Abstract
Background
Taenia solium, T. asiatica and T. saginata tapeworms cause human taeniasis and are the origin of porcine and bovine cysticercosis. Furthermore, T. solium eggs can cause human cysticercosis, with neurocysticercosis being the most serious form of the disease. These helminth infections are neglected tropical diseases and are endemic in several countries in the Americas, Asia and Africa. As a result of globalization, migration in particular, the infections have been extending to non-endemic territories. Species-specific diagnosis of taeniasis is subject to drawbacks that could be resolved using molecular approaches. In the present study, conventional and real-time amplification protocols (cPCR and qPCR) based on the T. saginata HDP2 sequence were applied in the differential diagnosis of taeniasis (T. saginata, T. solium) in both fecal samples and proglottids expelled by patients. The HDP2 homolog in T. solium was cloned and characterized.
Results
Semi-nested cPCR and qPCR (Sn-HDP2 cPCR and Sn-HDP2 qPCR) amplified T. saginata and T. solium DNA, with an analytical sensitivity of 40 and 400 fg, respectively, and identically in both protocols. Eighteen taeniasis patients were diagnosed directly with T. saginata or T. solium, either from proglottids or fecal samples with/without eggs (detected using microscopy), based on the optimized Sn-HDP2 qPCR. After cloning, the T. solium HDP2 homolog sequence was confirmed to be a ribosomal sequence. The HDP2 fragment corresponded to a non-transcribed sequence/external transcribed repeat (NTS/ETS) of ribosomal DNA. Compared with the T. saginata HDP2 homolog, the T solium HDP2 sequence lacked the first 900 nt at the 5′ end and showed nucleotide substitutions and small deletions.
Conclusions
Sn-HDP2 cPCR and Sn-HDP2 qPCR were set up for the diagnosis of human taeniasis, using proglottids and fecal samples from affected patients. The new Sn-HDP2 qPCR protocol was the best option, as it directly differentiated T. saginata from T. solium. The diagnosis of an imported T. solium-taeniasis case and nine European T. saginata cases was relevant. Finally, the cloning and sequencing of the T. solium HDP2 fragment confirmed that HDP2 was part of a ribosomal unit.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2646-6) contains supplementary material, which is available to authorized users.
Keywords: Taeniasis, Taenia solium, Taenia saginata, Diagnosis, cPCR, qPCR, Ribosomal DNA
Background
Taenia solium, T. asiatica and T. saginata tapeworms cause human taeniasis and are the origin of porcine and bovine cysticercosis [1]. In addition, T. solium eggs can cause cysticercosis in humans, with neurocysticercosis being the most serious form of the disease [2]. These helminth infections are neglected tropical diseases and are endemic in several countries in the Americas, Asia and Africa [2, 3]. As a result of globalization, human migration in particular, taeniasis and cysticercosis have been extending to other regions where they had been eliminated during the 20th century [4].
The impact of taeniasis/cysticercosis on health and socio-economic development in endemic regions is significant enough that several initiatives have been undertaken to control them [5, 6]. Sensitive and specific diagnostic tools are therefore of considerable importance in aiding these initiatives [7]. Species-specific identification of Taenia spp. is a fundamental component of the diagnosis of taeniasis, and suitable treatment and prevention of the transmission of cysticercosis play a key role, as human T. solium carriers are the origin of cysticercosis. In this regard, molecular techniques are excellent alternatives that complement immunodiagnosis and traditional parasitological methods, which are affected by specificity (e.g. Taenia coproantigen ELISA) and specificity/sensitivity (e.g. Taenia eggs and microscopy) [1]. The supporting information (Additional file 1: Table S1; Additional file 2: Table S2) summarizes the molecular targets used in the identification of Taenia spp. The targets include mitochondrial and ribosomal DNA, repetitive DNA sequences and/or genes encoding relevant antigens. Based upon these sequences, we investigated the T. saginata HDP2 repetitive sequence [8], which hybridized differentially with DNA from T. saginata (3 ng) and T. solium (25 ng) [8]. The T. saginata HDP2 fragment was sequenced and further characterized [9], and it was found that the 5′ end of HDP2 (5PHDP2, approximately 1000 bp) was not present in the homologous sequence of T. solium. The information extracted was used to design cPCRs, which were in turn used for species-specific identification of the taeniid [9, 10], and for the detection of T. solium DNA in cerebrospinal fluid from neurocysticercosis patients [11]. Next, several T. saginata and T. asiatica isolates were analyzed, and it was confirmed that the HDP2 fragment was polymorphic owing to a variable representation of partially repeated sequences, rather than to nucleotide sequence divergence, thus proving its relevance as a diagnostic target [12, 13]. Prior to this study, the PCRs derived from HDP2 had not been applied to samples from taeniasis patients. In addition, the homolog of HDP2 in T. solium had not been characterized.
