Abstract
Transforming growth factor β (TGFβ) signaling plays crucial roles in maintaining vascular integrity and homeostasis, and is established as a strong activator of vascular smooth muscle cell (VSMC) differentiation. Chronic inflammation is a hallmark of various vascular diseases. Although TGFβ signaling has been suggested to be protective against inflammatory aortic aneurysm progression, its exact effects on VSMC inflammatory process and the underlying mechanisms are not fully unraveled. Here we revealed that TGFβ1 suppressed the expression of a broad array of proinflammatory genes while potently induced the expression of contractile genes in cultured primary human coronary artery SMCs (HCASMCs). The regulation of TGFβ1 on VSMC contractile and proinflammatory gene programs appeared to occur in parallel and both processes were through a SMAD4-dependent canonical pathway. We also showed evidence that the suppression of TGFβ1 on VSMC proinflammatory genes was mediated, at least partially through the blockade of signal transducer and activator of transcription 3 (STAT3) and NF-κB pathways. Interestingly, our RNA-seq data also revealed that TGFβ1 suppressed gene expression of a battery of autophagy mediators, which was validated by western blot for the conversion of microtubule-associated protein light chain 3 (LC3) and by immunofluo-rescence staining for LC3 puncta. However, impairment of VSMC autophagy by ATG5 deletion failed to rescue TGFβ1 influence on both VSMC contractile and proinflammatory gene programs, suggesting that TGFβ1-regulated VSMC differentiation and inflammation are not attributed to TGFβ1 suppression on autophagy. In summary, our results demonstrated an important role of TGFβ signaling in suppressing proinflammatory gene program in cultured primary human VSMCs via the blockade on STAT3 and NF-κB pathway, therefore providing novel insights into the mechanisms underlying the protective role of TGFβ signaling in vascular diseases.
Keywords: Transforming growth factor β1, Vascular smooth muscle, Contractile phenotype, Inflammation, Autophagy
1. Introduction
The mature differentiated vascular smooth muscle cells (VSMCs) are the major structural and functional components of the vascular wall. These cells are highly specified by expressing a battery of genes encoding cyto-contractile proteins and ion channels, as well as a growing number of noncoding transcripts to together define the contractile VSMC phenotype for vascular homeostasis [1–3]. However, the differentiated phenotype of VSMCs is not terminal and can switch to a dedifferentiated synthetic mode in response to various pathophysiological stimuli, a process termed as VSMC phenotypic modulation. Synthetic VSMCs are highly proliferative, migratory, as well as proinflammatory. Their extensive accumulation underlies the pathogenesis of some prominent vascular diseases, such as atherosclerosis, in-stent restenosis, vein graft failure, and transplant arteriopathy [4–6]. Compared with the well-recognized role of VSMC phenotypic modulation in vascular pathologies, the hierarchical regulation among different gene programs during VSMC phenotypic modulation, remains largely unexplored.
Transforming growth factor β (TGFβ) superfamily members control diverse cellular processes including cell proliferation, differentiation, and apoptosis. TGFβ-triggered signals are transduced through multiple intracellular pathways, including the canonical SMAD-dependent and non-canonical SMAD-independent pathways, the latter involving other factors such as RhoA and mitogen-activated protein kinases (MAPKs) [7,8]. In addition, the canonical cascade can crosstalk with the non-canonical pathway, rendering the biological outcome of TGFβ signaling highly complex and context-dependent [9]. Therefore, in many disease contexts such as cancer, TGFβ signaling functions as a double-edged sword, making it difficult to target this pathway for therapies [10]. In VSMCs, TGFβ has been established as a strong activator of SMC differentiation involving both canonical and non-canonical pathways [11–14], therefore playing an essential role in maintaining vascular integrity and homeostasis. Consistent with this notion, disruption of the key components of this pathway such as TGFβ receptor II (TβRII) [15,16], SMAD3 [17] or SMAD4 [18,19] in VSMCs leads to vascular defects, including enhanced neointimal hyperplasia in response to injury, spontaneous aortic dilation, dissection, and aneurysm formation, which is largely attributed to the perturbation in contractile apparatus and/or the activation of proinflammatory gene program. Further, systemic blockade of TGFβ activity by a TGFβ neutralizing antibody promotes Angiotensin II (Ang II) [20] and elastase [21] induced abdominal aortic aneurysm (AAA) formation. Similarly, attenuation of TGFβ signaling has been recently reported to contribute to the progression of atherosclerosis via crosstalk with FGF pathway [22]. These studies underscore the beneficial role of TGFβ signaling in maintaining vessel homeostasis and protecting against aneurysm formation and atherosclerosis, regardless of the detrimental influence of this pathway in other vascular pathologies such as calcification, matrix deposition, arterial stiffening, and pulmonary hypertension [23–25], However, in contrast to the well-known action of TGFβ signaling to turn on VSMC contractile gene transcription, the precise role of this signal cascade in VSMC inflammation and the involved mechanisms are incompletely understood.
The signal transducer and activator of transcription 3 (STAT3) has been indicated as one of the key transcription factors participating in pathological vascular remodeling [26]. Activated STAT3 has been reported to be highly expressed in atherosclerotic lesions and medial VSMCs from injured vessels, regulating key inflammatory genes which are differentially expressed in pathological vessels [26–28]. Thus far, the major consequence of STAT3 activation in VSMCs has been demonstrated to promote cell proliferation, migration, survival [29,30], and inhibit MYOCD-activated contractile gene expression [31]. However, it remains unclear whether STAT3 is involved in TGFβ-regulated VSMC contractile and proinflammatory gene programs. In addition, TGFβ signaling has been shown to crosstalk with nuclear factor κB (NF-κB) pathway [32–37], but the interplay of these two integral pathways and the resulted functional consequences upon the activation of TGFβ cascade in VSMCs are not fully unraveled.
There is an increasing interest in surveying the role of autophagy in vascular diseases. Generally, autophagy is considered as cytoprotective, by which long-lived cytoplasmic components are sequestered in double-membrane autophagosomes and degraded on fusion with lysosomal compartments [38]. Defective autophagy is related to increased inflammation, as characterized by the activation of the inflammasome. Autophagy has been shown to be activated in early atherosclerotic lesions to protect plaque cells against inflammation [39,40]. In VSMCs, autophagy has been shown to promote cell survival [41] and regulate phenotype switching [42,43], and impaired autophagy accelerates the stress-induced premature senescence and atherogenesis [44]. However, thus far virtually nothing is known with respect to the role of TGFβ in VSMC autophagy and how it links to other TGFβ-controlled processes such as VSMC differentiation and inflammation.
In order to dissect the full transcriptome regulated by TGFβ signaling in VSMCs and therefore gain more insights into the mechanism underlying its protective role in vascular diseases, we performed RNA-deep sequencing (RNA-seq) in human coronary arterial SMCs (HCASMCs) stimulated by TGFβ1. We found that, beyond the expected highly induced expression of VSMC contractile genes, there is a marked reduction in a broad array of proinflammatory genes such as proinflammatory cytokines/chemokines and critical activators of inflammation, a finding supporting the protective role initiated by TGFβ signaling in the progression of vascular diseases. Mechanistically, though TGFβ1-activated VSMC differentiation and -suppressed inflammation are both mediated by SMAD4-dependent canonical pathway, these two processes appear to occur in parallel. Further, we found that the suppression on VSMC proinflammatory gene expression exerted by TGFβ1 is mediated partially by the blockade on both STAT3 and NF-κB pathways. Interestingly, TGFβ1 also suppresses gene expression of a repertoire of autophagy mediators. Though depletion of ATG5, one of the major autophagy mediators promotes VSMC contractile phenotype while inhibiting proinflammatory gene program, it fails to influence TGFβ1 regulated VSMC contractile and proinflammatory gene programs. Our results therefore revealed two important pathways required by TGFβ signaling to suppress VSMC inflammation, providing mechanistic insight into the protective role of TGFβ signaling in inflammatory vascular diseases such as aortic aneurysm and atherosclerosis.
2. Materials and methods
2.1. Cell culture and treatment
Primary HCASMCs were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Human aortic SMCs (HASMCs) and mouse aortic SMCs (MASMCs) were prepared by the cell culture core in the Department of Molecular and Cellular Physiology at Albany Medical College. HCASMCs and HASMCs were maintained in Medium 231 (Gibco, Gaithersburg, MD, USA) at 37 °C, 5% CO2 supplemented with smooth muscle growth supplement (SMGS, Gibco). MASMCs were maintained in Medium DMEM/F-12 (Gibco) at 37 °C, 5% CO2 supplemented with 10% fetal bovine serum.
For TGFβ1 treatment, subconfluent HCASMCs or HASMCs were serum starved overnight and then treated with TGFβ1 (2 ng/ml, 5 ng/ml for MASMCs, R&D, Minneapolis, MN, USA) for different time periods. For inflammatory induction by IL1β, subconfluent HCASMCs were serum starved 24 h and then treated with TGFβ1 (2 ng/ml) overnight followed by stimulation with IL1β (4 ng/ml, R&D) for 15 min.
