Abstract
Background
Physical activity (PA) and smoking have been reported to be associated with the duration and severity of common cold symptoms. However, few studies have addressed the associations between the frequency of leisure-time exercise, cigarette smoking status and the frequency of the common cold in a cold area. This study was designed to investigate these issues in northeastern China.
Methods
This cross-sectional study included individuals who participated in a regular health examination conducted in Jilin Province, China. Information on episodes of the common cold, the frequency of leisure-time exercise and cigarette smoking status in the past year were collected by self-administered health questionnaires. Ordinal logistic regression models were used to analyse the associations between the frequency of leisure-time exercise, cigarette smoking status and the retrospective frequency of common cold.
Results
A total of 1413 employees participated in the study, with an average age of 38.92 ± 9.04 years and 44.4% of them were male. Of all participants, 80.8% reported having experienced the common cold in the past year. After adjustment, the risk of suffering from the common cold more than once (odds ratios (ORs), 1.59; 95% confidence interval (CI), 1.27–1.99) in passive smokers was 1.59 times as high as that in non-smokers. Nevertheless, the results of the adjusted analysis showed no statistically significant relation between current smoking and the frequency of the common cold. A high frequency of leisure-time exercise (≥3 days/week) was associated with a 26% reduced risk of having at least one episode of the common cold (OR, 0.74; 95% CI, 0.55–0.98) compared with a low frequency group (< 4 days/month). For current and passive smokers, the protective effect of a high frequency of leisure-time exercise appears not to be obvious (current smokers: OR, 0.68; 95% CI, 0.33–1.43; passive smokers: OR, 1.15; 95% CI, 0.69–1.93).
Conclusion
Passive smoking was associated with a higher risk of having self-reported common cold at least once, while a high frequency of leisure-time exercise was related to a lower risk of reporting more than one episode of the disease in Chinese.
Keywords: The common cold, Leisure-time exercise, Passive smoking, Smoking
Background
As one of the most prevalent infectious illnesses, the common cold is estimated to occur an average of 2–5 times for adults and 6–10 times for children each year in the general healthy population [1]. It is usually thought that the common cold, although not a severe disease, is an infection attributed to more than 200 types of viruses or bacteria [2]. Causes of the common cold are difficult to be detected because of its various clinical symptoms and the variability of microbial virulence caused by antibiotic resistance [3, 4]. Therefore, effective prevention towards reduced clinical symptoms and duration of the common cold is still limited. The common cold also has the potential to bring those suffering from respiratory diseases a certain degree of difficulty in controlling and relieving their clinical symptoms, such as worsening asthma [5, 6] and chronic obstructive pulmonary disease (COPD) [7, 8]. Furthermore, the common cold is an issue that accounts for a substantial economic burden [9] and influences normal daily activities, such as low work productivity and absenteeism due to sickness [10–12].
Smoking and exercise are modifiable lifestyle factors of maintaining health. Both smoking and passive smoking were not only related to an increased risk of various malignancies [13–15] and degenerative disorders of the cardiovascular system [16, 17] but also associated with an increased predisposition to developing the upper respiratory tract infections (URTIs) by microbial pathogens [18–24]. Although previous studies have examined the relationship between passive smoking and the incidence of the common cold in women and children [21–23], few studies have examined the association in the general population. The relationships of active smoking, severity of active smoking and frequency of common cold remain controversial and remain to be confirmed.
Physical activity (PA) was also associated with a susceptibility to URTI, but most existing studies have been performed in the elderly [25–27] and athletes [28, 29]. It has been suggested that the risk of URTI was increased for individuals engaging in extreme physical exertion [29, 30]. However, little is known about effects of moderate-intensity levels of exercise on the frequency of the common cold in predominately nonathletic population. In comparison with athletes and elders, individuals in the general individuals, especially the occupational population, are more likely to engage in moderate levels of leisure-time exercise and have less leisure-time to exercise regularly. Thus, examining the role of leisure-time exercise could urge young and middle-aged individuals to exercise, to some extent.
Northeastern of China is thought to be at high risk of respiratory disease, mainly due to exposure to the cold air in the long winter season [31], air pollutants caused by winter coal-fired heating [32–34] and inadequate vitamin D, which can affect immune function due to fewer outdoor activities in winter [35]. Therefore, the main objective of this study was to examine the influences of active or passive cigarette smoking and the frequency of leisure-time exercise on the retrospective frequency of the common cold in a cold area in northeastern China. Furthermore, we further explore the potential interaction between leisure-time exercise and cigarette smoking status on the common cold.
Methods
Participants
This cross-sectional study was conducted at the medical examination centre of the First Hospital of Jilin University, northeastern China, from June to September in 2016. All staff members of banks participating in regular physical examinations were invited to participate during the study period. The study followed a common set of procedures, which included physical examinations, blood and urine screenings and self-administered health questionnaires. A total of 1530 employees accepted the invitation to participated in the questionnaire survey. Of these 1530 employees, the data of 69 employees were excluded because of missing data, and that of 48 retirees were also excluded, which resulted in a final sample size of 1413 for the present analyses. The data were collected from regular medical examination reports and self-administered questionnaires in these employees. All participants were given informed consent prior to being enrolled in the study. Characteristics of the participants are reported in Tables 1 and 2.
Table 1.
