Abstract
Purpose
We conducted a preliminarily exploration of the role and possible mechanism of the non-tight junction-related function of claudin-7 in the occurrence and development of colorectal cancer.
Methods
We selected the colorectal cancer cell line HCT116, constructed a stably transfected claudin-7 knockdown cell line via RNAi and lentiviral infection, and determined the claudin-7 knockdown efficiency. We assessed the biological behavior changes (cell viability, apoptosis, and migration) in the stably transfected HCT116 cells and observed structural changes in the tight junction by transmission electron microscopy. We used a subcutaneous tumor formation model to assess the tumorigenicity of HCT116 cells after claudin-7 knockdown. We assessed the expression and localization of integrinβ1 in the stably transfected cell line by immunofluorescence staining and investigated the interaction between integrinβ1 and claudin-7 by co-immunoprecipitation.
Results
After the knockdown of claudin-7 the expression, the viability and migration ability of HCT116 cells increased and apoptosis decreased. Transmission electron microscopy indicated that the intercellular tight junction structure did not change substantially. Furthermore, the tumor growth in nude mice was enhanced. Immunofluorescence staining showed that integrinβ1 and claudin-7 were co-expressed and co-localized on the cell membrane, and immunoprecipitation suggested that claudin-7 interacts with integrinβ1.
Conclusion
Claudin-7 may inhibit the proliferation and migration of tumor cells by interacting with integrinβ1, subsequently participating in the development of colorectal cancer.
Keywords: Claudin-7, non-tight junction, integrinβ1, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the common human malignant tumors. As a cancer with high incidence and high mortality,1 CRC greatly affects human life and health; and patients with CRC generally present high recurrence, high mortality and low cure rates, and no effective treatment methods currently exist. Thus, exploring the possible molecular mechanism underlying the occurrence and development of CRC and finding new therapeutic targets are paramount.
Tight junctions (TJs), the most common intercellular connection, are located at the apical cell junction complex, a special structure formed by the close binding of adjacent cells; TJs are composed primarily of occludins, claudins, adhesion molecules (junctional adhesion molecules, JAMs) and the zonula occludens proteins (ZO-1, ZO-2, and ZO-3), which play an important role in regulating transport and the permeability of adjacent cells by maintaining the barrier function of epithelial cells and controlling the horizontal diffusion of proteins in the lipid bilayer.2–4 The claudin family is a protein family important in the formation of TJs. Twenty-seven claudin family members have been found to date;5 the molecular weight of these proteins is between 20 and 27 kDa, and they are widely expressed among epithelial cells.6 Claudins play an important role in intercellular exchange, barrier function maintenance and cell polarity. Recently, the claudin family has been found to participate not only in classical tight junction-related functions such as barrier and fence functions but also in non-tight junction-related functions such as inflammation initiation and tumor development processes; for example, the expression of claudin-1, claudin-2 and claudin-7 in invasive breast cancer is decreased.7–10 The upregulated expression of claudin-3 and claudin-7 and the downregulation of claudin-18 expression might be related to the occurrence of gastric cancer; indeed, the upregulation of claudin-7 expression and the downregulation of claudin-18 expression might be an indicator of poor prognosis in gastric cancer patients.11 Furthermore, in cervical cancer tissues, the expression of claudin-5 and claudin-9 was downregulated and that of claudin-8 was upregulated; this expression pattern was associated with lymph node metastasis.12
Claudin-7 is an important member of the claudin family and is widely distributed in the intestines, stomach, lung, bladder, skin and kidney. In addition, claudin-7 plays an important role in maintaining the normal physiological function of various organs. The general claudin-7 gene knockout mouse model constructed by Lei Ding exhibited inflammatory responses, intestinal epithelial cell exfoliation and mucosal ulcers, suggesting that claudin-7 may play a non-tight junction-related role involved in the initiation of intestinal inflammation and the maintenance of environmental homeostasis in the intestine.13 Moreover, the study had confirmed that the non-junction of claudin-7 was related to the location. And many researches also had the same conclusion that basolateral membrane claudins-regulation of epithelial-mesenchymal transformation, cell migration, invasion, and tumorigenesis.14 Integrins are heterodimers with α and β subunits, which mainly mediate the interaction of cells with the extracellular matrix via functions such as the regulation of cell attachment, activity, proliferation and invasion, along with signal transduction.15–17 Integrinβ1 is an important member of the integrin family. Integrinβ1 has been found to be abnormal in many tumors and is involved in tumor occurrence and development.
