Abstract
The somatic activation of PI3K/AKT pathway mutations, PIK3CA and AKT1, and ESR1 mutations in plasma cell-free DNA (cfDNA) has been studied as a non-invasive procedure to quickly assess and monitor disease progression or therapeutic effect in breast cancer (BC) patients, but the clinical significance of these mutations in late treatment lines (TLs) remains unclear. The subjects of this study were a total of 251 plasma samples from 128 estrogen receptor-positive (ER+) BC patients. Of these plasma samples, 133 were from 73 primary BC (PBC) patients, and 118 plasma samples were from 68 metastatic BC (MBC) patients. We developed droplet digital PCR (ddPCR) assays to verify the clinical significance of PIK3CA, AKT1, and ESR1 mutations in these patients. cfDNA PIK3CA mutations were observed in 15.1% of the PBC patients, while a cfDNA AKT1 mutation was observed in 1.4% of patients, and cfDNA ESR1 mutations were observed in 2.7% of patients. Patients with detectable cfDNA PIK3CA mutations were not associated with clinical outcomes. According to the TL, the prevalence of the PIK3CA and ESR1 mutations in cfDNA were lower in early TLs compared with late TLs. In the early TL group, patients with cfDNA PIK3CA mutations had a shorter time to treatment failure (TTF) than patients without mutations (P = 0.035). However, there was no statistically significant difference between patients with or without cfDNA ESR1 mutations. However, in the late TL group, patients with cfDNA ESR1 mutations had a shorter TTF than patients without mutations (P = 0.048). However, there was no statistically significant difference between patients with or without cfDNA PIK3CA mutations. Since the prevalence of cfDNA AKT1 mutation is low in both PBC and MBC patients, the impact of AKT1 mutations on the prognosis remains unclear. We have demonstrated the difference in the clinical significance of the hotspot PIK3CA, AKT1, and ESR1 mutations in cfDNA for each TL in ER+ BC patients.
Electronic supplementary material
The online version of this article (10.1186/s12943-018-0808-y) contains supplementary material, which is available to authorized users.
Keywords: Estrogen receptor-positive breast cancer, Cell-free DNA, PIK3CA mutations, AKT1 mutation, ESR1 mutations
Endocrine therapy (ET) resistance occasionally occurs during the treatment of primary breast cancer (PBC) and inevitably results in metastatic BC (MBC). Recently, the focused mechanisms of ET resistance include hyperactivation of PI3K/AKT pathway. Importantly, somatic activating mutations of PIK3CA and AKT1 affect the magnitude of PI3K/AKT activation [1]. Meta-analysis of the BC literature shows that the PIK3CA mutation is present in 20%-40% of all BCs, making this gene the second most frequently mutated in BC, with most mutations being expressed in ‘hotspots’ in the helical domain (exon (Ex) 9) or the catalytic domain (Ex20) [2]. A somatic mutation in the plekstrin homology domain of AKT1:p.Glu17Lys is found in approximately 4% of breast tumors [3]. Conversely, recent evidence describing next generation sequencing showed that another key potential mechanism of the failure of ET involves activating mutations in the ligand-binding domain of the ESR1 gene [4]. Those mutations cause ligand-independent estrogen receptor (ER) transcriptional activity that does not respond to endocrine manipulation. The estimated frequency from comprehensive studies was 20%-50% for ESR1 mutations in MBC but that were minimally present in PBC. The potential of using PI3K/AKT pathway mutations and ESR1 mutations as biomarkers to predict clinical response of ET is currently the focus of many preclinical and clinical studies such as the BOLERO-2 cohort [5]. Additionally, recent digital PCR assays on plasma cell-free DNA (cfDNA) of several cohorts demonstrated the difference in the clinical features between the representative hotspot mutations in MBC, PIK3CA and ESR1 mutations [6–10]. In the BOLERO-2 study, Chandarlapaty and colleagues demonstrated that progression free survival (PFS) benefit of mammalian target of rapamycin (mTOR) inhibitor everolimus was maintained irrespective of PIK3CA mutations, but that was decreased according to the presence of ESR1 mutations [6, 7]. In another two phase III randomized trials, Fribbens and colleagues reported the effectiveness of the target drug by having the mutations or not. In the SoFEA study, fulvestrant improved PFS of patients with ESR1 mutations compared to exemestane. Meanwhile, in the PALOMA3 study, fulvestrant plus the CDK4/6-inhibitor palbociclib improved PFS regardless of the genomic status of ESR1 or PIK3CA [8, 9]. We demonstrated the clinical significance of the burden of on-treatment hotspot ESR1 mutations, both in a snapshot and serially in MBC patients in comparison with PIK3CA hotspot mutation status [10]. Although these studies revealed the clinical significance of the target mutations in the early treatment lines (TLs), the clinical significance of PIK3CA, AKT1, and ESR1 mutations in late line treatment in ER+ BC patients is still controversial. In this retrospective study, we demonstrated the clinical significance of the burden of on-treatment hotspot mutations: PIK3CA Ex9:p.Glu542Lys/Val, Glu545Val/Gly/Ala/Gln/Lys and Gln546Leu/Arg/Pro/Glu/Lys and PIK3CA Ex20:p.His1047Leu/Arg/Tyr and Gly1049Arg/Ser and AKT1:p.Glu17Lys, and ESR1:p.Tyr537Ser/Asn and Asp538Gly in cfDNA in comparison with ER+ PBC patients in each TL of ER+ MBC patients using multiplex droplet digital PCR (ddPCR) assays.