Sample basic characteristics according to the categorized cigarette smoking status
| Variables | Sample (n = 1413) |
Cigarette smoking categories | ||||
|---|---|---|---|---|---|---|
| Non-smokers (n = 641) |
Passive smokers (n = 476) | Past smokers (n = 82) |
Light current smokers (n = 113) |
Heavy current smokers (n = 101) | ||
| Gender, n (%)** | ||||||
| Male | 627(44.4) | 185(28.9) | 155(32.6) | 78(95.1)△ | 108(95.6)△ | 101(100.0)△ |
| Age, y** | 38.92 ± 9.04 | 38.07 ± 8.83 | 38.63 ± 9.32 | 43.95 ± 8.0△ | 38.81 ± 8.81 | 41.75 ± 8.36△ |
| Ethnicity, n (%) | ||||||
| Han | 1293(91.5) | 591(92.2) | 426(89.5) | 75(91.5) | 107(94.7) | 94(93.1) |
| Education levels, n (%)* | ||||||
| Senior school or less | 83(5.9) | 33(5.1) | 23(4.8) | 11(13.4) | 8(7.1) | 8(7.9) |
| Junior college | 326(23.1) | 137(21.4) | 113(23.7) | 23(28.0) | 24(21.2) | 29(28.7) |
| Bachelor degree | 799(56.5) | 365(56.9) | 270(56.7) | 42(51.2) | 64(56.6) | 58(57.4) |
| Master degree or greater | 205(14.5) | 106(16.5) | 70(14.7) | 6(7.3)△ | 17(15.0) | 6(5.9) |
| BMI** | 23.90 ± 5.36 | 23.70 ± 6.89 | 23.20 ± 3.52 | 25.90 ± 3.2△ | 25.60 ± 3.21△ | 25.40 ± 3.26△ |
| Frequency of drinking (days/week), n (%)** | ||||||
| 0 | 859(60.8) | 481(75.0) | 293(61.6) | 26(31.7) | 24(21.2) | 35(34.7) |
| 1–2 | 468(33.1) | 143(22.3) | 165(34.7) | 47(57.3) | 69(61.1) | 44(43.6) |
| ≥ 3 | 86(6.1) | 17(2.7) | 18(3.8)△ | 9(11.0)△ | 20(17.7)△ | 22(21.8)△ |
| Sleep duration, n (%)** | ||||||
| < 7 hours | 402(28.5) | 168(26.2) | 130(27.3) | 22(26.8) | 52(46.0) | 30(29.7) |
| 7–8 hours | 561(39.7) | 243(37.9) | 195(41.0) | 37(45.1) | 38(33.6) | 48(47.5) |
| ≥ 8 hours | 450(31.8) | 230(35.9) | 151(31.7) | 23(28.0) | 23(20.4)△ | 23(22.8) |
| SRSS, n (%) | ||||||
| ≥ 23 | 594(42.0) | 257(40.1) | 215(45.2) | 25(30.5) | 52(46.0) | 45(44.6) |
| Self-rated health, n (%) | ||||||
| Poor | 168(11.9) | 67(10.5) | 61(12.8) | 7(8.5) | 15(13.3) | 18(17.8) |
| Fair | 718(50.8) | 317(49.5) | 244(51.3) | 45(54.9) | 66(58.4) | 46(45.5) |
| Good | 527(37.3) | 257(40.1) | 171(35.9) | 30(36.6) | 32(28.3) | 37(36.6) |
| Self-perceived life stress, n (%)** | ||||||
| Low | 217(15.4) | 108(16.8) | 69(14.5) | 14(17.1) | 12(10.6) | 14(13.9) |
| Fair | 751(53.1) | 370(57.7) | 243(51.1) | 43(52.4) | 49(43.4) | 46(45.5) |
| High | 445(31.5) | 163(25.4) | 164(34.5)△ | 25(30.5) | 52(46.0)△ | 41(40.6)△ |
| Self-perceived work stress, n (%)* | ||||||
| Low | 165(11.7) | 76(11.9) | 56(11.8) | 12(14.6) | 12(10.6) | 9(8.9) |
| Fair | 615(43.5) | 306(47.7) | 183(38.4) | 40(48.8) | 42(37.2) | 44(43.6) |
| High | 633(44.8) | 259(40.4) | 237(49.8)△ | 30(36.6) | 59(52.2) | 48(47.5) |
| SAS, n (%)* | ||||||
| ≥ 50 | 219(15.5) | 87(13.6) | 89(18.7) | 7(8.5) | 15(13.3) | 21(20.8) |
| SDS, n (%)* | ||||||
| ≥ 50 | 561(39.7) | 236(36.8) | 200(42.0) | 28(34.1) | 45(39.8) | 52(51.5)△ |
| NE** | 3.78(3.10, 4.56) | 3.70(3.07, 4.49) | 3.73(3.00, 4.47) | 3.56(3.09, 4.33) | 3.94(3.22, 4.69) | 4.78(3.84, 5.76)△ |
| MO** | 0.29(0.21, 0.39) | 0.28(0.21, 0.37) | 0.29(0.22, 0.39) | 0.30(0.21, 0.39) | 0.30(0.21, 0.42) | 0.42(0.28, 0.51)△ |
| LY** | 2.15(1.83, 2.54) | 2.13(1.81, 2.52) | 2.10(1.77, 2.43) | 2.14(1.80, 2.50) | 2.25(1.97, 2.77)△ | 2.47(2.10, 2.87)△ |
Values are shown as the mean ± SD or median (interquartile range) for continuous data and number and proportions for categorical data
P-values were calculated by analysis of variance or Kruskal-Wallis H tests for continuous variables and χ2-tests for categorical variables
Superscript numbers denote statistically significant post hoc tests: △≤ 0.05/4 for the difference between current, past, passive smokers and non-smokers. Current, past and passive smokers were compared with non-smokers
*Statistical significance: P < 0.05
**Statistical significance: P < 0.001
Table 2.
Sample basic characteristics according to the categorized frequency of leisure-time exercise
| Variables | Sample (n = 1413) |
Frequency of leisure-time exercise | ||
|---|---|---|---|---|
| Low (< 4 days/month) (N = 375) | Medium (1–2 days/week) (N = 624) | High (≥3 days/week) (N = 414) | ||
| Gender, n (%)** | ||||
| Male | 627(44.4) | 141(34.1) | 285(45.7) | 201(53.6) |
| Age, y** | 38.92 ± 9.04 | 35.25 ± 7.55 | 38.44 ± 8.73 | 43.79 ± 8.94 |
| Ethnicity, n (%)* | ||||
| Han | 1293(91.5) | 370(89.4) | 571(91.5) | 352(93.9) |
| Education levels, n (%)** | ||||
| Senior school or less | 83(5.9) | 10(2.4) | 36(5.8) | 37(9.9) |
| Junior college | 326(23.1) | 80(19.3) | 147(23.6) | 99(26.4) |
| Bachelor degree | 799(56.5) | 268(64.7) | 336(53.8) | 195(52.0) |
| Master degree or greater | 205(14.5) | 56(13.5) | 105(16.8) | 44(11.7) |
| BMI** | 23.90 ± 5.36 | 23.10 ± 3.95 | 24.30 ± 6.92 | 24.40 ± 3.18 |
| Drinking categories (days/week), n (%)* | ||||
| 0 | 859(60.8) | 270(65.2) | 365(58.5) | 224(59.7) |
| 1–2 | 468(33.1) | 125(30.2) | 223(35.7) | 120(32.0) |
| ≥ 3 | 86(6.1) | 19(4.6) | 36(5.8) | 31(8.3) |
| Sleep duration, n (%)* | ||||
| < 7 hours | 402(28.5) | 115(27.8) | 168(26.9) | 119(31.7) |
| 7–8 hours | 561(39.7) | 153(37.0) | 256(41.0) | 152(40.5) |
| ≥ 8 hours | 450(31.8) | 146(35.3) | 200(32.1) | 104(27.7) |
| SRSS, n (%)* | ||||
| ≥ 23 | 594(42) | 189(45.7) | 260(41.7) | 145(38.7) |
| Self-rated health, n (%) | ||||
| Poor | 168(11.9) | 82(19.8) | 59(9.5) | 27(7.2) |
| Fair | 718(50.8) | 216(52.2) | 353(56.6) | 149(39.7) |
| Good | 527(37.3) | 116(28.0) | 212(34.0) | 199(53.1) |
| Self-perceived life stress, n (%)* | ||||
| Low | 217(15.4) | 52(12.6) | 82(13.1) | 83(22.1) |
| Fair | 751(53.1) | 217(52.4) | 356(57.1) | 178(47.5) |
| High | 445(31.5) | 145(35.0) | 186(29.8) | 114(30.4) |
| Self-perceived work stress, n (%)** | ||||
| Low | 165(11.7) | 30(7.2) | 56(9.0) | 79(21.1) |
| Fair | 615(43.5) | 171(41.3) | 292(46.8) | 152(40.5) |
| High | 633(44.8) | 213(51.4) | 276(44.2) | 144(38.4) |
| SAS, n (%)** | ||||
| ≥ 50 | 219(15.5) | 92(22.2) | 87(13.9) | 40(10.7) |
| SDS, n (%)* | ||||
| ≥ 50 | 561(39.7) | 188(45.4) | 238(38.1) | 135(36.0) |
| NE* | 3.78(3.10, 4.56) | 3.83(3.21, 4.72) | 3.82(3.10, 4.57) | 3.64(2.97, 4.36) |
| MO | 0.29(0.21, 0.39) | 0.29(0.21, 0.38) | 0.30(0.21, 0.40) | 0.29(0.21, 0.40) |
| LY | 2.15(1.83, 2.54) | 2.16(1.81, 2.52) | 2.15(1.84, 2.56) | 2.12(1.81, 2.51) |
Values are shown as the mean ± SD or median (interquartile range) for continuous data and number and proportions for categorical data
P-values were calculated by analysis of variance or Kruskal-Wallis H tests for continuous variables and trend χ2-tests for categorical variables
*Statistical significance: P < 0.05
**Statistical significance: P < 0.001
Common cold
The common cold was assessed with a single item: “How many times were you infected with the common cold in the previous year?” Response options included none, one, two, and three or more times, similar to prior studies [21, 36, 37]. We told participants about the definition and general clinical symptoms of the common cold [2] to help participants distinguish the common cold from flu-like illness and allergies before the health questionnaire survey. The frequency of the common cold was divided into four categories (none, one, two, three or more times) for analysis.