The precise roles of claudin-7 in intestinal tumorigenesis are largely unknown. In their study, Bhat et al, came to the conclusion that claudin-7 expression induced mesenchymal to epithelial transformation (MET) to inhibit colon tumori-genesis.18 But we found that claudin-7 co-localized and formed a protein complex with integrinβ1 in CRC cells. Interference with claudin-7 expression not only inhibited apoptosis but also disrupted the localization and down-regulated the expression of integrinβ1 at the protein level. Claudin-7 was confirmed to participate in biological behavior changes in CRC cells through this interaction with integrinβ1. Hence, claudin-7 exhibits non-tight junction-related activity in regulating the biological behavior of CRC cells through integrinβ1.
Material and methods
Cell culture, lentiviral shRNA knockdown of claudin-7 and transfection
The CRC cell line HCT116 which was purchased commercially from Shanghai Genechem Co, Ltd (Shanghai, China) was grown in RPMI-1640 culture medium containing 10% FBS in a humidified air (5% CO2) atmosphere at 37°C. Three lentiviral claudin-7 shRNA vectors (sequence#1: 5′-ATGGGTGGAGGCATAATTT-3′; sequence#2: 5′-CTAAGTCCAACTCTTCCAA-3′; and sequence#3: 5′-GCTCCTATGCGGGTGACAA-3′) or one control vector (sequence: 5′-TTCTCCGAACGTGTCACGT-3′) were transfected into HCT116 cells. All lentiviral vectors contained an EGFP (enhanced green fluorescent protein) expression sequence. After 16 hours of infection, the cell culture medium was replaced with fresh medium. After 72 hours of incubation, transfected cells were selected by 2 µg/mL puromycin.
RNA isolation and real-time quantitative PCR
Total RNA was isolated from HCT116 control, claudin-7 negative control and claudin-7 knockdown cells using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. Real-time quantitative PCR was performed using SYBR Green PCR Master Mix (TOYOBO, Japan) and an ABI7500 system (Thermo Fisher Scientific) according to the manufacturers’ instructions. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as the endogenous control for all cell lines. Each sample was analyzed in triplicate, and the relative changes in gene expression were analyzed using the comparative threshold cycle (2−ΔΔCt) method.
Western blot analysis
Total protein was extracted from HCT116 control, claudin-7 negative control and claudin-7 knockdown cells, and the protein concentration was measured using a BCA (protein assay kit) assay (Thermo Fisher Scientific). Equal amounts of protein (20 µg/well) were separated on 10% or 8% SDS polyacrylamide gels and transferred to nitrocellulose membranes. After the nitrocellulose membranes were blocked with 5% nonfat dry milk in TBST buffer (0.1% TWEEN-20) for 2 hours at room temperature, they were incubated with antibodies against claudin-7 (1:1000, Abcam, Cambridge, MA, USA), integrinβ1(1:1000, Abcam), GAPDH (1:10000, CST, [cell signaling technology, Danvers, MA, USA]) and β-actin (1:10,000, CST) at 4°C overnight. The membranes were then incubated with a secondary antibody for 2 hours at room temperature. Finally, the membranes were visualized using an Odyssey infrared imaging system (LI-COR, Lincoln, NE, USA). GAPDH and β-actin were used as the internal references.
Cell viability assay (CCK-8 assay)
Cell viability was determined by a cell counting kit (CCK-8) assay (Dojindo Laboratories, Japan). The transfected cells were plated into 96-well plates at a density of 2,000 cells/well. At 24, 48, 72, and 96 hours, the cells were incubated with CCK-8 reagent (10 µL/well) for 2–4 hours at 37°C, after which the absorbance at 450 nm was measured with a microplate reader SPARK® 10M, (Tecan Group Ltd, Männedorf, Switzerland).
Wound healing (migration) assay
HCT116 cells stably transfected with claudin-7 or empty vector were seeded evenly in 96-well plates and allowed to grow to full confluence. The cell monolayer was scratched using a 10 µL pipette tip to create a wound. The plates were washed three times with PBS to remove the floating cells before serum-free medium was added. The cell migration distances were imaged at 0, 8, and 24 hours after the scratches were made. Three fields were randomly selected for imaging in each experiment, and three independent experiments were performed to calculate the migration distance.
In vivo tumor xenograft model
Six-week old male and female athymic nude mice were obtained from Beijing Weitong Lihua Experimental Animal Technology Co. Ltd (Beijing, China) and used for human tumor xenografting. A total of 5×106 HCT116 control, claudin-7 negative control and claudin-7 knockdown cells were suspended in the culture medium and injected subcutaneously into the left and right flanks of each nude mouse. All mice were sacrificed four weeks after injection, and the tumors were removed and weighed. The animal experiments were performed according to the animal use protocol approved by Beijing Shijitan Hospital and had passed the approval of the animal ethics committee of Beijing Shijitan Hospital.
