ABSTRACT
The cytoskeleton is crucially important for the assembly of cell-cell junctions and the homeostatic regulation of their functions. Junctional proteins act, in turn, as anchors for cytoskeletal filaments, and as regulators of cytoskeletal dynamics and signalling proteins. The cross-talk between junctions and the cytoskeleton is critical for the morphogenesis and physiology of epithelial and other tissues, but is not completely understood. Microtubules are implicated in the delivery of junctional proteins to cell-cell contact sites, in the differentiation and spatial organization of the cytoplasm, and in the stabilization of the barrier and adhesive functions of junctions. Here we focus on the relationships between microtubules and junctions of vertebrate epithelial cells. We highlight recent discoveries on the molecular underpinnings of microtubule-junction interactions, and report new data about the interaction of cingulin and paracingulin with microtubules. We also propose a possible new role of junctions as “molecular sinks” for microtubule-associated signalling proteins.
KEYWORDS: Microtubules, adherens junctions, tight junctions, desmosomes, epithelial, cadherin, desmoglein, occludin, cingulin, paracingulin, PLEKHA7, kinesin, dynein, centrosome
Introduction
One of the central questions of cell biology is to understand the molecular mechanisms through which cells and tissues achieve their bewildering variety of shapes and functions, and how this relationship is dynamically regulated. How do cells generate, maintain, and alter their shapes? How does form translate into function? How do, in turn, cellular components regulate the shape-generating forces? Decades of research have established that the cytoskeleton, i.e. actin filaments, microtubules (MTs) and intermediate filaments, plays a fundamental role in determining cell shape, and provides cells with resistance to mechanical stresses. This dynamic network of filamentous proteins has many additional functions. It supports whole cell and tissue movement, cell division, precise segregation of genetic material into daughter cells, cytoplasm organization, intracellular vesicular traffic, regulation of gene transcription, and much more. In vertebrates, tissue and organ physiology also require a precise architectural organization and regulation of cell-cell interactions. In epithelial and endothelial tissues, such interactions are largely dependent on highly specialized cell-cell junctions, such as tight junctions (TJ), adherens junctions (AJ), and desmosomes. Importantly, the formation, dynamics and functions of cell-cell junctions crucially depend on their interaction with cytoskeletal filaments, providing an example of cytoskeleton-dependent structure-function relationship. In this review, we discuss the role of a subset of cytoskeletal polymers, e.g. MTs, in the regulation of epithelial junctions. We first briefly describe the composition, organization and distribution of MTs in vertebrate epithelial cells, and introduce a few basic concepts about epithelial junctions. In the second part, we discuss the roles of MTs in the regulation of junctions, and, on the other hand, the roles of specific junctional proteins in regulating MT organization. We also report new data about the interaction of the junctional proteins cingulin and paracingulin with MTs. Finally, we propose the new concept that junctions can function as “molecular sinks” for MT-associated signalling proteins, by focusing on a number of examples of such proteins, that bind to MTs and localize at junctions through known and unknown mechanisms.
Microtubules
Among different types of cytoskeletal polymers, microtubules (MTs), along with actin filaments, are the most evolutionarily conserved, since they are present in all eukaryotes, where they promote the generation of mechanical force and movement through kinesin and dynein (for MTs), and myosin (for actin filaments) motors, respectively. Although proteins similar to tubulin and actin are also found in prokaryotes, the associated protein motors appear to be missing.1 MTs are hollow cylindrical polymers of heterodimeric subunits made of α- and β-tubulin, and are typically made up of 13 parallel protofilaments.2 They are polarized, with plus ends, which are highly dynamic, undergoing either rapid polymerization or rapid depolymerization (catastrophe), and minus ends, which are typically either stabilized or acting as sites of depolymerization.3 Polymerizing MTs are nucleated and stabilized at their minus ends by the γ-tubulin ring complex (γ-TuRC). The γ-TuRC is the main structural unit of microtubule organizing centers (MTOCs), which are found both at centrosomes, and at non-centrosomal sites, such as the Golgi apparatus.4 Tubulins are targets for numerous types of post-translational modifications (PTMs) affecting their C-terminal sequences, including de-tyrosination, Δ2-tubulin generation, polyglutamylation, polyglycylation, and acetylation.5 The functional significance and mechanisms of tubulin PTMs have been investigated in neuronal cells, where PTMs regulate MTs organization and interactions with motors, but their role in epithelial cells is less clear.
MTs have many functions. Besides their role in the formation of the mitotic spindle and chromosome segregation in mitosis, they serve as tracks that facilitate targeted vesicular transport, they interact with different membrane-bound organelles to compartmentalize the cytoplasm, and they underlie the formation of apical cilia in epithelial cells (Figure 1). The organization of the MT cytoskeleton is cell-type dependent, and is related to the shape, cytoplasmic organization, differentiation and function of each cell. In unpolarized, non-differentiated epithelial cells, that do not have mature junctions, most MTs are nucleated by the centrosomes, the Golgi apparatus and other non-centrosomal sites, and their plus ends are directed towards the cell periphery (Figure 1(a)). In contrast, number of mechanisms account for the asymmetric organization of non-centrosomal MTs in differentiated cells.6 In polarized, differentiated epithelial cells with stable junctions, the majority of MTs lose their connection with perinuclear MTOCs, and non-centrosomal MTs align along the apico-basal axis, with the minus ends oriented towards the apical region, and the plus ends oriented towards the basal region, where they form a scattered network7 (Figure 1(b)). Thus, as epithelial cells become polarized and form junctions, the MT nucleating activity becomes more spatially disperse, and MTs become more stable.8 Importantly, the plus-end dynamic instability of MTs is suppressed in cells that form junctions, and individual MTs exhibit an extended state of pause, suggesting that they become capped.9 In stratified epithelia, such as the skin epidermis, keratinocytes drastically reorganize their MT cytoskeleton during differentiation, and radial MTs emanated from the centrosomes become non-centrosomal, and align along the cell cortex.6 In some cell types, such as cultured mouse mammary epithelial cells (Eph4), structured illumination microscopy reveals a new organization of apical non-centrosomal MTs, in the form of planar apical networks (PANs).10
Figure 1.

The organization and functional interactions of MTs in polarizing and polarized epithelial cells. (a) Schematic cartoon of two polarizing epithelial cells, with MTs anchored at their minus-ends at centrosomes and at non-centrosomal sites, and kinesin-mediated transport of junctional molecules and membrane vesicles. (b) Schematic cartoon of polarized differentiated cells, each displaying an apical cilium, and MTs oriented with their minus-ends apically, and plus-ends basally. Cell-cell junctions are indicated on the right, and selected junctional molecules are also schematically drawn (out of scale). (c) Graphical legend for structures and proteins implicated in MTs-junctions functional interactions, shown in A and B.
The plus-ends of microtubules are highly dynamic, switching between polymerization and depolymerization cycles, and interact with different plus-end tracking proteins (+TIPs): EB family proteins (e.g. EB1), CAP-Gly proteins (e.g. CLIP170), proteins containing basic and Serine-rich sequences (e.g. APC), HEAT and WD40-repeat proteins (e.g. LIS1), and MT motor proteins (e.g. dynein).4 + TIPs function to link MTs plus-ends to cellular structures, to regulate MT dynamics, to recruit signaling factors, and to generate pushing and pulling forces, via interaction with motors. The minus-ends of MTs are either stabilized by binding to capping proteins, or undergo either depolymerization, or, more rarely, slow polymerization.4 The balance between stabilization and minus-end depolymerization is important to control MT organization both in interphase cells and during mitosis, where minus-end depolymerization at the spindle poles allows poleward MT flux. Two classes of proteins bind to the minus-ends of MTs. The γ-tubulin ring complex (γ-TURC), mentioned above, is present at centrosomes and at Golgi MTOCs.4 However, in differentiated cells most minus-ends are either free in the cytoplasm or tethered to different cytoplasmic structures. In this case, the minus-ends bind to the second class of proteins, belonging to the CAMSAP/Patronin/nezha family, which are exclusive of animal cells.4 While CAMSAP1 exclusively tracks the growing MT minus-ends, CAMSAP2 and CAMSAP3/nezha are not only recruited by the growing minus-ends, but can also stay bound laterally to MTs, as they elongate. CAMSAP2 and Nezha/CAMSAP3 suppress the MT-organizing activity of the centrosome, through unknown mechanisms, so that the γ-TURC is no longer required for MT nucleation.11 Depletion of CAMSAP3/nezha in cultured cells promotes increased MT nucleation from centrosomes, and causes a reduction in MTs with polymerizing plus-ends, as well as inhibition of cell migration.11,12 The disruption of MT organization resulting from depletion of minus-end capping proteins has consequences on cellular morphogenesis. For example, in cultured cells depletion of CAMSAP3/nezha affects junction assembly, Golgi and nucleus positioning and polarization, and lumen formation in 3D.13 In contrast, upon KO of CAMSAP3/nezha in mice, the organization of the MT network and the positioning of the nuclei and Golgi are disrupted in intestinal cells, and some apical markers are mis-localized, but brush border morphology and epithelial junctions are not affected, suggesting redundant mechanism to promote apico-basal polarization.13
Cell-cell junctions
In vertebrate epithelial cells, the junctional complex comprises the tight junction (TJ, also known as zonula occludens or ZO), the adherens junction (AJ), and desmosomes (Figure 1(b)).14 TJ seal the apico-lateral borders of polarized cells, to prevent the free diffusion of solutes across the paracellular space (barrier function), and to define the border between the apical and lateral domains of the plasma membrane, which have a different composition (fence function). AJs are primarily involved in cell-cell adhesion and sensing of mechanical forces, and comprise two spatially distinct domains. The apical region, called zonula adhaerens (ZA), is a circumferential continuous junction, which is found immediately basal to the TJ. Together, the TJ and the ZA constitute the “zonular” apical junction (also denoted as “apical junctional complex”-AJC), which forms a continuous belt around the apico-lateral regions of polarized epithelial cells, and is connected to a subcortical bundle of contractile actin filaments. The basal part of epithelial AJ, referred to as “lateral contacts”, is constituted by a looser arrangement of cell-cell adhesive structures, which are uniformly distributed along the lateral surfaces, and are associated with a less contractile cortical actomyosin cytoskeleton.15 Thus, clustering of adhesion receptors distinguishes ZA from lateral contacts, and lateral contacts may be viewed as a “reservoir” of junctional and signaling molecules that can eventually be clustered at zonular junctions during differentiation. Desmosomes are hyper-adhesive button-like structures distributed on the lateral surfaces of epithelial cells, and they provide tissues with a strong resistance to mechanical stress.16 In endothelial cells, since the height of the lateral region is very small, TJ and AJ are intermingled, instead of being spatially separated, as they are in epithelial cells.17 Furthermore, unlike TJ and desmosomes, which are typical of epithelial cells, cadherin-based AJ can be found in most cell types, including fibroblasts, muscle cells and neurons.