Health-related behaviours
On the health questionnaires, participants were asked to choose their smoking status in the past year from the following options: non-active smoker, past smoker and current smoker. In addition, current smokers were required to answer an open-ended item: “How many cigarettes did you smoke per day in the past year?” Participants were also required to answer whether they were passively exposed to tobacco smoke by inhaling smog for more than 15 min at least once a week. In our study, we categorized current smokers as light smokers (< 15 cigarettes/day) and heavy smokers (≥15 cigarettes/day). Past smokers were considered to be those who quit more than one year ago, while those who quit less than one year ago were regarded as current smokers. Passive smokers were defined as those who had never smoked but were exposed to tobacco smoke. Current, past and passive smokers were compared with non-smokers who were non-active smokers without exposure to passive smoking. The status of cigarette smoking and the number of cigarettes were self-reported by the participants investigated in the study.
Leisure-time exercise was assessed according to an item designed by a reference to the National Health Interview Survey (NHIS) [38]: “Outside of work or daily activities, how often have you exercised per week in the past year that at least moderately increased your breathing and heart rate for at least 20 minutes?” The frequency of leisure-time exercise was divided into three categories: high (≥3 days/week), medium (1–2 days/week) and low (< 4 days/month). Intensity and patterns of leisure-time exercise were not recorded.
In the survey, participants were also required to report on drinking alcohol and engaging in alcohol abstinence, as well as on their frequency of drinking. With regard to the frequency of alcohol consumption, these participants chose one of the following four options: 0 days/week, 1–2 days/week, 3–5 days/week and 6–7 days/week. We combined data on the frequency of alcohol intake into three categories (0 days/week, 1–2 days/week and ≥3 days/week) for analysis. Average sleep duration at night was assessed by asking participants how many hours they slept on average at night. Sleep duration was categorized into three groups: < 7, 7–8 and ≥8 h. Sleep quality was measured by a continuous score based on 10 five-point items on the Self-Rating Scale of Sleep (SRSS). A score of more than 23 points indicates poor sleep according to the standard in SRSS (Cornbash’s alpha = 0.64) [39]. Previous study have confirmed the reliability and validity of SRSS for evaluating sleep quality in the Chinese population [40].
Psychological measures
The Chinese versions of the Self-Rating Depression Scale (SDS) [41] and the Self-Rating Anxiety Scale (SAS) [42], prepared by Zung in 1965 and 1971, respectively, were used to measure depressive and anxious symptoms. Both measures comprised 20 self-reported items, and each item was scored on a 4-point scale. Increasing scores suggested an increasing severity of anxiety or depression symptoms. The criterion of having depressive or anxious symptoms was determined as a standardized score of 50 or more out of 80 points. Previous studies have confirmed the reliability and validity of the SDS and the SAS (Cronbach’s alpha = 0.85) for used in the Chinese population [43, 44].
Self-rated health (SRH) was measured by asking participants what they think of their health status in the previous year, with response options of very poor, poor, fair, good and very good. In addition, life and work stresses were evaluated with two questions as follows: 1) How do you feel about your work stress in the previous year? 2) How do you feel about your life stress in the previous year? Response options for the two questions included very low, low, fair, high and very high. Due to small numbers in several categories, we combined data on groups of SRH, self-rated life stress and self-rated work stress into three categories, respectively (SRH: poor, fair and good; life or work stress: low, fair and high).
Biomarkers
Biomarkers (lymphocyte (LY), neutrophil (NE) and monocyte (MO)) of the participants were collected from regular medical examination reports.
Standard control variables
Information on gender (female/male), age (continuous), ethnicity (dichotomized as Han/other), education levels, weight and height were collected using health questionnaires and health examinations. Educational levels were categorized into senior high school or less, junior college, bachelor’s degree and master’s degree or greater. Body mass index (BMI) was calculated as weight/height2 (kg/m2).
Statistical analysis
The data were presented as means ± standard deviations (SD), medians (interquartile ranges) or number (proportions). Trend χ2-tests or χ2-tests for categorical variables and analyses of variance (ANOVA) or Kruskal-Wallis H tests for continuous variables were used to assess the differences in basic characteristics according to the categorized cigarette smoking status and frequency of leisure-time exercise. Post hoc analysis was conducted by the Bonferroni correction. Because the dependent variable of the frequency of the common cold had been classified into four categories according to their order of magnitude, ordinal logistic regression models were used to analyse the associations between cigarette smoking status, the frequency of leisure-time exercise and self-reported frequency of the common cold in the previous year. The odds ratios (ORs) and 95% confidence intervals (CI) were adjusted with the following potential confounding factors: gender, age, ethnicity, education levels, BMI, drinking status, sleep duration, SRSS, SRH, self-perceived work stress, self-perceived life stress, SAS, SDS, LY, NE and MO. The rationale for including these covariates in the model is that they could potentially affect the relationship of main variables (cigarette smoking status and the frequency of leisure-time exercise) and the common cold. ORs and 95%CI were calculated as exp.(b). The criterion for statistical significance was set at P < 0.05. The data were analysed using SPSS Statistics for Windows, version 22.0 (IBM Corp, Armonk, New York, USA).
Results
Basic characteristics
In this study, male employees accounted for 44.4% of the sample. Of all participants, 80.8% reported catching a common cold in the past year, and 37.2%, 31.6% and 12.1% experienced the common cold during the previous year once, twice, and three or more times, respectively. The prevalence of the common cold was almost similar between genders (79.7% for males and 81.7% for females, P = 0.359). Similarly, there were no statistically significant gender differences in the frequency of the common cold (P = 0.274).