Immunofluorescence
HCT116 control, claudin-7 negative control and claudin-7 knockdown cells grown in confocal chambers (Nest 801006) were fixed in 4% paraformaldehyde for 20–30 minutes at room temperature and washed with PBS for 10 minutes before being blocked in 1% BSA for 60 minutes at room temperature. After blocking, the cells were incubated with primary antibodies. All antibodies were diluted in PBS containing 1% BSA. After washing, the cells were incubated with the corresponding secondary antibodies for 60 minutes at room temperature in the dark. A drop of DAPI was added to each well of the confocal chambers, and the chambers were kept at room temperature for 5 minutes in the dark. The samples were imaged using a laser confocal microscope, Nikon A1 (Nikon Corporation, Tokyo Japan).
Co-immunoprecipitation
HCT116 claudin-7 negative control and claudin-7 knockdown cells were washed three times with ice-cold PBS and then lysed in RIPA buffer. After centrifugation, the protein concentration was measured using a BCA assay (Thermo Fisher Scientific). The protein samples were divided into 4 tubes of 1,200 µg each, and 10% of the sample was used for the input control. A 10 µL volume of protein A/G beads were added to each tube, and the tubes were incubated for 1 hour at 4°C with rotation. After centrifugation, the supernatants were incubated with either the anti-claudin-7 or anti-integrinβ1 antibody at 4°C overnight. Protein A/G beads were then added to the mixture, which was then incubated at 4°C for 4 hours. After centrifugation, the supernatants were discarded. The beads were washed six times with RIPA buffer and were then added to 50 µL loading buffer and boiled at 100°C for 10 minutes. Bound proteins were eluted from the beads in SDS sample buffer and analyzed by Western blotting.
Statistical analysis
At least three independent experiments were performed for each in vitro experiment. Statistical analysis was performed using GraphPad Prism 6.02 software (GraphPad Software Inc., La Jolla, CA, USA). All data are presented as the mean values ±SD. The differences between two groups were analyzed using Student’s t-test. A value of P<0.05 was considered statistically significant.
Results
Construction of the claudin-7 stable knockdown HCT116 CRC cell line
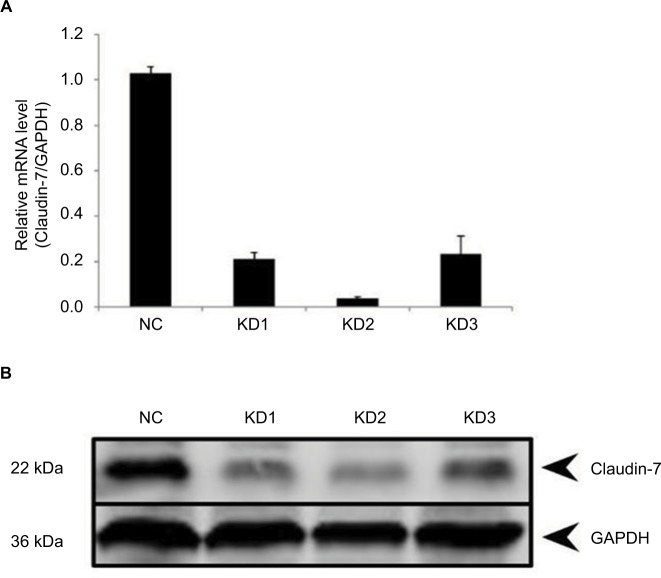
We used three RNAi loci to knock down the expression of claudin-7. The packaged lentivirus was used to infect with the target cells. The optimal viral titre of the HCT116 negative control cells was 5×108 TU/ml and that of the HCT116 claudin-7 knockdown cells was 3×108 TU/ml. The infected cells were imaged under a fluorescence microscope to assess the infection efficiency (Figure 1). Verification by qPCR (Real-time Quantitative PCR Detecting System, Life Technologies, Singapore) and Western blotting showed that the knockdown efficiency of interference vector #2, which was as high as 96.2%, was the highest among the three RNAi constructs. Therefore, we selected the HCT116 cells transfected with RNAi constructs #2 to establish the claudin-7 stable knockdown cell line (Figure 2).
Figure 1.