From a molecular standpoint, TJ, AJ and desmosomes are organized in a similar fashion (Figure 1(b)). Transmembrane molecules, many of which act as cell-cell adhesion molecules, interact in cis to cluster at junctions, and in trans to confer adhesive (TJ, AJ, desmosomes) and barrier (TJ) properties to junctions. These molecules comprise Ig-like adhesion molecules such as JAM-A and CAR at TJ, cadherins and nectins at AJ, and desmogleins and desmocollins (which belong to the cadherin superfamily) at desmosomes. In addition, the 4-pass transmembrane molecules claudins, occludin and tricellulin are critical to set up and regulate the paracellular barrier at the TJ. On the cytoplasmic side, the intracellular domains of the transmembrane junctional proteins interact with complexes of cytoplasmic scaffolding and adaptor proteins. The cytoplasmic proteins (indicated by colour-coded “clouds” in Figure 1) have multiple functions. They cluster transmembrane proteins at the junctional sites, thus making it possible, for example, to generate intramembrane continuous fibrils of claudins.18 They can also regulate the turnover and membrane association of transmembrane proteins. They can either directly or indirectly connect the transmembrane proteins to the actin, MT and intermediate filament cytoskeletons, thus stabilizing the respective junction. They can bind to transcription factors, RNA-associated molecules, kinases, GEFs, GAPs and other signaling molecules, thus either sequestering and inactivating them, or directing the site of their function at junctions.19 Among the most prominent cytoplasmic scaffolding/adaptor proteins are ZO proteins (ZO-1, ZO-2 and ZO-3) and cingulin-family proteins (cingulin and paracingulin) at TJ, catenins (p120-catenin, α-catenin, β-catenin), afadin and PLEKHA7 at AJ, and desmoplakin and plakoglobin at desmosomes. In addition, two protein complexes which are involved in signaling to direct the establishment of apico-basal polarity, the Par (Par3-Par6-apKC) and Crumbs (Crumbs-Pals1-PATJ) complexes, are associated apically with the cytoplasmic region of TJ, whereas the Lgl/Scribble/Dlg complex identifies the lateral membrane.20 The actin and intermediate filament cytoskeletons are crucial to allow tissues to adapt to physiological mechanical stresses, and specific junctional adaptor proteins, such as α-catenin, vinculin and ZO-1, have been shown to respond to force with changes in their conformation and interactions,21,22 to transduce mechanical signals. The reader is referred to additional excellent reviews for a more detailed description of the molecular organization of TJ, AJ and desmosomes, and the functional significance of the interaction of these junctions with the cytoskeleton.17,23-31
Regulation of junction assembly and homeostasis by microtubules
Tight junctions
The importance of MTs for TJ physiology and organization was first revealed by studies using colchicine, a drug that disrupts MTs organization. Treatment of MDCK cells with colchicine caused transient changes in trans-epithelial electrical resistance (TER), a measure of TJ barrier function, and in the structure of TJ, as determined by electron microscopy.32 Colchicine also affected TJ ultrastructure and permeability to lanthanum in hepatocytes in vivo.33 These early findings were confirmed by subsequent studies on cultured thyroid epithelial cells, showing that the barrier function of TJ was decreased, and the junctional accumulation of ZO-1 was disrupted, following colchicine treatment.34 Furthermore, treatment of MDCK cells with nocodazole, a drug which interferes with the polymerization of MTs, perturbs the junctional localization of the TJ/AJ protein paracingulin.35 Also, in human intestinal cells TJ barrier formation requires MTs integrity and the minus-end motor protein dynein,36 indicating that plus-end directed transport is involved in delivering TJ proteins to the membrane. Another evidence that MTs are required for TJ formation is the observation that either nocodazole treatment or depletion of Dual leucine zipper-bearing kinase (DLK), which is required for MT reorganization, significantly decreases the levels of ZO-1 and claudins at the cell periphery in keratinocytes.37,38 Conversely, the MT-stabilizing drug paclitaxel promotes keratinocyte differentiation, cortical accumulation of junctional proteins, and barrier formation.38
MTs likely contribute to TJ assembly by functioning as tracks for the delivery of TJ proteins through MT-associated vesicles. During the first division of the Xenopus oocyte, conspicuous ZO-1-stained vesicles are aligned in the cytoplasm, likely along MT tracks, in a plane orthogonal to the plane of cleavage, and appear to fuse with the basolateral membrane.39 In contrast, in dividing Xenopus oocytes occludin-containing granules are found near the tip of oolemma ingression,39 suggesting that at least two types of MT-associated membrane vesicles deliver TJ proteins to the sites of cell-cell contact. In agreement, recent studies confirm that occludin moves along MT tracks.36 The notion that MTs are also involved in epithelial morphogenesis is supported by studies showing that during epithelial lumen formation, which is associated with the formation of new junctions between the apicolateral regions of epithelial cells, the anterograde MT motor kinesin-2 mediates the plus-end directed transport of apical endosomes.40 More recent studies show that the fusion of the apically targeted endosomes with the plasma membrane requires Rab11 and its effector FIP5, as well as the TJ protein cingulin, which interacts with FIP5 and with MTs.41
In addition to the organization and maintenance of junctional integrity, MTs are involved in disassembly of TJ. This role of MTs was first characterized using the calcium depletion model of junction disassembly in intestinal and renal epithelial cells, which affects both TJ and AJ.42 In the absence of nocodazole, calcium depletion leads to disruption of junctions, formation of intracellular contractile actin rigs, and internalization of the TJ proteins occludin and ZO-1, and the AJ proteins E-cadherin and β-catenin, which associate with the actin rings. Depolymerization of MTs with nocodazole prevents the translocation of junctional proteins into these cytosolic ring-like structures.42 Conversely, drug-mediated stabilization of MTs attenuates the disassembly of the apical junctions, and blocks the contraction of the actin rings.42 MT-associated motor activity is implicated in ring formation, since the plus-end directed motor kinesin-1 is localized at junctions, where it associates with E-cadherin and catenins, and treatment with the kinesin inhibitor AMP-PNP blocks the contraction of F-actin rings and the translocation of E-cadherin and occludin into the subapical cytosolic rings.42 Actomyosin contractility is crucial for junction disassembly following calcium depletion.43-46 Indeed, the RhoA-ROCK pathway, mediated by the MT-associated GEF GEF-H1, was shown to be implicated in the formation and contractility of actin rings following calcium depletion, suggesting that MTs control junction disassembly by regulating factors that affect actomyosin contractility.47 GEF-H1 is also implicated in the restoration of functional junctions in adhesion-defective cancer cells, through which inhibition of MT polymerization activates GEF-H1, inducing actomyosin contractility, and a mechanosensitive response which stabilizes junctions.48 Another evidence that MT are implicated in TJ and AJ assembly and stability was the observation that overexpression of the β-tubulin chaperone cofactor D, which acts as a GTPase activating protein for β-tubulin, induces disassembly and prevents assembly of TJ and AJ in MDCK cells.49 In summary, these results indicate that MT dynamics is essential to regulate both assembly and calcium-depletion-mediated disassembly of apical junctions, and that MT operate, at least in part, through mechanisms that affect RhoA and downstream regulation of actomyosin contractility.
Adherens junctions
The first study addressing the role of MTs in the assembly of the AJC reported that colchicine treatment of thyroid epithelial cell monolayers reduced the junctional accumulation of E-cadherin.34 Furthermore, in lung epithelial cells inhibition of MT plus-end growth induced breakage of AJ, loss of F-actin from AJ, and rearrangement of catenins into puncta, and eventually their disappearance from junctions.9 It was proposed that junction breakage was not due to the acute formation of cytoplasmic stress fibers, that is observed following treatment with nocodazole, but rather to the decrease in junctional actin filaments occurring after MT depolymerization.9 These observations reinforce the notion that MT organization and dynamics has multiple effects on actomyosin organization.
