ABSTRACT
Body mass index (BMI) is widely used to define obesity. In studies of pancreatic beta-cell/islet mass, BMI is also a common standard for matching control subjects in comparative studies along with age and sex, based on the existing dogma of their significant positive correlation reported in the literature. We aimed to test the feasibility of BMI and BSA to assess obesity and predict beta-cell/islet mass. We used National Health and Nutrition Examination Survey (NHANES) data that provided dual-energy Xray absorptiometry (DXA)-measured fat mass (percent body fat; %BF), BMI, and BSA for adult subjects (20-75y; 4,879 males and 4,953 females). We then analyzed 152 cases of islet isolation performed at our center for correlation between islet yields and various donor anthropometric indices. From NHANES, over 50% of male subjects and 60% of female subjects with BMI:20.1–28.1 were obese as defined by %BF, indicating a poor correlation between BMI and %BF. BSA was also a poor indicator of %BF, as broad overlap was observed in different BSA ranges. Additionally, BMI and BSA ranges markedly varied between sex and race/ethnicity groups. From islet isolation, BMI and BSA accounted for only a small proportion of variance in islet equivalent (IEQ; r2 = 0.09 and 0.11, respectively). BMI and obesity were strongly correlated in cases of high BMI subjects. However, the critical populations were non-obese subjects with BMI ranging from 20.1–28.1, in which a substantial proportion of individuals may carry excess body fat. Correlations between BMI, BSA, pancreas weight and beta-cell/islet mass were low.
KEYWORDS: adiposity, beta cell mass, body mass index, body surface area, islets, obesity
Introduction
In the human pancreas, there is distinct intra-individual regional variability in beta cell/islet mass.1 In general, endocrine cell mass gradually increases from head, body and toward tail region, roughly 1:1:2 in ratio, respectively.1,2 However, this ratio can vary such as 2:1:2 in some cases.3 Throughout the pancreas, beta-cell/islet mass markedly fluctuates. We have shown the preferential loss of beta-cells in the head region of patients with type 2 diabetes2, whereas in type 1 diabetes, residual beta-cells were exclusively spared in the head region.4 The head region is developmentally and anatomically distinct from the rest of the pancreas, with its proximity to the duodenum, distinct blood supply, and the localization of a pancreatic polypeptide cell rich area.1 Furthermore, inter-individual heterogeneity is far more complex. Altogether, this suggests the importance of analyzing the whole pancreas with unbiased quantification. Three major sources of biased measurement of beta-cell/islet mass in the human pancreas are: [1] Sampling specific pancreatic region(s) of the whole pancreas; [2] Restricted selection of islet-rich areas of the entire section; and [3] Reference to the population-based pancreas volume data, instead of individual pancreas weight. These could lead to up to 14-fold overestimation of beta-cell/islet mass.3 On the other hand, although whole pancreas analysis is labor-intensive, it provides accurate quantification of each cell type as well as spatial distributions of several cell types within the same tissue section.3 Using a stereological approach to examine the large size of the human pancreas, we have shown the underlying heterogeneity of beta-cell/islet mass in healthy individuals.5 In contrast to previous studies and general belief of the close association between obesity and beta-cell mass and/or islet numbers, we found no clinically relevant correlation between beta-cell/islet mass and age, BMI or pancreas weight, with large differences in beta-cell/islet mass and islet number among the individuals.
Regarding BMI and pancreatic beta-cell mass, statistically significant correlation was reported by two well-known studies, one conducted in the US by Saisho et al.6 and another in Europe by Rahier et al.7 Interestingly, in Asian populations, while one study conducted in Korea showed positive correlation8, another in Japan reported a negative correlation.9 Unfortunately, none of these studies was based on the whole pancreas analysis, and all four studies included two to three sources of biased measurements of beta-cell/islet mass in the human pancreas described above. Nonetheless, it is noted that both Saisho et al.6 and Rahier et al.7 fairly and accurately reported their results. Saisho et al. provided a linear correlation coefficient value (r) of 0.5, which makes a coefficient of determination value (r2) of 0.25. The authors described the correlation as “considerable variance in beta-cell mass not explained by BMI”. Rahier et al. reported a correlation between beta-cell mass and BMI (r = 0.3, r2= 0.09), concluding that “obesity has a modest impact on beta-cell mass when compared with rodents.” Comparably, in clinical islet transplantation, it is believed that pancreas weight correlates with the body size9,10, and further that the larger a pancreas is, the more beta-cells/islets it contains.6–8 Therefore, donors with larger BMI are favored. North America Islet Donor Score (NAIDS), a new scoring system used for optimal pancreas donor selection for islet transplantation, included BSA11 in addition to the original Edmonton scoring system.12 BMI and BSA strongly influence NAIDS, encompassing up to 35 out of 100 points.
