Abstract
Both renin–angiotensin systems and insulin participate in kidney-involved blood pressure regulation. Activation of angiotensin II type 2 receptor (AT2R) decreases sodium reabsorption in renal proximal tubule (RPT) cells, whereas insulin produces the opposite effect. We presume that AT2R has an inhibitory effect on insulin receptor expression in RPT cells, which may affect renal sodium transport and therefore be of physiological or pathological significance. Our present study found that activation of AT2R inhibited insulin receptor expression in a concentration and time-dependent manner in RPT cells from Wistar-Kyoto (WKY) rats. In the presence of a protein kinase C (PKC) inhibitor (PKC inhibitor peptide 19–31, 10−6 mol/L) or a phosphatidylinositol 3 kinase inhibitor (wortmannin, 10−6 mol/L), the inhibitory effect of AT2R on insulin receptor was blocked, indicating that both PKC and phosphatidylinositol 3 kinase were involved in the signaling pathway. There was a linkage between AT2R and insulin receptor which was determined by both laser confocal microscopy and coimmunoprecipitation. However, the effect of AT2R activation on insulin receptor expression was different in RPT cells from spontaneously hypertensive rats (SHRs). Being contrary to the effect in WKY RPT cells, AT2R stimulation increased insulin receptor in SHR RPT cells. Insulin (10−7 mol/L, 15 minutes) enhanced Na+-K+-ATPase activity in both WKY and SHR RPT cells. Pretreatment with CGP42112 decreased the stimulatory effect of insulin on Na+-K+-ATPase activity in WKY RPT cells, whereas pretreatment with CGP42112 increased it in SHR RPT cells. It is suggested that activation of AT2R inhibits insulin receptor expression and function in RPT cells. The lost inhibitory effect of AT2R on insulin receptor expression may contribute to the pathophysiology of hypertension.
Keywords: Angiotensin II type 2 receptor, hypertension, insulin receptor, renal proximal tubule cells, kidney
Introduction
Kidney contributes importantly to the long-term blood pressure control via regulating sodium homeostasis.1–3 Renal proximal tubule (RPT) is one of the most critical segments for sodium reabsorption within kidney and therefore involved in blood pressure adjustment. This process is regulated by numerous hormones and humoral factors, including renin–angiotensin system (RAS) and insulin/insulin receptor.4–6
Angiotensin II (Ang II), as the major effector peptide of RAS, regulates sodium balance via two main receptor sub-types, angiotensin II type 1 receptor (AT1R) and angiotensin II type 2 receptor (AT2R).7,8 AT1R and AT2Rs are expressed throughout the vascular and tubule sites in adult kidney. Stimulation of AT1R increases sodium (Na+) reabsorption in RPT and induces antinatriuresis. AT2R, as an antagonist of AT1R, can oppose or offset this action.9–11 Activation of AT2R decreases sodium reabsorption, induces natriuresis, maintains negative Na+ balance, and lowers blood pressure chronically in angiotensin-dependent hyper-tension via reducing Na+ reabsorption in RPT.1
Insulin/insulin receptor is another critical signaling pathway involved in sodium balance in RPT. Insulin receptor was expressed throughout the nephron.12,13 Studies have demonstrated that insulin/insulin receptor has the potential of increasing sodium retention through stimulating sodium transporters Na+-H+-exchanger and Na+-K+-ATPase in RPT.14–16
Some researches have been performed to elucidate the interaction between AT2R and insulin in different aspects. AT2R can impact the β-cell to α-cell ratio of the neonate islets, insulin secretory function, and the glucose tolerance in pups.17 Activation of AT2R leads to inhibition of insulin-induced extracellular signal-regulated protein kinase 2 (ERK2) activity and cell proliferation in transfected Chinese hamster ovary cells.18 In rat pheochromocytoma cell line, AT2R inhibits insulin-associated phosphoinositide 3-kinase and Akt phosphorylation and induces cell apoptosis.19 Although relationship between AT2R and insulin receptor has been revealed, the detailed mechanisms remain unclear. Both AT2R and insulin receptor exist in RPT and they execute the opposite functions in sodium metabolism,5,6,20 we presume that activation of AT2R in hibits insulin receptor in RPT. To confirm this hypothesis, we explored the interaction between AT2R and insulin receptor in RPT cells from Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs). We found that activation of AT2R was capable of decreasing insulin receptor expression and function in RPT cells from WKY rats, whereas this inhibitory effect was disturbed in RPT cells from SHRs. We suggested that an aberrant interaction between AT2R and insulin receptor may play an important role in the abnormal regulation of sodium excretion and consequently be implicated in hypertension.
Methods
Cell Culture
Immortalized RPT cells from WKY rats and SHRs were cultured as previously described.4 Briefly, cells were cultured at 37°C in 95% O2 and 5% CO2 atmosphere in Dulbecco’s Modified Eagle Medium/F-12 containing 10% fetal bovine serum, 5 μg/mL transferrin, 5 μg/mL insulin, 4 μg/mL dexamethasone, and 10 ng/mL epidermal growth factor. The cells were diluted to 1 × 106 cells/mL and plated in different culture dishes according to the specific experimental requirements. Before the treatment of cells, the medium was replaced with serum-free medium for 2 hours.