Several AJ proteins are delivered to the cell surface through the dynamic plus-ends of MTs and associated motors (Figure 1(a)). In fibroblasts, kinesin is required for the formation of N-cadherin-dependent cell-cell contacts,50 and p120-catenin association with kinesin promotes the transport of the catenin-cadherin complex to junctions.51 In agreement, the KO of KAP3, a subunit of kinesin-2, results in decreased levels of N-cadherin and β-catenin in embryonic mouse neural precursors,52 whereas the overexpression of another kinesin, KIF17, promotes the apical and junctional accumulation of actin and E-cadherin, respectively.53 In epithelial Pkt2 cells cytoplasmic dynein transiently tethers MTs at sites of cell-cell contact during junction biogenesis, providing a track for the kinesin-dependent delivery of junctional components.54 Another plus-end binding protein, CLIP170, targets MTs to AJs prior to apico-basal array assembly.55 Either expression of mutant CLIP170, which affects MT dynamics, or depolymerization of dynamic MTs by nocodazole, reduces the accumulation of E-cadherin at junctions, as well as the accumulation of myosin-IIA and phosphorylated myosin light chain.56 Since surface expression of E-cadherin was not affected by nocodazole, it was suggested that dynamic MTs stabilize and regulate the junctional clustering of E-cadherin, rather than its surface expression, by modulating the actomyosin cytoskeleton.56 Cortical MT-associated transfer of junction-associated proteins can also orchestrate the localization of cell fate determinants. For example, β-catenin is transported towards the plus-ends of MTs, and acts as a dorsal determinant in the Xenopus egg.57
In polarized epithelial cells, multiple mechanisms account for the apicolateral anchoring of MTs minus-ends and the formation of non-centrosomal MTOCs (Figure 1(b)). First, different centrosomal proteins, including ninein and CAP350, move along MT tracks to reach AJ, where they act as non-centrosomal MT anchoring sites, at least in some cell types.58-60 In MDCK cells CAP350 is recruited to AJ by α-catenin, and its depletion induces an enlargement of the apical domain and a reduction in cell height, and prevents the formation of cell-cell contacts in the calcium switch model of junction assembly.59 Second, MT minus-ends are anchored to AJ by the interaction of CAMSAP3/nezha with PLEKHA7.61 This anchoring has a stabilizing effect on AJ, since depletion of either PLEKHA7 or CAMSAP3/nezha decreases the accumulation of E-cadherin at the ZA.61 The minus-end directed kinesin KIFC3 is targeted to the ZA in a MT-, PLEKHA7- and CAMSAP3-dependent manner, but it does not colocalize with E-cadherin,61 suggesting that it is not involved in E-cadherin transport to the junction. On the other hand, KIFC3 transports to the junction the ubiquitin-specific protease 47 (USP47), which deubiquitinates E-cadherin, and thus prevents its degradation.62 Thus, cadherin stabilization by PLEKHA7/nezha involves its decreased ubiquitination and degradation. As indicated above, the anchoring of MTs minus-ends to AJ and then to the apical membrane plays a role in epithelial morphogenesis, since depletion of CAMSAP3 leads to cell flattening, a wider apical cell surface, although normal cell height is restored after full polarization.13
In summary, these studies show that dynamic MTs are required for the efficient assembly of AJ and delivery of factors that regulate junction dynamics, and multiple mechanisms contribute to tethering MTs to AJ during epithelial differentiation and polarization.
Desmosomes
The first evidence for MT-desmosome interaction was the co-localization between desmoglein-1 and MTs in cultured cells.63 The importance of MT organization for desmosome assembly was further demonstrated by the observation that desmoglein-2 and desmocollin-2 depend on MTs for their rapid accumulation at junctions, requiring kinesin-1 and kinesin-2, respectively, for their transport to the surface.64 Desmosome function requires MTs, because depletion of these kinesin motors dramatically weakens cell-cell adhesive strength in confluent epithelial sheets, despite the observation that desmosomal plaque proteins are not affected.64 In addition, the genetic loss of the centrosomal protein LIS1, which re-localizes to desmosomes upon differentiation to reorganize non-centrosomal MTs, leads not only to MT organization defects, but also to severe loss of desmosome stability and epidermal barrier activity, and decreased expression of desmosomal proteins, resulting in embryonic lethality.65 In a tissue-engineered skin model, nocodazole treatment reduces the number and size of desmosomes, and the accumulation of desmoglein-1 at the cell cortex.37 The mitogen-activated protein kinase DLK (dual leucine zipper-bearing kinase) is expressed in granular layer of the epidermis and is required for terminal differentiation of keratinocytes.66 This kinase acts through MTs, since in the epidermis of DLK KO mice, which die soon after birth, MTs fail to align along the cell cortex, and decreased localization of desmoplakin and LIS1 at junctions leads to MT disorganization and to short desmosomes with increased intercellular spaces, similar to nocodazole-treated tissues.37 In summary, in keratinocytes MTs allow trafficking of desmosome transmembrane proteins (desmogleins and desmocollins) to the sites of junction formation, and are critical for keratinocyte differentiation. Finally, MTs are not only involved in desmosome assembly, but also in their disassembly. Desmosomes are internalized following removal of extracellular calcium in MDCK cells, and their intracellular transport is regulated by MTs, leading to lysosomal degradation of internalized desmosomal proteins.67
Regulation of MTs organization by junctional proteins
While MTs are important in the regulation of junction assembly and disassembly and in epithelial differentiation, the cross-talk between MTs and junctions also involves the interaction of several junctional proteins with either MTs or MT-associated proteins, resulting, for example, in the tethering of MTs to TJ, AJ and desmosomes.
Cingulin (CGN), a specific TJ protein, was initially discovered as a protein associated with the actomyosin fraction from intestinal cells lysates,68,69 and was later found to organize the planar apical network (PAN) of MTs in cultured mammary epithelial (Eph4) cells.10 Cingulin controls tethering of the PAN to TJ, based on the observation that depletion of cingulin leads to disruption of the association of organized MT with TJ.10 PANs have not been detected in all epithelial cell types, thus it remains to be seen how cingulin promotes MT organization in other cell types. Phosphorylation of the CGN head domain on Ser 132 and Ser 150 by AMPK is required for cingulin anchoring of MTs and for cyst formation in 3D cultures, implying that the cingulin-MT association is important for epithelial morphogenesis.10 In agreement with this idea, cingulin was found to be involved in the proper establishment of the Apical Membrane Initiation Site (AMIS) and subsequent formation of apical lumen in epithelial cysts.41 The interaction with MTs is relevant, because cingulin mutants that bind less well to tubulin were localized ectopically and failed to rescue the multi-lumen phenotype of cingulin-KO cysts.41 Proteolytic digestion of the C-terminus of tubulin and mutation of the cingulin head domain reduces the MT-cingulin interaction, indicating that a basic patch within the cingulin head binds to an acidic patch within the unstructured C-terminal tail of tubulin.41 Cingulin participates in lumen formation by interacting with FIP5 (Rab11 family interacting protein 5), which is an effector of the Rab11 GTPase, and is required for apical endosome transport and targeting.41 AMPK kinase, which phosphorylates cingulin,10 is in turn activated by phosphorylation by the LKB1 kinase.70 Interestingly, in LKB1-KO mice the level of phosphorylated AMPK is highly reduced, and CGN junctional accumulation in hepatocytes is lost, correlating with changes in cell morphology, and TJ integrity and permeability.71 This suggests that the LKB1-AMPK kinase axis controls the apical accumulation of cingulin through its phosphorylation.
The evidence that cingulin binds to MTs is three-fold. First, a gel overlay assay using tubulin as a ligand binds to cingulin among different proteins present in lysate of a junction-enriched liver membrane fraction.10 Second, the recombinant cingulin head domain co-immunoprecipitates with α-tubulin.10 Third, the same domain co-pellets with microtubules.41 To confirm and extend these studies, we examined the interaction of full-length recombinant cingulin and paracingulin (also known as JACOP or cingulin-like-1, CGNL1), expressed in baculovirus-infected insect cells, with MTs. We found that not only full-length cingulin, but also paracingulin co-pellet specifically with MTs (Figure 2), thus providing further evidence for cingulin MT-interaction, and reporting for the first time an interaction of paracingulin with MTs.
Figure 2.

Full-length cingulin and paracingulin co-sediment with MTs in vitro. Top: Immunoblot analysis, using antibodies against cingulin (CGN, left) and paracingulin (CGNL1, right) of supernatant (S) and pellet (P) fractions following centrifugation (100,000 x g) of cingulin and paracingulin (His-tagged, purified from baculovirus-transduced insect cells72), either in the absence (-) or in the presence (+) of taxol-stabilized brain MTs (cat n. BK029, Cytoskeleton). Bottom: Coomassie blue staining of corresponding gels, showing sedimented tubulin in pellet fractions. Numbers on the left indicate approximate Mr, based on the migration of pre-stained markers. Densitometric analysis of immunoblots from three experiments show that the presence of MTs promotes the sedimentation of a statistically significant fraction of both cingulin and paracingulin.
The interaction of paracingulin with MTs suggests that it may also function to tether MTs to junctions. However, no experimental evidence is available so far in this sense, except for the observation that disruption of MTs alters CGNL1 localization.35 Unlike cingulin, which has been detected only at apical TJ, paracingulin has been detected both at TJ and AJ, depending on cell type,73 and both ZO-1 and PLEKHA7 contribute to its junctional recruitment.74 On the other hand, ZO-1 is solely responsible for cingulin recruitment to TJ.75,76 Thus, both cingulin and paracingulin may anchor MTs to TJ and AJ, respectively. Interestingly, paracingulin (e.g. hypothetical protein FLJ14957) was identified among human centrosomal proteins detected through mass-spectrometry-based proteomic analysis.77 Furthermore, antibodies against cingulin and paracingulin label centrosomes in fixed cells.35 Although this labeling could be due to cross-reaction of the antibodies with coiled-coil proteins, we also observed that GFP-tagged forms of cingulin and paracingulin dynamically localize both at centrosomes and junctions in live cultured epithelial cells.35 This evidence, and proteomic data, indicate that cingulin and paracingulin are bona fide centrosomal components. The potential role of cingulin and paracingulin at centrosomes, and their interactors at the centrosome, are not known. However, the centrosomal localization of cingulin and paracingulin suggests that they may interact with centrosomal proteins, and possibly recruit them to junctions in confluent monolayers. As discussed below, cingulin and paracingulin are also implicated in the junctional recruitment of MT-associated signaling proteins (GEFs and GAPs) that regulate the organization and contractility of the actomyosin cytoskeleton. In summary, the identification of both cingulin and paracingulin as MT-binding protein, and their centrosomal localization reinforce the notion of a dynamic re-localization of centrosome-associated proteins to apical junctions, once these are formed following epithelial differentiation (Figure 1(b)).
Another potential regulator of MTs at the TJ is the apical polarity complex protein Par3, which in mammalian systems is involved in epithelial apicobasal polarization, and in the junctional recruitment of the exocyst complex.78-81 In invertebrate model systems and in neuronal cells and fibroblasts Par3 regulates MT stability and organization, and forms cortical docking sites for centrosomes, subjected to modulation by Par1 and aPKC.82-84 Par3 associates with the TJ transmembrane protein occludin during polarized migration of MDCK cells, and in this context occludin is required for the organization of the MT network and the orientation of the MTOC during migration.85 However, the specific roles of Par3 and occludin in the regulation of MT organization at epithelial junctions have not been investigated.