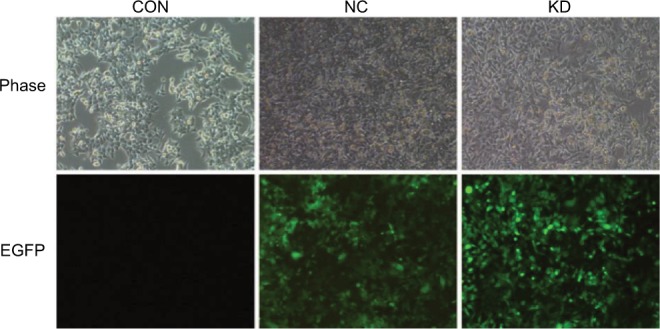
Colorectal cancer cell line HCT116CON and the stable transfected cell lines HCT116NC and HCT116KD screened by puromycin.
Notes: Representative phase and green fluorescence images of live control, negative control and claudin-7 knockdown cells. Both control and claudin-7 shRNA lentivirus constructs contain an EGFP expression sequence. (Puromycin concentration: 2 µg/mL, magnification: 100×).
Abbreviations: CON, control; NC, negative control; KD, knockdown; EGFP, enhanced green fluorescent protein.
Figure 2.
The expression level of claudin-7 in stable transfected cell lines HCT116NC and HCT116KD.
Notes: (A) Relative mRNA expression levels of claudin-7 in HCT116NC, HCT116KD1, HCT116 KD2, HCT116KD3. (B) Protein expression levels of claudin-7 in HCT116NC, HCT116KD1, HCT116 KD2, HCT116KD3.
Abbreviations: NC, negative control; KD, knockdown.
Claudin-7 knockdown decreased apoptosis, enhanced cell viability and migration and few substantial changes in tight junctions
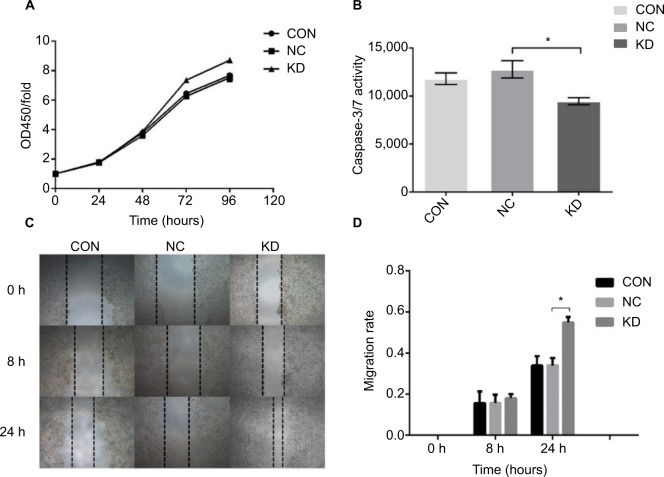
The viability of the different groups of cells was determined by measuring the absorbance values at 450 nm at 0, 24, 48, 72, and 96 hours, and the relative proliferation rate of each group was calculated. The results showed that the viability of the HCT116 claudin-7 knockdown (HCT116KD) cells was higher than that of the HCT116 negative control (HCT116KC) cells (P<0.05, Figure 4). The apoptotic ability of the different groups of cells was assessed by determining the activity of the apoptotic proteases caspase-3/7. The apoptotic ability of the HCT116KD cells was weaker than that of the HCT116NC cells (P<0.05, Figure 4). The migration ability of the different groups of cells was assessed by scratching the cell monolayers; imaging the wounds at 0, 8, and 24 hours; and calculating the mobility of the cells in each group. The migration ability of the HCT116KD cells was higher than that of the HCT116NC cells (P<0.05, Figure 4). The cell biological behavior assays described above showed that after the knockdown of claudin-7 expression in the CRC cell line HCT116, the cell viability and migration ability were enhanced and the apoptotic ability was weakened, suggesting that claudin-7 is a tumor suppressor gene. HCT116 negative control and knockdown cells were harvested to prepare the transmission electron microscopy samples. No obvious change in the tight junction structure at the top and sides of the cells was observed microscopically between the two groups (as shown in Figure 3).
Figure 4.
Increased cell proliferation and migration and decreased cell apoptosis in claudin-7 KD cells.