In the present study, we first explored a general trend in the relationship between BMI and obesity based on a large NHANES cohort data set. We aimed to determine BMI and BSA trends at the population level and to learn the distribution of relevant BMI and BSA ranges. The NAIDS framework, which was based on over 1,000 clinical islet isolations, provided an existing guidance in assessing potential islet donors, which includes assessment of BSA and BMI, in the context of a large donor population such as the one sampled from NHANES. We then examined the correlation of islet yields in clinical islet isolation from our center with BMI, BSA, and various other donor anthropometric factors.
Subjects and methods
NHANES data analysis
We examined adult subjects over 20 years old from the US NHANES 1999–2004 data set, containing in vivo measurements of regional body fat distributions using DXA scanners. The data was retrieved using the R package “NHANES”13 and analyzed with the R base package.14 Following exclusion criteria based on NAIDS (age:<20y and >75y, weight<55kg) and incomplete anthropometric data, 9,832 (4,879 male and 4,953 female) of 31,126 subjects (15,184 males and 15,942 females) from the NHANES data set were analyzed.
Islet isolation data analysis
In parallel, we reviewed 152 cases of human islet isolations performed in our center. Nine individuals were excluded from pancreas weight analysis due to missing data. Islet isolations were performed according to the Edmonton Protocol with subsequent improvement.15,16 The time from the cross clamp to digestive enzyme perfusion was limited to less than 12 hours. The islet isolation time was usually 6–7 hours. Isolations were considered successful for post-purification IEQ measuring greater than 400,000, which cover recipients with 80 kg body weight (5,000 IEQ/kg body weight). Qualitatively, islet isolation was considered successful when isolated islets were digested optimally, which results from administration of proper enzyme concentration, technical timing, and high quality of donor pancreata. Optimal digestion can be identified by high islet yield, high quality dithizone (DTZ) stain intensity, rounded islet borders without fragmented islets, and high purity.17 The information about the donors and islet isolation characteristics is summarized in Table 1.
Table 1.
Donor and islet isolation characteristics.
| Data Summary | Range | Total number/Mean ± SD |
|---|---|---|
| Age (year) | 1–71 | 45.0 ± 13.7 |
| Sex | Male | 94 |
| Female | 62 | |
| Race | American Indian/ Alaska Native |
1 |
| Asian | 1 | |
| Black/ African American |
23 | |
| Hispanic/Latino | 17 | |
| White | 114 | |
| Height (cm) | 60.0– 196.0 | 173.1 ± 14.8 |
| Weight (kg) | 8.0– 179.6 | 92.9 ± 24 |
| BMI | 17.6– 56.9 | 30.6 ± 6.4 |
| BSA | 0.4– 3.1 | 2.1 ± 0.3 |
| Total Post-Purification IEQ | 2,842– 857,630 | 255,486 ± 180,851 |
| Final Trimmed Pancreas Weight (g) | 6.7– 207.8 | 99.8 ± 32.7 |
Statistical analyses
Statistical analyses were performed using the Pearson’s correlation method for evaluating continuous variables. Correlations were analyzed using the same data set repeatedly in comparing various anthropometric indices with pancreas metrics/islet yields. The non-normally distributed data from calculating partial NAIDS population scores was tested for significance using Mood’s median test. Differences were considered to be significant at P< 0.05.
Results
Distribution of %BF by BMI and BSA range
In order to first define feasibility of BMI in assessing adiposity, we examined the correlation between BMI and DXA-measured %BF in non-diabetic adults (Figure1(a).a). Coefficient of determination (r2) was modest for both sexes (0.57 in males and 0.59 in females). The population was classified into BMI groups used in NAIDS; BMI<20.1, BMI:20.1–28.1, BMI:28.1–32.5, BMI:32.5–52.0, and BMI>52.0. Distribution of %BF was plotted according to these BMI ranges (Figure1(a).b). The overall distribution of %BF in each group is shown by a red line. According to the American Association of Clinical Endocrinology/American College of Endocrinology and the World Health Organization, cutoffs for obesity are 25% and 35% of %BF for males and females, respectively.18,19 The area under the gray bar indicates a fraction of obese individuals based on %BF. Overall, almost all individuals with BMI over 30 (97.9% of males and 99.8% of females) were defined to be obese. Similarly, a marked proportion of those with BMI under 20 were not obese as defined by %BF (93.8% of males and 92.9% of females). The critical population for this study appeared to be non-obese subjects defined by BMI:20–28.1. In males, over half had %BF greater than 25. Over 60% of females were obese as defined by %BF above 35. Both males and females had median %BF beyond the cutoff for obesity (28.0% and 41.5%, respectively).