The cadherin-catenin complex, which is clustered at AJ of epithelial and non-epithelial cells, has been implicated in MT anchoring through different types of catenin-dependent molecular interactions. Early studies showed that exogenous expression of either E-cadherin or N-cadherin in CHO fibroblasts, which do not express either cadherin, stabilizes non-centrosomal MTs.86 Experiments on astrocytes confirmed that classical cadherins control nucleus and centrosome positioning.87 It was further shown that only membrane targeting of α-catenin, but not β-catenin or p120-catenin (p120-ctn), led to a significant increase in MT length and density in centrosome-free cytoplasts, suggesting that α-catenin can regulate MT dynamics in a centrosome-independent manner.88 This could be due to the fact that α-catenin recruits the centrosomal component CAP350 to the AJ, and can thus bind to MTs through CAP350.59 β-catenin is another AJ protein involved in MT interaction, since it has been identified at centrosomes of non-epithelial cells, where it may anchor MTs,89 and regulate centrosome cohesion.90,91 In addition, β-catenin overexpression disrupts the localization of the minus-end motor dynein, and dramatically perturbs the organization of MT arrays, suggesting that β-catenin may anchor MTs to AJ through dynein.92 Finally, several observations support the idea that p120-ctn interacts and cross-talks with MTs. In COS7 cells, mutated exogenous p120-ctn localizes along MTs in interphase, and recruitment to MT suppresses the ability of p120-ctn to regulate Rho GTPases.93 In MDCK cells p120-ctn binding to either cadherin or MTs is mutually exclusive, and promoting p120-ctn association to MTs by overexpressing its indirect linker kinesin reduces the nuclear pool of p120-ctn.94 MTs plus-ends undergo more frequent rapid depolymerization in p120-ctn-depleted cells, suggesting that p120-ctn can regulate MT dynamics.95 Finally, in mouse primary keratinocytes p120-ctn interacts directly with the plus-end capping protein CLASP2, and recruits it to AJ of basal epidermal progenitors, where CLASP2 functions to promote AJ formation and stability.96
An important mechanism of MTs-AJ interaction was discovered with the identification of PLEKHA7, which binds to p120-ctn and to the minus-end MT-binding protein nezha/CAMSAP3, thus promoting the stabilization of the ZA through the accumulation of E-cadherin.61 In agreement, overexpression of PLEKHA7 attenuates, in a MT-dependent manner, the disruption of junctions and the increase in paracellular TJ-dependent permeability induced by depletion of extracellular calcium.97 PLEKHA7 binds to additional ZA proteins, such as paracingulin,74,98 afadin99 and PDZD11.100 PLEKHA7 is also involved in recruiting mRNAs and miRNA processing factors to the ZA, modulating cellular resistance to staphylococcal α-toxin and controlling the pathogenesis of hypertension and angle closure glaucoma, through mechanisms that remain to be clarified (reviewed in101). However, the role of MT-tethering in all these additional interactions and functions of PLEKHA7 is not clear.
Desmoplakin (DP), which links intermediate filaments to the desmoglein/desmocollin complex (Figure 1(b)) (reviewed in102,103), plays a crucial role in MTs rearrangement, through the desmosomal recruitment of MT-binding centrosome proteins, such as ninein, LIS1, Ndel1, and CLIP170.104,105 The recruitment of these MT-binding proteins by desmoplakin during keratinocyte differentiation facilitates MTs reorganization, and provides new nucleation sites, thus promoting MT alignment along the cell cortex.
In summary, several junctional proteins interact either directly or indirectly with MTs, and thus provide cortical sites for the MT-dependent delivery of additional junctional components, as well as tethering MTs during their spatial reorganization of differentiating cells. These interactions are crucial to stabilize junctions, and to establish an architectural framework for the cross-talk between MT and actin cytoskeleton.
Junctions as molecular sinks and regulators for MT-associated signaling proteins
Zonular junctions (TJ and ZA) are sites where transmembrane proteins, cytoplasmic adaptors and signaling proteins are highly concentrated and clustered in a thin circumferential plaque, which is intimately associated with the actin and MT cytoskeletons. Contractility of the actin cytoskeleton affects the conformation of selected TJ and ZA proteins within the cluster, such as ZO-1, α-catenin, and vinculin,21,22 resulting in mechanical reinforcement and enhanced junctional recruitment of signaling and regulatory proteins.21,106 Among proteins found in the cytoplasmic region of zonular junctions there are several proteins that bind either directly or indirectly to MTs, including proteins that redistribute from centrosomes to junctions during epithelial differentiation, and proteins involved in the regulation of Rho family GTPases and actomyosin contractility, such as GEFs and GAPs19,106 (Figure 3). The recruitment of these signaling, adaptor and regulatory proteins to zonular junctions can result in their functional inhibition, or, alternatively, may spatially restrict their activity at junctions. We propose that zonular junctions act as “sinks”, e.g. either storage, and/or spatial restriction, and/or silencing of signaling and adaptor proteins, in a manner which couples the integrity of cell-cell contacts to the dynamic regulation of the actin and MT cytoskeletons. Indeed, the observation that several junctional proteins, including cingulin, paracingulin,35 β-catenin90,107 and occludin108 are localized at centrosomes suggests that epithelial polarization and junction assembly results in the redistribution of a subset of centrosomal proteins to the zonular “sink”. Below we provide a few examples of proteins that have been functionally and/or spatially linked to both junctions and MTs (Figure 3), and review the current knowledge about the molecular basis and functional implications of their localization at junctions, and/or association with MTs.
Figure 3.
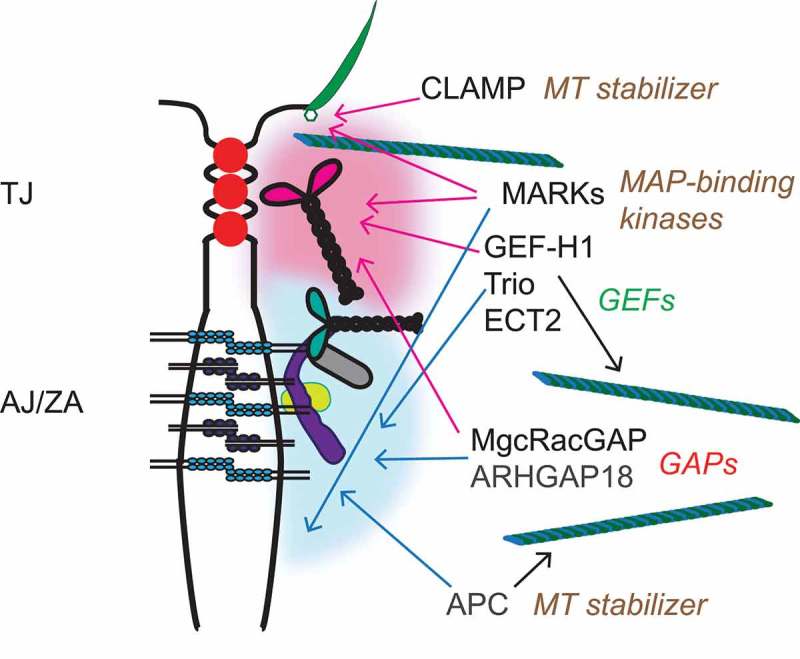
Zonular junctions as sinks for MT-associated signaling proteins. TJ and ZA are shown in a schematic cartoon of an apical junction between epithelial cell, with an apical cilium shown on the right. Cytoplasmic plaque “clusters” of junctional proteins are indicated by diffuse red (TJ) and blue (ZA/AJ) “clouds”, showing only a few of the junctional molecules, for simplicity (see Fig. 1(c) for graphical legend). On the right, the signaling proteins described in the text are indicated, with color-coded arrows (red-TJ, blue-AJ/ZA) linking them to the junctional structures with which they have been associated, based on current literature. For MARKs, only MARK2 and MARK4 have been localized at apical junctions and in cilia basal bodies, respectively. Each protein or group of proteins is also associated with a text, on the right, describing their function in relationship to either MTs or Rho family GTPases.
GEF-H1
GEF-H1 (ARHGEF2) is a Dbl family guanidine exchange factor (GEF), which was originally identified as a MT-associated activator of RhoA.109 The activity of GEF-H1 is inhibited when it is bound to MTs, since mutants that do not bind to MTs constitutively activate RhoA.110 GEF-H1 is a key molecule involved in the cross-talk between MTs and the actin cytoskeleton.111 For example, in endothelial cells GEF-H1 plays a critical role in RhoA activation and downstream actomyosin contractility and endothelial barrier disruption caused by MT disassembly, induced either by thrombin or by mechanical strain.112,113 RhoA activation by GEF-H1 is involved not only in promoting the formation of contractile cytoplasmic stress fibers, but also in inducing apical constriction during development,114 establishing epithelial polarity,115 regulating cell proliferation, transcription factors and other signaling molecules.116-118 Because of its multiple roles in disease, it was proposed that GEF-H1 could be a therapeutic target for pathologies where RhoA activity is dysregulated.119
Besides binding to MTs, GEF-H1 is also regulated by phosphorylation and by recruitment to junctions. The interaction with MTs, and hence the inhibition of GEF-H1 catalytic activity, is promoted by phosphorylation at Ser886, either by The Rac1/Cdc42 effector p21-activated kinase (PAK1), or by protein kinase A.120,121 In contrast, GEF-H1 phosphorylation at Ser151 by the MARK3 kinase disrupts the association of GEF-H1 with MTs, and thus activates it, resulting in development of epithelial polarity and cyst lumen formation.115 During mitosis the catalytic activity of GEF-H1 is inhibited, prior to cytokinesis, through phosphorylation by the mitotic kinases Aurora A/B and Cdk1/Cyclin B.122 Another mechanism through which GEF-H1 activity is inhibited is its recruitment to junctions, which depends on cingulin123 (Figure 3). Upon either depletion of cingulin in cultured cells, or KO of cingulin in either embryoid bodies or in mice, GEF-H1 loses its junctional localization, resulting in RhoA activation, and RhoA-dependent transcriptional changes, such as increased expression in the TJ transmembrane protein claudin-2.123-126 Thus, the junctional sequestration of GEF-H1 by cingulin provides a simple mechanism to couple cell confluency to downregulation of RhoA activity, both in epithelial and endothelial cells.127 However, it is not clear whether direct binding to cingulin, which has been proposed based on in vitro experiments,123 functions as a regulator of either the catalytic activity or the phosphorylation of GEF-H1. Another TJ protein, ZO-2, was recently shown to have a role in promoting GEF-H1 inhibition through phosphorylation.128
MgcRacGAP and ECT2
MgcRacGAP, together with the kinesin-6-family member MKLP1 (mitotic kinesin-like protein), forms the centralspindlin complex, which organizes antiparallel arrays of MTs at the spindle midzone and midbody, controls the activity of RhoA and Rac1 during furrow ingression, and links the mitotic spindle to the plasma membrane during cytokinesis.129 ECT2 is a Rho GEF, which concentrates on the central spindle by binding to centralspindlin, and is essential for RhoA activation in the cleavage furrow.130,131 MgcRacGAP localizes to MT plus-ends at the equatorial cortex as cytokinesis initiates, and it tracks with EB3 at growing MT plus-ends.132 In contrast, in interphase confluent epithelial cells, MgcRacGAP accumulates at apical junctions, where it is recruited by cingulin, paracingulin and α-catenin133,134 (Figure 3). In some but not all cell types the centralspindlin complex recruits the ECT2 to the ZA133,134 (Figure 3). MgcRacGAP regulates AJ but not TJ structure, through its function as a GTPase activating protein (GAP) activity, and signaling via the RhoA pathway.135 The C-terminal region of MgcRacGAP is involved to its targeting both to AJ, via a SxIP motif,132 and to TJ, via its interaction with cingulin and paracingulin.134 These observations suggest that initially MgcRacGAP is delivered to AJ through plus-end directed motors, and then is redistributed at both TJ and AJ through multiple interactions.