Notes: (A) The detection of cell viability of CRC cell line HCT116CON and stable transfected cell lines HCT116NC and HCT116KD. Cell viability in group HCT116KD was significantly higher than that in group HCT116NC. *P<0.05. (B) The detection of apoptosis ability of CRC cell line HCT116CON and stable transfected cell lines HCT116NC and HCT116KD. The apoptosis ability in group HCT116KD was significantly lower than that in group HCT116NC. *P<0.05. (C, D) The detection of migration ability of CRC cell line HCT116CON and stable transfected cell lines HCT116NC and HCT116KD. Compared with group HCT116NC, the migration ability of cells in HCT116KD group was enhanced. *P<0.05 (magnification: 40×).
Abbreviations: CON, control; NC, negative control; KD, knockdown; CRC, colorectal cancer.
Figure 3.
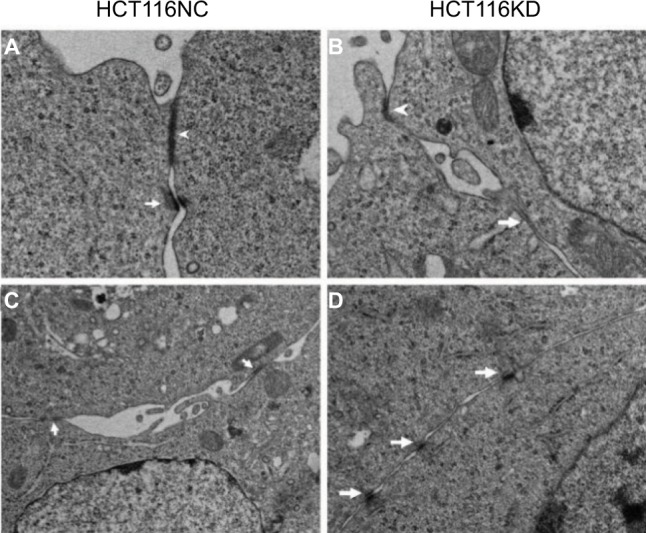
Electron microscope results of HCT116NC and HCT116KD cells.
Notes: (A, B) The arrowhead indicates the apical tight junction between cells in HCT116NC and HCT116KD (magnification: 8000×). (C, D) The arrowhead indicates the lateral tight junction between cells (magnification: 5000×).
Claudin-7 suppressed tumor growth in vivo
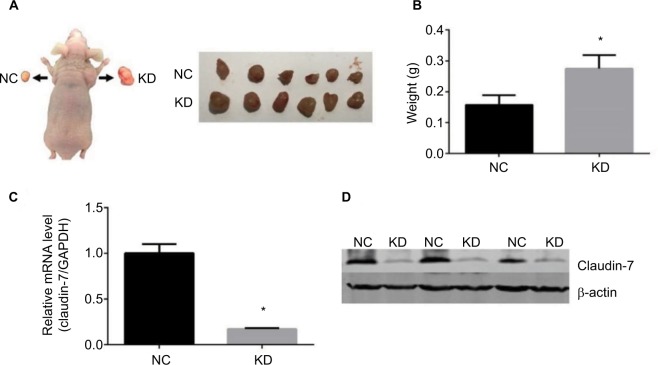
To detect the effect of interfering with claudin-7 expression on the ability of HCT116 cells to form subcutaneous tumors in nude mice, HCT116NC and HCT116KD cells were injected subcutaneously in the flanks of nude mice. Compared with the tumors formed by the HCT116NC cells, the tumors formed in the nude mice by the HCT116KD cells were larger and exhibited a higher tumor formation ability (Figure 5). The tumors were separated from both flanks of the nude mice. The expression of claudin-7 in the tumor tissues was detected by qPCR and Western blotting. Claudin-7 expression was significantly lower in the tissues from the HCT116KD group than in the tissues from the HCT116NC group (Figure 5), further suggesting that claudin-7 is a tumor suppressor gene and could inhibit tumor growth.
Figure 5.
The growth of stable transfected cell lines HCT116NC and HCT116KD subcutaneously in nude mice.
Notes: (A, B) Comparison of the quality of groups HCT116NC and HCT116KD of implanted tumors in nude mice. Compared with group HCT116NC, the ability of subcutaneous tumor formation in nude mice was enhanced in group HCT116KD. (C, D) The expression of claudin-7 in subcutaneous tumor tissue of nude mice which was transfected with stable transfected cell lines HCT116NC and HCT116KD. Compared with group HCT116NC, the expression levels of claudin-7 in mRNA and protein levels in group HCT116KD were significantly decreased.
Abbreviations: NC, negative control; KD, knockdown.