Immunoblotting
The RPT cells were treated with different reagents or the vehicle (dH2O) at the indicated concentrations and times according to the specific experimental requirements. Immunoblotting was performed as previously reported except that the transblots were probed with the rabbit anti-insulin receptor antibody (1:400, Santa Cruz Biotechnology, Inc, Santa Cruz, CA), rabbit anti-phospho-ERK1/2 antibody, or rabbit anti-ERK1/2 antibody (1:400, Cell Signaling Technology, Beverly, MA).4,21 The amount of protein transferred onto the membranes was verified by immunoblotting for α-actin (Santa Cruz Biotechnology Inc) and used for the normalization of the receptor densities.
RNA Isolation, Reverse Transcription, and Real-Time Polymerase Chain Reaction
Total RNA from RPT cells was extracted using Trizol re-agent, and 1 ng RNA was used to synthesize cDNA following the manufacturer’s instructions. For glyceralde-hyde-3-phosphate dehydrogenase (GAPDH), the forward primer was 5′- CAGGGCTGCCTTCTCTTGTG-3′ and the reverse primer was 5′-GGTGATGGGTTTCCCGTTGA-3’ (GeneBank Accession No.: NM_017008.4). For insulin receptor, the forward primer was 5′-TTCAGGAAGACCTTCGAGGATTACCTGCAC-3′ and the reverse primer was 5′-AGGCCAGAGATGACAAGTGACTCCTTGTT-3’ (Gene-Bank Accession No.: NM_017071.2). Insulin receptor mRNA expression was normalized for GAPDH mRNA.
Analysis of the Second Messengers Involved in the Regulation of AT2R on Insulin Receptor Expression
To determine the second messenger(s) involved in the AT2R-mediated regulation of insulin receptor expression in RPT cells from WKY rats and SHRs, several inhibitors or agonists were used: protein kinase C (PKC) inhibitor (19–31, 10−6 mol/L), PKC activator (phorbol 12-myristate 13-acetate, PMA, 10−7 mol/L), protein kinase A (PKA) inhibitor (14–22, 10−6 mol/L), PKA activator (Sp-cAMP-S, 10−7 mol/L), calcium channel blocker (nicardipine, 10−6 mol/L), calcium channel agonist (BAY-K8644, 10−6 mol/L), and phosphatidylinositol 3 kinase (PI3K) inhibitor (wortmannin, 10−6 mol/L). These reagents were added into the incubation medium 15 minutes before the addition of the AT2R agonist CGP42112 (10−6 mol/L). The PKC inhibitor 19–31, PMA, Sp-cAMPs, BAY-K8644, nicardipine, and CGP42112 were purchased from Sigma-Aldrich (St. Louis, MO,). PKA inhibitor 14–22 was purchased from Calbiochem Company (Darmstadt, Germany). Wortmannin was purchased from Millipore (Billerica, MA).
Immunoprecipitation
RPT cells were cultured with vehicle or CGP42112 (10−6 mol/L) for 24 hours and then were extracted with ice-cold lysis buffer for 1 hour, centrifuged at 16,000g for 30 minutes. Equal amounts of lysates were incubated with rabbit anti-AT2R antibody (Santa Cruz Biotechnology Inc, 1.0 μg/mL) for 24 hours at 4°C and G-protein agarose for 1 hour at room temperature. The immunoprecipitates were pelleted and washed four times with lysis buffer. The pellets were suspended in sample buffer, boiled for 10 minutes, and subjected to immunoblotting with the rabbit anti-insulin receptor antibody. The bands were quantified by densitometry through Quantiscan. To determine the specificity of the bands found on the immunoblots, IgG was used instead of AT2R antibody as the immunoprecipitant (negative control). Moreover, we also exchanged the antibodies for immunoprecipitation and immunoblotting to confirm the results. The specificity of AT2R antibody has been checked in our previous published paper.22
Confocal Microscopy of Double-Stained Kidney Sections and RPT Cells
Fixing with 4% paraformaldehyde for 24 hours, kidneys from WKY rats were embedded in paraffin, sectioned and mounted on slides. Then, sections were deparaffinized, rehydrated, and subjected to antigen retrieval. After incubating with goat anti-AT2R and rabbit anti-insulin receptor antibodies (1: 100 dilution, Santa Cruz Biotechnology Inc) overnight at 4°C, sections were stained with Alexa Fluor 568-rabbit antigoat IgG antibody (1:100 dilution, red; Molecular Probes, Eugene, OR) and fluorescein isothiocyanate–conjugated goat anti-rabbit IgG antibody (1:100 dilution, green; Molecular Probes). Immunofluorescence images were acquired at excitation wavelengths of 480 nm and 560 nm; emission was detected at 535 and 645 nm (Olympus AX70 laser confocal microscopy).
To determine the colocalization of AT2R and insulin receptor in cells, RPT cells were fixed (3% paraformaldehyde) for 30 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes. The subsequent immunofluorescence experiment was the same as above.
Na+-K+-ATPase Activity Assay
RPT cells were treated with vehicle or indicated re-agents, and then, the membranes were obtained as previously reported method.5 To measure Na+-K+-ATPase activity, 100 μL aliquots of the membrane fraction was added to 800 μL reaction mixture (75 mM NaCl, 5 mM KCl, 5 mM MgCl2, 6 mM sodium azide, 1 mM Na4EGTA, 37.5 mM imidazole, 75 mM Tris HCl, and 30 mM histidine; pH 7.4) with or without 1 mM ouabain to make the final volume to 1 mL. After preincubating the mixtures for 5 minutes at 37°C in a water bath, reactions were initiated by adding 4 mM Tris-ATP and terminated after 15 minutes by adding 50 μL of 50% trichloroacetic acid at 37 °C. For the determination of ouabain-insensitive ATPase activity, NaCl and KCl were omitted from the reaction mixtures with ouabain. The amount of phosphate produced was quantified by adding 1-mL coloring reagent (10% ammonium molybdate in 10N sulfuric acid and ferrous sulfate). After mixture and centrifugation (3000g, 10 minutes), the amount of phosphomolybdate in the supernatant was quantified spectrophotometrically at 740 nm against a standard curve prepared from K2HPO4. The difference between total and ouabain-insensitive ATPase activity was used for Na+-K+-ATPase activity.