In the present study, both Sn-HDP2 cPCR and Sn-HDP2 qPCR were performed based on the T. saginata HDP2 sequence, and their analytical sensitivities were determined using purified T. saginata and T. solium genomic DNA (gDNA). These protocols were applied to carry out the species-specific diagnosis of taeniasis in both fecal samples and proglottids expelled by infected humans. Furthermore, the HDP2 homologous sequence from T. solium was cloned and characterized.
Methods
Parasite material
A frozen T. solium adult was provided by Professor Elizabeth Ferrer, Universidad de Carabobo, Valencia, Venezuela. Taenia solium cysticerci were a gift from Professor Edda L. Sciutto, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México DF, México. A T. saginata adult was supplied by Dr Sabino Puente Puente, Hospital La Paz-Carlos III, Madrid, Spain. The parasitic material was stored at -80 °C until use; genomic DNA (gDNA) from T. saginata and T. solium samples was isolated as described elsewhere [14].
Clinical samples
The study samples comprised 8 fecal samples and 16 proglottids (fragmented or non-preserved, unidentifiable by morphology) from 18 individuals with taeniasis (Table 1). In the case of stool, 1–3 serial samples per patient were used. The clinical samples belonged to collection C.0003989, which is registered at the ISCIII Biobank, and were managed following current ethical recommendations, according to Spanish Royal Decree 1716/2011 about the requirements of biobanks in biomedical research.
Table 1.
Species-specific diagnosis of human taeniosis using Sn-HDP2 cPCRs and Sn-HDP2 qPCR. In serial fecal samples, the comparison of both PCR and microcopy observation (MO) results is included
| Case | Origin | Sex | Age (yrs) | Species | Proglottids | Fecal samples | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sn HDP2 cPCRa | Sn HDP2 qPCR | Sn HDP2 cPCRa & qPCR | MO | |||||||||
| 1 | 3 | 3 | 1 | 2 | 3 | |||||||
| 1 | Spain | Female | 18 | T. saginata | + | + | ||||||
| 2 | France | Male | 47 | T. saginata | + | + | ||||||
| 3 | Spain | Female | 34 | T. saginata | + | + | + | + | – | – | ||
| 4 | Spain | Male | 33 | T. saginata | + | + | ||||||
| 5 | Spain | Male | 37 | T. saginata | + | + | ||||||
| 6 | Spain | Female | 29 | T. saginata | + | + | ||||||
| 7 | Spain | Female | 32 | T. saginata | + | + | ||||||
| 8 | Spain | Female | 33 | T. saginata | + | + | + | + | ||||
| 9 | Spain | Female | 84 | T. saginata | + | + | ||||||
| 10 | Paraguay | Female | 48 | T. saginata | + | + | + | + | + | + | – | – |
| 11 | Bolivia | Female | 29 | T. saginata | + | + | ||||||
| 12 | Nicaragua | Female | 37 | T. saginata | + | + | ||||||
| 13 | Colombia | Female | 43 | T. solium | + | + | ||||||
| 14 | Ethiopia | Female | 65 | T. saginata | + | + | ||||||
| 15 | Unknown | Male | 35 | T. saginata | + | + | ||||||
| 16 | Unknown | Female | 34 | T. saginata | + | + | ||||||
| 17 | Unknown | Male | 15 | T. saginata | + | + | ||||||
| 18 | Unknown | Male | 47 | T. saginata | + | + | ||||||
aSn-HDP2 cPCR amplicons were sequenced
Key: + positive; − negative
Prior to DNA extraction, 1 g of each stool sample was suspended in 8 ml of saline solution and concentrated using Bioparapred-Midicolumns (Leti Diagnostics, Barcelona, Spain). The supernatants were then discarded, and 200 mg of the pellets was used for total DNA extraction [15]. DNA from serial fecal samples and proglottids was purified using QIAamp DNA extraction kits (QIAamp Fast DNA Stool Mini Kit and QIAamp DNA Mini Kit, respectively) (Qiagen, Venlo, the Netherlands), following the manufacturer’s recommendations. DNA samples were eluted in 200 μl of water.