ARHGAP18
The RhoGAP ARHGAP18/SENEX was identified in a screen for genes involved in angiogenesis, and is highly expressed in endothelial cells, where it is downregulated in the early migration phase, and upregulated in the stabilization phase of tube formation, when junctions are formed.136 Indeed, ARHGAP18 translocates to and stabilizes junctions during angiogenesis137 (Figure 3). Consistent with its role in stabilizing endothelial junctions, loss of ARHGAP18 promotes tumor angiogenesis,137 and, conversely, its expression is associated with good breast cancer prognosis.138 In HUVEC endothelial cells, ARHGAP18 is associated with MTs, and ARHGAP18 depletion decreases total MT network density and acetylation levels,139 which regulate the ability of the MT lattice to cope with mechanical stress and self-repair.140 However, nothing is known about the mechanisms through which ARHGAP18 associates with junctions, and how it may affect tubulin acetylation.
Trio
Trio (ARHGEF23) is a RhoGEF, which promotes localized Rac1 activation, and is targeted to the plus-ends of MTs during neurite extension, through its interaction with the +TIPs NAV and EB1.141 In endothelial cells Trio is involved in the stabilization of VE-cadherin.142 In epithelial cells it interacts with E-cadherin and mediates the junctional recruitment of Tara, which regulates E-cadherin transcription143 (Figure 3). However, it is not known whether Trio activity as a GEF is modulated by its MT versus junctional localizations.
APC
APC is a gene mutated in familial colorectal cancer, which encodes a large protein involved in the regulation of the cytoplasmic pool of β-catenin.144 APC binds to MTs, either directly or through the +TIPs EB1 or CLIP170, and its binding to MTs is regulated by phosphorylation, and results in MT polymerization and stabilization145-149 (Figure 3). Studies on cultured cells show that two spatially separate populations of APC exist at the cell periphery, one which is highly dynamic and associates with MTs near free edges, and a second comparatively static and closely associated with actin at sites of cell-cell contact150,151 (Figure 3). However, the molecular basis for APC association with junctions, and how the junctional localization of APC is related to its signaling activity is not clear.
MARK proteins
Microtubule affinity-regulating kinases (MARKs) are an evolutionarily conserved family of kinases that comprise MARK1(PAR-1c), MARK2 (PAR-1b/EMK), MARK3 (PAR-1a/C-TAK1) and MARK4 (PAR-1d/MARKL1).152 Mammalian MARKs are homologous to the invertebrate partitioning gene product Par-1, which regulates development of cell polarity,153 and belong to the same family of energy sensing kinases as AMPK.154 MARKs are indirectly associated with MTs, since they were identified as kinases that phosphorylate the MT-regulating proteins tau and MAPs, to regulate MT dynamics and MT-dependent transport.155 There is evidence that MARK proteins are involved in different aspects of epithelial differentiation. For example, MARK4 localizes to the basal body and promotes ciliogenesis in ciliated cells,156 and MARK2 functions downstream of aPKC in the establishment and maintenance of epithelial cell polarity in mammalian cells157 (Figure 3). Importantly, MARK2 (PAR1b) has been localized both at apical and lateral epithelial junctions,157 and is required for the formation of apical lumens in epithelial cells grown in 3D, by promoting the apicobasal alignment of MTs, and by modulating myosin-II and E-cadherin dependent signaling158,159 (Figure 3). MARKs are involved in regulation of different signaling pathways, for example through the phosphorylation of proteins involved in membrane recycling,160 RhoA/Rac1 signaling,115,161,162 inflammation,163 and the Hippo pathway.164 However, although MARK2 has been localized at junctions,157,158 little is known about the junctional localization of other MARKs (MARK1, MARK3 and MARK4), the mechanisms that regulate the subcellular localizations of MARKs, and the effect of junctional localization on the enzymatic activity and signaling functions of MARK proteins.
CLAMP
CLAMP/Spef1 is a MT-associated protein which is highly expressed in ciliated epithelia, stabilizes MTs, and has been implicated in the apical accumulation of stable acetylated MTs.165 Recently, it was shown that CLAMP/Spef1 accumulates not only at MT-rich structures, but also at junctions, and it controls planar polarity and MT asymmetry in Xenopus ciliated epithelia166 (Figure 3). CLAMP co-localizes with components of the Par complex and interacts with aPKC, suggesting that the Par complex directs the junctional localization of CLAMP.165 However, it is not clear how the junctional localization of CLAMP affects the PTMs and dynamics of MTs.
Conclusions
The dynamics and the reorganization of the MT network are fundamentally important in the formation of cell-cell junctions and in the biogenesis of the epithelial phenotype. MTs serve as tracks for the delivery of vesicles containing junctional and signaling proteins to the cell periphery, contributing to generation of apical lumens, efficient assembly of junctions, apico-basal polarization, and differentiation. In a feed-forward loop, the formation of junctions promotes the local clustering of cytoplasmic plaque proteins, several of which interact either with MT or MT-binding proteins, and help to redundantly orient, tether, and stabilize MTs along the apicobasal axis of polarized cells. Among these proteins, the MT-binding proteins cingulin and paracingulin may form docking sites for apical MT structures, such as the planar apical network. Other junctional proteins, such as PLEKHA7, can represent transient docking sites for MT-binding proteins, such as CAMSAP3/nezha, which redistribute apically in fully differentiated cells. The junctional localization of several centrosome-associated proteins underlines the crucial role of junctions as sites that orchestrate MT organization in polarizing cells. Besides nucleating the formation of a molecular environment rich in MT-binding proteins, cytoplasmic junctional proteins also promote the junctional recruitment and storage of MT-associated signaling proteins, several of which play crucial roles in the regulation of the actomyosin cytoskeleton. The release of these signaling proteins during junction disassembly contributes to the reorganization of both actin and MT cytoskeleton, leading to changes in cell motility and behavior. Thus, junctions act both as organizers of MTs, as “molecular sinks” for MT-associated proteins, and as signaling hubs, which modulate the assembly and dynamics of both MT and actin cytoskeletons locally and globally in the cell.
Funding Statement
This work was supported by the Novartis Stiftung für Medizinisch-Biologische Forschung [17B072];Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung [31003A_172809].