2.2. RNA deep sequencing (RNA-seq)
RNA-seq analysis of HCASMCs and detailed information of library construction was described previously [45]. Briefly, total RNA from HCASMCs treated with TGF β1 or vehicle control for 24 h was isolated using the miRNeasy RNA extraction kit (Qiagen, Valenica, CA, USA) and submitted for RNA-seq at the University of Rochester Medical Center’s Genomics Research Center. RNA samples were initially analyzed by bioanalyzer for quality control confirmation. RNA-seq was done with the polyadenylated RNA fraction at a depth of 20 million reads per replicate using the Illumina HISeq 2500. Raw sequence reads were pre-processed by using CASAVA 1.8.2 for demultiplexing, Sequence Cleaner (http://sourceforge. net/projects/seqclean/) for transcript trimming of contaminating sequences, UniVec database (http://www.ncbi.nlm.nih.gov/VecScreen-/UniVec.html) for removal of vector sequences, and FASTX Toolkit (http://cancan.cshl.edu/labmembers/gordon/fastx_-toolkit/index.html) for FASTQ quality trimming. Sequence reads were aligned to annotated transcripts on the UCSC Reference Genome (build GRCh37/hg19) by using SHRiMP2.2.3. Cufflinks 2.0.2 and Cuffdiff2 (http://cufflinks.cbcb.umd.edu) were utilized for quantitative analysis. Finally, the expression value of the all transcripts was presented as FPKM (fragments per kilobase of exon per million fragments mapped). Volcano plot was generated with cummerbund (http://compbio.mit.edu/cummeRbund/). RNA-seq data were deposited in the NIH Gene Expression Omnibus (GEO) under the accession number of GSE85910.
2.3. RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA from cultured HCASMCs/HASMCs/MASMCs was isolated using the miRNeasy Kit (Qiagen). cDNA was synthesized using an iScript cDNA kit (Bio-Rad, Hercules, CA, USA). IQ SYBR Green based qRT-PCR was performed in a MyiQ real-time PCR detection system (Bio-Rad). mRNA levels were expressed relative to 18S ribosomal RNA or GUSB which were used as internal loading control. Technical duplicates of each sample were examined. PCR primers were included in Supplementary Table 1.
2.4. Enzyme-linked immunosorbent assay (ELISA)
CXCL1 and CXCL6 protein levels in cell culture supernatants were determined using commercial ELISA kits (Thermo Fisher Scientific) according to the manufacturer’s instructions. Absorbance was read at 450 nm using an iMark™ Microplate Absorbance Reader (Bio-Rad). Concentrations of cytokines were calculated based on a standard curve for each cytokine.
2.5. Small interfering RNA (siRNA) transfection
Silence selective siRNA to human SMAD4 (siRNA ID: s8404) and ATG5 (siRNA ID: s18160) were purchased from Thermo Fisher Scientific. Silencer® Select Negative Control No. 1 (cat# 4390843) was used as siRNA negative control. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to deliver siRNA at a dose of 25 nM to HCASMCs according to the manufacturer’s instruction. Following 5 h siRNA transfection, cells were refed with fresh growth medium for 24 h before treatment as indicated. RNA or protein was extracted 48–72 h after transfection, and qRT-PCR or western blot was used to determine knockdown efficiency.
2.6. Adenoviral transduction
Adenovirus-STAT3C and Adenovirus-LacZ (negative control) were obtained as previously reported [46]. Briefly, murine STAT3-C construct was obtained from Addgene (Addgene plasmids #8722) and TOPO cloned into pCR-Blunt II-Topo vector. An EcoRI and AgeI fragment was then subcloned into a Gateway® TurboGFP-N vector (Addgene plasmid #20754). The resulting vector pANTHR-STAT3-C was utilized for constructing Adenovirus by using pAdCMV/V5-DEST acceptor vector system (Invitrogen). Viral particles were collected from QBI-HEK-293A cells (Qbiogene) and purified through two consecutive centrifugations in CsCl gradients and titrated by Adeno-X™ Rapid Titer Kit from Clontech. HCASMCs were fed in fresh growth medium (Medium 231 supplemented with smooth muscle growth supplement SMG as described above) containing equal amount of adenovirus-STAT3C or adenovirus-Lacz (MOI = 100) for 24 h. Cells were then refreshed with growth medium for another 24 h before protein or RNA isolation.
2.7. Whole cell lysate preparation and western blot
HCASMCs were rinsed in cold phosphate-buffered saline (PBS) twice and total protein was extracted with ice-cold lysis buffer (Cell Signaling Technology, Danvers, MA, USA) supplemented with a protease inhibitor cocktail (1%; Sigma-Aldrich, St. Louis, MO, USA), PMSF (1 mM; Sigma-Aldrich), Na3VO4 (1 mM), and NaF (50 mM). Protein concentration was measured by a detergent-compatible protein assay kit (Bio-Rad).
Equal amounts of protein were resolved by SDS-PAGE, transferred onto PVDF membranes, blocked with 5% nonfat milk for 1 h, and then incubated with the indicated primary antibody overnight at 4 °C. After 1 h incubation with the appropriate secondary antibody, specific signals were detected by SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific).
Primary antibodies used were as follows: TUBA (Sigma-Aldrich, #T5168), ACTB (Sigma-Aldrich, #A5441), GAPDH (Thermo Fisher Scientific, #MA5–15738), ACTA2 (Sigma-Aldrich, #A2547), CNN1 (Dako, Carpinteria, CA, USA, #M3556), TAGLN (Abcam, Cambridge, MA, USA, #ab10135), TGFB1I1(BD Biosciences, Franklin Lakes, NJ, USA, #611165), IL1B (Cell Signaling, #12242), IL1RI (Abcam, #ab106278), MYD88 (Cell Signaling, #4283), p-SMAD2 (Cell Signaling, #3101), p-MAPK8 (Cell Signaling, #9251), SMAD4 (Cell Signaling, #9515), p-STAT3 (Santa Cruz, Dallas, TX, USA, #sc-8059), STAT3 (Santa Cruz, #sc-482), p-RELA (Cell Signaling, #3033), RELA (Cell Signaling, #8242), p-IKBKB (Cell Signaling, #2697), NFKBIA (Cell Signaling, #4814), MAP1LC3A (Cell Signaling, #4599) and ATG5 (Santa Cruz, #sc-133158).
2.8. Immunofluorescence staining and quantification
HCASMCs were seeded in 35 mm plates mounted with coverslips. Cells were then washed twice with PBS and fixed in freshly prepared 4% paraformaldehyde for 10 min at room temperature (RT). After rinsing 3 times with PBS-Tween20, cells were permeabilized with 0.5% Triton X-100 for 10 min and then blocked with Protein Block buffer (Dako, #0909) for 30 min at RT. Cells were then incubated with a 1:400 diluted of RELA rabbit mAb (Cell Signaling, #8242) or MAP1LC3A rabbit mAb (Cell Signaling, #4599) at 37 °C for 1 h followed by the incubation with 1:300 diluted goat anti-rabbit IgG Alexa Fluor 488 secondary antibody (Invitrogen) for another 1 h. Fluorescent signals were captured by a confocal microscope (Leica Microsystems, Wet-zlar, German) and processed by Photoshop (Adobe, San Jose, CA, USA). All images were captured and processed under equivalent conditions. ≥100 cells were randomly selected from 3 separate experiments for quantitative analysis. Image J was utilized to analyze the relative intensity (nuclear/cytoplasmic) of RELA signal, and the puncta number of MAP1LC3A.
2.9. Chromatin immunoprecipitation (ChIP) assay for RELA
ChIP assay was described previously [45]. Briefly, 2 × 107 HCASMCs were cross-linked with 1% formaldehyde, lysed with Farnham lysis buffer, and sonicated with Bioruptor UCD-200 (Diagenode, Denville, NJ, USA) to obtain chromatin fragments with 300–1000 bp in length. 1/10 of the total chromatin was included as input. Chromatin complexes were precipitated with RELA rabbit mAb (Cell Signaling, #8242), while the same amount of ChIP-grade rabbit IgG control (Abcam, Cambridge, MA, USA) was used as negative control. After reverse cross-linking, precipitated DNA samples were subjected to qPCR that amplified the putative NF-κB binding site within the promoter region of IL1β. PCR primers were included in Supplementary Table 1.
2.10. Statistics
Data were presented as mean ± S.E.M. and were representative of at least 3 independent experiments. GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. Differences were assessed by 2-tailed Student’s t-test or ANOVA followed by post hoc tests for multiple comparisons. The details of the statistical analysis for each experiment was shown in figure legends. P < 0.05 was considered statistically significant and indicated by asterisk(s) in figures. P < 0.05 and P < 0.01 were indicated by *and **, respectively.