Microscopy analysis
Clinical samples were analyzed by optical microscopy according to standard protocols [16].
Amplification protocols (PCR): Sn-HDP2 cPCR and Sn-HDP2 qPCR
The Sn-HDP2 cPCR used F2,2 (5′-CTT CTC AAT TCT AGT CGC TGT GGT CAG-3′) and R10 (5′-GAG GAA TAG ATG GAT GAA GGG-3′) as primers for the first amplification and F2,2 and R1m (5′-GAC GAA GAA TGG AGT TGA AGG-3′) as primers for the second amplification. The cPCR was performed as previously described [11]. The first cPCR reaction was performed using 40 μl of fecal DNA, or 15 μl of proglottid DNA as template. Negative and positive controls were performed for each assay. For the second amplification, 2 μl of a 1:500 dilution in water from the first amplification was used. Amplicons were sequenced, as described below.
The primers used in Sn-HDP2 qPCR were the same as for Sn-HDP2 cPCR. The first reaction was performed in a total volume of 20 μl, containing 2 μl of 10× LightCycler® FastStart DNA Master SYBR Green I (Roche Diagnostics, Barcelona, Spain). In addition, 1 μl of each primer at 10 μM, DNA template (15 μl fecal DNA plus 1 μl of sterile water and 5 μl of DNA template from proglottids plus 11 μl of sterile water). Negative and positive controls were performed for each assay. The thermocycler conditions were as follows: 95 °C for 10 min; 40 cycles of 95 °C for 10 s, 56 °C for 15 s, 72 °C for 20 s; 72 °C to 99 °C (continuous acquisition). For the second amplification, the reaction was carried out in a total volume of 20 μl using the same reagents as for the first qPCR and 2 μl of a 1:500 dilution in water from the first reaction. The thermocycler conditions of the second amplification were as follows: 95 °C for 10 min; 35 cycles of 95 °C for 10 s, 58 °C for 15 s, 72 °C for 20 s; 72 °C to 99 °C (continuous acquisition). Amplicons were sequenced, and melt curves were estimated.
Analytical sensitivity of Sn-HDP2 cPCR and Sn-HDP2 qPCR
Serial dilutions (1:10) from purified T. saginata and T. solium gDNA prepared from 1 ng/μl to 1 fg/μl were amplified using the two amplification protocols.
Construction of a T. solium genomic library
gDNA from T. solium cysticerci was used to prepare a parasite DNA library with Lambda Fix II vector following a previously described protocol [14].
Cloning of the T. solium HDP2 sequence
Two strategies were used to clone the T. solium HDP2 sequence: (i) T. solium gDNA library screening with the T. saginata HDP2 homolog probe [9, 13] and (ii) cPCR-cloning using T. solium gDNA as a template and ribosomal DNA-specific primers derived from T. solium 28S and 18S ribosomal DNA sequences. The supporting information (Additional file 3: Table S3) includes the characteristics of both universal and walking primers used to complete the sequencing of T. solium HDP2 subclones, which were obtained by library screening. In addition, cPCR ribosomal primers and walking primers (Additional file 3: Table S3) were used to directly clone and sequence the T. solium HDP2 amplification product. The DNA from recombinant plasmids was prepared using standard protocols. Sequencing, sequence data alignments and other analyses were performed as previously described [14, 17, 18].
Results and discussion
Sn-HDP2 cPCR and Sn-HDP2 qPCR amplified T. saginata and T. solium DNA with an analytical sensitivity of 40 fg and 400 fg, respectively, which was maintained in both protocols. In the case of T. saginata DNA in particular, sensitivity was comparable to that reported for other molecular markers [19–21].