Statistical Analysis
The data were analyzed individually and expressed as mean ± standard error of the mean. Student unpaired t test was used to compare two independent groups, and one-way analysis of variance was used for a comparison of ≥3 groups. GraphPad Prism 5.0 and SPSS 17.0 were used to perform the statistical analyses. A value of P less than .05 was considered significant.
Results
Activation of AT2R Decreases Insulin Receptor Expression in WKY RPT Cells but Increases It in SHR RPT Cells
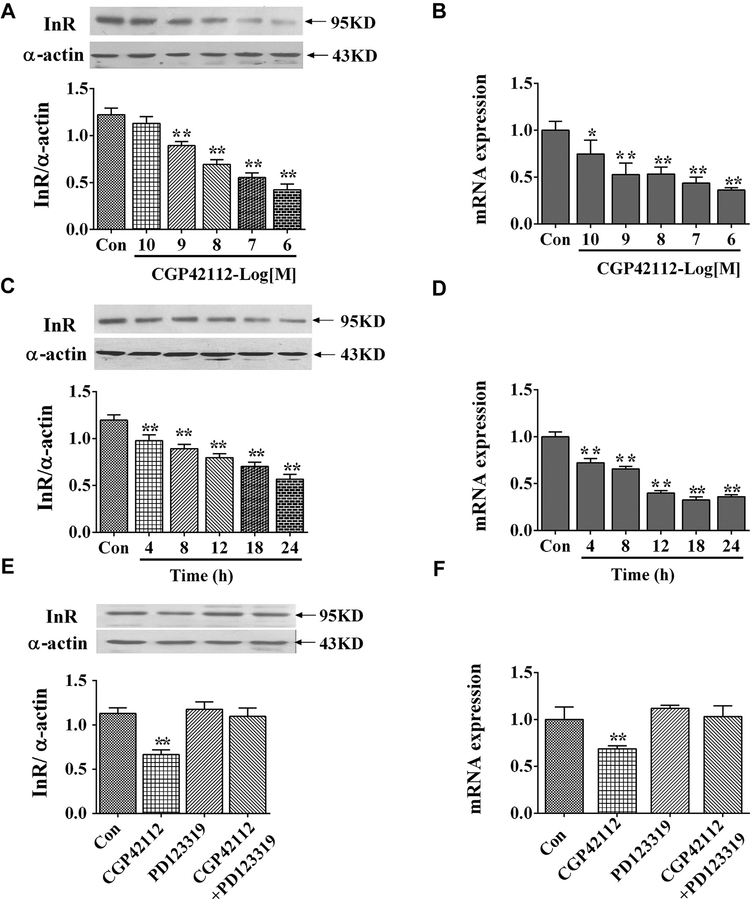
Administration of an AT2R agonist CGP42112 decreased insulin receptor expression in a concentration- and time-dependent manner in WKY RPT cells. The inhibition effect was found not only at protein level, but also at mRNA level (Figure 1A–D).
Figure 1.
Effect of AT2R agonist, CGP42112, on insulin receptor expression in RPT cells from WKY rats. Concentration response of insulin receptor protein expression (A) and mRNA expression (B) in RPT cells after treatment with CGP42112 for 24 hours. Results are expressed as the ratio of insulin receptor and α-actin densities (n = 6; *P < .05; **P < .01 vs. control). Time course of insulin receptor protein expression (C) and mRNA expression (D) in RPT cells after treatment with CGP42112 (10−6 mol/L) for varying time periods. Results are expressed as the ratio of insulin receptor and α-actin densities (n = 6; **P < .01 vs. control [0 time point]). (E and F) Effect of the AT2R agonist, CGP42112 (10−6 mol/L), and antagonist, PD123319 (10−6 mol/L), on insulin receptor protein (E) and mRNA expression (F). The cells were incubated for 24 hours. Results are expressed as the ratio of insulin receptor and α-actin densities (n = 6; **P < .01 vs. others). AT2R, angiotensin II type 2 receptor; RPT, renal proximal tubule; WKY, Wistar-Kyoto.
To confirm the specificity of CGP42112 as an AT2R agonist, PD123319 (Sigma-Aldrich), the AT2R antagonist was used. Consistent with the previous results, PD123319 (10−6 mol/L), by itself, did not affect insulin receptor expression but reversed the inhibitory effect of CGP42112 on insulin receptor (Figure 1E, F).