3. Results
3.1. TGFβ1 suppresses inflammatory genes and induces SMC differentiation marker genes in human VSMCs
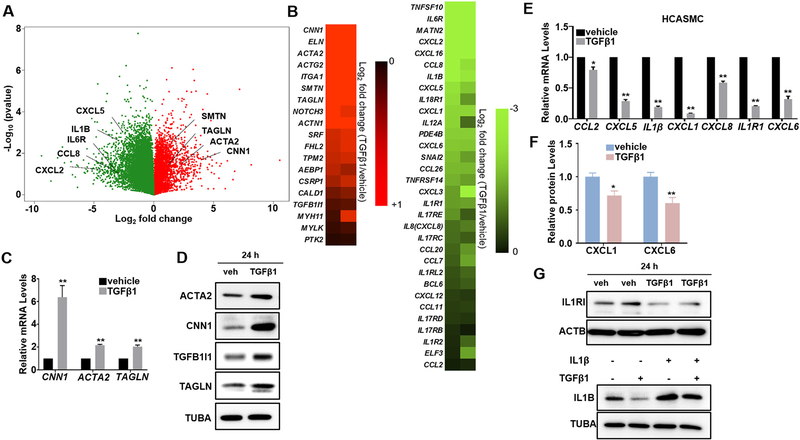
In order to define the complete transcriptome regulated by TGFβ signaling in VSMCs, we performed RNA-seq study in HCASMCs stimulated with TGFβ1 for 24 h. Analysis of each set of duplicate samples showed hundreds of genes were markedly induced or suppressed by TGFβ1 (Fig. 1A). Carefully examining these significantly regulated genes uncovered that most VSMC contractile genes were markedly induced by TGFβ1 (Fig. 1A, B Left, Supplementary Table 3A). As expected, the positive regulators of VSMC differentiation, SRF and MKL1 were induced while some established negative regulators, KLF4/5 and FOXO4 were suppressed by TGFβ1 (Supplementary Table 2). These results are consistent with the notion that TGFβ is an activator of VSMC differentiation. Of note, we also found that an array of inflammatory genes such as proinflammatory cytokines, chemokines, and adhesion molecules were dramatically decreased upon TGFβ1 stimulation (Fig. 1A, B Right, Supplementary Table 3B). We further validated the induction of TGFβ1 on VSMC contractile genes in HCASMCs by qRT-PCR (Fig. 1C) and western blot (Fig. 1D), as well as its suppression on proinflammatory genes, including CCL2, CXCL5, IL1β, CXCL1, IL8, IL1R1 and CXCL6 by qRT-PCR in HCASMCs (Fig. 1E). Similar results were found in human and mouse aortic SMCs (HASMCs, MASMCs) (Supplementary Fig. 1), suggesting that the repression of TGFβ1 on VSMC inflammation is general phenomena in VSMCs. To test if TGFβ1 suppression on the expression of proinflammatory genes occurs at protein levels, we performed enzyme-linked immunosorbent assay (ELISA) for supernatants collected from HCASMC cultures treated with TGFβ1 for 24 h. Consistently, we found that protein levels of CXCL1 and CXCL6 were significantly downregulated by TGFβ1 treatment (Fig. 1F). Western blot further confirmed that protein levels of IL1R1 and IL1B were decreased by TGFβ1 treatment (Fig. 1G). Taken together, these data demonstrate that beyond its activation on VSMC contractile gene program, TGFβ1 is also a suppressor of proinflammatory gene program in VSMCs.
Fig. 1.
TGFβ1 induces contractile genes and suppresses inflammatory genes in human VSMCs. Subconfluent human coronary arterial smooth muscle cells (HCASMCs) were starved overnight, followed by TGFβ1 (2 ng/ml) treatment for 24 h (A–F). For G, cells were starved for 24 h, treated with TGFβ1 (2 ng/ml) overnight and then IL1β (4 ng/ml) for 15 min. Total RNA was isolated for RNA-seq analysis. Volcano plot (A) illustrating gene expression changes by TGFβ1 treatment and heat map (B) illustrating contractile genes (left) and proinflammatory genes (right) modulated by TGFβ1 are shown. Changes in gene expression are displayed as the Log2 fold change of transcript levels in TGFβ1 relative to vehicle control treated cells. Biological replicates are included, with upregulated genes in red and downregulated genes in green (A, B). Total RNA was isolated for qRT-PCR validation of the indicated contractile and proinflammatory genes (C, E); whole cell lysates were collected for western blot of the indicated contractile proteins (D, G); supernatants from HCASMC cultures were collected for ELISA of the indicated chemokines (F). Representative western blot from at least 3 independent experiments is shown. ACTB/TUBA is used as internal loading control. Quantitative data are expressed as means ± S.E.M. of at least 3 independent experiments, reflecting fold changes of TGFβ1 treated to vehicle control values (set to 1). *P < 0.05, **P < 0.01 compared to vehicle control, unpaired two-tailed Student’s t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. TGFβ1 suppression on proinflammatory genes is parallel to its induction on VSMC contractile genes
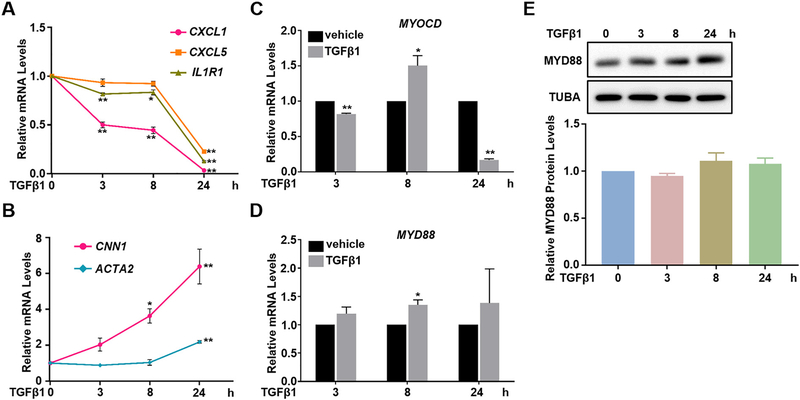
To examine the dynamics of TGFβ1 effect on VSMC proinflammatory and contractile genes in VSMCs, we treated HCASMCs with TGFβ1 for different time periods followed by qRT-PCR analysis of both gene programs. We found that an evident suppression on proinflammatory genes such as CXCL1, CXCL5, and IL1R1 was seen as early as 3 h, sustained at 8 h, and maximized at 24 h upon TGFβ1 treatment (Fig. 2A). Similar to the dynamic suppression on proinflammatory genes, the induction on contractile genes such as CNN1 also occurred as early as 3 h, whereas it further increased in a time-dependent manner. Compared with CNN1, obvious mRNA induction of ACTA2 was delayed to 24 h (Fig. 2B). These results suggest that the suppression on proinflammatory genes by TGFβ1 likely occurs in parallel and not secondary to its activation on contractile genes in VSMCs.
Fig. 2.
TGFβ1 suppression on inflammatory genes is parallel to its induction on VSMC contractile genes. Subconfluent HCASMCs were starved overnight, followed by TGFβ1 (2 ng/ml) treatment for the indicated time periods. Total RNA was isolated for qRT-PCR of the indicated genes (A–D); whole cell lysates were collected for western blot of MYD88 and its quantification (E). Representative western blot from at least 3 independent experiments is shown. TUBA is used as internal loading control. Quantitative data are expressed as means ± S.E.M. of at least 3 independent experiments, reflecting fold changes of TGFβ1 treated to vehicle control values (set to1; for A and B, the value of each time point was normalized to its own vehicle control value). *P < 0.05, **P < 0.01 compared to vehicle control, unpaired two-tailed Student’s t-test.
MYOCD, a potent co-activator of serum response factor (SRF) for the transcription of VSMC contractile gene program has been reported as a negative regulator of VSMC inflammation [47,48]. To test whether TGFβ1 suppression on proinflammatory genes was achieved through MYOCD, we performed qRT-PCR to assess the dynamics of MYOCD mRNA expression after TGFβ1 stimulation. Our result showed that TGFβ1-induced MYOCD mRNA occurred later than 3 h (Fig. 2C). The induction peaked at 8 h but declined at 24 h, a pattern in contrast to the early (earlier than 3 h) and sustained effects on both proinflammatory and contractile genes (Fig. 2A, B). In this context, we are unsure of MYOCD protein changes influenced by TGFβ1 due to the unavailability of authenticated MYOCD antibody for detecting endogenous MYOCD protein (ATVB [49]). Collectively, though MYOCD has been reported as a repressor of VSMC inflammation in vivo [47], it is inconsistently involved in TGFβ1-suppressed proinflammatory gene program in HCASMCs at least at mRNA level.
On the other hand, myeloid differentiation primary response 88 (MYD88) is the key adaptor protein functioning as an essential signal transducer in both interleukin 1 receptor (IL1R) and Toll-like receptor (TLR) signaling pathways to activate a variety of proinflammatory gene expression [49]. Previous studies have shown that TGFβ1 decreases MYD88 protein levels through a ubiquitination-dependent proteolysis pathway to suppress the expression of inflammatory genes in human dermal microvessel endothelial cells (HMEC), primary peritoneal macrophages and CMT-93 mouse epithelial cells [50,51]. We therefore asked whether similar mechanism occurs in HCASMCs. However, qRT-PCR showed that TGFβ1 caused a subtle upregulation, rather than downregulation, of MYD88 mRNA (Fig. 2D) whereas MYD88 protein levels were not affected throughout 48 h treatment (Fig. 2E, Supplementary Fig. 2). This indicates that the regulation of MYD88 protein stability is not involved in TGFβ1 suppression on proinflammatory genes in VSMCs.