Acknowledgments
We thank members of the Citi laboratory and Paul Guichard for their comments on the manuscript, and the Swiss National Foundation (n. 31003A_172809, SC), and the Novartis Foundation for Medical and Biological Research (#17B072, SC) for financial support.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Pollard TD, Goldman RD.. Overview of the cytoskeleton from an evolutionary perspective. Cold Spring Harb Perspect Biol. 2018;10(7):a030288. doi: 10.1101/cshperspect.a030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH. Microtubules: evidence for 13 protofilaments. J Cell Biol. 1973;59(2 Pt 1):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Martin M, Akhmanova A. Coming into Focus: mechanisms of Microtubule Minus-End Organization. Trends Cell Biol. 2018;28(7):574–588. doi: 10.1016/j.tcb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Gadadhar S, Bodakuntla S, Natarajan K, Janke C. The tubulin code at a glance. J Cell Sci. 2017;130(8):1347–1353. doi: 10.1242/jcs.199471. [DOI] [PubMed] [Google Scholar]
- 6.Muroyama A, Lechler T. Microtubule organization, dynamics and functions in differentiated cells. Development. 2017;144(17):3012–3021. doi: 10.1242/dev.153171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacallao R, Antony C, Dotti C, Karsenti E, Stelzer EH, Simons K. The subcellular organization of Madin-Darby Canine Kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989;109:2817–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bré MH, Pepperkok R, Hill AM, Levilliers N, Ansorge W, Stelzer EH, Karsenti E. Regulation of microtubule dynamics and nucleation during polarization in MDCK II cells. J Cell Biol. 1990;111(6 Pt 2):3013–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterman-Storer CM, Salmon WC, Salmon ED. Feedback interactions between cell-cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol Biol Cell. 2000;11(7):2471–2483. doi: 10.1091/mbc.11.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yano T, Matsui T, Tamura A, Uji M, Tsukita S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol. 2013;203(4):605–614. doi: 10.1083/jcb.201304194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka N, Meng W, Nagae S, Takeichi M. Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial-specific organization of noncentrosomal microtubules. Proc Natl Acad Sci U S A. 2012;109(49):20029–20034. doi: 10.1073/pnas.1218017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang K, Hua S, Mohan R, Grigoriev I, Yau KW, Liu Q, Katrukha EA, Altelaar AFM, Heck AJR, Hoogenraad CC, et al. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev Cell. 2014;28(3):295–309. doi: 10.1016/j.devcel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Toya M, Kobayashi S, Kawasaki M, Shioi G, Kaneko M, Ishiuchi T, Misaki K, Meng W, Takeichi M. CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc Natl Acad Sci U S A. 2016;113(2):332–337. doi: 10.1073/pnas.1520638113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15(6):397–410. doi: 10.1038/nrm3802. [DOI] [PubMed] [Google Scholar]
- 16.Godsel L.M., et al. The molecular composition and function of desmosomes. Handb Exp Pharmacol. 2004;165:137–193. [DOI] [PubMed] [Google Scholar]
- 17.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26(5):441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126(4):741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Citi S, Guerrera D, Spadaro D, Shah J. Epithelial junctions and Rho family GTPases: the zonular signalosome. Small GTPases. 2014;5(4):1–15. doi: 10.4161/21541248.2014.973760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen W, Zhang M. Protein complex assemblies in epithelial cell polarity and asymmetric cell division. J Mol Biol. 2017;430:3504–3520. [DOI] [PubMed] [Google Scholar]
- 21.Charras G, Yap AS. Tensile forces and mechanotransduction at cell-cell junctions. Curr Biol. 2018;28(8):R445–R457. doi: 10.1016/j.cub.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Spadaro D, Le S, Laroche T, Mean I, Jond L, Yan J, Citi S. Tension-dependent stretching activates ZO-1 to control the junctional localization of its interactors. Curr Biol. 2017;27(24):3783–3795.e8. doi: 10.1016/j.cub.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Hatzfeld M, Keil R, Magin TM. Desmosomes and intermediate filaments: their consequences for tissue mechanics. Cold Spring Harb Perspect Biol. 2017;9(6):a029157. doi: 10.1101/cshperspect.a029157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillemot L, Paschoud S, Pulimeno P, Foglia A, Citi S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta Biomembr. 2008;1778(3):601–613. doi: 10.1016/j.bbamem.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2(2):a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157–165. doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899–a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778(3):562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandai K, Rikitake Y, Mori M, Takai Y. Nectins and nectin-like molecules in development and disease. Curr Top Dev Biol. 2015;112:197–231. doi: 10.1016/bs.ctdb.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16(4):181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17(5):533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- 32.Meza I, Ibarra G, Sabanero M, Martínez-Palomo A, Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980;87:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rassat J, Robenek H, Themann H. Alterations of tight and gap junctions in mouse hepatocytes following administration of colchicine. Cell Tissue Res. 1982;223(1):187–200. [DOI] [PubMed] [Google Scholar]
- 34.Yap AS, Stevenson BR, Abel KC, Cragoe EJ, Manley SW. Microtubule integrity is necessary for the epithelial barrier function of cultured thyroid cell monolayers. Exp Cell Res. 1995;218(2):540–550. doi: 10.1006/excr.1995.1189. [DOI] [PubMed] [Google Scholar]
- 35.Paschoud S, Yu D, Pulimeno P, Jond L, Turner JR, Citi S. Cingulin and paracingulin show similar dynamic behaviour, but are recruited independently to junctions. Mol Membr Biol. 2011;28(2):123–135. doi: 10.3109/09687688.2010.538937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glotfelty LG, Zahs A, Iancu C, Shen L, Hecht GA. Microtubules are required for efficient epithelial tight junction homeostasis and restoration. Am J Physiol Cell Physiol. 2014;307(3):C245–54. doi: 10.1152/ajpcell.00336.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simard-Bisson C, Bidoggia J, Larouche D, Guérin SL, Blouin R, Hirai S-I, Germain L. A role for DLK in microtubule reorganization to the cell periphery and in the maintenance of desmosomal and tight junction integrity. J Invest Dermatol. 2017;137(1):132–141. doi: 10.1016/j.jid.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Hsu C-Y, Lecland N, Pendaries V, Viodé C, Redoulès D, Paul C, Merdes A, Simon M, Bierkamp C. Stabilization of microtubules restores barrier function after cytokine-induced defects in reconstructed human epidermis. J Dermatol Sci. 2018;91(1):87–96. doi: 10.1016/j.jdermsci.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Fesenko I, Kurth T, Sheth B, Fleming TP, Citi S, Hausen P. Tight junction biogenesis in the early Xenopus embryo. Mech Dev. 2000;96:51–65. [DOI] [PubMed] [Google Scholar]
- 40.Li D, Kuehn EW, Prekeris R. Kinesin-2 mediates apical endosome transport during epithelial lumen formation. Cell Logist. 2014;4(1):e28928. doi: 10.4161/cl.28928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangan AJ, Sietsema DV, Li D, Moore JK, Citi S, Prekeris R. Cingulin and actin mediate midbody-dependent apical lumen formation during polarization of epithelial cells. Nat Commun. 2016;7:12426. doi: 10.1038/ncomms12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanov AI, McCall IC, Babbin B, Samarin SN, Nusrat A, Parkos CA. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol. 2006;7:12. doi: 10.1186/1471-2121-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Citi S. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Cell Biol. 1992;117(1):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Citi S, Volberg T, Bershadsky AD, Denisenko N, Geiger B. Cytoskeletal involvement in the modulation of cell-cell junctions by the protein kinase inhibitor H-7. J Cell Sci. 1994;107(3):683–692. [PubMed] [Google Scholar]
- 45.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15(6):2639–2651. doi: 10.1091/mbc.e04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA, Cordes N. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One. 2007;2(7):e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell. 2007;18(9):3429–3439. doi: 10.1091/mbc.e07-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito S, Okuda S, Abe M, Fujimoto M, Onuki T, Nishimura T, Takeichi M. Induced cortical tension restores functional junctions in adhesion-defective carcinoma cells. Nat Commun. 2017;8(1):1834. doi: 10.1038/s41467-017-01945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shultz T, Shmuel M, Hyman T, Altschuler Y. Beta-tubulin cofactor D and ARL2 take part in apical junctional complex disassembly and abrogate epithelial structure. FASEB J. 2008;22(1):168–182. doi: 10.1096/fj.06-7786com. [DOI] [PubMed] [Google Scholar]
- 50.Mary S, Charrasse S, Meriane M, Comunale F, Travo P, Blangy A, Gauthier-Rouvière C. Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol Biol Cell. 2002;13(1):285–301. doi: 10.1091/mbc.01-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Kojima S-I, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163(3):547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teng J, Rai T, Tanaka Y, Takei Y, Nakata T, Hirasawa M, Kulkarni AB, Hirokawa N. The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat Cell Biol. 2005;7(5):474–482. doi: 10.1038/ncb1249. [DOI] [PubMed] [Google Scholar]
- 53.Acharya BR, Espenel C, Libanje F, Raingeaud J, Morgan J, Jaulin F, Kreitzer G. KIF17 regulates RhoA-dependent actin remodeling at epithelial cell-cell adhesions. J Cell Sci. 2016;129(5):957–970. doi: 10.1242/jcs.173674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ligon LA, Holzbaur ELF. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8(7):808–819. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 55.Bellett G, Carter JM, Keynton J, Goldspink D, James C, Moss DK, Mogensen MM. Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil Cytoskeleton. 2009;66(10):893–908. doi: 10.1002/cm.20393. [DOI] [PubMed] [Google Scholar]
- 56.Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Parton RG, Yap AS. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci. 2006;119(Pt 9):1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- 57.Rowning BA, Wells J, Wu M, Gerhart JC, Moon RT, Larabell CA. Microtubule-mediated transport of organelles and localization of beta-catenin to the future dorsal side of Xenopus eggs. Proc Natl Acad Sci U S A. 1997;94(4):1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moss DK, Bellett G, Carter JM, Liovic M, Keynton J, Prescott AR, Lane EB, Mogensen MM. Ninein is released from the centrosome and moves bi-directionally along microtubules. J Cell Sci. 2007;120(Pt 17):3064–3074. doi: 10.1242/jcs.010322. [DOI] [PubMed] [Google Scholar]