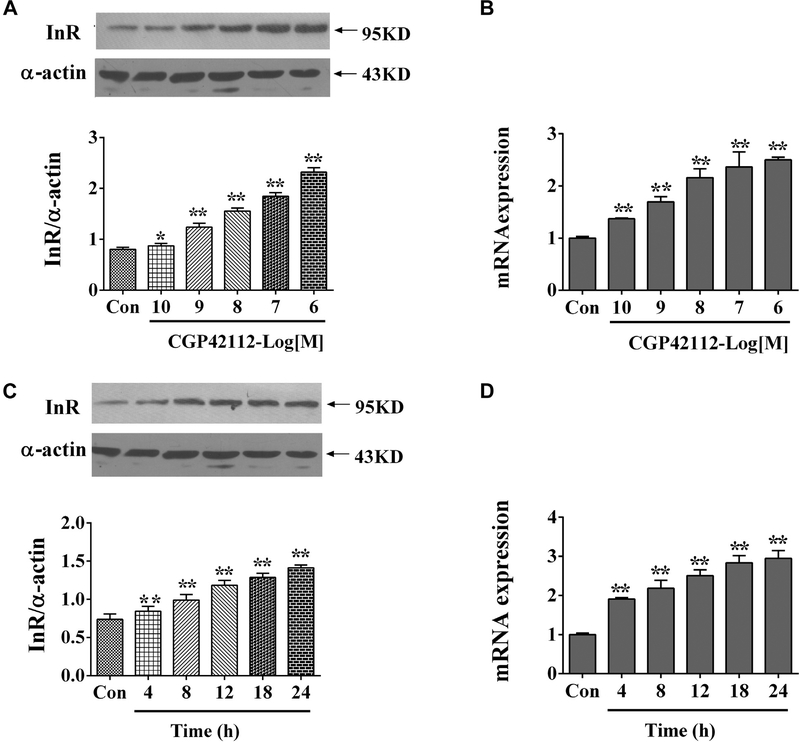
In contrast to the inhibition effect of AT2R on insulin receptor in RPT cells from WKY rats, AT2R activation increased both mRNA and protein expression of insulin receptor in SHR RPT cells (Figure 2).
Figure 2.
Effect of AT2R agonist, CGP42112, on insulin receptor expression in SHR RPT cells. Concentration response of insulin receptor protein expression (A) and mRNA expression (B) in RPT cells after treatment with CGP42112 for 24 hours. Results are expressed as the ratio of insulin receptor and α-actin densities (n = 7; *P < .05; **P < .01 vs. control). Time course of insulin receptor protein expression (C) and mRNA expression (D) in RPT cells after treatment with CGP42112 (10−6 mol/L) for varying time periods. Results are expressed as the ratio of insulin receptor and α-actin densities (n = 7; **P < .01 vs. control [0 time point]). AT2R, angiotensin II type 2 receptor; RPT, renal proximal tubule; SHR, spontaneously hypertensive rats.
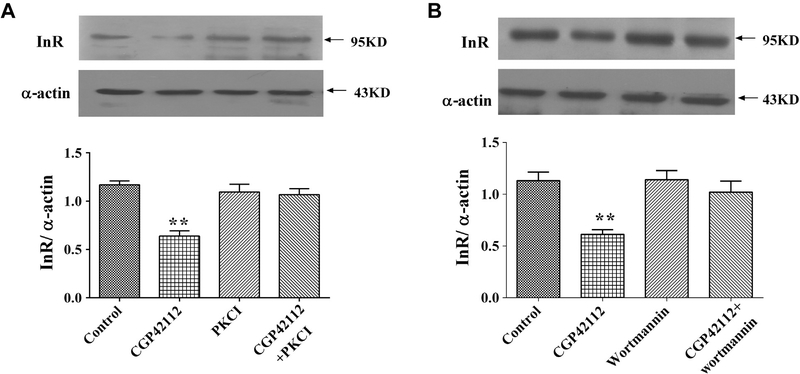
Roles of PKC and PI3K in the Inhibition of AT2R on Insulin Receptor in WKY RPT Cells
Treating RPT cells from WKY rats with PKC inhibitor (19–31, 10−6 mol/L) or PI3K inhibitor wortmannin (10−6 mol/L) blocked the inhibitory effect of AT2R on insulin receptor expression (Figure 3A, B), which had no effect on insulin receptor expression by themselves, indicating that both PKC and PI3K were involved in the AT2R-mediated signal transduction pathway. Some other key cell signaling proteins, including PKC activator (phorbol 12-myristate 13-acetate, PMA, 10−7 mol/L), PKA inhibitor (14–22, 10−6 mol/L), PKA activator (Sp-cAMP-S, 10−7 mol/L), calcium channel blocker (nicardipine, 10−6 mol/L), and calcium channel agonist (BAY-K8644, 10−6 mol/L), have also been tested in the present study. None of these reagents blocked effect on AT2R on insulin receptor expression (data not shown). To determine whether or not PKC and PI3K were involved in the upregulation of AT2R on insulin receptor expression in SHR cells, PKC inhibitor (19–31, 10−6 mol/L) or wortmannin (10−6 mol/L) was used. It resulted that neither PKC inhibitor nor wortmannin could blocked the upregulation of AT2R on insulin receptor expression in SHR cells (Figure S1).
Figure 3.
Role of PKC and PI3K in the effect of AT2R on insulin receptor expression in RPT cells from WKY rats. (A) Effect of CGP42112 and the PKC inhibitor 19–31 on insulin receptor protein expression in RPT cells from WKY rats. The cells were incubated with CGP42112 (10−6 mol/L) or PKC inhibitor 19–31 (10−6 mol/L) for 24 hours. Results are expressed as the ratio of insulin receptor to α-actin densities (n = 5; **P < .01 vs. control). (B) Effect of CGP42112 and the PI3K inhibitor wortmannin on insulin receptor protein expression in RPT cells from WKY rats. The cells were incubated with CGP42112 (10−6 mol/L) or wortmannin (10−6 mol/L) for 24 hours. Results are expressed as the ratio of insulin receptor to α-actin densities (n = 5; **P < .01 vs. control). AT2R, angiotensin II type 2 receptor; RPT, renal proximal tubule; PI3K, phosphatidylinositol 3 kinase; PKC, protein kinase C; WKY, Wistar-Kyoto.