3.3. TGFβ1-induced contractile and -suppressed inflammatory gene expression is SMAD4-dependent
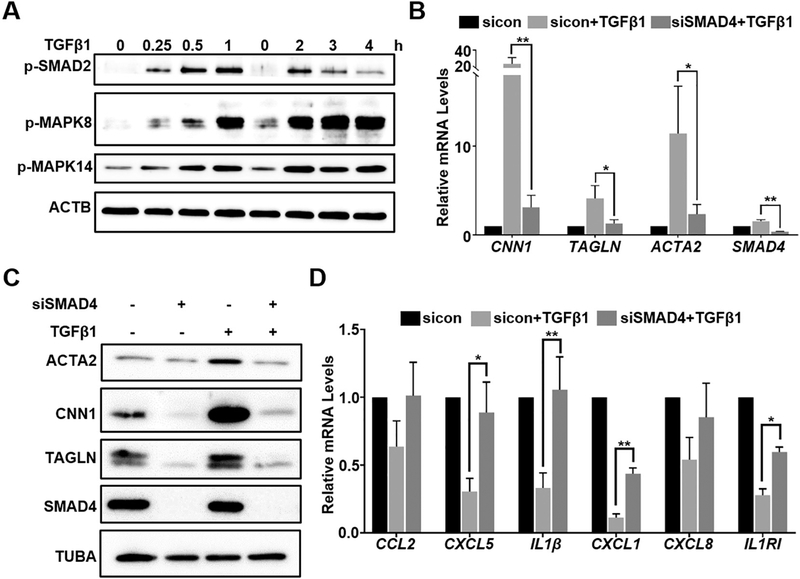
To identify the critical pathways underlying TGFβ1 suppression on proinflammatory gene program in HCASMCs, we first evaluated the time course activity of different pathways triggered by TGFβ1 signaling, including SMAD-dependent canonical and SMAD-independent non-canonical pathways including Jun N-terminal kinase (JNK, MAPK8) and p38 (MAPK14). Western blot showed that the phosphorylation of SMAD2 (p-SMAD2) was induced as early as 15 min, peaked at 1 h, and declined afterwards (Fig. 3A). The activation of MAPK8 and MAPK14 occurred similarly as early as 15 min, such activation was further enhanced up to 4 h following TGFβ1 treatment (Fig. 3A). Since both MAPK8 and MAPK14 pathways have been established to positively regulate proinflammatory genes [52,53], it is unlikely that the activation of these two non-canonical pathways was involved in TGFβ1-suppressed proinflammatory gene program. Since the activation of SMAD pathway preceded the changes of VSMC contractile and proinflammatory gene expression by TGFβ1, we then asked if SMAD pathway participates in TGFβ1 regulation on both gene programs. To test this, we used siRNA to deplete the endogenous common SMAD (co-SMAD), SMAD4 and then treated cells with TGFβ1 for 24 h. SMAD4 was effectively knocked down by around 70% as shown in Fig. 3B. siRNA-mediated depletion of SMAD4 caused a dramatic decrease in TGFβ1-induced expression of VSMC contractile genes, including CNN1, TAGLN, and ACTA2, at both mRNA and protein levels (Fig. 3B, C). This result is consistent with our previous published data [11]. Of note, knockdown of SMAD4 markedly rescued the suppression of TGFβ1 on proinflammatory genes such as CXCL5, IL1β, CXCL1, and IL1R1 (Fig. 3D). Collectively, these results suggest that the opposing regulation of TGFβ1 on VSMC contractile and proinflammatory gene programs is via SMAD-dependent canonical pathway.
Fig. 3.
TGFβ1-induced contractile and -suppressed inflammatory gene expression is SMAD4-dependent. Subconfluent HCASMCs were starved overnight, followed by TGFβ1 (2 ng/ml) treatment for the indicated time periods. Whole cell lysates were collected for western blot of the indicated signaling proteins (A). Growing HCASMCs were transfected with siRNA to SMAD4 (siSMAD4) or the same amount of negative control scramble RNA (sicon) for 48–72 h. Cells were then starved overnight followed by TGFβ1 (2 ng/ml) treatment for 24 h. Total RNA was isolated for qRT-PCR of SMAD4 and the indicated contractile/inflammatory genes (B, D); whole cell lysates were collected for western blot of the indicated contractile proteins (C). Representative western blot from at least 3 independent experiments is shown (For A, each “0” represents the vehicle control for its following time points). ACTB/TUBA is used as internal loading control. Quantitative data are expressed as means ± S.E.M. of at least 3 independent experiments, reflecting fold changes of (TGFβ1 + sicon/siSMAD4) to (sicon + vehicle control) values (set to 1). *P < 0.05, **P < 0.01, two-way ANOVA with Sidak’s post hoc test for multiple comparison correction.
3.4. TGFβ1-induced contractile and -suppressed proinflammatory genes expression is partially STAT3-dependent
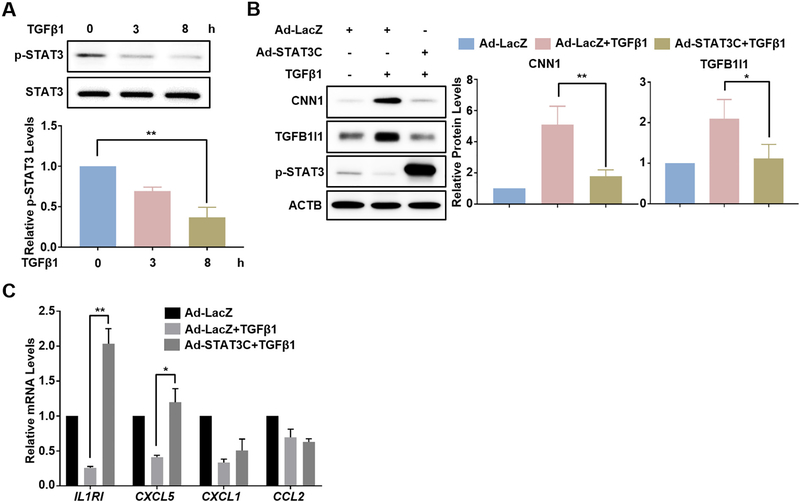
The JAK-STAT3 signaling pathway has been reported as an important pathway to negatively regulate VSMC differentiation [31]. Activated STAT3 is also known as an important proinflammatory mediator in vascular diseases [26–28]. To find out if STAT3 could mediate the regulation of TGFβ1 on VSMC contractile and proinflammatory gene programs, we first examined the influence of TGFβ1 on STAT3 activation. Western blot showed a robust suppression on the phosphorylation of STAT3 (p-STAT3) at 3 h and 8 h following TGFβ1 treatment in HCASMCs whereas total STAT3 protein levels were not altered (Fig. 4A). To test if such reduction on p-STAT3 could account for the influences of TGFβ1 on VSMC contractile and proinflammatory gene programs, we sought to perform rescue experiments by over-expressing a constitutively active form of STAT3 (STAT3C) in HCASMCs by adenovirus transduction. We first confirmed a marked increase in p-STAT3 followed by the transduction of adenovirus carrying STAT3C (Ad-STAT3C) (Fig. 4B). Interestingly, overexpression of STAT3C by Ad-STAT3C transduction almost abolished TGFβ1 induction on CNN1 and TGFB1I1 protein expression (Fig. 4B), whereas protein levels of other contractile genes such as ACTA2 and TAGLN were not significantly changed (Supplementary Fig. 3). This suggests that the suppression on p-STAT3 by TGFβ1 may partially account for the induction of TGFβ1 on VSMC contractile program. We then asked if the suppression on proinflammatory gene expression in VSMCs by TGFβ1 is also attributed to the reduction of p-STAT3. qRT-PCR showed that though forced activation of STAT3 by Ad-STAT3C transduction completely rescued TGFβ1-suppressed IL1R1 and CXCL5 as well as insignificantly rescued TGFβ1-suppressed CXCL1 gene expression (Fig. 4C), this rescue was not applied to other proinflammatory genes, such as IL1B, CXCL6, and CXCL8 (Supplementary Fig. 4). Taken together, these results suggest that TGFβ1-suppressed STAT3 activity underlies only partially the mechanism responsible for both TGFβ1 induction on VSMC contractile and suppression on proinflammatory genes in VSMCs.
Fig. 4.