Sn-HDP2 cPCR had previously been successfully used for the diagnosis of neurocysticercosis based on cerebrospinal fluid samples from infected Mexican patients [11]. At that time, a species-specific amplification protocol was not needed, as T. solium larva is the only taeniid that can invade the human central nervous system. However, in the case of taeniasis, which is produced by both taeniids, Sn-HDP2 cPCR yielded an amplicon with an identical size for both T. saginata and T. solium DNA [9, 10]; sequencing of the amplification product was essential for the specific identification of the taeniid [11, 12]. A new diagnostic amplification protocol was set up to circumvent the sequencing step by taking advantage of the differences in HDP2 in nucleotides between the two species. The novel Sn-HDP2 qPCR showed a distinct melting temperature (Tm) according to the Taenia species analyzed (Tm of 83.30 °C for T. saginata and 86.00 °C for T. solium), and the difference in Tm had already been detected in the first amplification (Fig. 1b). Furthermore, for proglottids, the first reaction usually yielded conclusive diagnostic results in both amplification protocols, as was the case in fecal samples when the parasite material was sufficient. In previous years, other HDP2 cPCRs protocols were applied for identification of T. saginata in fecal samples artificially spiked with known numbers of T. saginata eggs [22]. Moreover, as indicated above, amplifications of several molecular targets such as mitochondrial genes (cox1, nad5, nad1, 12S), and ribosomal ITS1 region and 28S and 5.8S genes [19, 21, 23] were described in various amplification protocols (cPCR, qPCR, LAMP) using mono- or multiplex systems, with very good results for the identification of Taenia spp. [19–21, 23, 24]. In comparison with Triplex Taq-Man (T3qPCR) [21], Sn-HDP2 qPCR did not need a labeled probe, although it did not include an internal PCR control and was not checked with T. asiatica DNA.
Fig. 1.
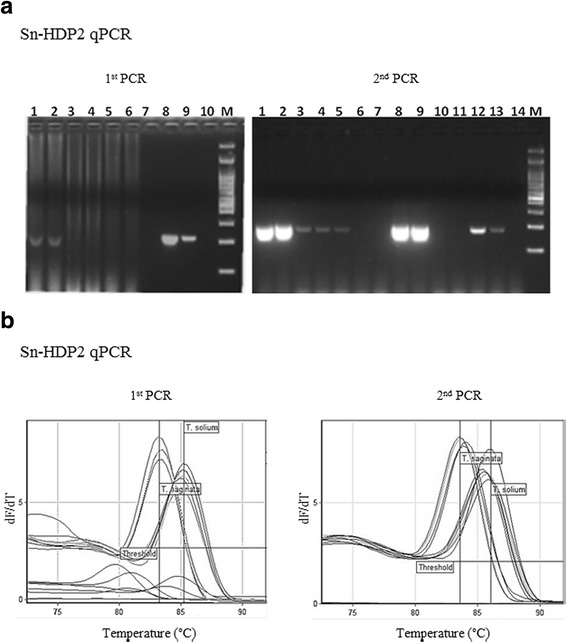
Diagnosis of human taeniosis using Sn-HDP2 cPCR and Sn-HDP2 qPCR. a Sn-HDP2 cPCR applied to 3× fecal samples from a patient with taeniasis. Amplification products fractionated on 2% agarose gels and stained by GelRed. First PCR: Lanes 1, 2: fecal sample 1 (positive by microscopy); Lanes 3, 4: fecal sample 2; Lanes 5, 6: fecal sample 3 (negative by microscopy); Lane 8: T. saginata DNA (positive control); Lane 9: T. solium DNA (positive control); Lane 10: no DNA (negative control); Lane M: 100 bp DNA Ladder (NIPPON Genetics Europe, Dueren, Germany). Second PCR: Lanes 1, 2: fecal sample 1; Lanes 3, 4: fecal sample 2; Lanes 5, 6: fecal sample 3; Lanes 8, 9: T. saginata DNA (positive control); Lanes 12, 13: T. solium DNA (positive control); Lane 14: no DNA (negative control); Lane M: 100 bp DNA Ladder (Genetics). Sn-HDP2 cPCR amplification products were sequenced. b Melting curves corresponding to the new Sn-HDP2 qPCR (both first and second PCR runs), applied to both T. saginata and T. solium DNA
In order to confirm the applicability of Sn-HDP2 cPCR and Sn-HDP2 qPCR in the diagnosis of infected human samples, proglottids and fecal samples from taeniasis patients were examined using both PCR assays. The results obtained are summarized in Table 1. With proglottids, the two PCRs were able to diagnose taeniasis in the first amplification as follows: T. saginata in patients from Europe (n = 9), South America (n = 3), Africa (n = 1) and from undetermined regions (n = 4), with ages ranging from 15 to 84 years; T. solium in a 43 year-old Latin American individual. In the case of fecal samples, the PCRs yielded positive results, and T. saginata was identified, even in stools negative for taeniid eggs (Case 3 and Case 10, Table 1). The identification was confirmed by agarose gel electrophoresis of the cPCR amplification product (Fig. 1a) followed by amplicon sequencing. Molecular approaches have already been used in human samples for the diagnosis of taeniasis in endemic regions, with excellent specificity and sensitivity [20, 21, 23]. The molecular identification of a T. solium tapeworm carrier in the present study is noteworthy, given the epidemiological consequences of the transmission of cysticercosis [25] and the possible need for surveillance measures in countries where the parasite has been eliminated. Today, migration from taeniid-endemic regions could extend transmission [26]. In addition, the frequent diagnosis of T. saginata in the taeniasis patients studied, in accordance with recent data from Europe [27], suggests the need for better reporting of taeniasis/bovine cysticercosis if the disease is to be better controlled [25].