Table 1 shows the basic characteristics (standard controls variables, health-related behaviours, psychological measures and biomarkers) for the participants according to the categorized cigarettes smoking status. The results of the study indicate that almost all current smokers were males (97.7%), and the number of passive smokers in women was almost twice as high as that in men. Past, heavy current smokers (≥15cigarettes/day) were older than non-smokers. Current smokers reported a higher proportion of high-frequency daily alcohol intake (≥3 days/week) and high life stress than non-smokers, and a relative increase in having depressive symptoms (SDS ≥ 50) appeared only for the heavy/non-smokers. They had higher values for BMI and LY. Additionally, higher prevalences of high-frequency daily alcohol intake, high work stress and high life stress were found in passive smokers than in non-smokers.
Table 2 presents characteristics of the sample across the low, medium and high leisure-time exercise groups. Groups differed on the standard control variables. BMI, age, the proportion of males, and the number of participants of Han ethnicity, Junior college or lower education level and high frequency of daily alcohol intake (≥3 days/week) increased across low to high groups. In contrast, there were reductions in the percentages of participants having psychological problems (having high life or work stress and having anxious (SAS ≥ 50) or depressive (SDS ≥ 50) symptoms), reporting poor quality and sleeping more than 8 h with an increase in the frequency of leisure-time exercise. Higher-frequency exercisers reported a lower value of NE.
Cigarettes smoking status and the common cold
The proportions of participants who had the common cold more than once by cigarette smoking status (non-smokers, passive smokers, past smokers, light current smokers, heavy current smokers) were 78.5%, 85.7%, 78.0%, 78.8%, and 77.2%, respectively (data not shown). Table 3 and Fig. 1 show that the percentages of having more than two episodes of the common cold in passive, past and current smokers were higher than that in non-smokers, while the relationship between past or current smoking and the frequency of the common cold was not significant.
Table 3.
Smoking status and frequency of leisure-time exercise according to the categorized frequency of common cold
| Variables | Sample (n = 1413) | Frequency of common colda | P-value | |||
|---|---|---|---|---|---|---|
| 0 (n = 271) | 1 (n = 525) | 2 (n = 446) | ≥3 (n = 171) | |||
| Cigarette smoking categories | ||||||
| Non-smokers | 641 | 138(21.5) | 256(39.9) | 178(27.8) | 69(10.8) | – |
| Passive smokers | 476 | 68(14.3) | 163(34.2) | 178(37.4) | 67(14.1) | < 0.001 |
| Past smokers | 82 | 18(22.0) | 30(36.6) | 24(29.3) | 10(12.2) | 0.715 |
| Light current smokers | 113 | 24(21.2) | 42(37.2) | 37(32.7) | 10(8.8) | 0.790 |
| Heavy current smokers | 101 | 23(22.8) | 34(33.7) | 29(28.7) | 15(14.9) | 0.443 |
| Frequency of leisure-time exercise | ||||||
| Low < 4 days/month | 414 | 63(15.2) | 155(37.4) | 132(31.9) | 64(15.5) | – |
| Medium 1–2 days/week | 624 | 110(17.6) | 233(37.3) | 205(32.9) | 76(12.2) | 0.206 |
| High ≥3 days/week | 375 | 98(26.1) | 137(36.5) | 109(29.1) | 31(8.3) | < 0.001 |
aEpisodes of self-reported common cold in the previous year
Values are shown as numbers and proportions for categorical data
P-values were calculated by ordinal logistic regression
Statistical significance: P < 0.05
Fig. 1.
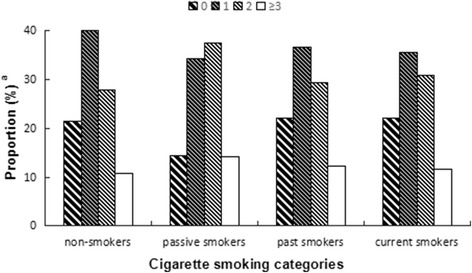
Observed association between frequency of common cold and cigarette smoking status. aRepresent the proportion of self-reported common cold in the categorized cigarette smoking
Table 4 presents ORs and their 95% CI from ordinal logistic regression analyses, with the frequency of the common cold as the dependent variable. After adjustment for gender and age, the likelihood of suffering from at least one episode of the common cold was higher in passive smokers than in non-smokers (Table 4, Model 1: OR, 1.64; 95%CI, 1.32–2.03; P < 0.001). To determine whether other potential factors accounted for the association, we entered SRSS, SRH, health-related behaviours, psychological variables and biomarkers into ordinal logistic regression model, including cigarette smoking status and standard control variables. We observed that passive smokers had 1.59 times odds of experiencing the common cold more than once compared with non-smokers (Table 4, Model 2: OR, 1.59; 95%CI, 1.27–1.99; P < 0.001), and the addition of these variables did not appreciably influence the association. Nevertheless, the relationship between past or current smoking and the disease appears to be not obvious (Model 2: past smokers: OR, 1.32; 95%CI, 0.84–2.09; P = 0.229; light current smokers: OR, 1.05; 95%CI, 0.70–1.59; P = 0.801; heavy current smokers: OR, 1.26; 95%CI, 0.81–1.96; P = 0.295).
Table 4.
Results of ordinal logistic regression analyses for the related factors of the common colda
| Variables | Statistical ordinal logistic parameters | |||||
|---|---|---|---|---|---|---|
| Model 1b | Model 2c | |||||
| β | OR(95% CI) | P-value | β | OR(95% CI) | P-value | |
| Cigarette smoking categories | ||||||
| Non-smokers | Ref | Ref | ||||
| Passive smokers | 0.49 | 1.64(1.32–2.03) | < 0.001 | 0.46 | 1.59(1.27–1.99) | < 0.001 |
| Past smokers | 0.29 | 1.34(0.85–2.10) | 0.207 | 0.28 | 1.32(0.84–2.09) | 0.229 |
| Light current smokers | 0.18 | 1.19(0.80–1.77) | 0.384 | 0.05 | 1.05(0.70–1.59) | 0.801 |
| Heavy current smokers | 0.33 | 1.39(0.92–2.11) | 0.121 | 0.24 | 1.26(0.81–1.96) | 0.295 |
| Frequency of leisure-time exercise | ||||||
| Low (< 4 days/month) | Ref | Ref | ||||
| Medium (1–2 days/week) | − 0.11 | 0.89(0.71–1.13) | 0.344 | − 0.04 | 0.96(0.76–1.22) | 0.764 |
| High (≥3 days/week) | − 0.45 | 0.64(0.48–0.84) | 0.001 | −0.31 | 0.74(0.55–0.98) | 0.035 |
aEpisodes of self-reported common cold in the previous year
Ordinal logistic regression models were used to analyse the association of cigarette smoking status, frequency of leisure-time exercise and self-reported frequency of common cold
bAdjusted models control for gender and age
cAdjusted models control for gender, age, ethnicity, education levels, BMI, frequency of drinking, sleep duration, SRSS, SRH, self-perceived life stress, self-perceived work stress, SAS, SDS, LY, NE and MO
Statistical significance: P < 0.05
Physical activity and the common cold
When retrospective episodes of the common cold were divided by leisure-time exercise groups, at least one episode of the common cold was reported by 84.8% of the low group (< 3 days/month), 82.4% of the medium group (1–2 days/week), and 73.9% of the high group (≥3 days/week) (data not shown). Table 3 and Fig. 2 show the trend of an increase in the percentage of people who had not caught a cold in the previous year with increasing frequency of leisure-time exercise, while there was an inverse trend for the proportion of individuals experiencing three or more episodes of the common cold.