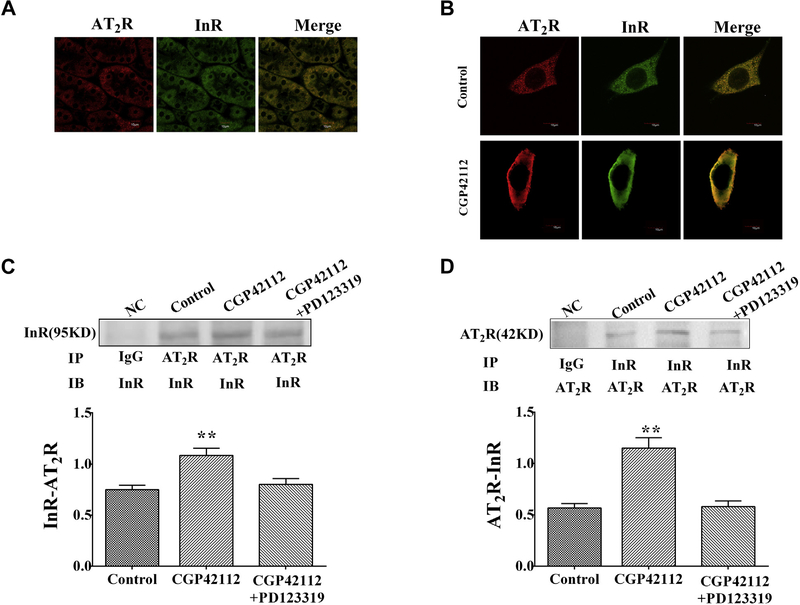
AT2R Colocalizes and Interacts With Insulin Receptor
To investigate whether or not AT2R and insulin receptor can interact directly, immunofluorescence laser confocal microscopy and coimmunoprecipitation were performed. Immunofluorescence staining showed that AT2R and insulin receptor colocalized in kidney and cells from WKY rats (Figure 4A, B). What’s more, stimulation of CGP42112 (10−6 mol/L per 24 hours) increased AT2R/insulin receptor colocalization, especially at plasma membrane (Figure 4B). Coimmunoprecipitation study indicated there was a linkage between AT2R and insulin receptor at the basal state, which was increased by CGP42112 (10−6 mol/L 24 hours) in WKY RPT cells (Figure 4C, D). Although there was AT2R/insulin receptor linkage in SHR RPT cells as well, CGP42112 (10−6 mol/L per 24 hours) had not effect on AT2R/insulin receptor coimmunoprecipitation and colocalization (Figure S2A, B).
Figure 4.
The linkage between AT2 and insulin receptors in kidney and RPT cells from WKY rats. (A and B) The colocalization of AT2 and insulin receptors in kidney and RPT cells from WKY rats. The samples were washed, fixed, and immunostained for AT2 and insulin receptors, as described in the Methods. Colocalization appears as yellow after merging the images of fluorescein isothiocyanate-tagged insulin receptor (green) and Alexa 568-tagged AT2 receptor (red). As for WKY RPT cells, the cells were treated with or without CGP42112 (10−6 mol/L) for 24 hours. (C and D) Effect of the AT2R agonist, CGP42112, on AT2/insulin receptor coimmunoprecipitation in RPT cells. After the cells were incubated with CGP42112 (10−6 mol/L) for 24 hours, samples were immunoprecipitated with anti-AT2R antibody and immunoblotted with anti-insulin receptor antibody (C) or immunoprecipitated with anti-insulin receptor antibody and immunoblotted with anti-AT2R antibody (D). Results are expressed as relative density units (DUs) (n = 5, **P < .01 vs. control). AT2R, angiotensin II type 2 receptor; RPT, renal proximal tubule; WKY, Wistar-Kyoto; SHRs, spontaneously hypertensive rats.
Pretreatment With AT2R Agonist Decreases the Stimulatory Effect of Insulin Receptor on Na+-K+-ATPase Activity in WKY RPT Cells but not in SHR RPT Cells
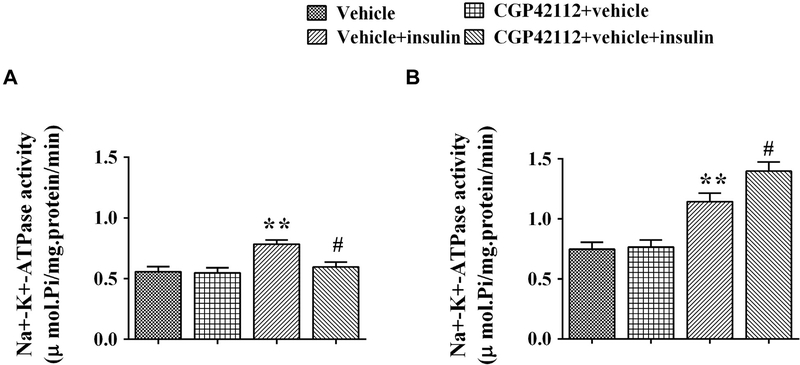
To further explore the effect of AT2R on insulin receptor function, the Na+-K+-ATPase activity in RPT cells from WKY rats and SHRs was checked with or without pretreatment with the AT2R agonist CGP42112. Activity of Na+-K+-ATPase was higher in SHR than WKY RPT cells at the basal level. Insulin (10−7 mol/L 15 minutes) increased Na+-K+-ATPase activity in both WKY and SHR RPT cells. However, pretreatment with CGP42112 inhibited the insulin-mediated stimulatory effect on Na+-K+-ATPase activity in WKY RPT cells but increased it in SHR RPT cells (Figure 5A, B), which was consistent with the regulation of AT2R on insulin receptor expression.