In-depth analysis and characterization of the properties of the HDP2 homolog of T. solium were performed through cloning of the DNA sequence by screening a T. solium gDNA library with the T. saginata HDP2 homolog [14] and through direct cPCR-cloning using T. solium gDNA and specific ribosomal DNA primers [28]. A positive recombinant phage (phage clone #1, c.14–15 kb) and a specific genomic amplicon (c.3.0 kb) were obtained (Fig. 2). To determine which region of the phage fragment corresponded to the HDP2 sequence, phage clone #1 DNA was digested by the SalI restriction enzyme. The digestion yielded four fragments (Fig. 2a: a.1), two of which were hybridized with the T. saginata HDP2 sequence probe, namely, subclones #1.1 and #1.2 (Fig. 2a: a.2). T. solium phage subclones #1.1 and #1.2 were 2081 bp and 2799 bp long, respectively (Fig. 2b), and showed high similarities in their DNA sequences (GenBank: subclone #1.1: KY750552; subclone #1.2: KY750553). They also exhibited a high similarity with the DNA sequence of the T. solium ribosomal amplicon (3122 bp) cloned by cPCR (Fig. 2c), with few nucleotide substitutions and small deletions (GenBank: amplicon: KY750551). The DNA similarities discovered and the BLAST analysis data confirmed that T. solium HDP2 (3.12 kb) was a ribosomal gene, as was previously suggested for other taeniid homologous HDP2 molecules [13], when significant similarities between T. saginata HDP2 and the repetitive ribosomal sequences Taiwan Taenia pTTr 3.1, T. saginata pTSgr 3.1 and pTSgr 2.4, were demonstrated [29]. The T. solium fragment (3.12 kb) and subclones #1.1 and #1.2, in comparison with the T. saginata HDP2, lacked the first 900 nt at the 5′ end, as described elsewhere [9], and showed nucleotide substitutions and small deletions. Specifically, the largest deletion was located in T. solium HDP2 clones (from 590 to 620 nt).
Fig. 2.
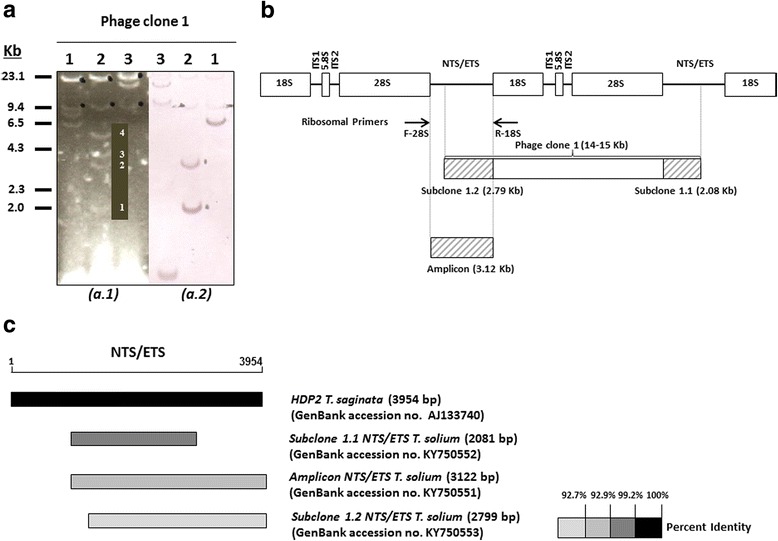
DNA sequence organization and similarities of the T. solium HDP2 fragment. a a.1: Restriction fragment patterns for T. solium recombinant phage clone 1 DNA digested by SacI (Lane 1); SalI (Lane 2); XbaI (Lane 3); a.2: Southern blot: T. saginata HDP2 DNA sequence digoxigenin-11-dUTP-labeled hybridization with T. solium recombinant phage clone 1 DNA digested by SacI (Lane 1); SalI (Lane 2); XbaI (Lane 3). b Diagram showing the genomic organization of the T. solium HDP2 subclones 1.1 and 1.2 and the T. solium HDP2 amplicon, with respect to the structure of the ribosomal DNA repeats (-18S-ITS1-5.8S-ITS2-28S-NTS/ETS-). c Physical alignment of Taenia HDP2 (NTS/ETS) fragments. The NTS/ETS ribosomal repeat is represented by a line, with the T. saginata HDP2 unit as the reference sequence. The boxes in grayscale correspond to the percent homology with the T. saginata HDP2 fragment (black box represents 100% identity). T. solium subclones 1.1 and 1.2 and the ribosomal amplicon are represented by boxes in different shades of gray according to similarities