Results and discussion
A total of 128 patients (251 plasma samples) with breast carcinoma who had an ECOG Performance scale status of 0 or 1 were enrolled in this study. The participants were comprised of 73 women (133 plasma samples) with PBC and 68 women (118 plasma samples) with MBC. Of these participants, 13 women had plasma samples with both PBC and MBC (Additional file 1: Table S1). Plasma PIK3CA mutations were found in 15.1% (11/73) of the PBC patients, the plasma AKT1 mutation was detected in 1.4% (1/73), and the plasma ESR1 mutations were observed in 2.7% (2/73) (Additional file 1: Table S2). One patient had co-mutations (PIK3CA mutations and AKT1 mutation). This finding agrees with previous studies in which PIK3CA mutations are particularly common, whereas AKT1 and ESR1 mutations occur less frequently in PBC patients [3–5]. The presence of plasma PIK3CA mutations were statistically associated with invasive lobular carcinoma (P = 0.036) and marginally associated with histological grade III carcinoma (P = 0.059) (Additional file 1: Table S3). In the PBC group for recurrence free survival and breast cancer specific survival analyses, the patients were not stratified for the PIK3CA mutation status (Fig. 1a, b and Additional file 1: Tables S4, S5). In general, among the ER+ PBC patients, the PIK3CA mutations had varying clinical outcomes, but they were associated with a favorable prognosis [2]. Due to the difference in the clinical effect of PIK3CA mutations in the helical versus kinase domains, the prognostic effect may not be consistent. This finding suggests that the presence of Ex9 mutations predicted a more favorable outcome, whereas the presence of the Ex20 mutations predicted a relatively poor prognosis [2]. In this study, we could not evaluate the difference in the prognosis for each PIK3CA mutation, since PIK3CA Ex20 mutations were observed in 90.9% (10/11) of patients with PIK3CA mutations, and PIK3CA Ex9 and Ex20 mutations were observed in 10.1% (1/11) of patients with PIK3CA mutations (Additional file 1: Table S2). Interestingly, out of all of the patients, two patients with plasma ESR1 mutations recurred. Investigations on the progression or survival by ESR1 mutations in PBC have not been conducted and are highly desirable. Rudolph and colleagues reported that initial follow-up survival data suggests that the AKT1:p.Glu17Lys mutant could be associated with increased mortality [3]; however, none of the two patients with plasma AKT1 mutation in this study showed recurrence.
Fig. 1.