Fig. 2.
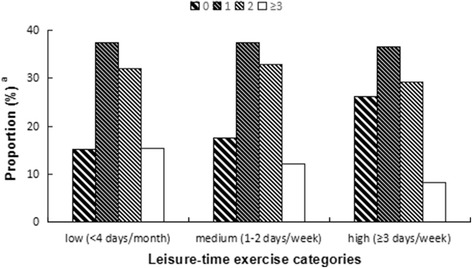
Observed association between frequency of common cold and frequency of leisure-time exercise. aRepresent the proportion of self-reported common cold in the categorized leisure-time exercise
Inspection of the ORs adjusted for gender and age reveals a significant association between the risk of the common cold and the frequency of leisure-time exercise. The risk of experiencing more than one episode of the common cold during a 12-month prospective period was 36% lower in the high vs. low exercise groups (Table 4, Model 1 high group: OR, 0.64; 95%CI, 0.48–0.84; P = 0.001). After multivariable adjustment, the high group of leisure-time exercise was associated with a 26% reduced risk of having at least one episode of the common cold in comparison to the low group. Inclusion of these variables resulted in a 10.0% increment in the OR of leisure-time exercise associated with self-reported common cold in the high vs. low groups for leisure-time exercise levels, but a relevant effect size remained robust (Model 2 high group: OR, 0.74; 95%CI, 0.55–0.98; P = 0.035).
The effect of the interaction between leisure-time exercise and cigarette smoking on the common cold
Table 5 presents the risk of the common cold for leisure-time exercise by cigarette smoking status. These results indicate that an inverse association between the high group of leisure-time exercise and the risk of experiencing at least one episode of the common cold was significant in non-smokers (OR, 0.54; 95%CI, 0.35–0.84; P = 0.006), especially in women (OR, 0.48; 95%CI, 0.28–0.80; P = 0.005). For past smokers, the protective effect of a high frequency of leisure-time exercise appears to approach marginal significance (OR, 0.22; 95%CI, 0.05–0.96; P = 0.045), while the frequency of leisure-time exercise was found to have no statistically significant influence on the risk of the common cold in current and passive smokers. (current smokers: OR, 0.68; 95%CI, 0.33–1.43; P = 0.313; passive smokers: OR, 1.15; 95%CI, 0.69–1.93; P = 0.595).
Table 5.
Leisure-time exercise and OR of common cold by cigarette smoking categories
| Frequency of leisure-time exercise | Non-smokers | Passive smokers | Past smokers | Current smokers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | OR(95% CI)a | P-value | β | OR(95%CI)a | P-value | β | OR(95% CI)a | P-value | β | OR(95% CI)a | P-value | |
| All sample | ||||||||||||
| Low (< 4 days/month) | Ref | Ref | Ref | Ref | ||||||||
| Medium (1–2 days/week) | −0.204 | 0.82(0.58–1.15) | 0.246 | 0.079 | 1.08(0.71–1.65) | 0.713 | 0.917 | 2.50(0.60–10.46) | 0.209 | −0.227 | 0.80(0.41–1.53) | 0.495 |
| High (≥3 days/week) | −0.615 | 0.54(0.35–0.84) | 0.006 | 0.140 | 1.15(0.69–1.93) | 0.595 | −1.529 | 0.22(0.05–0.96) | 0.045 | −0.381 | 0.68(0.33–1.43) | 0.313 |
| Men | ||||||||||||
| Low (< 4 days/month) | Ref | Ref | Ref | Ref | ||||||||
| Medium (1–2 days/week) | −0.003 | 1.00(0.49–2.02) | 0.994 | −0.724 | 0.48(0.20–1.20) | 0.116 | 0.814 | 2.26(0.53–9.71) | 0.274 | −0.217 | 0.80(0.41–1.57) | 0.523 |
| High (≥3 days/week) | −0.128 | 0.88(0.37–2.09) | 0.772 | −0.200 | 0.82(0.31–2.17) | 0.688 | −1.532 | 0.22(0.05–0.96) | 0.045 | −0.391 | 0.68(0.32–1.43) | 0.306 |
| Woman | ||||||||||||
| Low (< 4 days/month) | Ref | Ref | ||||||||||
| Medium (1–2 days/week) | −0.221 | 0.80(0.54–1.20) | 0.283 | 0.231 | 1.26(0.77–2.05) | 0.351 | – | – | ||||
| High (≥3 days/week) | −0.744 | 0.48(0.28–0.80) | 0.005 | 0.047 | 1.05(0.55–1.99) | 0.887 | – | – | ||||
Ordinal logistic regression models were used to analyse the association of leisure-time exercise and OR of the common cold, both globally and stratified by gender
aAdjusted models control for gender, age, ethnicity, education levels, BMI, frequency of drinking, sleep duration, SRSS, SRH, self-perceived life stress, self-perceived work stress, SAS, SDS, LY, NE and MO
Statistical significance: P < 0.05
Discussion
Poor sleep, poor self-rated health, mental stress, anxious symptoms and depressive symptoms are risk factors of URTI, as confirmed in other studies [27, 45–47]. However, compared with these factors, lifestyle strategies for URTI prevention can be relatively easy to implement and have received consistent scientific supports. We combined data from the cross-sectional study revealing that passive smoking and a high frequency of leisure-time exercise (≥3 days/week) were important correlates of the retrospective frequency of the common cold in the general population. The association was independent of socio-demographic, SRSS, SRH, health-related behaviours, psychological variables and biomarkers.
We found that the probability of suffering from at least one episode of the common cold during the previous year was higher in passive smokers than in non-smokers, while the impact of active smoking on the frequency of the common cold was not observed. The results were similar to other investigations of the association between cigarette smoking and RTI [21, 48]. In the trial by Bensenor and his colleagues in 2001 [21], the risk of more frequent colds was higher for women who were non-smokers with passively exposed to cigarette smoking (RR, 1.33; 95%CI, 1.18–1.51), while current light (<25cigarettes/day) (RR, 0.94; 95%CI, 0.81–1.10) and heavy (≥25cigarettes/day) (RR, 1.17; 95%CI, 0.81–1.10) smokers had no appreciable association with the frequency of common cold relative to women who were non-smokers without exposed to passive smoke. German and his colleagues also indicated that they did not find an association between smoking and symptomatic respiratory infection in a cohort of military recruits in Greece [48]. In addition, previous studies performed in children provide some supports for an impact, such that children who were exposed to environmental tobacco smoke had higher risk for URTI than children who lived in a smoke-free environment [22, 23].
Nevertheless, the findings presented should be interpreted with caution. Although we data fail to show that there was an increase in the frequency of the common cold in active smokers, previous studies indicated that increased damages by URTI in active smokers were attributable to increased duration and severity of the URTI symptoms [19, 21] and the restricted the efficacy of anti-infectious therapy [18, 49]. Furthermore, other earlier experimental and epidemiological studies observed that active smokers had a higher incidence of URTI [24, 50].