The T. solium HDP2 structure within the ribosomal tandem repeats was further studied by restriction enzyme mapping based on the characteristics of both phage subclones and amplicon sequences. Thus, the rough size of cloned phage 1 was 14–15 kb. Together with the characteristics of standard rRNA genes determined by BLAST analysis, this phage included two partial copies of the ribosomal amplicon HDP2 (3.12 kb) at its 5′ and 3′ ends and the phage subclones #1.2 (2.79 kb) and #1.1 (2.08 kb), which were located in two different and contiguous non-transcribed sequence/external transcribed repeat (NTS/ETS) regions (Fig. 2b). Since information on the structural organization of rDNA sequences in Taenia species is poor, these data make it possible to conclude that the phage 1 sequence corresponded to a T. solium ribosomal DNA repeat plus an NTS/ETS region (Fig. 2b), with the HDP2 fragment being an NTS/ETS unit, according to the description made for the sequence and structure of the Caenorhabditis elegans rDNA repeat [30]. Therefore, the genomic variations detected between the characteristics of HDP2 in both taeniids could explain the following: (i) the different hybridization profiles found when the T. saginata and T. solium gDNAs were hybridized with the labeled T. saginata HDP2 DNA sequence [8, 9]; (ii) the non-amplification of T. solium DNA by the R1F1-HDP2-PCR [9]; and (iii) the differential sensitivity of taeniids determined by the amplification protocols described in the present paper.
Finally, the repetitive nature of HDP2 sequences (ribosomal NTS/ETS units) and the differences in nucleotide composition between Taenia species would explain the diagnostic properties of the molecule as a target in high-sensitivity, species-specific amplification protocols.
Conclusions
In the present study, Sn-HDP2 cPCR and Sn-HDP2 qPCR based on proglottids and fecal samples were applied to diagnose taeniasis in humans, even though the fecal sample did not contain microscopy-proven parasitic forms and the proglottid was damaged. Sn-HDP2 qPCR proved to be the better diagnostic option. The finding of a T. solium tapeworm carrier and the eight cases of T. saginata was relevant, considering the crucial epidemiological consequences of transmission of cysticercosis. The cloning and sequencing of the HDP2 homolog in the T. solium genome confirmed that the DNA was ribosomal, specifically an NTS/ETS unit, and explained the differences in performance of the amplification protocols according to the specific composition of this genomic region in T. solium and T. saginata.
Additional files
DNA markers and molecular protocols used to identify the taeniid species involved in human taeniasis. (PDF 35 kb)
References included in Additional file 1: Table S1. (PDF 18 kb)
Universal and walking primers used for T. solium HDP2 sequencing. (PDF 60 kb)
Acknowledgements
We thank Professor Elizabeth Ferrer, Professor Edda Sciutto and Dr. Sabino Puente, who kindly provided us with the parasite material necessary for our study. We are particularly grateful to Dr Leslie Harrison and Dr Michael Parkhouse, with whom the HDP2 project was launched.
Funding
This study was supported by the Network of Biomedical Research on Tropical Diseases (RICET), RD12/0018/0001 and RD12/0018/0011, AESI (MPY1279/15) and MINECO (AGL2014-56193-R).
Availability of data and materials
Data supporting the conclusions of this article are included within the article.