Figure 5.
Effect of pretreatment with AT2R agonist on the stimulatory effect of insulin on Na+-K+-ATPase activity in WKY (A) and SHR (B) RPT cells. The cells were pretreated with AT2R agonist, CGP42112 (10−6 mol/L), or vehicle (dH2O) for 24 hours after washing three times (15 minutes/times) with serum-free culture medium and then treated with insulin (10−7 mol/L) for 15 minutes. Results are expressed as mmol phosphate released per mg protein per minute (n = 7, **P < .01 vs. control; #P < .05 vs. vehicle + insulin). AT2R, angiotensin II type 2 receptor; RPT, renal proximal tubule; SHRs, spontaneously hypertensive rats; WKY, Wistar-Kyoto.
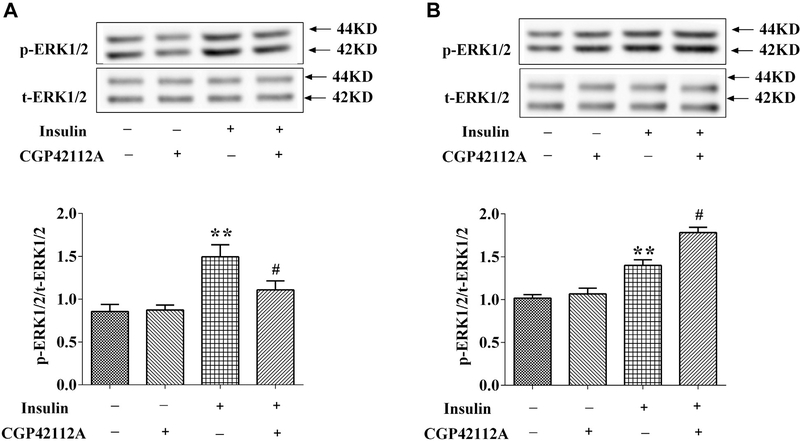
Besides of Na+-K+-ATPase activity, we also checked the signaling of insulin, that is, ERK1/2 phosphorylation (p-ERK). Consistent with the result regarding to Na+-K+-ATPase activity, insulin (10−7 mol/L, 15 min) increased p-ERK in both WKY and SHR RPT cells. Pretreatment with CGP42112 (10−6 mol/L) for 24 hours inhibited the stimulatory effect of insulin on ERK1/2 activity in WKY cells, but enhanced insulin induced ERK1/2 phosphorylation in SHR RPT cells (Figure 6A, B).
Figure 6.
Effect of insulin and AT2R agonists on phospho-ERK1/2 protein expression in RPT cells from WKY rats (A) and SHRs (B). The cells were pretreated with AT2R agonist, CGP42112 (10−6 mol/L), or vehicle (dH2O) for 24 hours after washing three times (15 minutes/times) with serum-free culture medium and then treated with insulin (10−7 mol/L) for 15 minutes. The cell lysates were immunoblotted with antiphosphorylation (p)-ERK antibody and antitotal (t)-ERK antibody. P-ERK expression was corrected by t-ERK protein expression. (n = 3, **P < .01 vs. control; #P < .05 vs. insulin). AT2R, angiotensin II type 2 receptor; RPT, renal proximal tubule; WKY, Wistar-Kyoto.
Discussion
The present study for the first time demonstrated that there is a link between AT2R and insulin receptor in RPT cells. AT2R is capable of inhibiting insulin receptor expression via PI3K and PKC pathways in RPT cells from WKY rats. Correspondingly, activation of AT2R in WKY cells reduces the insulin-mediated stimulatory effect on Na+-K+-ATPase activity, whereas this repression effect of AT2R on insulin receptor was aberrant in SHR cells. Altered interaction between AT2R and insulin receptor may contribute to the pathogenesis of hypertension.
RAS is one of the major regulatory systems in the modulation of cardiovascular and renal function.23,24 Ang II, the primary RAS effector peptide hormone, executes multiple physiological or pathological roles via binding to different receptor subtypes, including AT1R and AT2R. Studies have shown that AT1R predominately mediates Ang II–induced vasoconstriction, sympathetic nervous system activation, inflammation, sodium reabsorption in RPT, and aldosterone secretion.1,23 AT2R is antagonistic to the above effects under most circumstances.25,26 Kemp et al1 have demonstrated that AT2R activation initiates its translocation to the RPT apical membrane. Intrarenal AT2R activation prevents Na+ retention and lowers blood pressure in Ang II–dependent hypertension. Ali et al27 found that AT2R activation shifts the abnormal regulation of AT1R and Angiotensin-converting enzyme 2 (ACE2) which contributes to obesity-related hypertension. AT2R activation also prevents salt-sensitive hypertension in the obese Zucker rat model of obesity and the metabolic syndrome.