- 59.Gavilan MP, Arjona M, Zurbano A, Formstecher E, Martinez-Morales JR, Bornens M, Rios RM, Gumbiner BM. Alpha-catenin-dependent recruitment of the centrosomal protein CAP350 to adherens junctions allows epithelial cells to acquire a columnar shape. PLoS Biol. 2015;13(3):e1002087. doi: 10.1371/journal.pbio.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldspink DA, Rookyard C, Tyrrell BJ, Gadsby J, Perkins J, Lund EK, Galjart N, Thomas P, Wileman T, Mogensen MM. Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres. Open Biol. 2017;7(2):160274. doi: 10.1098/rsob.160274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008;135(5):948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 62.Sako-Kubota K, Tanaka N, Nagae S, Meng W, Takeichi M. Minus end-directed motor KIFC3 suppresses E-cadherin degradation by recruiting USP47 to adherens junctions. Mol Biol Cell. 2014;25(24):3851–3860. doi: 10.1091/mbc.E14-07-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasdar M, Krzeminski KA, Nelson WJ. Regulation of desmosome assembly in MDCK epithelial cells: coordination of membrane core and cytoplasmic plaque domain assembly at the plasma membrane. J Cell Biol. 1991;113:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nekrasova OE, Amargo EV, Smith WO, Chen J, Kreitzer GE, Green KJ. Desmosomal cadherins utilize distinct kinesins for assembly into desmosomes. J Cell Biol. 2011;195(7):1185–1203. doi: 10.1083/jcb.201106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sumigray KD, Chen H, Lechler T. Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J Cell Biol. 2011;194(4):631–642. doi: 10.1083/jcb.201104009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robitaille H, Proulx R, Robitaille K, Blouin R, Germain L. The mitogen-activated protein kinase kinase kinase dual leucine zipper-bearing kinase (DLK) acts as a key regulator of keratinocyte terminal differentiation. J Biol Chem. 2005;280(13):12732–12741. doi: 10.1074/jbc.M411619200. [DOI] [PubMed] [Google Scholar]
- 67.McHarg S, Hopkins G, Lim L, Garrod D, Klymkowsky M. Down-regulation of desmosomes in cultured cells: the roles of PKC, microtubules and lysosomal/proteasomal degradation. PLoS One. 2014;9(10):e108570. doi: 10.1371/journal.pone.0108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 69.Citi S, Sabanay H, Kendrick-Jones J, Geiger B. Cingulin: characterization and localization. J Cell Sci. 1989;93(1):107–122. [DOI] [PubMed] [Google Scholar]
- 70.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23(4):833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porat-Shliom N, Tietgens AJ, Van Itallie CM, Vitale-Cross L, Jarnik M, Harding OJ, Anderson JM, Gutkind JS, Weigert R, Arias IM. Liver kinase B1 regulates hepatocellular tight junction distribution and function in vivo. Hepatology. 2016;64(4):1317–1329. doi: 10.1002/hep.28724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Atri F, Citi S. Cingulin interacts with F-actin in vitro. FEBS Lett. 2001;507:21–24. [DOI] [PubMed] [Google Scholar]
- 73.Ohnishi H, Nakahara T, Furuse K, Sasaki H, Tsukita S, Furuse M. JACOP, a novel plaque protein localizing at the apical junctional complex with sequence similarity to cingulin. J Biol Chem. 2004;279(44):46014–46022. doi: 10.1074/jbc.M402616200. [DOI] [PubMed] [Google Scholar]
- 74.Pulimeno P, Paschoud S, Citi S. A role for ZO-1 and PLEKHA7 in recruiting paracingulin to tight and adherens junctions of epithelial cells. J Biol Chem. 2011;286(19):16743–16750. doi: 10.1074/jbc.M111.230862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279(43):44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- 76.D’Atri F, Nadalutti F, Citi S. Evidence for a functional interaction between cingulin and ZO-1 in cultured cells. J Biol Chem. 2002;277(31):27757–27764. doi: 10.1074/jbc.M203717200. [DOI] [PubMed] [Google Scholar]
- 77.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426(6966):570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 78.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2(8):540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 79.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2(8):531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 80.Mizuno K, Suzuki A, Hirose T, Kitamura K, Kutsuzawa K, Futaki M, Amano Y, Ohno S. Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J Biol Chem. 2003;278(33):31240–31250. doi: 10.1074/jbc.M303593200. [DOI] [PubMed] [Google Scholar]
- 81.Ahmed SM, Macara IG. The Par3 polarity protein is an exocyst receptor essential for mammary cell survival. Nat Commun. 2017;8:14867. doi: 10.1038/ncomms14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmoranzer J, Fawcett JP, Segura M, Tan S, Vallee RB, Pawson T, Gundersen GG. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol. 2009;19(13):1065–1074. doi: 10.1016/j.cub.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen S, Chen J, Shi H, Wei M, Castaneda-Castellanos DR, Bultje RS, Pei X, Kriegstein AR, Zhang M, Shi S-H. Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev Cell. 2013;24(1):26–40. doi: 10.1016/j.devcel.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang T, McKinley RFA, McGill MA, Angers S, Harris TJC. A Par-1-Par-3-Centrosome cell polarity pathway and its tuning for isotropic cell adhesion. Curr Biol. 2015;25(20):2701–2708. doi: 10.1016/j.cub.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 85.Du D, Xu F, Yu L, Zhang C, Lu X, Yuan H, Huang Q, Zhang F, Bao H, Jia L, et al. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell. 2010;18(1):52–63. doi: 10.1016/j.devcel.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 86.Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol. 2000;2(11):797–804. doi: 10.1038/35041037. [DOI] [PubMed] [Google Scholar]
- 87.Dupin I, Camand E, Etienne-Manneville S. Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol. 2009;185(5):779–786. doi: 10.1083/jcb.200812034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shtutman M, Chausovsky A, Prager-Khoutorsky M, Schiefermeier N, Boguslavsky S, Kam Z, Fuchs E, Geiger B, Borisy GG, Bershadsky AD. Signaling function of alpha-catenin in microtubule regulation. Cell Cycle. 2008;7(15):2377–2383. doi: 10.4161/cc.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang P, Senga T, Hamaguchi M. A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene. 2007;26(30):4357–4371. doi: 10.1038/sj.onc.1210217. [DOI] [PubMed] [Google Scholar]
- 90.Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH, O’Toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ, Barth AIM. beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev. 2008;22(1):91–105. doi: 10.1101/gad.1596308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bahmanyar S, Guiney EL, Hatch EM, Nelson WJ, Barth AIM. Formation of extra centrosomal structures is dependent on beta-catenin. J Cell Sci. 2010;123(Pt 18):3125–3135. doi: 10.1242/jcs.064782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3(10):913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 93.Franz CM, Ridley AJ. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279(8):6588–6594. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 94.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004;279(10):9512–9521. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- 95.Ichii T, Takeichi M. p120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells. 2007;12(7):827–839. doi: 10.1111/j.1365-2443.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 96.Shahbazi MN, Megias D, Epifano C, Akhmanova A, Gundersen GG, Fuchs E, Perez-Moreno M. CLASP2 interacts with p120-catenin and governs microtubule dynamics at adherens junctions. J Cell Biol. 2013;203(6):1043–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paschoud S, Jond L, Guerrera D, Citi S. PLEKHA7 modulates epithelial tight junction barrier function. Tissue Barriers. 2014;2(2):e28755. doi: 10.4161/tisb.28755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pulimeno P, Bauer C, Stutz J, Citi S, Arkowitz RA. PLEKHA7 is an adherens junction protein with a tissue distribution and subcellular localization distinct from ZO-1 and E-Cadherin. PLoS One. 2010;5(8):e12207. doi: 10.1371/journal.pone.0012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurita S, Yamada T, Rikitsu E, Ikeda W, Takai Y. Binding between the junctional proteins afadin and PLEKHA7 and implication in the formation of adherens junction in epithelial cells. J Biol Chem. 2013;288(41):29356–29368. doi: 10.1074/jbc.M113.453464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guerrera D, Shah J, Vasileva E, Sluysmans S, Méan I, Jond L, Poser I, Mann M, Hyman AA, Citi S. PLEKHA7 recruits PDZD11 to adherens junctions to stabilize nectins. J Biol Chem. 2016;291(21):11016–11029. doi: 10.1074/jbc.M115.712935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah J, Guerrera D, Vasileva E, Sluysmans S, Bertels E, Citi S. PLEKHA7: cytoskeletal adaptor protein at center stage in junctional organization and signaling. Int J Biochem Cell Biol. 2016;75:112–116. doi: 10.1016/j.biocel.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Franke WW, Cowin P, Schmelz M, Kapprell HP. The desmosomal plaque and the cytoskeleton. Ciba Found Symp. 1987;125:26–48. [DOI] [PubMed] [Google Scholar]
- 103.Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778(3):572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 104.Lechler T, Fuchs E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J Cell Biol. 2007;176(2):147–154. doi: 10.1083/jcb.200609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sumigray KD, Lechler T. Control of cortical microtubule organization and desmosome stability by centrosomal proteins. Bioarchitecture. 2011;1(5):221–224. doi: 10.4161/bioa.18403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sluysmans S, Vasileva E, Spadaro D, Shah J, Rouaud F, Citi S. The role of apical cell-cell junctions and associated cytoskeleton in mechanotransduction. Biol Cell. 2017;109(4):139–161. doi: 10.1111/boc.201600075. [DOI] [PubMed] [Google Scholar]
- 107.Mbom BC, Nelson WJ, Barth A. beta-catenin at the centrosome: discrete pools of beta-catenin communicate during mitosis and may co-ordinate centrosome functions and cell cycle progression. Bioessays. 2013;35(9):804–809. doi: 10.1002/bies.201300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Runkle EA, Sundstrom JM, Runkle KB, Liu X, Antonetti DA. Occludin localizes to centrosomes and modifies mitotic entry. J Biol Chem. 2011;286(35):30847–30858. doi: 10.1074/jbc.M111.262857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273(52):34954–34960. [DOI] [PubMed] [Google Scholar]
- 110.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4(4):294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 111.Chang Y-C, Nalbant P, Birkenfeld J, Chang Z-F, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell. 2008;19(5):2147–2153. doi: 10.1091/mbc.e07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290(3):L540–8. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- 113.Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L837–48. doi: 10.1152/ajplung.00263.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Itoh K, Ossipova O, Sokol SY. GEF-H1 functions in apical constriction and cell intercalations and is essential for vertebrate neural tube closure. J Cell Sci. 2014;127(Pt 11):2542–2553. doi: 10.1242/jcs.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sandí M-J, Marshall CB, Balan M, Coyaud É, Zhou M, Monson DM, Ishiyama N, Chandrakumar AA, La Rose J, Couzens AL, et al. MARK3-mediated phosphorylation of ARHGEF2 couples microtubules to the actin cytoskeleton to establish cell polarity. Sci Signal. 2017;10(503):eaan3286. doi: 10.1126/scisignal.aan3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nie M, Aijaz S, Leefa Chong San IV, Balda MS, Matter K. The Y-box factor ZONAB/DbpA associates with GEF-H1/Lfc and mediates Rho-stimulated transcription. EMBO Rep. 2009;10(10):1125–1131. doi: 10.1038/embor.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cullis J, Meiri D, Sandi MJ, Radulovich N, Kent OA, Medrano M, Mokady D, Normand J, Larose J, Marcotte R, et al. The RhoGEF GEF-H1 is required for oncogenic RAS signaling via KSR-1. Cancer Cell. 2014;25(2):181–195. doi: 10.1016/j.ccr.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 118.Dubois F, Keller M, Calvayrac O, Soncin F, Hoa L, Hergovich A, Parrini M-C, Mazières J, Vaisse-Lesteven M, Camonis J, et al. RASSF1A suppresses the invasion and metastatic potential of human non-small cell lung cancer cells by inhibiting YAP activation through the GEF-H1/RhoB pathway. Cancer Res. 2016;76(6):1627–1640. doi: 10.1158/0008-5472.CAN-15-1008. [DOI] [PubMed] [Google Scholar]