Figure 1.
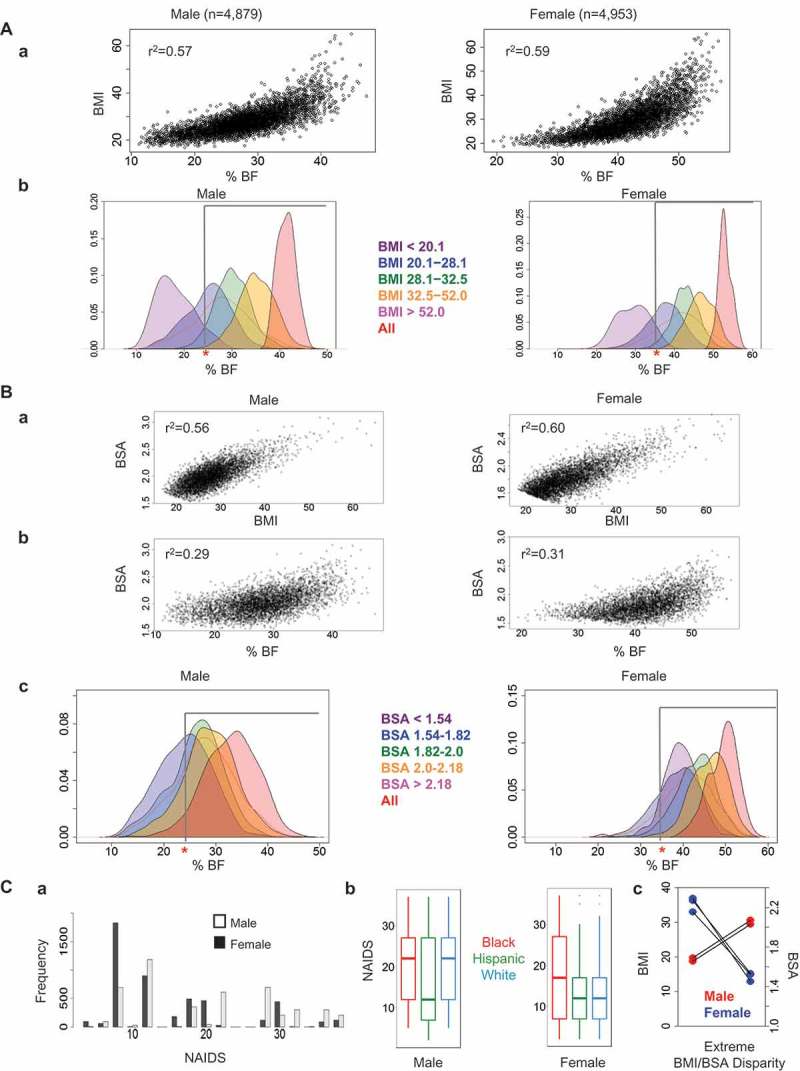
Relationship of %BF with BMI and BSA. A. Distribution of %BF by BMI range. (a) DXA-measured percentage of body fat (%BF) compared with BMI in non-diabetic adults; male adults (n = 4,879) and female adults (n = 4,953). (b) Distribution of %BF plotted according to BMI ranges. The population was classified into five BMI groups used in NAIDS; BMI<20.1 (purple), BMI:20.1–28.1 (blue), BMI:28.1–32.5 (green), BMI:32.5–52.0 (yellow), and BMI>52.0 (red). The red line depicts the proportion of individuals with a given %BF for all subjects. The cutoff for obesity as determined by %BF is shown by the gray bar (25% for males and 35% for females). (B) Distribution of %BF by BSA range. (a) Correlation between BMI and BSA. (b) DXA-measured %BF compared with BSA in male and female adults. c. Distribution of %BF by sex plotted according to BSA ranges. The population was classified into five BSA groups used in NAIDS; BSA<1.54 (purple), BSA:1.54–1.82 (blue), BSA:1.82–2 (green), BSA:2–2.18 (orange), and BSA>2.18 (red). (C) Comparison of NAIDS, BMI, and BSA by sex. a. Distribution of NAIDS for male and female subjects from NHANES data set. (b) Distribution of NAIDS across sex and race/ethnicity groups from NHANES data set. The center bar indicates the median of each group, the box contains the 25th-75th percentile of data, and the whiskers show the range of all data. (c) Cases of extreme BMI/BSA disparity in males and females from NHANES data set.