Insulin is the central hormone involved in the control of glucose and lipid metabolism. Other nonmetabolic effects of insulin are intensively studied as well. Activation of insulin receptor not only promotes glucose uptake, protein synthesis, and inhibits lipid metabolism, but also is crucial for renal function in glomeruli and tubules.28,29 Insulin receptor has been shown regulating sodium excretion in RPTs. Studies supported that renal-specific knockout of insulin receptor in mice results in impairment of sodium transport and increased blood pressure.30–32 Our previous study has found that insulin increased Na+-K+-ATPase activity in RPTs.5 When signaling is aberrant here, as may occur in insulin-resistant states, it may be responsible for a number of important renal complications including hypertension.
As two pivotal regulators in the regulation network of blood pressure, the cross talk between RAS and insulin has been described in many models. For example, Ang II can decrease the number of insulin receptors in the membrane in bovine aortic endothelial cells.33 Ang II leads to phosphorylation of insulin receptor substrates at Ser636/639 through activating mTOR/p70S6K and inhibits the insulin-stimulated phosphorylation of eNOS via AT1R.34 Insulin-induced capillary recruitment is abrogated, and glucose disposal is attenuated after the treatment with AT2R antagonist, PD123319.35 However, correlation between AT2R and insulin receptor on sodium transport in kidney remains undetermined. Our current study showed that AT2R agonist CGP42112 decreased insulin receptor mRNA and protein expressions in a concentration- and time-dependent manner in WKY rats RPT cells. Pretreatment with the AT2R agonist decreased the stimulatory effect of insulin on Na+-K+-ATPase activity in RPT cells from WKY rats. Therefore, we deduced that AT2R depresses the function of insulin receptor via inhibiting insulin receptor expression. Balance between AT2R and insulin receptor is important to keep the steady state for sodium reabsorption in the proximal tubule. However, the antagonism of AT2R on insulin receptor is lost in RPT cells from SHRs. Early studies have proved that WKY and SHRs RPT cells own different ways of physiological regulation.5,6 There are different responses even to the same treatment in WKY rats and SHRs. For example, intravenous infusion of losartan for 1 week significantly lowered systolic blood pressure and increased urine volume and sodium excretion in D4 dopamine receptor agonist treated SHRs, but not in WKY rats.36 We showed here that there was an aberrant regulation of AT2R on insulin receptor in SHRs which might be involved in the pathogenesis of hypertension.
The mechanism for the inhibitory effect of AT2R on insulin receptor expression in WKY rats was further investigated in this study. Both PKC and PI3K play important roles in the signal transduction in inhibition of AT2R on insulin receptor. Previous studies show that the signaling pathway for RAS and insulin involves the activation of PI3K and PKC. AT2R can inhibit the phosphorylation and the signal transduction of PKC.37,38 A previous study has found that inhibition of PKC prevents hepatic insulin resistance.39 Similarly, mutation or knockout of PKC can change the insulin sensitivity or insulin receptor phosphor-ylation.40,41 The present study showed that the inhibitory effect of AT2R on insulin receptor expression was blocked by PKC or PI3K inhibitor in WKY RPT cells, but not in SHR RPT cells, indicating that PKC and PI3K are involved in this signaling pathway in normotensive states, but not in hypertensive states. Besides insulin receptor protein expression, mRNA level was also decreased by stimulating AT2R in WKY RPT cells, indicating that the regulation may occur at the transcriptional level. However, the detailed mechanisms remain to be elucidated in the future.
In summary, we have demonstrated that AT2R inhibits insulin receptor expression in WKY cells, while increases it in SHR cells. PKC and PI3K are signaling pathways involved in the regulation of AT2R on insulin receptor in WKY cells, but not in SHR cells. The regulation is pathophysiological significance because pretreatment with CGP42112 inhibited the insulin-mediated stimula-tory effect on Na+-K+-ATPase activity or ERK1/2 phosphorylation in WKY RPT cells but increased it in SHR RPT cells.
Supplementary Material
Acknowledgments
This study was supported by research grants from the National Natural Science Foundation of China (31430043, 81570380), National Basic Research Program of China (2013CB531104), and grants from the National Institutes of Health (7R37HL023081–37, 5P01HL074940–11).
Footnotes
Conflict of interest: None.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jash.2017.11.009.
Supplemental Material can be found at www.ashjournal.com.
