found that the Karnofsky score had increased from Tzero to T3m by a mean of 28.28 (SE 2.11; 95% CI 23.95–32.60). Similarly, in the present study, we found that the performance status score had significantly decreased at 3 months (0.83±0.47) after IRE, indicating that treatment with IRE markedly increased the quality of life of patients with pancreatic cancer compared to that prior to treatment.
Multimodal treatment is needed to prolong the survival of pancreatic cancer patients. Moreover, although the ablation procedure was associated with certain complications, most of these complications were not serious and could be managed via conservative treatment. The long-term outcomes were satisfactory, and hence, IRE could serve as a new clinical treatment for unresectable pancreatic carcinoma.
Conclusion
Patients with pancreatic carcinoma could benefit from IRE, which improved the OS in certain patients who had also undergone chemotherapy. Although some severe complications were noted, IRE was generally well tolerated.
Acknowledgments
We would like to thank the native English-speaking scientists of Elixigen Company for editing our manuscript. This study was supported by four grants, from the National Natural Science Foundation of China (grant no. 81774376); Guangzhou Key Scientific Research Grant (grant no. 201803010059); Scientific and Technological Plan, Guangdong Province, China (grant no. 2016A020216018); and International Foundation for Sciences of Guangzhou Fuda Cancer Hospital (grant no. Y2018-ZD-001). SL and ZQ are co-first authors.
Abbreviations
- AJCC
American Joint Committee on Cancer
- CT
computed tomography
- IRE
irreversible electroporation
- MRI
magnetic resonance imaging
- OS
overall survival
- PFS
progression-free survival
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Baron TH, Kozarek RA. Preoperative biliary stents in pancreatic cancer – proceed with caution. N Engl J Med Overseas Ed. 2010;362(2):170–172. doi: 10.1056/NEJMe0908773. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Jooste V, Dejardin O, Bouvier V, et al. Pancreatic cancer: wait times from presentation to treatment and survival in a population-based study. Int J Cancer. 2016;139(5):1073–1080. doi: 10.1002/ijc.30166. [DOI] [PubMed] [Google Scholar]
- 4.Smeenk HG, Tran TC, Erdmann J, van Eijck CH, Jeekel J. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390(2):94–103. doi: 10.1007/s00423-004-0476-9. [DOI] [PubMed] [Google Scholar]
- 5.Kleeff J, Reiser C, Hinz U, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245(4):566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morganti AG, Massaccesi M, La Torre G, et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol. 2010;17(1):194–205. doi: 10.1245/s10434-009-0762-4. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosn M, Kourie HR, El Karak F, et al. Optimum chemotherapy in the management of metastatic pancreatic cancer. World J Gastroenterol. 2014;20(9):2352–2357. doi: 10.3748/wjg.v20.i9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol. 2013;24(10):2484–2492. doi: 10.1093/annonc/mdt239. [DOI] [PubMed] [Google Scholar]
- 11.Rombouts SJ, Vogel JA, van Santvoort HC, et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg. 2015;102(3):182–193. doi: 10.1002/bjs.9716. [DOI] [PubMed] [Google Scholar]
- 12.Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20(9):2267–2278. doi: 10.3748/wjg.v20.i9.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silk M, Tahour D, Srimathveeravalli G, Solomon SB, Thornton RH. The state of irreversible electroporation in interventional oncology. Semin Intervent Radiol. 2014;31(2):111–117. doi: 10.1055/s-0034-1373785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long G, Bakos G, Shires PK, et al. Histological and finite element analysis of cell death due to irreversible electroporation. Technol Cancer Res Treat. 2014;13(6):561–569. doi: 10.7785/tcrtexpress.2013.600253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belfiore MP, Ronza FM, Romano F, et al. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neo- adjuvant protocol in locally advanced pancreatic cancer: our preliminary experience. Int J Surg. 2015;21(Suppl 1):S34–S39. doi: 10.1016/j.ijsu.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23(12):1613–1621. doi: 10.1016/j.jvir.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Venkat S, Hosein PJ, Narayanan G. Percutaneous approach to irreversible electroporation of the pancreas: miami protocol. Tech Vasc Interv Radiol. 2015;18(3):153–158. doi: 10.1053/j.tvir.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Al Efishat M, Wolfgang CL, Weiss MJ. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ. 2015;350:h521. doi: 10.1136/bmj.h521. [DOI] [PubMed] [Google Scholar]
- 19.Martin RC. Irreversible electroporation of locally advanced pancreatic head adenocarcinoma. J Gastrointest Surg. 2013;17(10):1850–1856. doi: 10.1007/s11605-013-2309-z. [DOI] [PubMed] [Google Scholar]
- 20.Martin RC., 2nd Irreversible electroporation of locally advanced pancreatic neck/body adenocarcinoma. J Gastrointest Oncol. 2015;6(3):329–335. doi: 10.3978/j.issn.2078-6891.2015.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin RC, 2nd, Durham AN, Besselink MG, et al. Irreversible electroporation in locally advanced pancreatic cancer: a call for standardization of energy delivery. J Surg Oncol. 2016;114(7):865–871. doi: 10.1002/jso.24404. [DOI] [PubMed] [Google Scholar]
- 22.Martin RC, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262(3):486–494. doi: 10.1097/SLA.0000000000001441. discussion 492–494. [DOI] [PubMed] [Google Scholar]
- 23.Martin RCG, Mcfarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20(S3):443–449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 24.Paiella S, Butturini G, Frigerio I, et al. Safety and feasibility of irreversible electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg. 2015;32(2):90–97. doi: 10.1159/000375323. [DOI] [PubMed] [Google Scholar]
- 25.Scheffer HJ, Vroomen LG, de Jong MC, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: results of the phase I/II PANFIRE study. Radiology. 2017;282(2):585–597. doi: 10.1148/radiol.2016152835. [DOI] [PubMed] [Google Scholar]
- 26.Trueba-Arguinarena FJ, de Prado-Otero DS, Poves-Alvarez R. Pancreatic adenocarcinoma treated with irreversible electroporation case report: first experience and outcome. Medicine. 2015;94(26):e946. doi: 10.1097/MD.0000000000000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Månsson C, Bergenfeldt M, Brahmstaedt R, et al. Safety and preliminary efficacy of ultrasound-guided percutaneous irreversible electroporation for treatment of localized pancreatic cancer. Anticancer Res. 2014;34(1):289–293. [PubMed] [Google Scholar]
- 28.Belfiore G, Belfiore MP, Reginelli A, et al. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med Oncol. 2017;34(3):38. doi: 10.1007/s12032-017-0887-4. [DOI] [PubMed] [Google Scholar]
- 29.Vogel JA, Rombouts SJ, de Rooij T, et al. Induction chemotherapy followed by resection or irreversible electroporation in locally advanced pancreatic cancer (IMPALA): a prospective cohort study. Ann Surg Oncol. 2017;24(9):2734–2743. doi: 10.1245/s10434-017-5900-9. [DOI] [PubMed] [Google Scholar]
- 30.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 31.Conroy T, Desseigne F, Ychou M, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 32.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 33.Von Hoff DDV, Ervin TJ, Arena FP, et al. Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT) J Clin Oncol. 2013;31(4_Suppl) [Google Scholar]