TGFβ1-induced contractile and -suppressed inflammatory gene expression is partially STAT3-dependent. Subconfluent HCASMCs were starved overnight followed by TGFβ1 (2 ng/ml) treatment for the indicated time periods. Whole cell lysates were collected for western blot of STAT3 and p-STAT3 (A). Growing HCASMCs were transduced with constitutively active STAT3 adenovirus (Ad-STAT3C) or the same amount of negative control virus (Ad-LacZ) for 24 h. Cells were then starved overnight followed by TGFβ1 (2 ng/ml) treatment for 24 h. Whole cell lysates were collected for western blot of the indicated contractile proteins and the related quantifications (B); total RNA was isolated for qRT-PCR of the indicated inflammatory genes (C). Representative western blot from at least 3 independent experiments is shown. ACTB is used as internal loading control. Quantitative data are expressed as means ± S.E.M. of at least 3 independent experiments, reflecting fold changes of (TGFβ1 + Ad-LacZ/Ad-STAT3C) to (vehicle + Ad-LacZ) values (set to 1). *P < 0.05, **P < 0.01, one-way ANOVA with Dunnett’s post hoc test for multiple comparison correction (A); paired two-tailed Student’s t-test (B–C).
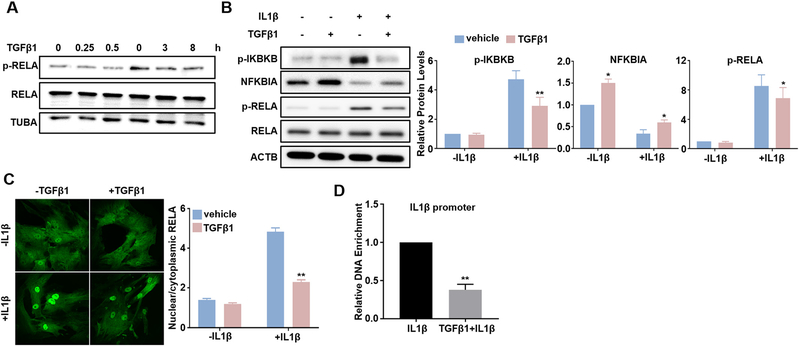
3.5. TGFβ1 inhibits the activation of p65/NF-κB pathway induced by IL1β in VSMCs
The NF-κB pathway has been established as a pivotal signaling pathway to transactivate proinflammatory genes. In general, proinflammatory stimuli phosphorylate the Inhibitor-κB Kinase (IKK), which in turn phosphorylates inhibitory κB protein (IκB) to facilitate its degradation, therefore releasing NF-κB to translocate into the nucleus to activate proinflammatory genes transcription [34,54]. Previous studies have reported that TGFβ signal can influence NF-κB pathway in multiple contexts [32–37]. To find out if NF-κB pathway involves in TGFβ1 suppression on proinflammatory genes in VSMCs, we evaluated the influence of TGFβ1 on NF-κB activation. At basal level, phosphorylation of p65 NF-κB (p-RELA) was relatively low and TGFβ1 only marginally suppressed p-RELA levels (Fig. 5A). We then treated HCASMCs with IL1β to activate NF-κB pathway. We observed a strong activation of NF-κB pathway in HCASMCs at 15 min after IL1β treatment, evidenced by robustly increased IKKβ (IKBKB) phosphorylation (p-IKBKB), decreased IκBα (NFKBIA) protein level, and increased p65 phosphorylation (p-RELA). Of note, pretreatment of TGFβ1 attenuated all the aforementioned effects exerted by IL1β (Fig. 5B). These results suggest that the inhibitory effect of TGFβ1 on NF-κB pathway is likely achieved through suppressing the upstream IKBKB activation, which ultimately leads to decreased RELA phosphorylation. To further confirm these results, we performed immunofluorescence staining of RELA in HCASMCs. Under the baseline level, RELA was distributed in both nucleus and cytoplasm of HCASMCs, and treatment of TGFβ1 caused little effect on RELA staining in both compartments (Fig. 5C, left). As expected, IL1β induced a striking nuclear translocation of RELA, and pretreatment of TGFβ1 attenuated the induction of nuclear-localized RELA triggered by IL1β (Fig. 5C, left). Quantitative analysis showed a 3-fold increase in the ratio of nuclear/cytoplasmic RELA at 15 min after IL1β treatment, and pretreatment of TGFβ1 decreased this ratio by around 50% (Fig. 5C, right). To further delineate the NF-κB-involved mechanism for TGFβ1 to suppress VSMC inflammation, we performed chromatin immunoprecipitation assays (ChIPs) in HCASMCs to test if TGFβ1 inhibits RELA binding to NF-κB site (s) in the regulatory region of proinflammatory genes. Human IL1β proximal promoter region harbors a computationally predicted and previously validated NF-kB site [55]. As expected, TGFβ1 significantly decreased the enrichment of RELA in the IL1β promoter region encompassing this NF-κB site induced by IL1β treatment for 15 min (Fig. 5D). Collectively, these data indicate that TGFβ1 blocked IL1β induced NF-κB pathway activation and suggest that the inhibition of TGFβ1 on VSMC proinflammatory gene program is at least partially through its blockade on RELA/NF-κB pathway.
Fig. 5.
TGFβ1 suppresses IL1β-activated RELA/NF-κB pathway. Subconfluent HCASMCs were starved overnight, followed by TGFβ1 (2 ng/ml) treatment for the indicated time periods. Whole cell lysates were collected for western blot of RELA and p-RELA (A). Subconfluent HCASMCs were starved for 24 h, treated with TGFβ1 (2 ng/ml) overnight and then IL1β (4 ng/ml) for 15 min. Whole cell lysates were collected for western blot of the indicated NF-κB signal proteins and the related quantifications (B); immunofluorescence staining of RELA (green) (C); Chromatin Immunoprecipitation (ChIP) assay for RELA binding to IL1β promoter encompassing a predicted NF-kB site, amplified DNA signal was normalized to isoform and species matched IgG negative control (D). Representative western blot and fluorescence images from at least 3 independent experiments are shown. TUBA/ACTB is used as internal loading control. Quantitative data are expressed as means ± S.E.M. of at least 3 independent experiments, reflecting fold changes of TGFβ1 and/or IL1β treated to vehicle control values (set to 1; for D, data reflect fold changes of (TGFβ1 + IL1β) treated to IL1β treated values (set to 1)). *P < 0.05, **P < 0.01 compared to vehicle control, two-way ANOVA with Sidak’s post hoc test for multiple comparison correction (B, C); **p < 0.01 compared to IL1β treated group, unpaired two-tailed Student’s t-test (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
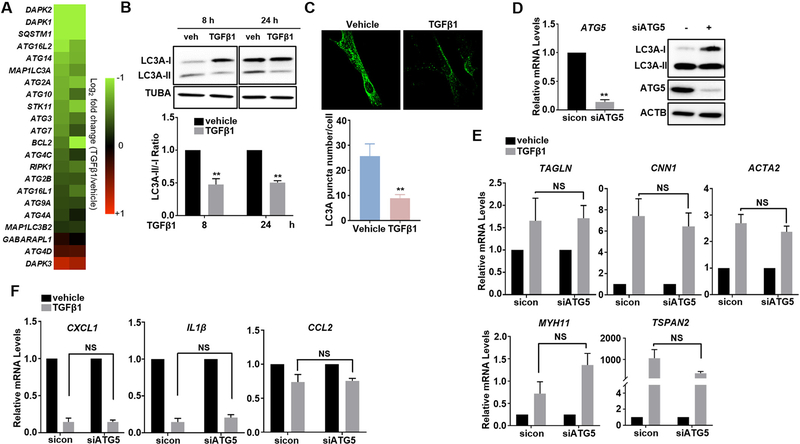
3.6. Regulation of contractile and proinflammatory gene programs by TGFβ1 is independent of its suppression on VSMC autophagy
Dysregulated autophagy has been frequently linked to vascular disorders such as restenosis and atherosclerosis [39,56], but the role of autophagy in VSMC phenotype determination and the regulation of TGFβ signaling on VSMC autophagy remain largely unexplored. An interesting finding while we analyzed our RNA-seq data was that TGFβ1 decreased gene expression of a wide array of autophagy mediators such as Microtubule Associated Protein 1 Light Chain 3A (MAP1LC3A, aka LC3A), autophagy-related (ATG) proteins, BCL2, Death Associated Protein Kinases (DAPKs) and Serine/Threonine Kinase 11 (STK11) (Fig. 6A). We validated this phenotype by western blot on LC3A conversion (LC3A-I to LC3A-II), the most widely used and also a more definitive method for measuring autophagic activity. TGFβ1-treated HCASMCs significantly decreased LC3A conversion, as quanti-fied by the LC3A-II/-I ratio. This reduction was sustained from 8 h till 24 h after TGFβ1 treatment (Fig. 6B). Similar results were also seen in HASMCs (Supplementary Fig. 5). Further, we performed immunostaining of endogenous LC3A to confirm the influence of TGFβ1 on VSMC autophagy. LC3 displays a distinct punctate distribution once autophagy is induced. Therefore, the number of LC3 puncta reflects the number of autophagosomes [57,58]. Our LC3A immunostaining showed that the fluorescent puncta in TGFβ1-treated HCASMCs significantly decreased compared with vehicle control treated cells (Fig. 6C). These results demonstrate that TGFβ1 suppresses autophagy in VSMCs. To determine if TGFβ1-suppressed autophagy is involved in TGFβ1-regulated VSMC contractile and proinflammatory gene programs, we performed siRNA studies to endogenous ATG5, one of the established primary autophagy mediators in VSMCs [59–61]. ATG5 was effectively depleted in HCASMCs by siATG5 at both mRNA and protein level, which caused an attenuation in LC3A conversion by western blot as expected (Fig. 6D). Interestingly, qRT-PCR showed that siRNA control and siRNA-ATG5 transfected HCASMCs exhibited equivalent induction on contractile gene expression (Fig. 6E) and reduction on proinflammatory gene expression (Fig. 6F) in response to TGFβ1 treatment, suggesting that regulation of TGFβ1 on VSMC contractile and inflammatory gene programs is independent of TGFβ1 suppression on VSMC autophagy.