References
- 1.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 receptor activation induces natriuresis and lowers blood pressure. Circ Res 2014;115:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Wang J, Guo C, Luo W, Kleiman K, Eitzman DT. Renal denervation attenuates progression of atherosclerosis in apolipoprotein E-deficient mice independent of blood pressure lowering. Hypertension 2015;65:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal 2014;20: 74–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng C, Wang Z, Li H, Yu P, Zheng S, Wu L, et al. D3 dopamine receptor directly interacts with D1 dopamine receptor in immortalized renal proximal tubule cells. Hypertension 2006;47:573–9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Ren H, Lu X, He D, Han Y, Wang H, et al. Inhibition of D4 dopamine receptors on insulin receptor expression and effect in renal proximal tubule cells. J Am Heart Assoc 2016;5:e002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Cui Z, He D, Ren H, Han Y, Yu C, et al. Insulin increases D5 dopamine receptor expression and function in renal proximal tubule cells from Wistar-Kyoto rats. Am J Hypertens 2009;22:770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey RM. The intrarenal renin-angiotensin system in hypertension. Adv Chronic Kidney Dis 2015;22: 204–10. [DOI] [PubMed] [Google Scholar]
- 8.Pontes RB, Girardi AC, Nishi EE, Campos RR, Bergamaschi CT. Crosstalk between the renal sympathetic nerve and intrarenal angiotensin II modulates proximal tubular sodium reabsorption. Exp Physiol 2015;100:502–6. [DOI] [PubMed] [Google Scholar]
- 9.Chow BS, Koulis C, Krishnaswamy P, Steckelings UM, Unger T, Cooper ME, et al. The angiotensin II type 2 receptor agonist compound 21 is protective in experimental diabetes-associated atherosclerosis. Diabetologia 2016;59:1778–90. [DOI] [PubMed] [Google Scholar]
- 10.Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Daviet L, et al. Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc Res 2001; 49:863–71. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara H Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res 1998;83:1182–91. [DOI] [PubMed] [Google Scholar]
- 12.Hale LJ, Coward RJ. The insulin receptor and the kidney. Curr Opin Nephrol Hypertens 2013;22:100–6. [DOI] [PubMed] [Google Scholar]
- 13.Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G. Selective insulin resistance in the kidney. Biomed Res Int 2016;2016:5825170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Yan Y, Malhotra D, Liu J, Xie Z, Najjar SM, et al. Ouabain and insulin induce sodium pump endocytosis in renal epithelium. Hypertension 2012;59: 665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura M, Satoh N, Suzuki M, Kume H, Homma Y, Seki G, et al. Stimulatory effect of insulin on renal proximal tubule sodium transport is preserved in type 2 diabetes with nephropathy. Biochem Biophys Res Commun 2015;461:154–8. [DOI] [PubMed] [Google Scholar]
- 16.Baquero AF, Gilbertson TA. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am J Physiol Cell Physiol 2011;300:C860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung KK, Liang J, Zhao S, Chan WY, Leung PS. Angiotensin II type 2 receptor regulates the development of pancreatic endocrine cells in mouse embryos. Dev Dyn 2014;243:415–27. [DOI] [PubMed] [Google Scholar]
- 18.Elbaz N, Bedecs K, Masson M, Sutren M, Strosberg AD, Nahmias C. Functional trans-inactivation of insulin receptor kinase by growth-inhibitory angiotensin II AT2 receptor. Mol Endocrinol 2000;14:795–804. [DOI] [PubMed] [Google Scholar]
- 19.Cui TX, Nakagami H, Nahmias C, Shiuchi T, Takeda-Matsubara Y, Li JM, et al. Angiotensin II subtype 2 receptor activation inhibits insulin-induced phosphoinositide 3-kinase and Akt and induces apoptosis in PC12W cells. Mol Endocrinol 2002;16:2113–23. [DOI] [PubMed] [Google Scholar]
- 20.Baum M Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest 1987; 79:1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, et al. Interaction of angiotensin II type 1 and D5 dopa-mine receptors in renal proximal tubule cells. Hyper-tension 2005;45:804–10. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, et al. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMPgmp/Sp1 in renal proximal tubule cells from wistar-kyoto rats. J Hypertens 2012;30:1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 2003; 24:261–71. [DOI] [PubMed] [Google Scholar]
- 24.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 2006;86: 747–803. [DOI] [PubMed] [Google Scholar]
- 25.Padia SH, Carey RM. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch 2013;465:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther 2008;120: 292–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int 2013;84:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int 2003;64: 2163–71. [DOI] [PubMed] [Google Scholar]
- 29.Brands MW, Manhiani MM. Sodium-retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol 2012;303:R1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari S, Singh RS, Li L, Tsukerman S, Godbole M, Pandey G, et al. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. J Am Soc Nephrol 2013;24:1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rash A, Tiwari S, Riazi S, Ecelbarger CM. Blood pressure and sodium transporter and channel regulation in mice with targeted knockout of the insulin receptor from collecting duct principal cells. J Anat 2015;226:215–23.25652795 [Google Scholar]
- 32.Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, et al. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci U S A 2008;105:6469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh SJ, Ha WC, Lee JI, Sohn TS, Kim JH, Lee JM, et al. Angiotensin II inhibits insulin binding to endothelial cells. Diabetes Metab J 2011;35:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab 2012; 302:E201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes 2011;60:2939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K, Deng K, Wang X, Wang Z, Zheng S, Ren H, et al. Activation of D4 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Hypertension 2015;65:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utsugisawa K, Kizawa H, Nagane Y, Kondoh R, Iwa Y, Akutsu H, et al. Biphasic effects of angiotensin II and receptor antagonism on aggregability and protein kinase C phosphorylation in human platelets. Thromb Haemost 2005;94:1012–8. [DOI] [PubMed] [Google Scholar]
- 38.Rabkin SW. The angiotensin II subtype 2 (AT2) receptor is linked to protein kinase C but not cAMP-dependent pathways in the cardiomyocyte. Can J Physiol Pharmacol 1996;74:125–31. [PubMed] [Google Scholar]
- 39.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, et al. Inhibition of protein kinase cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 2007; 117:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bezy O, Tran TT, Pihlajamaki J, Suzuki R, Emanuelli B, Winnay J, et al. Pkcdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J Clin Invest 2011;121:2504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waraich RS, Weigert C, Kalbacher H, Hennige AM, Lutz SZ, Haring HU, et al. Phosphorylation of Ser357 of rat insulin receptor substrate-1 mediates adverse effects of protein kinase c-delta on insulin action in skeletal muscle cells. J Biol Chem 2008;283: 11226–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.