The discrepancy shown in our study might be explained by the limitation that the proportion of active smokers was too low (15.1%), which may have led to a false negative association for current smokers. To determine whether active smoking is a risk factor for the frequency of common cold, a sufficiently large sample size is needed to be collected in our study.
Another possible explanation for the results is that passive smokers mainly absorbed side-stream tobacco smoke drifting from lit cigarettes, and there were higher concentrations of toxic gases, including ammonia and dimethylnitrosamine, in side-stream than in mainstream smoke inhaled by active smokers [18, 51]. It has been reported that fresh side-stream smoke at concentrations commonly encountered indoors is well above the 2 mg/m3 reference concentration, and the level can cause respiratory tract epithelium damage [52]. In addition, non-smokers were more vulnerable to effects of tobacco smoke than past and current smokers [21], because their cardiopulmonary function is probably less “adaptable to tobacco smoke”. Tobacco smoke has a suppressive effect on the protective functions of immunocyte and adaptive immune mechanisms, and had a direct effect on microbial pathogens to enhance the probability of infectious disease, specifically promoting microbial virulence and antibiotic resistance [18, 53].
Regular moderate PA enhanced resistance to URTI, which can reduce the number of episodes, clinical symptoms and duration of URTI [27, 36, 54]. A cohort study performed in healthy adults aged 20–70 years indicated that there was a “J”-shaped relationship of PA and the risk of URTI, and high levels of moderate-vigorous activity were associated with a reduced risk for URTI relative to low levels of activity [36]. An epidemiological study conducted by Nieman et al. showed that the frequency of near-daily aerobic activity was correlated with reduced severity and duration with URTI symptoms during a 12-week period [54]. By contrast, among the frail elderly nursing home population, a 32-week exercise intervention was not associated with a change in the incidence of URTI [26]. Moreover, an earlier study performed in elderly women also concluded that 12 weeks of moderate cardiorespiratory exercise did not bring about an improvement in immune function [25].
Our study supported the opinion that in the general healthy Chinese population, leisure-time moderate exercise at least three times per week was inversely related to the risk of experiencing at least one episode of the common cold, while a “J”-shaped association between leisure-time moderate and the risk of the common cold was not found. Given that the participants investigated in our study were staff members at a bank, a professional population, few individuals performed heavy physical labour or had an opportunity to participate in high levels of exercise training. Thus, the effects of high levels of exercise training on the risk of the common cold could not be found, as implied by the latter part of the “J”- shaped curve. Findings from the investigation demonstrated that the risk of having one or more episodes of the common cold was reduced by 26% in the high vs. low groups of leisure-time exercise after controlling for confounders. A four months follow-up period in the general population aged 20–60 years showed that high levels of PA (≥55 MET•h•d-1) were associated with an 18% reduced risk (IRR, 0.82; 95% CI, 0.69–0.98) of self-reported URTI compared with low level group(< 45 MET•h•d-1) [55]. A study of the symptomatology of URTI and habitual PA performed in elderly indicated that the risk for having at least three URTI episodes per year was 9% lower in subjects with higher 7-day recall moderate scores (OR, 0.91; 95% CI, 0.83–0.99) [27]. The reduction in the risk of the common cold with a high frequency of leisure-time exercise had more significance in our study is probably because the individuals we investigated are predominately nonathletic adults with sedentary lifestyle. Moderate PA has been deemed to improve immune competence in a manner, and the mechanisms for the effect of moderate exercise on the reduction of URTI risk are still being explored and debated. The physiological mechanism might be explained by the fact that immune system function is partially enhanced via increasing the activity of natural killer cells and the function of superior T cells [56]. Moreover, recent data suggest that moderate-intensity exercise can increase the secretion of immunoglobulin (Ig) A [57, 58], and salivary IgA plays a major role in the human mucosal immune system [59].
In addition, our data indicate that a high frequency of leisure-time exercise was associated with a reduced risk of experiencing at least one episode of the common cold in non-smokers, and the protective effect of a high level of leisure-time exercise appears to approach marginal significance in past smokers. However, we did not see that leisure-time exercise reduced the negative effects of cigarette smoke on common cold risk, as suggested by Hemila et al. [60]. The findings in their study showed that moderate PA had no material association with a lower common cold risk in middle-aged male smokers. A study performed in postmenopausal women compared moderate-intensity exercise individuals with those engaging in stretching sessions, finding that the incidence of URTI can be reduced in non-smoking women [61]. In contrast, the abovementioned study conducted by Fondell et al. indicated that a high level of PA was associated with a reduced risk of self-reported common cold for both smokers and non-smokers [55]. This difference is possibly explained by the fact that the association between leisure-time exercise and the common cold was modified by smoking status. Passive smokers were at a relatively high risk, irrespective of how much exercise they performed. Non-smokers and past smokers have eliminated the negative effects of cigarette smoke on the common cold. Therefore, the risk of the common cold was reduced as the frequency of moderate exercise increased. It is noteworthy that this phenomenon was not observed in current smokers. Considering that previous studies have confirmed the effect of active smoking on cold symptoms [19, 21], it is necessary to further explore the association between active smoking and the frequency of the common cold in our study.
Several shortcomings of the present study should be acknowledged. First, it was an observational study that relied on self-reports of the common cold without confirmation by laboratory examination. However, it is the subjective symptoms that affect the individual’s life and work status, and therefore the subjective outcome is more relevant for the purpose of public health [60]. Furthermore, episodes of the common cold and the exposure to main variables (leisure-time exercise and cigarettes smoking status) were also self-reported. Therefore, recall bias may exist in the process of information collection. Second, the associations among common cold risk and the intensity, duration and types of leisure-time exercise patterns, as well as the impact of daily activities, were not analysed. In fact, as mentioned before, considering the professional nature of participants investigated in our study, the likelihood of engaging in extreme intensity of leisure-time exercise would be slim. Third, we controlled for the SRH, SRSS, socio-demographic variables, psychological variables, biomarkers and other health-related behaviours, but there were still other possibilities we had not covered, for instance, the number of people with whom the participants may share an office or apartment and the health status of apartment mates and colleagues [62].
Conclusion
In summary, our study revealed that passive smoking was associated with higher risk of having at least one episode of a self-reported common cold during the previous year. Nevertheless, active smoking was not significantly related to the frequency of the common cold. In addition, a high frequency of leisure-time exercise (≥3 days/week) in the general population of healthy adults was associated with a lower risk of experiencing the self-reported common cold more than once than a low frequency group. For smokers and passive smokers, the protective effect of a high level of leisure-time exercise on the risk of the common cold does not appear to be obvious.
Acknowledgments
This work was supported by the medical examination centre of the First Hospital of Jilin University. We are grateful to all participants who participated in the health examination and agreed to be included in the study.