Abbreviations
- cPCR
Conventional polymerase chain reaction
- ETS
External transcribed spacer
- LAMP
Loop-mediated isothermal amplification
- NTS
Non-transcribed spacer
- qPCR
Real-time polymerase chain reaction
- WHO
World Health Organization
Authors’ contributions
Conceived and designed the experiments: MDF, LMG, CH, YMM, MJP and TG. Performed the experiments: MDF, LMG, CH, YMM and CD. Analyzed the data: MDF, LMG, CH, YMM, FJM, MJP and TG. Contributed reagents/materials/analysis tools: MDF, LMG, CH, YMM, FJM, MJP andTG. Wrote the paper: MDF, LMG, CH, YMM, CD, FJM, MJP and TG. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Samples were analyzed in anonymous form. They belonged to repository C.0003989, which is registered at ISCIII Biobank, and were managed following current ethical recommendations, according to Spanish Royal Decree 1716/2011. Current ethical recommendations about disclosure and anonymization of individual patient data were adhered to at all times.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
María D. Flores, Email: mflores@externos.isciii.es
Luis M. Gonzalez, Email: lmgonzal@isciii.es
Carolina Hurtado, Email: carolina.hurtadomarcos@ceu.es.
Yamileth Monje Motta, Email: ymonje@unimagdalena.edu.co.
Cristina Domínguez-Hidalgo, Email: cristina-dh@hotmail.com.
Francisco Jesús Merino, Email: franciscojesus.merino@salud.madrid.org.
María J. Perteguer, Email: chus.perteguer@isciii.es
Teresa Gárate, Email: tgarate@isciii.es.
References
- 1.Craig P, Ito A. Intestinal cestodes. Curr Opin Infect Dis. 2007;20:524–532. doi: 10.1097/QCO.0b013e3282ef579e. [DOI] [PubMed] [Google Scholar]
- 2.García HH, Gonzalez AE, Evans CA, Gilman RH, Cysticercosis Working Group in Peru. Taenia solium cysticercosis. Lancet. 2003;362(9383):547–56. [DOI] [PMC free article] [PubMed]
- 3.Martins-Melo FR, Ramos AN, Jr, Cavalcanti MG, Alencar CH, Heukelbach J. Neurocysticercosis-related mortality in Brazil, 2000–2011: epidemiology of a neglected neurologic cause of death. Acta Trop. 2016;153:128–136. doi: 10.1016/j.actatropica.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Zammarchi L, Strohmeyer M, Bartalesi F, Bruno E, Muñoz J, Buonfrate D, et al. Epidemiology and management of cysticercosis and Taenia solium taeniasis in Europe, systematic review 1990–2011. PLoS One. 2013;8:e69537. doi: 10.1371/journal.pone.0069537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World Health Organization estimates of the global and regional disease burden of 11 food-borne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001920. [DOI] [PMC free article] [PubMed]
- 6.Garcia HH, Gonzalez AE, Tsang VC, O'Neal SE, Llanos-Zavalaga F, Gonzalvez G, et al. Elimination of Taenia solium transmission in northern Peru. N Engl J Med. 2016;374(24):2335–2344. doi: 10.1056/NEJMoa1515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Taenia solium taeniasis/cysticercosis diagnostic tools report of a stakeholder meeting. Geneva 17–18 December 2015. Geneva: WHO Press; 2016. [Google Scholar]
- 8.Harrison LJ, Delgado J, Parkhouse RM. Differential diagnosis of Taenia saginata and Taenia solium with DNA probes. Parasitology. 1990;100(3):459–461. doi: 10.1017/S0031182000078768. [DOI] [PubMed] [Google Scholar]
- 9.González LM, Montero E, Harrison LJ, Parkhouse RM, Garate T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J Clin Microbiol. 2000;38:737–744. doi: 10.1128/jcm.38.2.737-744.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González LM, Montero E, Sciutto E, Harrison LJ, Parkhouse RM, Garate T. Differential diagnosis of Taenia saginata and Taenia solium infections: from DNA probes to polymerase chain reaction. Trans R Soc Trop Med Hyg. 2002;96(Suppl. 1):S243–S250. doi: 10.1016/S0035-9203(02)90083-0. [DOI] [PubMed] [Google Scholar]
- 11.Hernández M, Gonzalez LM, Fleury A, Saenz B, Parkhouse RM, Harrison LJ, et al. Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann Trop Med Parasitol. 2008;102:317–323. doi: 10.1179/136485908X278856. [DOI] [PubMed] [Google Scholar]