Fig. 6.
TGFβ1-induced contractile and -suppressed inflammatory gene expression is independent of its suppression on VSMC autophagy. Subconfluent HCASMCs were starved overnight, followed by TGFβ1 (2 ng/ml) treatment for 24 h. Total RNA was isolated for RNA-seq analysis. Heat map described in Fig. 1 illustrating autophagic genes modulated by TGFβ1 (A); whole cell lysates were collected for western blot of LC3A conversion and the quantification of LC3A-II/-I ratio (B); immunofluorescence staining of LC3A (green) and the quantification of LC3A puncta number (C). Growing HCASMCs were transfected with siRNA to ATG5 (siATG5) or the same amount of negative control scramble RNA (sicon) for 48–72 h. Total RNA was isolated for qRT-PCR of ATG5 and whole cell lysates were collected for western blot of LC3A conversion and ATG5 protein expression (D). siATG5 and sicon transfected cells were then starved overnight followed by TGFβ1 (2 ng/ml) treatment for 24 h. Total RNA was isolated for qRT-PCR of the indicated contractile/inflammatory genes (E, F). Representative western blot and fluorescence images from at least 3 independent experiments are shown. TUBA/ACTB is used as internal loading control. Quantitative data are expressed as means ± S.E.M. of at least 3 independent experiments, reflecting fold changes of TGFβ1 treated to vehicle control values (for B, the value of each time point was normalized to its own vehicle control value (set to 1); for E–F, the value of each siRNA group was normalized to its own vehicle control value (set to 1)). *P < 0.05, **P < 0.01 compared to vehicle control, unpaired two-tailed Student’s t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Chronic inflammation is a critical hallmark of various prominent vascular diseases, including atherosclerosis and aortic aneurysm [47,62]. Though the protective role of TGFβ signaling in aneurysm and atherosclerosis has been reported by different groups using different knockout mouse models and neutralizing TGFβ antibody, the underlying mechanism is incompletely understood [18–22,63]. In this study, we first interrogated data from an unbiased genome wide RNA-seq study in human primary VSMCs treated with TGFβ1, and revealed that beyond the well-recognized role as an activator of VSMC differentiation, TGFβ1 also suppressed a wide range of proinflammatory genes. We validated the suppressive effect of TGFβ1 in primary VSMC cultures derived from different species and arterial sources (Fig. 1, and Supplementary Fig. 1). Further, we showed that the regulation of TGFβ1 on VSMC contractile and proinflammatory gene programs was SMAD4-dependent (Fig. 3) and occurred likely in parallel (Fig. 2). Mechanistically, we found that TGFβ1 negatively regulated VSMC proinflammatory gene program partially by suppressing the activities of STAT3 (Fig. 4) and NF-κB (Fig. 5) signaling pathways. Finally, we demonstrated that TGFβ1-treated HCASMCs exhibited notably decreased autophagic activity; however, such suppression was not involved in TGFβ1 regulation on both VSMC differentiation and inflammation (Fig. 6). To our knowledge, our study is the first to identify TGFβ signaling as a guardian to protect VSMCs from entering the detrimental proinflammatory program, therefore providing novel insights into the protective role of TGFβ signaling in chronic inflammatory vascular diseases.
It has been recognized that the synthetic VSMCs are proinflammatory, with high levels of expression of proinflammatory genes. However, it is unclear whether this proinflammatory feature of the synthetic VSMCs is attributed to the less contractile phenotype in these cells. We therefore aimed to determine if the suppressive effect of TGFβ1 on VSMC inflammation is secondary to TGFβ1 activation on the VSMC contractile phenotype. Our results showed that reduction of proinflammatory genes expression was in a time-dependent manner within the 24 h period of treatment, which was similar to the induction on the contractile gene CNN1 (Fig. 2). This finding revealed that there was no obvious temporal order of TGFβ1 effects on VSMC proinflammatory and contractile programs. This indicates that these two events are possibly parallel and mutually independent. In support of this notion, we observed that mRNA levels of MYOCD, a master transcription coactivator of VSMC differentiation, was transiently induced at a later time-point (8 h) but declined at 24 h upon TGFβ1 treatment (Fig. 2), suggesting that MYOCD may not be involved in the negative regulation of TGFβ1 on VSMC inflammation. Taken together, we consider that the suppression of the proinflammatory gene program is not the consequence of the induction of the contractile VSMC phenotype at least in the context of TGFβ1 stimulation in cultured primary VSMCs.
We further delved into the underlying molecular mechanisms, and provided several lines of evidence supporting that TGFβ1 suppresses NF-κB activity. It has been shown that crosstalk exists between components of TGFβ and NF-κB pathways in multiple contexts [32–37], and our work uncovered, for the first time, a link between these two pathways in human VSMCs. We observed that TGFβ1 markedly inhibited IL1β-induced phosphorylation of IKK and enhanced the protein stability of IκB (Fig. 5), both of which are upstream mediators in RELA/NF-κB pathway. MYD88 is essential for recruiting signaling components in IL1R/TLR-triggered activation of RELA/NF-κB [64–66]. However, in our studies we did not find significant influence of TGFβ1 on MYD88 protein levels (Fig. 2) regardless of the observed consistent striking reduction on p-IKBKB (Fig. 5). This implies that there exists unknown upstream mediator(s) directly controlled by TGFβ1 to converge the suppressive signal of TGFβ1 upon the RELA/NF-kB pathway. It should be noted that, the downstream RELA phosphorylation and nuclear translocation in response to IL1β stimulation were only reduced by 22% and 52%, respectively (Fig. 5). This suggests that the suppression of NF-κB pathway is only a partial mechanism through which TGFβ1 down-regulates proinflammatory gene expression. Therefore, we believe there might exist additional levels of regulation on proinflammatory gene programs. Indeed, our data implied that STAT3 antagonized TGFβ1 in VSMC differentiation and inflammation (Fig. 4). Since JAK/STAT3 signaling is known as an important inflammatory mediator in vascular diseases, and enhanced STAT3 activation can lead to induced inflammatory damage [26–28], we propose that inhibition of STAT3 activity is another mechanism through which TGFβ1 inhibits VSMC inflammatory responses. Whether there is crosstalk between NF-κB and STAT3 pathway warrants future investigation.
Despite the protective actions of autophagy in almost every organ system and an expanding list of diseases, the role of autophagy in VSMCs is poorly understood. Thus far, our limited understanding is that, many vascular disease-related stimuli, such as metabolic stress, reactive oxygen species (ROS), and oxidized lipids can trigger VSMC autophagy [39]; and activation of VSMC autophagy has been implicated as promoting cell survival by removing damaged organelles, especially defective mitochondria, and attenuating endoplasmic reticulum (ER) stress [67]. The relationship between TGFβ signaling and VSMC autophagy remains unclear, prompting us to investigate how TGFβ signaling contributes to this process, and how it links to other TGFβ-controlled events including VSMC differentiation and inflammation. In the present study, we showed that TGFβ1 counteracted VSMC autophagy primarily through the repressing mRNA levels of a broad range of autophagic mediator genes and decreasing the number of autophagosomes (Fig. 6). It has been reported that Platelet-Derived Growth Factor (PDGF), a well-known stimulus to induce VSMC proliferation and migration, augmented VSMC autophagic flux, which was important for removal of the contractile apparatus and transition to the synthetic VSMC phenotype, suggesting that autophagy negatively regulates the VSMC contractile phenotype [42]. In contrast, rapamycin, a well-known inducer of autophagy [68], has been reported to induce VSMC contractile phenotype by increasing contractile gene expression and inhibiting cell proliferation [69], though it remains unclear if rapamycin-induced autophagy contributes to its induction on VSMC differentiation. Therefore, the influence of autophagy on VSMC contractile phenotype remains to be enigmatic. Interestingly, we found that dysfunctional autophagy through ATG5 knockdown was unable to influence TGFβ1-mediated upregulation of VSMC contractile genes (Fig. 6). With regard to inflammation, defective autophagy in macrophages has been reported to correlate to inflammasome activation. Activation of autophagy has been implicated as protecting endothelial cells (ECs) against inflammation, and autophagy has been shown to be activated in early atherosclerotic lesions to protect plaque cells against inflammation [39]. These studies underscore the protective role of autophagy in vascular disease progression via antagonizing inflammation in ECs and macrophages. However, similar to contractile gene program, depletion of ATG5 failed to influence TGFβ1 suppression on VSMC proinflammatory genes in our context (Fig. 6). Intriguingly, despite that the regulation of TGFβ1 on VSMC differentiation and inflammation may not be attributable to the impaired autophagy, ATG5 gene depletion caused a significant augmentation of VSMC contractile and attenuation of proinflammatory genes expression (Supplementary Fig. 6). This suggests that autophagy per se in cultured VSMC may be necessary to maintain the synthetic VSMC phenotype though this awaits further investigation.