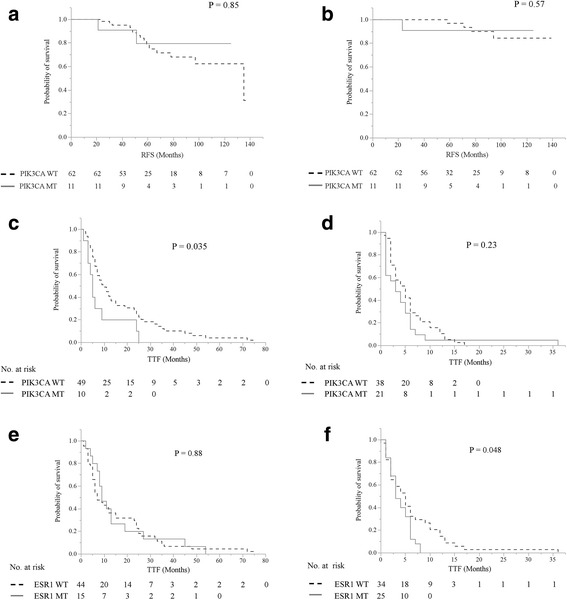
Kaplan-Meier plots of the association of the presence of cfDNA PIK3CA mutations with RFS; a and BCSS; b in the entire cohort and the presence of cfDNA PIK3CA mutations with TTF in less than the 5th line; c more than the 5th line; d, and the presence of cfDNA ESR1 mutations with TTF in less than the 5th line; e and more than the 5th line; f in the entire cohort. The presence of cfDNA PIK3CA mutations and cfDNA ESR1 mutations were defined as either positive or negative. Abbreviations: cfDNA, cell-free DNA; RFS, relapse-free survival; BCSS, breast cancer-specific survival; TTF, time to treatment failure
In the total TL analysis, as the TL increased, the frequency of PIK3CA and ESR1 mutations increased due to the accumulation of genetic alterations. PIK3CA and ESR1 mutations were specifically found to be lower in the 4th TL or less (PIK3CA vs ESR1 mutations: 16.7% vs 25% in the 1st/2nd line and 17% vs 26% in the 3rd/4th line), compared with those in more than the 5th TL (PIK3CA vs ESR1 mutations: 39% vs 43% in the 5th–7th line and 32% vs 42% in the 8th line or more) (Fig. 2a). In the ET line analysis of the MBC patients, PIK3CA, AKT1, and ESR1 mutations were found to be highest in the 4th/5th line (PIK3CA mutations: 39% (11/28), AKT1 mutation: 7.1% (2/28), and ESR1 mutations: 61% (17/28)) (Fig. 2b). In the chemotherapy line analysis of the MBC patients, PIK3CA and ESR1 mutations were found to be highest in the 6th line or more (PIK3CA mutations: 36% (4/11) and ESR1 mutations: 64% (7/11)) (Fig. 2c). To the best of our knowledge, this result that PIK3CA and ESR1 mutations were more frequent in the late TL is a new finding. Of these mutations, ESR1 mutations were slightly more frequent than PIK3CA mutations. Meanwhile, plasma AKT1 mutation was rare in MBC and it did not correlate with the TL (3.6% in the 5th–7th line and 3.2% in the 8th line or more) (Fig. 2). AKT1 mutation coexisted with PIK3CA mutations in one MBC patient.
Fig. 2.
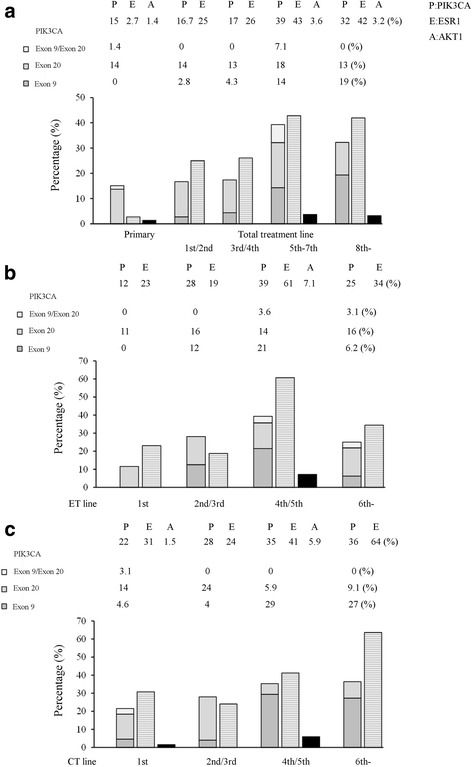
Distributions of PIK3CA, AKT1, and ESR1 mutations according to each TL in ER-positive breast cancer. The bar graphs show PIK3CA mutations (gray monochrome), ESR1 mutations (gray horizontal stripes), and AKT1 mutation (black monochrome) from the left in each category. a The subgroup in the primary and each TL (the 1st/2nd line, the 3rd/4th line, the 5th–7th line, and the 8th line or more line) were omitted. The subgroup in each endocrine therapy line; b, and in each chemotherapy line; c, (the 1st line, the 2nd/3rd line, the 4th/5th line, and the 6th line or more line) were omitted. Abbreviations: ER, estrogen receptor; P, PIK3CA mutations; E, ESR1 mutations; A, AKT1 mutation
Next, we analyzed whether PIK3CA and ESR1 mutations detected in cfDNA were associated with the differential benefit of time to treatment failure (TTF) of the early or late TL (Fig. 1c–f). By examining the difference in the TTF of the early or late TL with cfDNA PIK3CA and ESR1 mutations, we could create subgroups of patients whose TL was either less than or more than the 5th line, since patients in less than the 5th line had a longer TTF than patients in more than the 5th line (P < 0.0001) (Additional file 1: Figure S1). Since the prevalence of the AKT1 mutation is low in both PBC and MBC patients, the impact of AKT1 mutation on prognosis remains unclear. Our data indicated that patients with cfDNA PIK3CA mutations had a shorter TTF than patients without mutations in the early TL group (P = 0.035), but there were no statistically significant differences in the patients with or without cfDNA PIK3CA mutations (P = 0.23) in