Funding
This study was supported by Jilin Provincial Science and Technology Grant, China (No. 201105074). Xiumin Zhang received the funding. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI
Body mass index
- LY
Lymphocyte
- MO
Monocyte
- NE
Neutrophil
- PA
Physical activity
- SAS
Self-Rating Anxiety Scale
- SDS
Self-Rating Depression Scale
- SRH
Self-rated health
- SRSS
Self-Rating Scale of Sleep
Authors’ contributions
XZ and HL conceived and designed the study. GZ, MY, PG, FW, XL, YP, XY, JM and ML conducted the field study and data management. GZ, HL and MH analysed and interpreted the data. GZ drafted the manuscript. XZ, HL, MH and JL participated in revising the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of Jilin University School of Public Health (No.2015-12-08), and written informed consent was obtained from all subjects before they participated in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112(Suppl 6A):4S–12S. doi: 10.1016/S0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 2.Heikkinen T, Jarvinen A. The common cold. Lancet (London, England) 2003;361(9351):51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinogradova KA, Bulgakova VG, Polin AN, Kozhevin PA. Microbial antibiotic resistance: resistome, its volume, diversity and development. Antibiot Khimioter. 2013;58(5–6):38–48. [PubMed] [Google Scholar]
- 4.Alpuche C, Garau J, Lim V. Global and local variations in antimicrobial susceptibilities and resistance development in the major respiratory pathogens. Int J Antimicrob Agents. 2007;30(Suppl 2):S135–S138. doi: 10.1016/j.ijantimicag.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Walter MJ, Castro M, Kunselman SJ, Chinchilli VM, Reno M, Ramkumar TP, Avila PC, Boushey HA, Ameredes BT, Bleecker ER, et al. Predicting worsening asthma control following the common cold. Eur Respir J. 2008;32(6):1548–1554. doi: 10.1183/09031936.00026808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet (London, England) 2002;359(9309):831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 7.Varkey JB, Varkey B. Viral infections in patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14(2):89–94. doi: 10.1097/MCP.0b013e3282f4a99f. [DOI] [PubMed] [Google Scholar]
- 8.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet (London, England) 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramley TJ, Lerner D, Sames M. Productivity losses related to the common cold. J Occup Environ Med. 2002;44(9):822–829. doi: 10.1097/00043764-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Dicpinigaitis PV, Eccles R, Blaiss MS, Wingertzahn MA. Impact of cough and common cold on productivity, absenteeism, and daily life in the United States: ACHOO survey. Curr Med Res Opin. 2015;31(8):1519–1525. doi: 10.1185/03007995.2015.1062355. [DOI] [PubMed] [Google Scholar]
- 11.Roelen CA, Koopmans PC, Notenbomer A, Groothoff JW. Job satisfaction and short sickness absence due to the common cold. Work (Reading, Mass). 2011;39(3):305–13. [DOI] [PubMed]
- 12.Nakata A, Takahashi M, Irie M, Ray T, Swanson NG. Job satisfaction, common cold, and sickness absence among white-collar employees: a cross-sectional survey. Ind Health. 2011;49(1):116–121. doi: 10.2486/indhealth.MS1202. [DOI] [PubMed] [Google Scholar]
- 13.Zhi XY, Zou XN, Hu M, Jiang Y, Jia MM, Yang GH. Increased lung cancer mortality rates in the Chinese population from 1973-1975 to 2004-2005: an adverse health effect from exposure to smoking. Cancer. 2015;121(Suppl 17):3107–3112. doi: 10.1002/cncr.29603. [DOI] [PubMed] [Google Scholar]
- 14.Stayner L, Bena J, Sasco AJ, Smith R, Steenland K, Kreuzer M, Straif K. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am J Public Health. 2007;97(3):545–551. doi: 10.2105/AJPH.2004.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Jee SH, Shin HR, Park EH, Shin A, Jung KW, Hwang SS, Cha ES, Yun YH, Park SK, et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer. 2014;14:406. doi: 10.1186/1471-2407-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura K, Nakagawa H, Sakurai M, Murakami Y, Irie F, Fujiyoshi A, Okamura T, Miura K, Ueshima H, Group E-JR Influence of smoking combined with another risk factor on the risk of mortality from coronary heart disease and stroke: pooled analysis of 10 Japanese cohort studies. Cerebrovasc Dis. 2012;33(5):480–491. doi: 10.1159/000336764. [DOI] [PubMed] [Google Scholar]
- 17.Lightwood JM, Coxson PG, Bibbins-Domingo K, Williams LW, Goldman L. Coronary heart disease attributable to passive smoking: CHD policy model. Am J Prev Med. 2009;36(1):13–20. doi: 10.1016/j.amepre.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect. 2013;67(3):169–184. doi: 10.1016/j.jinf.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health. 1993;83(9):1277–1283. doi: 10.2105/AJPH.83.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gryczynska D, Kobos J, Zakrzewska A. Relationship between passive smoking, recurrent respiratory tract infections and otitis media in children. Int J Pediatr Otorhinolaryngol. 1999;49(Suppl 1):S275–S278. doi: 10.1016/S0165-5876(99)00178-0. [DOI] [PubMed] [Google Scholar]
- 21.Bensenor IM, Cook NR, Lee IM, Chown MJ, Hennekens CH, Buring JE, Manson JE. Active and passive smoking and risk of colds in women. Ann Epidemiol. 2001;11(4):225–231. doi: 10.1016/S1047-2797(00)00214-3. [DOI] [PubMed] [Google Scholar]
- 22.Chatzimicael A, Tsalkidis A, Cassimos D, Gardikis S, Spathopoulos D, Tripsianis GA, Kambouri K, Aivazis V, Vaos G, Bouros D. Effect of passive smoking on lung function and respiratory infection. Indian J Pediatr. 2008;75(4):335–340. doi: 10.1007/s12098-008-0034-5. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Plata R, Rojas-Martinez R, Martinez-Briseno D, Garcia-Sancho C, Perez-Padilla R. Effect of passive smoking on the growth of pulmonary function and respiratory symptoms in schoolchildren. Rev Invest Clin. 2016;68(3):119–127. [PubMed] [Google Scholar]
- 24.Galvan Fernandez C, Suarez Lopez de Vergara RG, Oliva Hernandez C, Domenech Martinez E. Respiratory disease in young people and smoking. Archivos de bronconeumologia. 2000;36(4):186–190. doi: 10.1016/S0300-2896(15)30179-4. [DOI] [PubMed] [Google Scholar]
- 25.Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsen-Cannarella SL. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25(7):823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kapasi ZF, Ouslander JG, Schnelle JF, Kutner M, Fahey JL. Effects of an exercise intervention on immunologic parameters in frail elderly nursing home residents. J Gerontol A Biol Sci Med Sci. 2003;58(7):636–643. doi: 10.1093/gerona/58.7.M636. [DOI] [PubMed] [Google Scholar]
- 27.Kostka T, Praczko K. Interrelationship between physical activity, symptomatology of upper respiratory tract infections, and depression in elderly people. Gerontology. 2007;53(4):187–193. doi: 10.1159/000100017. [DOI] [PubMed] [Google Scholar]