In summary, we reported here an important role of TGFβ1 in suppressing VSMC inflammation via the blockade of STAT3 and NF-kB pathways. These studies therefore extended our knowledge of TGFβ1 from a known activator of the VSMC contractile phenotype to a repressor of VSMC inflammation. This work will provide novel insights into the molecular mechanisms underlying the protective role of TGFβ signaling in inflammatory vascular diseases.
5. Conclusion
We reported here that TGFβ1 suppresses proinflammatory gene program while it induces VSMC differentiation via SMAD4-dependent canonical pathway in cultured human VSMCs. Mechanistically, TGFβ1 suppression on VSMC inflammation occurs likely in parallel to its induction on VSMC differentiation, and partially through the inhibition of STAT3 and NF-κB pathways. Though TGFβ1 inhibits autophagic activity in VSMCs, such an effect is not involved in its opposing regulations on VSMC differentiation and inflammation. These findings are timely, because they provide mechanistic insights into the protective role of TGFβ signaling in inflammatory vascular diseases such as atherosclerosis and abdominal aortic aneurysm, which have been recently reported.
Supplementary Material
Funding
This work is supported by National Institutes of Health [grant number R01HL122686], Paul Teschan Research Fund [grant number #2017–07], and Albany Medical College faculty startup funding to XL; National Institutes of Health [grant number R01HL49426] to HAS, American Heart Association [grant number 13SDG17100110] to APA.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A.: Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellsig.2018.07.002.
References
- [1].Owens GK, Kumar MS, Wamhoff BR, Molecular regulation of vascular smooth muscle cell differentiation in development and disease, Physiol. Rev 84 (3) (2004) 767–801. [DOI] [PubMed] [Google Scholar]
- [2].Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D, miR-145 and miR-143 regulate smooth muscle cell fate and plasticity, Nature 460 (7256) (2009) 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Long X, Tharp DL, Georger MA, Slivano OJ, Lee MY, Wamhoff BR, Bowles DK, Miano JM, The smooth muscle cell-restricted KCNMB1 ion channel subunit is a direct transcriptional target of serum response factor and myocardin, J. Biol. Chem 284 (48) (2009) 33671–33682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK, KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis, Nat. Med 21 (6) (2015) 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Herring BP, Hoggatt AM, Burlak C, Offermanns S, Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury, Vasc. Cell 6 (2014) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jorgensen HF, Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to Neointimal formation in mouse injury and atherosclerosis models, Circ. Res 119 (12) (2016) 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Derynck R, Zhang YE, Smad-dependent and Smad-independent pathways in TGF-beta family signalling, Nature 425 (6958) (2003) 577–584. [DOI] [PubMed] [Google Scholar]
- [8].Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ, RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation, J. Biol. Chem 281 (3) (2006) 1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu M, Chen G, Li YP, TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease, Bone Res. 4 (2016) 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Akhurst RJ, Derynck R, TGF-beta signaling in cancer–a double-edged sword, Trends Cell Biol. 11 (11) (2001) S44–S51. [DOI] [PubMed] [Google Scholar]
- [11].Long X, Miano JM, Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells, J. Biol. Chem 286 (34) (2011) 30119–30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang Y, Urs S, Boucher J, Bernaiche T, Venkatesh D, Spicer DB, Vary CP, Liaw L, Notch and transforming growth factor-beta (TGFbeta) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation, J. Biol. Chem 285 (23) (2010) 17556–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen S, Lechleider RJ, Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line, Circ. Res 94 (9) (2004) 1195–1202. [DOI] [PubMed] [Google Scholar]
- [14].Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK, Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells, Am. J. Physiol. Cell Physiol 287 (6) (2004) C1560–C1568. [DOI] [PubMed] [Google Scholar]
- [15].Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G, Tgfbr2 disruption in post-natal smooth muscle impairs aortic wall homeostasis, J. Clin. Invest 124 (2) (2014) 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu JH, Wei H, Jaffe M, Airhart N, Du L, Angelov SN, Yan J, Allen JK, Kang I, Wight TN, Fox K, Smith A, Enstrom R, Dichek DA, Postnatal deletion of the type II transforming growth factor-beta receptor in smooth muscle cells causes severe aortopathy in mice, Arterioscler. Thromb. Vasc. Biol 35 (12) (2015) 2647–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kobayashi K, Yokote K, Fujimoto M, Yamashita K, Sakamoto A, Kitahara M, Kawamura H, Maezawa Y, Asaumi S, Tokuhisa T, Mori S, Saito Y, Targeted disruption of TGF-beta-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury, Circ. Res 96 (8) (2005) 904–912. [DOI] [PubMed] [Google Scholar]
- [18].Zhang P, Hou S, Chen J, Zhang J, Lin F, Ju R, Cheng X, Ma X, Song Y, Zhang Y, Zhu M, Du J, Lan Y, Yang X, Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm, Circ. Res 118 (3) (2016) 388–399. [DOI] [PubMed] [Google Scholar]
- [19].Da Ros F, Carnevale R, Cifelli G, Bizzotto D, Casaburo M, Perrotta M, Carnevale L, Vinciguerra I, Fardella S, Iacobucci R, Bressan GM, Braghetta P, Lembo G, Carnevale D, Targeting interleukin-1beta protects from aortic aneurysms induced by disrupted transforming growth factor beta signaling, Immunity 47 (5) (2017) 959–973 (e9). [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z, TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice, J. Clin. Invest 120 (2) (2010) 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lareyre F, Clement M, Raffort J, Pohlod S, Patel M, Esposito B, Master L, Finigan A, Vandestienne M, Stergiopulos N, Taleb S, Trachet B, Mallat Z, TGFbeta (transforming growth factor-beta) blockade induces a human-like disease in a nondissecting mouse model of abdominal aortic aneurysm, Arterioscler. Thromb. Vasc. Biol 37 (11) (2017) 2171–2181. [DOI] [PubMed] [Google Scholar]
- [22].Chen PY, Qin L, Li G, Tellides G, Simons M, Smooth muscle FGF/TGFbeta cross talk regulates atherosclerosis progression, EMBO Mol. Med 8 (7) (2016) 712–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krohn JB, Hutcheson JD, Martinez-Martinez E, Irvin WS, Bouten CV, Bertazzo S, Bendeck MP, Aikawa E, Discoidin domain Receptor-1 regulates calcific extracellular vesicle release in vascular smooth muscle cell Fibrocalcific response via transforming growth factor-beta signaling, Arterioscler. Thromb. Vasc. Biol 36 (3) (2016) 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, Demarco VG, Martinez-Lemus LA, Regional variation in arterial stiffening and dysfunction in western diet-induced obesity, Am. J. Physiol. Heart Circ. Physiol 309 (4) (2015) H574–H582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu A, Zuo C, He Y, Chen G, Piao L, Zhang J, Xiao B, Shen Y, Tang J, Kong D, Alberti S, Chen D, Zuo S, Zhang Q, Yan S, Fei X, Yuan F, Zhou B, Duan S, Yu Y, Lazarus M, Su Y, Breyer RM, Funk CD, Yu Y, EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-beta1 signaling, J. Clin. Invest 125 (3) (2015) 1228–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dutzmann J, Daniel JM, Bauersachs J, Hilfiker-Kleiner D, Sedding DG, Emerging translational approaches to target STAT3 signalling and its impact on vascular disease, Cardiovasc. Res 106 (3) (2015) 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kovacic JC, Gupta R, Lee AC, Ma M, Fang F, Tolbert CN, Walts AD, Beltran LE, San H, Chen G, St Hilaire C, Boehm M, Stat3-dependent acute Rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice, J. Clin. Invest 120 (1) (2010) 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang R, Zhang Y, Xu L, Lin Y, Yang X, Bai L, Chen Y, Zhao S, Fan J, Cheng X, Liu E, Protein inhibitor of activated STAT3 suppresses oxidized LDL-induced cell responses during atherosclerosis in apolipoprotein E-deficient mice, Sci. Rep 6 (2016) 36790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang D, Liu Z, Li Q, Karpurapu M, Kundumani-Sridharan V, Cao H, Dronadula N, Rizvi F, Bajpai AK, Zhang C, Muller-Newen G, Harris KW, Rao GN, An essential role for gp130 in neointima formation following arterial injury, Circ. Res 100 (6) (2007) 807–816. [DOI] [PubMed] [Google Scholar]