the late TL group (Fig. 1c, d). Paradoxically, as the TL advanced, there was a tendency for PIK3CA Ex9 mutations, a more favorable mutation, to increase. PIK3CA Ex20 mutations, a more aggressive mutation, were expressed stably over the course of treatment (Fig. 2). Meanwhile, there was no statistically significant differences in patients with cfDNA ESR1 mutations or not in the early TL group (P = 0.88), but patients with cfDNA ESR1 mutations had a shorter TTF than patients without mutations in the late TL group (P = 0.048) (Fig. 1e, f). Additionally, as the TL advanced, there was a tendency for patients treated with ET to decrease (83% in the 1st/2nd line, 74% in the 3rd/4th line, 64% in the 5th–7th line, and 68% in the 8th line or more) (Additional file 1: Figure S2A) and there was a tendency for the best overall response for the treatment to be worse (the frequency of progressive disease: 33% in the 1st/2nd line, 30% in the 3rd/4th line, 50% in the 5th–7th line, and 45% in the 8th line or more) (Additional file 1: Figure S2B). These results suggested the presence of plasma ESR1 mutations, which may be relevant for the choice of treatment of the ER+ MBC patients even in the higher TLs.
Recently, potential interest in the mutations accompanying disease progression or treatment has been increasing, since they may be a clue for the further treatment. Our data indicated that of the 65 PBC patients treated with neoadjuvant therapy, 9 patients (13.8%) acquired or maintained PIK3CA mutations, but 8 patients (12.3%) lost PIK3CA mutations after neo-adjuvant therapy. Meanwhile, one patient (1.5%) lost AKT1 mutation and one patient (1.5%) acquired ESR1 mutations after neo-adjuvant therapy. Of the 13 BC patients with both primary and recurrence blood samples, 6 patients (46.2%) acquired or maintained PIK3CA mutations, but 1 patient (7.7%) lost PIK3CA mutations during relapse. Meanwhile, 4 patients (30.8%) acquired or maintained ESR1 mutations during relapse (Table 1). These results were very interesting, but we could not determine their clinical relevance due to the small sample size.
Table 1.
Details of the change of PIK3CA mutations, AKT1 mutation, and ESR1 mutations
| No. of patients (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changes of the mutations due to neo-adjuvant therapies (N = 65) | Changes of the mutations on relapse (N = 13) | ||||||||||||||
| PIK3CA | E542x/ G1049x →H1047x |
WT→ E542x /E545x, Q546x |
WT→ H1047x |
H1047x/ G1049x →WT |
H1047x →WT |
N/A→ H1047x |
N/A →WT |
WT →WT |
H1047x→ H1047x |
WT→ E542x/ E545x, Q546x /H1047x |
WT→ E545x, Q546x |
WT→ H1047x |
WT→ G1049x |
H1047x→WT | WT →WT |
| 1 (1.5) | 2 (3.1) | 6 (9.2) | 1 (1.5) | 7 (10.8) | 1 (1.5) | 4 (6.1) | 43 (66.1) | 1 (7.7) | 1 (7.7) | 1 (7.7) | 2 (15.4) | 1 (7.7) | 1 (7.7) | 6 (46.1) | |
| AKT1 | E17K→ WT |
N/A→ WT |
WT→ WT |
WT→WT | |||||||||||
| 1 (1.5) | 5 (7.7) | 59 (90.8) | 13 (100) | ||||||||||||
| ESR1 | N/A→ Y537S /Y537N /D538G |
N/A→ WT |
WT→ WT |
Y537S/ Y537N /D538G→ Y537S /Y537N /D538G |
WT→Y537S | WT→ D538G |
WT→ WT |
||||||||
| 1 (1.5) | 4 (6.1) | 60 (92.3) | 1 (7.7) | 1 (7.7) | 2 (15.4) | 9 (69.2) | |||||||||
Abbreviations: WT wild-type, N/A not available, E542x E542K/V, E545x Q546x E545V/G/A/Q/K Q546L/R/P/E/K, H1047x H1047L/R/Y, G1049x, G1049R/S
Conclusions
Our study demonstrates the difference in the clinical significance of PIK3CA, AKT1, and ESR1 hotspot mutations for each TL in ER+ BC patients using multiplex ddPCR assays. To the best of our knowledge, limited data exist on whether the detection of these mutations may be useful as a biomarker for predicting the effect of late line treatment.