Next, we examined a correlation between BSA and %BF (Figure1(b)). Interestingly, although the equations used to calculate both BSA and BMI use only height and weight as their parameters, coefficient of determination was modest (0.56 for males and 0.60 for females) (Figure1(b).a). Surprisingly, the correlation between BSA and %BF had far more variability (r2:0.29 and 0.31) (Figure1(b).b) as compared to that between BMI and %BF (Figure1(a).a). Distribution of %BF was plotted according to BSA ranges in NAIDS; BSA<1.54, BSA:1.54–1.82, BSA:1.82–2, BSA:2–2.18, and BSA>2.18 (Figure1(b).c). Notably, a considerable proportion of populations in different BSA ranges had similar %BF distributions. In males with BSA:1.54–1.82, the interquartile range (IQR, 25th-75th percentile) for %BF overlapped appreciably with the %BF IQR of individuals with BSA:1.82–2.0 and BSA:2.0–2.18 (20.3–27.8, 23.2–29.8, 25.7–32.3, respectively). This trend was observed to a lesser degree in females in the same BSA ranges (IQR’s 35.4–42.7, 40.0–46.2, and 43.1–49.2, respectively). The NAIDS was then used as a guiding framework for a simulation using NHANES, the aim being to examine population data in the context of individual body metrics, and how they might relate to obesity, and by extension to pancreas parameters. The total NAIDS score includes BMI (0–10), BSA (0–25), vasopressor type used (0–15), unfavorable factors (0–35), and favorable factors (0–15). A partial NAIDS was calculated for each subject from the NHANES database according to BMI (0–10), BSA (0–25), and weight>120kg (+2 for favorable factor) (Figure1(c).a). Scores ranged from 0 to 37, and mean scores differed by more than five points between males and females (19.6 and 14.3, respectively; p < 0.05 with Mood’s median test). Of all NHANES subjects examined, 4.4% of males and 2.5% of females had a maximum score of 37. Similarly, 17.0% of male subjects and 4.5% of female subjects had a score greater than 30. The distribution of %BF compared to BMI was also examined in three race/ethnicity groups: Black or African-American (Black), Hispanic or Latino (Hispanic) and non-Hispanic White (White). Black and White male individuals tended to have higher partial NAIDS points as compared to Hispanic subjects (mean scores 20.2, 21.2, and 16.6, respectively). Black females had the highest NAIDS points as compared to Hispanic and White females (mean points 17.6, 12.8, and 13.9, respectively) (Figure1(c).b). Individual extreme cases from NHANES, in which BMI and BSA were incongruent, were plotted by sex (Figure1(c).c). Two male individuals scored in the second-highest BSA range and the lowest BMI range as determined by NAIDS. These individuals had an average height of 1.96 m (1.960 m and 1.956 m) and weight 73.7 kg (71.8 kg and 75.5 kg) with an average %BF of 15.6% (17.36% and 13.84%). Three female subjects scored in the lowest BSA range, while earning the most possible points for BMI, with an average height of 1.36 m, average weight of 65.6 kg, and average %BF of 45.9%. Lastly, we examined the distribution of BMI and BSA by sex based on ranges from NAIDS for individuals from NHANES (Table 2). We observed marked variation in BSA for a given BMI between males and females, with male subjects tending to have a larger BSA than females.
Table 2.
BMI and BSA distribution by sex among NHANES subjects.