- 28.Robson-Ansley P, Howatson G, Tallent J, Mitcheson K, Walshe I, Toms C, DUT G, Smith M, Ansley L. Prevalence of allergy and upper respiratory tract symptoms in runners of the London marathon. Med Sci Sports Exerc. 2012;44(6):999–1004. doi: 10.1249/MSS.0b013e318243253d. [DOI] [PubMed] [Google Scholar]
- 29.Nieman DC. Immune function responses to ultramarathon race competition. Medicina Sportiva. 2009;13(4):189–196. doi: 10.2478/v10036-009-0031-4. [DOI] [Google Scholar]
- 30.Tiollier E, Gomez-Merino D, Burnat P, Jouanin JC, Bourrilhon C, Filaire E, Guezennec CY, Chennaoui M. Intense training: mucosal immunity and incidence of respiratory infections. Eur J Appl Physiol. 2005;93(4):421–428. doi: 10.1007/s00421-004-1231-1. [DOI] [PubMed] [Google Scholar]
- 31.Mourtzoukou EG, Falagas ME: Exposure to cold and respiratory tract infections. International Journal of Tuberculosis & Lung Disease the Official Journal of the International Union Against Tuberculosis & Lung Disease 2007, 11(9):938–943. [PubMed]
- 32.Teng B, Zhang X, Yi C, Zhang Y, Ye S, Wang Y, Tong DQ, Lu B. the association between ambient air pollution and allergic rhinitis: further epidemiological evidence from Changchun, northeastern China. Int J Environ Res Public Health. 2017;14(3) [DOI] [PMC free article] [PubMed]
- 33.Dong GH, Qian Z, Liu MM, Wang D, Ren WH, Fu Q, Wang J, Simckes M, Ferguson TF, Trevathan E. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the seven northeastern cities study. Int J Obes (Lond) 2013;37(1):94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 34.Tam WW, Wong TW, Ng L, Wong SY, Kung KK, Wong AH. Association between air pollution and general outpatient clinic consultations for upper respiratory tract infections in Hong Kong. PLoS One. 2014;9(1):e86913. doi: 10.1371/journal.pone.0086913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleeson M. Immunological aspects of sport nutrition. Immunol Cell Biol. 2016;94(2):117–123. doi: 10.1038/icb.2015.109. [DOI] [PubMed] [Google Scholar]
- 36.Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, Hebert JR. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34(8):1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Ouchi E, Niu K, Kobayashi Y, Guan L, Momma H, Guo H, Chujo M, Otomo A, Cui Y, Nagatomi R. Frequent alcohol drinking is associated with lower prevalence of self-reported common cold: a retrospective study. BMC Public Health. 2012;12:987. doi: 10.1186/1471-2458-12-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons VL, Moriarity C, Jonas K, Moore TF, Davis KE, Tompkins L. Design and estimation for the national health interview survey, 2006-2015. Vital Health Stat 2. 2014(165):1–53. [PubMed]
- 39.JM L, SF Y, JX D, QB Z: Analysis rating of sleep state of 13273 normal persons. Health Psychol J 2000(03):351–353. (in Chinese).
- 40.Liu C, Xie H, Zhang X, Yu Y, Zhang X, Sun Y, Zhou L, Su B, Wang H. Health related management plans improve sleep disorders of patients with chronic liver disease. Int J Clin Exp Med. 2015;8(6):9883–9889. [PMC free article] [PubMed] [Google Scholar]
- 41.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 42.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Yang Y, Qiu X, Yang X, Pan H, Ban B, Qiao Z, Wang L, Wang W. Relationship between anxiety and burnout among Chinese physicians: a moderated mediation model. PLoS One. 2016;11(8):e0157013. doi: 10.1371/journal.pone.0157013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu L, Ren J, Cheng M, Tang K, Dong M, Hou X, Sun L, Chen L. Depressive symptoms and risk factors in Chinese persons with type 2 diabetes. Arch Med Res. 2004;35(4):301–307. doi: 10.1016/j.arcmed.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SG, Kim HC, Min JY, Hwang SH, Park YS, Min KB. A prospective study of work stressors and the common cold. Occup Med (Lond) 2011;61(1):53–56. doi: 10.1093/occmed/kqq141. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S, Janickideverts D, Doyle WJ. Self-rated health in healthy adults and susceptibility to the common cold. Psychosom Med. 2015;77(9):959. doi: 10.1097/PSY.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.German V, Kopterides P, Poulikakos P, Giannakos G, Falagas ME. Respiratory tract infections in a military recruit setting: a prospective cohort study. J Infect Public Health. 2008;1(2):101–104. doi: 10.1016/j.jiph.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohren DC, Swaen GM, Borm PJ, Bast A, Galama JM. Psychological job demands as a risk factor for common cold in a Dutch working population. J Psychosom Res. 2001;50(1):21–27. doi: 10.1016/S0022-3999(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 51.Behera SN, Xian H, Balasubramanian R. Human health risk associated with exposure to toxic elements in mainstream and sidestream cigarette smoke. Sci Total Environ. 2014;472:947–956. doi: 10.1016/j.scitotenv.2013.11.063. [DOI] [PubMed] [Google Scholar]
- 52.Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control. 2005;14(6):396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 54.Nieman DC, Henson DA, Austin MD, Sha W. Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med. 2011;45(12):987–992. doi: 10.1136/bjsm.2010.077875. [DOI] [PubMed] [Google Scholar]
- 55.Fondell E, Lagerros YT, Sundberg CJ, Lekander M, Balter O, Rothman KJ, Balter K. Physical activity, stress, and self-reported upper respiratory tract infection. Med Sci Sports Exerc. 2011;43(2):272–279. doi: 10.1249/MSS.0b013e3181edf108. [DOI] [PubMed] [Google Scholar]
- 56.Nieman DC, Henson DA, Austin MD, Brown VA. Immune response to a 30-minute walk. Med Sci Sports Exerc. 2005;37(1):57–62. doi: 10.1249/01.MSS.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- 57.Sloan CA, Engels HJ, Fahlman MM, Yarandi HE, Davis JE. Effects of exercise on S-IGA and URS in postmenopausal women. Int J Sports Med. 2013;34(1):81–86. doi: 10.1055/s-0032-1314817. [DOI] [PubMed] [Google Scholar]
- 58.Martins RA, Cunha MR, Neves AP, Martins M, Teixeira-Verissimo M, Teixeira AM. Effects of aerobic conditioning on salivary IgA and plasma IgA, IgG and IgM in older men and women. Int J Sports Med. 2009;30(12):906–912. doi: 10.1055/s-0029-1237389. [DOI] [PubMed] [Google Scholar]
- 59.Bishop NC, Gleeson M. Acute and chronic effects of exercise on markers of mucosal immunity. Front Biosci (Landmark Ed). 2009;(14):4444–56. [DOI] [PubMed]
- 60.Hemila H, Virtamo J, Albanes D, Kaprio J. Physical activity and the common cold in men administered vitamin E and beta-carotene. Med Sci Sports Exerc. 2003;35(11):1815–1820. doi: 10.1249/01.MSS.0000093616.60899.92. [DOI] [PubMed] [Google Scholar]
- 61.Chubak J, McTiernan A, Sorensen B, Wener MH, Yasui Y, Velasquez M, Wood B, Rajan KB, Wetmore CM, Potter JD, et al. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. 2006;119(11):937–942. doi: 10.1016/j.amjmed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 62.Sun Y, Wang Z, Zhang Y, Sundell J. In China, students in crowded dormitories with a low ventilation rate have more common colds: evidence for airborne transmission. PLoS One. 2011;6(11):e27140. doi: 10.1371/journal.pone.0027140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