- [30].Daniel JM, Dutzmann J, Bielenberg W, Widmer-Teske R, Gunduz D, Hamm CW, Sedding DG, Inhibition of STAT3 signaling prevents vascular smooth muscle cell proliferation and neointima formation, Basic Res. Cardiol 107 (3) (2012) 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liao XH, Wang N, Zhao DW, Zheng DL, Zheng L, Xing WJ, Ma WJ, Bao LY, Dong J, Zhang TC, STAT3 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin, J. Biol. Chem 290 (32) (2015) 19641–19652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jensen-Jarolim E, Fazekas J, Singer J, Hofstetter G, Oida K, Matsuda H, Tanaka A, Crosstalk of carcinoembryonic antigen and transforming growth factor-beta via their receptors: comparing human and canine cancer, Cancer Immunol. Immunother 64 (5) (2015) 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lu T, Tian L, Han Y, Vogelbaum M, Stark GR, Dose-dependent cross-talk between the transforming growth factor-beta and interleukin-1 signaling pathways, Proc. Natl. Acad. Sci. U. S. A 104 (11) (2007) 4365–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shiou SR, Yu Y, Guo Y, Westerhoff M, Lu L, Petrof EO, Sun J, Claud EC, Oral administration of transforming growth factor-beta1 (TGF-beta1) protects the immature gut from injury via Smad protein-dependent suppression of epithelial nuclear factor kappaB (NF-kappaB) signaling and proinflammatory cytokine production, J. Biol. Chem 288 (48) (2013) 34757–34766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Freudlsperger C, Bian Y, Contag Wise S, Burnett J, Coupar J, Yang X, Chen Z, Van Waes C, TGF-beta and NF-kappaB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers, Oncogene 32 (12) (2013) 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li Z-Y, Chen Z-L, Zhang T, Wei C, Shi W-Y, TGF-β and NF-κB signaling pathway crosstalk potentiates corneal epithelial senescence through an RNA stress response, Aging (Albany NY) 8 (10) (2016) 2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rautava S, Lu L, Nanthakumar NN, Dubert-Ferrandon A, Walker WA, TGF-beta2 induces maturation of immature human intestinal epithelial cells and inhibits inflammatory cytokine responses induced via the NF-kappaB pathway, J. Pediatr. Gastroenterol. Nutr 54 (5) (2012) 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Marino G, Niso-Santano M, Baehrecke EH, Kroemer G, Self-consumption: the interplay of autophagy and apoptosis, Nat. Rev. Mol. Cell Biol 15 (2) (2014) 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Meyer GR, Grootaert MO, Michiels CF, Kurdi A, Schrijvers DM, Martinet W, Autophagy in vascular disease, Circ. Res 116 (3) (2015) 468–479. [DOI] [PubMed] [Google Scholar]
- [40].Nussenzweig SC, Verma S, Finkel T, The role of autophagy in vascular biology, Circ. Res 116 (3) (2015) 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kyriakakis E, Frismantiene A, Dasen B, Pfaff D, Rivero O, Lesch KP, Erne P, Resink TJ, Philippova M, T-cadherin promotes autophagy and survival in vascular smooth muscle cells through MEK1/2/Erk1/2 axis activation, Cell. Signal 35 (2017) 163–175. [DOI] [PubMed] [Google Scholar]
- [42].Salabei JK, Cummins TD, Singh M, Jones SP, Bhatnagar A, Hill BG, PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress, Biochem. J 451 (3) (2013) 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Salabei JK, Hill BG, Autophagic regulation of smooth muscle cell biology, Redox Biol. 4 (2015) 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grootaert MO, da Costa Martins PA, Bitsch N, Pintelon I, De Meyer GR, Martinet W, Schrijvers DM, Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis, Autophagy 11 (11) (2015) 2014–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao J, Wu W, Zhang W, Lu YW, Tou E, Ye J, Gao P, Jourd’heuil D, Singer HA, Wu M, Long X, Selective expression of TSPAN2 in vascular smooth muscle is independently regulated by TGF-beta1/SMAD and myocardin/serum response factor, FASEB J. 31 (6) (2017) 2576–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alsaffar H, Martino N, Garrett JP, Adam AP, Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphor-ylation and de novo protein synthesis, Am. J. Phys. Cell Phys 314 (5) (May 1 2018) C589–C602. [DOI] [PubMed] [Google Scholar]
- [47].Ackers-Johnson M, Talasila A, Sage AP, Long X, Bot I, Morrell NW, Bennett MR, Miano JM, Sinha S, Myocardin regulates vascular smooth muscle cell inflammatory activation and disease, Arterioscler. Thromb. Vasc. Biol 35 (4) (2015) 817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Miano JM, Myocardin in biology and disease, J Biomed. Res 29 (1) (2015) 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lyu Q, Dhagia V, Han Y, Guo B, Wines-Samuelson ME, Christie CK, Yin Q, Slivano OJ, Herring P, Long X, Gupte SA, Miano JM, CRISPR-Cas9 mediated epitope tagging provides accurate and versatile assessment of myocardin, Arterioscler. Thromb. Vasc. Biol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M, Transforming growth factor-beta differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling, J. Biol. Chem 280 (7) (2005) 5491–5495. [DOI] [PubMed] [Google Scholar]
- [51].Lee YS, Park JS, Kim JH, Jung SM, Lee JY, Kim SJ, Park SH, Smad6-specific recruitment of Smurf E3 ligases mediates TGF-beta1-induced degradation of MyD88 in TLR4 signalling, Nat. Commun 2 (2011) 460. [DOI] [PubMed] [Google Scholar]
- [52].Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, Han QY, Wang H, Chen Y, Li HY, Li AL, Zhang XM, Zhou T, Li T, NLRP3 phosphorylation is an essential priming event for inflammasome activation, Mol. Cell 68 (1) (2017) 185–197 (e6). [DOI] [PubMed] [Google Scholar]
- [53].Gupta J, Nebreda AR, Roles of p38alpha mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer, FEBS J. 282 (10) (2015) 1841–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lawrence T, The nuclear factor NF-kappaB pathway in inflammation, Cold Spring Harb. Perspect. Biol 1 (6) (2009) a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yu L, Fang F, Dai X, Xu H, Qi X, Fang M, Xu Y, MKL1 defines the H3K4Me3 landscape for NF-kappaB dependent inflammatory response, Sci. Rep 7 (1) (2017) 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY, Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis, Cardiovasc. Res 114 (4) (2018) 622–634. [DOI] [PubMed] [Google Scholar]
- [57].Mizushima N, Yoshimori T, Levine B, Methods in mammalian autophagy research, Cell 140 (3) (2010) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Korah J, Canaff L, Lebrun JJ, The retinoblastoma tumor suppressor protein (pRb)/E2 promoter binding factor 1 (E2F1) pathway as a novel mediator of TGFbeta-induced autophagy, J. Biol. Chem 291 (5) (2016) 2043–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N, Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice, Nature 441 (7095) (2006) 885–889. [DOI] [PubMed] [Google Scholar]
- [60].Torisu T, Torisu K, Lee IH, Liu J, Malide D, Combs CA, Wu XS, Rovira II MM, Fergusson R, Weigert PS, Connelly MP, Daniels M, Komatsu L, Finkel Cao T, Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor, Nat. Med 19 (10) (2013) 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW, Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation, Circ. Res 112 (8) (2013) 1159–1170. [DOI] [PubMed] [Google Scholar]
- [62].Grainger DJ, Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis, Arterioscler. Thromb. Vasc. Biol 24 (3) (2004) 399–404. [DOI] [PubMed] [Google Scholar]
- [63].Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A, Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice, Circ. Res 89 (10) (2001) 930–934. [DOI] [PubMed] [Google Scholar]
- [64].Jeon J, Lee JH, Park KA, Byun HS, Lee H, Lee Y, Zhang T, Kang K, Seok JH, Kwon HJ, Han MD, Kang SW, Hong JH, Hur GM, Brazilin selectively disrupts proximal IL-1 receptor signaling complex formation by targeting an IKK-upstream signaling components, Biochem. Pharmacol 89 (4) (2014) 515–525. [DOI] [PubMed] [Google Scholar]
- [65].Yang Y, Yang WS, Yu T, Yi YS, Park JG, Jeong D, Kim JH, Oh JS, Yoon K, Kim JH, Cho JY, Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases, Biochem. Pharmacol 88 (2) (2014) 201–215. [DOI] [PubMed] [Google Scholar]
- [66].Cohen P, The TLR and IL-1 signalling network at a glance, J. Cell Sci 127 (11) (2014) 2383–2390 Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Salabei JK, Hill BG, Implications of autophagy for vascular smooth muscle cell function and plasticity, Free Radic. Biol. Med 65 (2013) 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Klionsky DJ, et al. , Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition), Autophagy 12 (1) (2016) 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Marx SO, Jayaraman T, Go LO, Marks AR, Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells, Circ. Res 76 (3) (1995) 412–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.