Additional file
Methods and Supplementary information. (DOCX 407 kb)
Acknowledgements
Not applicable
Funding
This work was supported in part by a grant-in-aid (project numbers 17 K16511 and 15 K10057) for scientific research from the Ministry of Education, Science and Culture of Japan.
Availability of data and materials
Please contact the corresponding author for all data requests.
Abbreviations
- cfDNA
cell-free DNA
- ddPCR
droplet dPCR
- ER
Estrogen receptor
- ET
Endocrine therapy
- Ex
Exon
- MBC
Metastatic BC
- PBC
Primary breast cancer
- PFS
Progression free survival
- TL
Treatment line
- TTF
Time to treatment failure
Authors’ contributions
The study was conceived by TT and HI. TT performed ddPCR assay, generated and assembled the data. TT, YY, MY-I, MT, AS, KM, and HI developed and performed the data analysis and wrote the manuscript. All authors have read and approved of the final version of this manuscript.
Ethics approval and consent to participate
The Ethics Committee of Kumamoto University Graduate School of Medicine (Kumamoto, Japan) approved the study protocol (#1857). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
All of the authors declare that they have no actual, potential, or perceived conflict of interest regarding the manuscript submitted for review.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takashi Takeshita, Email: henamon@gmail.com.
Yutaka Yamamoto, Email: ys-yama@triton.ocn.ne.jp.
Mutsuko Yamamoto-Ibusuki, Email: mibusuki@kumamoto-u.ac.jp.
Mai Tomiguchi, Email: toma5889@yahoo.co.jp.
Aiko Sueta, Email: aikos0214@yahoo.co.jp.
Keiichi Murakami, Email: keiichimurakami.surgeon@gmail.com.
Hirotaka Iwase, Phone: +81-96-373-5521, Email: hiwase@kumamoto-u.ac.jp.
References
- 1.Loi S, Haibe-Kains B, Majjaj S, Lallemand F, Durbecq V, Larsimont D, Gonzalez-Angulo AM, Pusztai L, Symmans WF, Bardelli A, et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci U S A. 2010;107:10208–10213. doi: 10.1073/pnas.0907011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirican E, Akkiprik M, Ozer A. Mutation distributions and clinical correlations of PIK3CA gene mutations in breast cancer. Tumour Biol. 2016;37:7033–7045. doi: 10.1007/s13277-016-4924-2. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph M, Anzeneder T, Schulz A, Beckmann G, Byrne AT, Jeffers M, Pena C, Politz O, Kochert K, Vonk R, Reischl J. AKT1 (E17K) mutation profiling in breast cancer: prevalence, concurrent oncogenic alterations, and blood-based detection. BMC Cancer. 2016;16:622. doi: 10.1186/s12885-016-2626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus L, Beije N, Jager A, Martens JW, Sleijfer S. ESR1 mutations: moving towards guiding treatment decision-making in metastatic breast cancer patients. Cancer Treat Rev. 2017;52:33–40. doi: 10.1016/j.ctrv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA, 3rd, Pritchard KI, Campone M, Noguchi S, Perez AT, Deleu I, et al: Correlative analysis of genetic alterations and Everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol 2016, 34:419–426. [DOI] [PMC free article] [PubMed]
- 6.Moynahan ME, Chen D, He W, Sung P, Samoila A, You D, Bhatt T, Patel P, Ringeisen F, Hortobagyi GN, et al. Correlation between PIK3CA mutations in cell-free DNA and everolimus efficacy in HR+, HER2- advanced breast cancer: results from BOLERO-2. Br J Cancer. 2017;116:726–730. doi: 10.1038/bjc.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, Bhatt T, Patel P, Voi M, Gnant M, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016;2:1310–1315. doi: 10.1001/jamaoncol.2016.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 9.Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, Cristofanilli M, Andre F, Loi S, Loibl S, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34:2961–2968. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 10.Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, Tomiguchi M, Sueta A, Murakami K, Omoto Y, Iwase H. Analysis of ESR1 and PIK3CA mutations in plasma cell-free DNA from ER-positive breast cancer patients. Oncotarget. 2017;8:52142–52155. doi: 10.18632/oncotarget.18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods and Supplementary information. (DOCX 407 kb)
Data Availability Statement
Please contact the corresponding author for all data requests.