- 119.Birkenfeld J, Nalbant P, Yoon S-H, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008;18:210–219. doi: 10.1016/j.tcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem. 2004;279(18):18392–18400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- 121.Meiri D, Greeve MA, Brunet A, Finan D, Wells CD, LaRose J, Rottapel R. Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation. Mol Cell Biol. 2009;29(21):5963–5973. doi: 10.1128/MCB.01268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12(5):699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8(5):777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Guillemot L, Hammar E, Kaister C, Ritz J, Caille D, Jond L, Bauer C, Meda P, Citi S. Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression. J Cell Sci. 2004;117(Pt 22):5245–5256. doi: 10.1242/jcs.01399. [DOI] [PubMed] [Google Scholar]
- 125.Guillemot L, Citi S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell. 2006;17(8):3569–3577. doi: 10.1091/mbc.e06-02-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guillemot L, Schneider Y, Brun P, Castagliuolo I, Pizzuti D, Martines D, Jond L, Bongiovanni M, Citi S. Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury. J Cell Sci. 2012;125:5005–5012. doi: 10.1242/jcs.101261. [DOI] [PubMed] [Google Scholar]
- 127.Tian Y, Gawlak G, Tian X, Shah AS, Sarich N, Citi S, Birukova AA. Cingulin role in agonist-induced vascular endothelial permeability. J Biol Chem. 2016;291:23681–23692. doi: 10.1074/jbc.M116.720763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raya-Sandino A, Castillo-Kauil A, Domínguez-Calderón A, Alarcón L, Flores-Benitez D, Cuellar-Perez F, López-Bayghen B, Chávez-Munguía B, Vázquez-Prado J, González-Mariscal L. Zonula occludens-2 regulates Rho proteins activity and the development of epithelial cytoarchitecture and barrier function. Biochim Biophys Acta. 2017;1864(10):1714–1733. doi: 10.1016/j.bbamcr.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 129.White EA, Glotzer M. Centralspindlin: at the heart of cytokinesis. Cytoskeleton (Hoboken). 2012;69(11):882–892. doi: 10.1002/cm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170(4):571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Su KC, Takaki T, Petronczki M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev Cell. 2011;21(6):1104–1115. doi: 10.1016/j.devcel.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 132.Breznau EB, Murt M, Blasius TL, Verhey KJ, Miller AL. The MgcRacGAP SxIP motif tethers Centralspindlin to microtubule plus ends in Xenopus laevis. J Cell Sci. 2017;130(10):1809–1821. doi: 10.1242/jcs.195891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14(8):818–828. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guillemot L, Guerrera D, Spadaro D, Tapia R, Jond L, Citi S. MgcRacGAP interacts with cingulin and paracingulin to regulate Rac1 activation and development of the tight junction barrier during epithelial junction assembly. Mol Biol Cell. 2014;25(13):1995–2005. doi: 10.1091/mbc.E13-11-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Breznau EB, Semack AC, Higashi T, Miller AL. MgcRacGAP restricts active RhoA at the cytokinetic furrow and both RhoA and Rac1 at cell-cell junctions in epithelial cells. Mol Biol Cell. 2015;26(13):2439–2455. doi: 10.1091/mbc.E14-11-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coleman PR, Hahn CN, Grimshaw M, Lu Y, Li X, Brautigan PJ, Beck K, Stocker R, Vadas MA, Gamble JR. Stress-induced premature senescence mediated by a novel gene, SENEX, results in an anti-inflammatory phenotype in endothelial cells. Blood. 2010;116(19):4016–4024. doi: 10.1182/blood-2009-11-252700. [DOI] [PubMed] [Google Scholar]
- 137.Chang GHK, Lay AJ, Ting KK, Zhao Y, Coleman PR, Powter EE, Formaz-Preston A, Jolly CJ, Bower NI, Hogan BM, et al. ARHGAP18: an endogenous inhibitor of angiogenesis, limiting tip formation and stabilizing junctions. Small GTPases. 2014;5(3):1–15. doi: 10.4161/21541248.2014.975002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aleskandarany MA, Sonbul S, Surridge R, Mukherjee A, Caldas C, Diez-Rodriguez M, Ashankyty I, Albrahim KI, Elmouna AM, Aneja R, et al. Rho-GTPase activating-protein 18: a biomarker associated with good prognosis in invasive breast cancer. Br J Cancer. 2017;117(8):1176–1184. doi: 10.1038/bjc.2017.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lovelace MD, Powter EE, Coleman PR, Zhao Y, Parker A, Chang GH, Lay AJ, Hunter J, McGrath AP, Jormakka M, et al. The RhoGAP protein ARHGAP18/SENEX localizes to microtubules and regulates their stability in endothelial cells. Mol Biol Cell. 2017;28(8):1066–1078. doi: 10.1091/mbc.E16-05-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Janke C, Montagnac G. Causes and consequences of microtubule acetylation. Curr Biol. 2017;27(23):R1287–R1292. doi: 10.1016/j.cub.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 141.van Haren J, Boudeau J, Schmidt S, Basu S, Liu Z, Lammers D, Demmers J, Benhari J, Grosveld F, Debant A, et al. Dynamic microtubules catalyze formation of navigator-TRIO complexes to regulate neurite extension. Curr Biol. 2014;24(15):1778–1785. doi: 10.1016/j.cub.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 142.Timmerman I, Heemskerk N, Kroon J, Schaefer A, van Rijssel J, Hoogenboezem M, van Unen J, Goedhart J, Gadella TWJ, Yin T, et al. A local VE-cadherin and Trio-based signaling complex stabilizes endothelial junctions through Rac1. J Cell Sci. 2015;128(18):3514. doi: 10.1242/jcs.179424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yano T, Yamazaki Y, Adachi M, Okawa K, Fort P, Uji M, Tsukita S, Tsukita S. Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling. J Cell Biol. 2011;193(2):319–332. doi: 10.1083/jcb.201009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gumbiner BM. Carcinogenesis: a balance between beta-catenin and APC. Curr Biol. 1997;7(7):R443–6. [DOI] [PubMed] [Google Scholar]
- 145.Munemitsu S, Souza B, Müller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54(14):3676–3681. [PubMed] [Google Scholar]
- 146.Zumbrunn J, Kinoshita K, Hyman AA, Näthke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol. 2001;11(1):44–49. [DOI] [PubMed] [Google Scholar]
- 147.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJS, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6(9):820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 148.Morrison EE. The APC-EB1 interaction. Adv Exp Med Biol. 2009;656:41–50. [DOI] [PubMed] [Google Scholar]
- 149.Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7(6):871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 150.Rosin-Arbesfeld R, Ihrke G, Bienz M. Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. Embo J. 2001;20(21):5929–5939. doi: 10.1093/emboj/20.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Langford KJ, Askham J, Lee T, Adams M, Morrison E. Examination of actin and microtubule dependent APC localisations in living mammalian cells. BMC Cell Biol. 2006;7:3. doi: 10.1186/1471-2121-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu Y, Griffin EE. Regulation of Cell Polarity by PAR-1/MARK Kinase. Curr Top Dev Biol. 2017;123:365–397. doi: 10.1016/bs.ctdb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81(4):611–620. [DOI] [PubMed] [Google Scholar]
- 154.Hurov J, Piwnica-Worms H. The Par-1/MARK family of protein kinases: from polarity to metabolism. Cell Cycle. 2007;6(16):1966–1969. doi: 10.4161/cc.6.16.4576. [DOI] [PubMed] [Google Scholar]
- 155.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89(2):297–308. [DOI] [PubMed] [Google Scholar]
- 156.Kuhns S, Schmidt KN, Reymann J, Gilbert DF, Neuner A, Hub B, Carvalho R, Wiedemann P, Zentgraf H, Erfle H, et al. The microtubule affinity regulating kinase MARK4 promotes axoneme extension during early ciliogenesis. J Cell Biol. 2013;200(4):505–522. doi: 10.1083/jcb.201206013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14(16):1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 158.Cohen D, Brennwald PJ, Rodriguez-Boulan E, Müsch A. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J Cell Biol. 2004;164(5):717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cohen D, Tian Y, Musch A. Par1b promotes hepatic-type lumen polarity in Madin Darby canine kidney cells via myosin II- and E-cadherin-dependent signaling. Mol Biol Cell. 2007;18(6):2203–2215. doi: 10.1091/mbc.e07-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ducharme NA, Hales CM, Lapierre LA, Ham A-JL, Oztan A, Apodaca G, Goldenring JR. MARK2/EMK1/Par-1Balpha phosphorylation of Rab11-family interacting protein 2 is necessary for the timely establishment of polarity in Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17(8):3625–3637. doi: 10.1091/mbc.e05-08-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cohen D, Fernandez D, Lázaro-Diéguez F, Müsch A. The serine/threonine kinase Par1b regulates epithelial lumen polarity via IRSp53-mediated cell-ECM signaling. J Cell Biol. 2011;192(3):525–540. doi: 10.1083/jcb.201007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamahashi Y, Saito Y, Murata-Kamiya N, Hatakeyama M. Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J Biol Chem. 2011;286(52):44576–44584. doi: 10.1074/jbc.M111.267021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li X, Thome S, Ma X, Amrute-Nayak M, Finigan A, Kitt L, Masters L, James JR, Shi Y, Meng G, et al. MARK4 regulates NLRP3 positioning and inflammasome activation through a microtubule-dependent mechanism. Nat Commun. 2017;8:15986. doi: 10.1038/ncomms15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Heidary Arash E, Shiban A, Song S, Attisano L. MARK4 inhibits Hippo signaling to promote proliferation and migration of breast cancer cells. EMBO Rep. 2017;18(3):420–436. doi: 10.15252/embr.201642455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Werner ME, Mitchell JW, Putzbach W, Bacon E, Kim SK, Mitchell BJ. Radial intercalation is regulated by the Par complex and the microtubule-stabilizing protein CLAMP/Spef1. J Cell Biol. 2014;206(3):367–376. doi: 10.1083/jcb.201312045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim SK, Zhang S, Werner ME, Brotslaw EJ, Mitchell JW, Altabbaa MM, Mitchell BJ. CLAMP/Spef1 regulates planar cell polarity signaling and asymmetric microtubule accumulation in the Xenopus ciliated epithelia. J Cell Biol. 2018;217(5):1633–1641. doi: 10.1083/jcb.201706058. [DOI] [PMC free article] [PubMed] [Google Scholar]