Interaction between claudin-7 and integrinβ1 in HCT116 cells
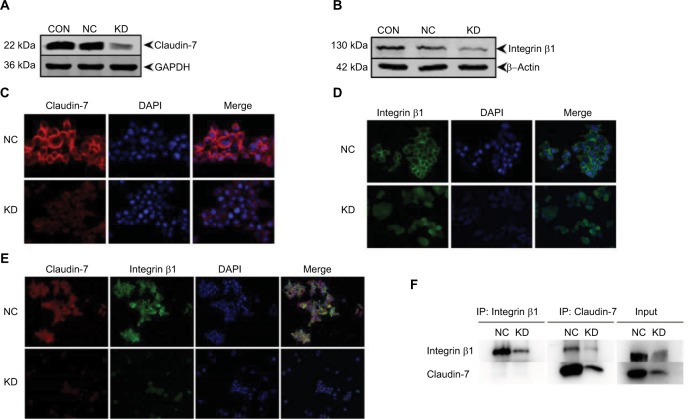
The expression of the integrinβ1 protein in the stably transfected cell lines HCT116NC and HCT116KD was detected by Western blotting. The expression of the integrinβ1 protein was lower in the HCT116KD cells than in the HCT116NC cells, which was in accordance with the expression trend of the claudin-7 protein in HCT116KD cells and HCT116NC cells. The protein expression of both claudin-7 and integrinβ1 was lower in HCT116KD cells than in the HCT116NC cells (Figure 6). Furthermore, the HCT116NC and HCT116KD cells were examined for immunofluorescence by confocal microscopy. The expression of integrinβ1 was significantly different between HCT116NC cells and HCT116KD cells. The amount of integrinβ1 on the cell membrane was appreciably lower in HCT116KD cells than in HCT116NC cells, consistent with the trend of claudin-7 protein localization and expression in the two groups of cells. Again, HCT116NC and HCT116KD cells were examined for immunofluorescence, and the claudin-7 protein and integrinβ1 protein were partially co-localized on the membrane of HCT116 cells. Co-immunoprecipitation showed an interaction between claudin-7 and integrinβ1. Considering the input control results, claudin-7 protein expression in the HCT116KD cells was significantly lower than that in the HCT116NC cells, which indicated that the knockdown of claudin-7 in the two cell lines was still very effective in the setting of immunoprecipitation (IP). Because we used the claudin-7 antibody for IP, the claudin-7 protein could be immunoprecipitated and the integrinβ1 protein could be recovered from the immunoprecipitate; the amount of both claudin-7 and integrinβ1 in the immunoprecipitate was reduced after claudin-7 knockdown, indicating that claudin-7 and integrinβ1 could interact with each other. However, the reverse use of the integrinβ1 antibody for IP resulted in the pull-down of only integrinβ1; claudin-7 was not detected in the immunoprecipitate.
Figure 6.
Decreased protein levels and disrupted localization of integrinβ1 in claudin-7 KD cells.
Notes: (A, B) The expression of claudin-7 and integrinβ1 protein in the stable transfected cell lines HCT116NC and HCT116KD. Compared with group HCT116NC, the expression of integrinβ1 protein decreased in group HCT116KD and was consistent with the expression of claudin-7 protein. (C) The expression and location of claudin-7 protein in stable transfected cell lines HCT116NC and HCT116KD were detected by cellular immunofluorescence assay. Compared with group HCT116NC, the expression of claudin-7 protein in cell membrane of HCT116KD group was significantly reduced (magnification: 200×). (D) The expression and localization of integrinβ1 protein in stable transfected cell lines HCT116NC and HCT116KD. The expression of integrinβ1 protein in cell membrane of HCT116KD group decreased significantly compared with group HCT116NC (magnification: 200×). (E) The co-expression and co-localization of claudin-7 protein and integrinβ1 protein in stable transfected cell lines HCT116NC and HCT116KD. According to the diagram, there were partial co-localization and co-expression of claudin-7 and integrinβ1 on the cell membrane in HCT116 cells (magnification: 100×). (F) The interaction between claudin-7 protein and integrinβ1 protein in stable transfected cell lines HCT116NC and HCT116KD was detected by co-immunoprecipitation assay. According to the graph, claudin-7 and integrinβ1 interact with each other in HCT116 cells.
Abbreviations: NC, negative control; KD, knockdown.