- 12.González LM, Montero E, Morakote N, Puente S, Díaz De Tuesta JL, Serra T, et al. Differential diagnosis of Taenia saginata and Taenia saginata asiatica taeniasis through PCR. Diagn Microbiol Infect Dis. 2004;49:183–188. doi: 10.1016/j.diagmicrobio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 13.González LM, Bailo B, Ferrer E, García MD, Harrison LJ, Parkhouse MR, et al. Characterization of the Taenia spp HDP2 sequence and development of a novel PCR-based assay for discrimination of Taenia saginata from Taenia asiatica. Parasit Vectors. 2010;3:51. doi: 10.1186/1756-3305-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montero E, González LM, Harrison LJ, Parkhouse RM, Gárate T. Taenia solium cDNA sequence encoding a putative immunodiagnostic antigen for human cysticercosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;786:255–269. doi: 10.1016/S1570-0232(02)00909-1. [DOI] [PubMed] [Google Scholar]
- 15.Saugar JM, Merino FJ, Martín-Rabadán P, Fernández-Soto P, Ortega S, Gárate T, Rodríguez E. Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Trop. 2015;142:20–25. doi: 10.1016/j.actatropica.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Garcia LS. Diagnostic medical parasitology. Washington DC: ASM Press; 2001. [Google Scholar]
- 17.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 18.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roelfsema JH, Nozari N, Pinelli E, Kortbeek LM. Novel PCRs for differential diagnosis of cestodes. Exp Parasitol. 2016;161:20–26. doi: 10.1016/j.exppara.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Praet N, Verweij JJ, Mwape KE, Phiri IK, Muma JB, Zulu G, et al. Bayesian modelling to estimate the test characteristics of coprology, coproantigen ELISA and a novel real-time PCR for the diagnosis of taeniasis. Tropical Med Int Health. 2013;18:608–614. doi: 10.1111/tmi.12089. [DOI] [PubMed] [Google Scholar]
- 21.Ng-Nguyen D, Stevenson MA, Dorny P, Gabriël S, Vo TV, Nguyen VT, et al. Comparison of a new multiplex real-time PCR with the Kato Katz thick smear and copro-antigen ELISA for the detection and differentiation of Taenia spp. in human stools. PLoS Negl Trop Dis. 2017;11:e0005743. doi: 10.1371/journal.pntd.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunes CM, Lima LG, Manoel CS, Pereira RN, Nakano MM, Garcia JF. Taenia saginata: polymerase chain reaction for taeniasis diagnosis in human fecal samples. Exp Parasitol. 2003;104:67–69. doi: 10.1016/S0014-4894(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, et al. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–553. doi: 10.1128/JCM.42.2.548-553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nkouawa A, Sako Y, Nakao M, Nakaya K, Ito A. Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species. J Clin Microbiol. 2009;47:168–174. doi: 10.1128/JCM.01573-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murrell KD. Epidemiology of taeniosis and cysticercosis. In: Murrell KD, editor. WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniosis/cysticercosis. Paris: World Health Organisation for Animal Health (OIE); 2005. p. 27–43.
- 26.Gómez-Morales MA, Gárate T, Blocher J, Devleesschauwer B, GSA S, Schmidt V, et al. Present status of laboratory diagnosis of human taeniosis/cysticercosis in Europe. Eur J Clin Microbiol Infect Dis. 2017;36:2029–2040. doi: 10.1007/s10096-017-3029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laranjo-González M, Devleesschauwer B, Trevisan C, Allepuz A, Sotiraki S, Abraham A, et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Western Europe. Parasit Vectors. 2017;10:349. doi: 10.1186/s13071-017-2280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hancock K, Broughel DE, Moura IN, Khan A, Pieniazek NJ, Gonzalez AE, et al. Sequence variation in the cytochrome oxidase I, internal transcribed spacer 1, and Ts14 diagnostic antigen sequences of Taenia solium isolates from South and Central America, India, and Asia. Int J Parasitol. 2001;31:1601–7. [DOI] [PubMed]
- 29.Zarlenga DS, DP MM, Fan PC, Cross JH. Characterization and detection of a newly described Asian taeniid using cloned ribosomal DNA fragments and sequence amplification by the polymerase chain reaction. Exp Parasitol. 1991;72:174–183. doi: 10.1016/0014-4894(91)90135-J. [DOI] [PubMed] [Google Scholar]
- 30.Ellis RE, Sulston JE, Coulson AR. The rDNA of C. elegans: sequence and structure. Nucleic Acids Res. 1986;14:2345–2364. doi: 10.1093/nar/14.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA markers and molecular protocols used to identify the taeniid species involved in human taeniasis. (PDF 35 kb)
References included in Additional file 1: Table S1. (PDF 18 kb)
Universal and walking primers used for T. solium HDP2 sequencing. (PDF 60 kb)
Data Availability Statement
Data supporting the conclusions of this article are included within the article.