Discussion
We demonstrated that after the successful and stable interference with the expression of claudin-7, the intercellular TJs, including the apical and lateral junctions, were not appreciably changed. However, cell proliferation was increased, apoptosis-related protease activity was weakened, and the cell migration ability was enhanced. Furthermore, in the in vivo xenograft experiment assessing subcutaneous tumor formation in nude mice, the tumors induced by the negative control cells were much smaller than those induced by the claudin-7 knockdown cells. Our results suggested that claudin-7 could be a tumor suppressor in CRC, inhibiting tumor cell growth both in vitro and in vivo. The abnormal expression of claudin-7 was found in various tumor tissues. For example, the expression of claudin-1, claudin-2 and claudin-7 decreased in invasive breast cancer.7–10 The occurrence of gastric cancer might be related to the upregulated expression of claudin-3 and claudin-7 and the downregulated expression of claudin-18; in particular, the upregulation of claudin-7 expression and the downregulation of claudin-18 expression might be an indicator of poor prognosis in gastric cancer patients.11
As the main member of the cell surface receptors, integrins not only mediate the interaction between the cell and the extracellular matrix but also participate in processes of signal transduction, proliferation, differentiation, invasion and metastasis in various tumors. Various tumor cells from epithelial sources express integrins such as α6β4, α6β1, αvβ5, α2β1 and α3β1, and the expression of integrins is different in different tumors; for example, integrins αvβ3, αvβ5 and αvβ6 are upregulated in the brain metastases of lung cancer.19 After the expression of integrinsα2β1 was silenced in the human breast cancer cell line MCF-7, the expression of the apoptotic protein p53 increased and the cell proliferation ability decreased, which suggested that α2β1 might be an oncogene in breast cancer.20 Integrinβ1 is an important member of the integrin family and is abnormally expressed in many tumors. For example, the high expression of galectin-1 in carcinoma-associated fibroblasts might promote gastric cancer cell migration and invasion by upregulating the expression of integrinβ.21 The expression of integrinβ1 was increased significantly in hypopharyngeal squamous cell carcinoma and was correlated with pathological grading and cervical lymph node metastasis.22 In addition, high integrinβ1 expression, which increased with increases in clinical stage and malignancy, was found in cervical squamous cell carcinoma.23
Numerous studies have shown that claudin family proteins could participate in the development of tumors by interacting with integrins. An indirect regulatory role of in the integrin/FAK signaling pathway in human CRC tissue specimens was found by Ding et al.24 Bologna et al found that claudins and integrins were involved in the invasion and metastasis of primary malignant melanoma in the oral mucosa.25 Tabariès et al26 showed that claudin-2 expression was low in primary breast cancer and that claudin-2 was highly expressed in specimens from breast cancer and liver metastases, and in vitro experiments confirmed that claudin-2 could promote the interaction between breast cancer cells and the extracellular matrix by increasing the expression of the integrinα2β1 and integrinα5β1 complexes. In the study by Hong et al27 the expression of integrinβ1 in EGF-stimulated HCT116 CRC cells was mainly localized on the cell membrane, but in EGF-stimulated Rab-25 knockout HCT116 cells, integrinβ1 accumulated in the cell membrane, and EGF, integrinβ1 and Rab-25 were proposed as molecular targets for proliferation and metastasis, which further explained the important role of integrinβ1 in the pathogenesis and development of CRC. Lu et al28 found that claudin-7 could inhibit the proliferation and detachment of lung cancer cells through interaction with integrinβ1. All these findings suggested that integrinβ1 might be involved in tumor development. Our experiment further confirmed that in CRC, claudin-7 might regulate the proliferation and migration of tumor cells through interaction with integrinβ1. The immunofluorescence assay showed partial co-expression and co-localization of claudin-7 and integrinβ1 on the cell membrane of HCT116NC cells and a decrease in integrinβ1 expression with the decrease in claudin-7 expression in HCT116KD cells; furthermore, the IP experiment showed that claudin-7 and integrinβ1 could interact with each other. Thus, claudin-7 may participate in the proliferation and migration of CRC cells through interaction with integrinβ1. However, the IP experiment showed that although claudin-7 could interact with integrinβ1, when the integrinβ1 antibody was used for IP, claudin-7 was not detected in the immunoprecipitate, possibly due to the indirect interaction between the two proteins. The other possible explanation for this result is that the binding sites between the integrinβ1 antibody and the integrinβ1 protein and the binding sites between the claudin-7 protein and the integrinβ1 protein conflict with each other. Thus, the specific mechanism remains to be further studied.
Conclusion
Our study indicates that Claudin-7 may inhibit the proliferation and migration of tumour cells by interacting with Integrinβ1, subsequently participating in the development of colorectal cancer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81372585, 81772557), and Beijing Health System High Level Training Plan of Health Technical Personnel (2014-3-048).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17(2):375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 1986;322(6080):639–641. doi: 10.1038/322639a0. [DOI] [PubMed] [Google Scholar]
- 4.Runkle EA, Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337(1):41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mineta K, Yamamoto Y, Yamazaki Y, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585(4):606–612. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117(Pt 12):2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- 7.Kim TH, Huh JH, Lee S, Kang H, Kim GI, An HJ. Down-regulation of claudin-2 in breast carcinomas is associated with advanced disease. Histopathology. 2008;53(1):48–55. doi: 10.1111/j.1365-2559.2008.03052.x. [DOI] [PubMed] [Google Scholar]
- 8.Morohashi S, Kusumi T, Sato F, et al. Decreased expression of claudin-1 correlates with recurrence status in breast cancer. Int J Mol Med. 2007;20(2):139–143. [PubMed] [Google Scholar]
- 9.Tokés AM, Kulka J, Paku S, et al. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res. 2005;7(2):R296–305. doi: 10.1186/bcr983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22(13):2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 11.Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg. 2014;12(2):156–162. doi: 10.1016/j.ijsu.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Wang R, Cao H, et al. Expression of claudin-5, -7, -8 and -9 in cervical carcinoma tissues and adjacent non-neoplastic tissues. Int J Clin Exp Pathol. 2015;8(8):9479–9486. [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Lu Z, Foreman O, et al. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 2012;142(2):305–315. doi: 10.1053/j.gastro.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagen SJ. Non-canonical functions of claudin proteins: beyond the regulation of cell-cell adhesions. Tissue Barriers. 2017;5(2):e1327839. doi: 10.1080/21688370.2017.1327839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 16.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3(3):a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhat AA, Pope JL, Smith JJ, et al. Claudin-7 expression induces mesenchymal to epithelial transformation (MET) to inhibit colon tumorigenesis. Oncogene. 2015;34(35):4570–4580. doi: 10.1038/onc.2014.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berghoff AS, Kovanda AK, Melchardt T, et al. αvβ3, αvβ5 and αvβ6 integrins in brain metastases of lung cancer. Clin Exp Metastasis. 2014;31(7):841–851. doi: 10.1007/s10585-014-9675-0. [DOI] [PubMed] [Google Scholar]
- 20.Morozevich GE, Kozlova NI, Susova OY, Karalkin PA, Berman AE. Implication of α2β1 integrin in anoikis of MCF-7 human breast carcinoma cells. Biochemistry. 2015;80(1):97–103. doi: 10.1134/S0006297915010113. [DOI] [PubMed] [Google Scholar]
- 21.He XJ, Tao HQ, Hu ZM, et al. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin β1. Cancer Sci. 2014;105(11):1402–1410. doi: 10.1111/cas.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong YM, Gan WG, Xu ZH. Significance of the expression of integrin β1, VEGF and MVD in hypopharyngeal squamous cell carcinoma. Genet Mol Res. 2014;13(3):6455–6465. doi: 10.4238/2014.August.25.9. [DOI] [PubMed] [Google Scholar]
- 23.Zhan P, Liu L, Liu B, Mao XG. Expression of integrin β1 and its significance in squamous cell carcinoma of the cervix. Mol Med Rep. 2014;9(6):2473–2478. doi: 10.3892/mmr.2014.2134. [DOI] [PubMed] [Google Scholar]
- 24.Ding L, Wang L, Sui L, et al. Claudin-7 indirectly regulates the integrin/FAK signaling pathway in human colon cancer tissue. J Hum Genet. 2016;61(8):711–720. doi: 10.1038/jhg.2016.35. [DOI] [PubMed] [Google Scholar]
- 25.Bologna SB, Nico MM, Hsieh R, et al. Adhesion molecules in primary oral mucosal melanoma: study of claudins, integrins and immunoglobulins in a series of 35 cases. Am J Dermatopathol. 2013;35(5):541–554. doi: 10.1097/DAD.0b013e318276cab3. [DOI] [PubMed] [Google Scholar]
- 26.Tabariès S, Dong Z, Annis MG, et al. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene. 2011;30(11):1318–1328. doi: 10.1038/onc.2010.518. [DOI] [PubMed] [Google Scholar]
- 27.Hong KS, Jeon EY, Chung SS, Kim KH, Lee RA. Epidermal growth factor-mediated Rab25 pathway regulates integrin β1 trafficking in colon cancer. Cancer Cell Int. 2018;18:32. doi: 10.1186/s12935-018-0526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Z, Kim DH, Fan J, et al. A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment. Mol Cancer. 2015;14:120. doi: 10.1186/s12943-015-0387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]