| Male | BMI < 20.1 | BMI 20.1–28.1 | BMI 28.1–32.5 | BMI 32.5–52.0 | BMI > 52.0 | Total |
|---|---|---|---|---|---|---|
| BSA < 1.54 | 0 | 2 | 0 | 0 | 0 | 2 |
| BSA 1.54–1.82 | 103 | 745 | 64 | 3 | 0 | 915 |
| BSA 1.82–2.0 | 32 | 1,161 | 366 | 36 | 0 | 1,595 |
| BSA 2.0–2.18 | 2 | 616 | 595 | 212 | 0 | 1,425 |
| BSA > 2.18 | 0 | 104 | 307 | 525 | 13 | 949 |
| Total | 137 | 2,628 | 1,332 | 776 | 13 | 4,886 |
| Female | ||||||
| BSA < 1.54 | 0 | 98 | 13 | 3 | 0 | 114 |
| BSA 1.54–1.82 | 55 | 1,889 | 596 | 186 | 0 | 2,726 |
| BSA 1.82–2.0 | 1 | 327 | 502 | 467 | 0 | 1,297 |
| BSA 2.0–2.18 | 0 | 22 | 92 | 459 | 4 | 577 |
| BSA > 2.18 | 0 | 0 | 2 | 216 | 26 | 244 |
| Total | 56 | 2,336 | 1,205 | 1,331 | 30 | 4,958 |
Relationship between post-purification IEQ and various anthropometric parameters from donors
To validate an existing paradigm of the association between islet numbers and obesity, we first examined correlations of pancreas weight with height, weight, BMI and BSA data from islet isolations performed at our center (Figure 2(a)). There were significant positive correlations with all four parameters (P < 0.05), however, r2 values were low (range:0.11–0.27). In fact, when we examined a correlation between pancreas weight and IEQ, only 11% of the variability in IEQ was explained by donor pancreas weight (Figure 2(b)). As expected, BMI and BSA similarly showed low correlations with IEQ (r2 = 0.09 and 0.11, respectively). In anticipation of possible technical failure in cases of low islet yields, we also exclusively examined successful cases of islet isolation (IEQ>300,000). The correlations of IEQ with three parameters remained low; with pancreas weight: r2= 0.21, BMI: r2= 0.05, and BSA: r2= 0.12 (Figure 2(c)). In addition, we examined correlations of IEQ with height and weight, but r2 values were low (r2= 0.03 and 0.12, respectively) (Figure 2(d)). Age showed a negative correlation with IEQ (r2= 0.03). Sexual dimorphism was recognized as males and females were differentially plotted (open circles for males and closed circles for females). This finding aligns with the well-known observation that males are taller and heavier than females in general (average height from NHANES cohort: 1.75 ± 0.08 m in males and 1.62 ± 0.07 m in females, P < 0.05; average weight: 86.0 ± 18.2 kg in males and 77.8 ± 17.8 kg in females, P < 0.05).
Figure 2.
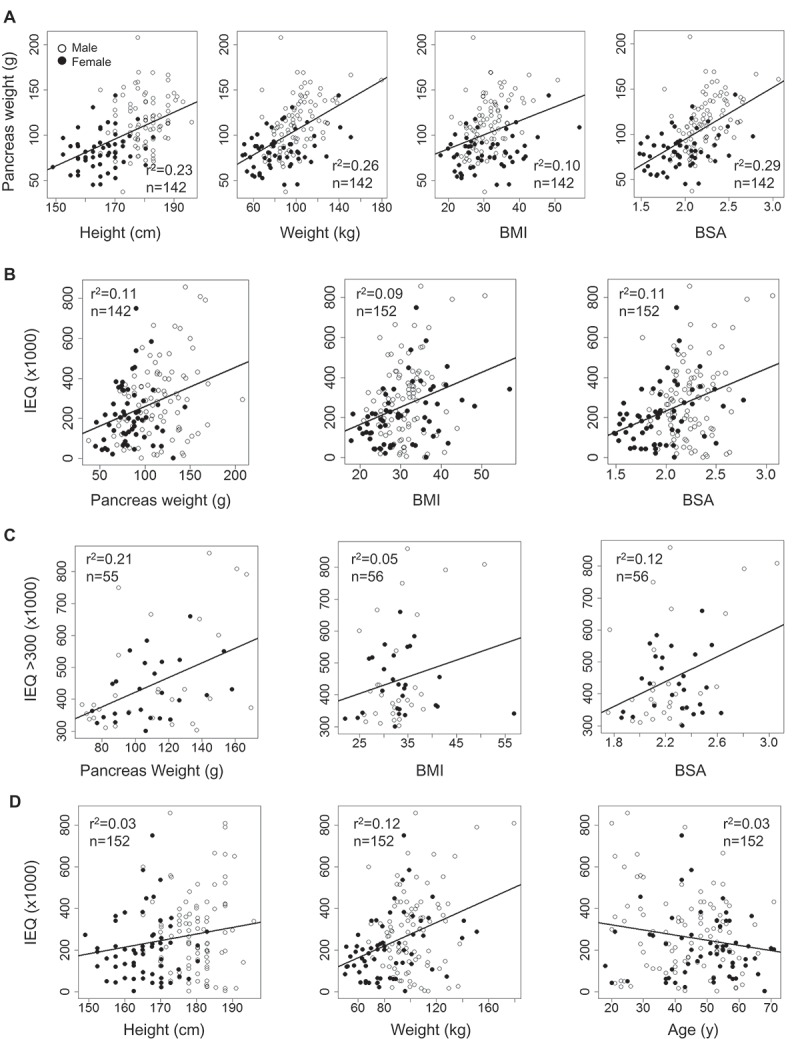
Relationship between post-purification IEQ and various parameters from donors. (A) Pancreas weight: height, weight, BMI and BSA. (B) IEQ: pancreas weight, BMI and BSA. (C) IEQ>300K: pancreas weight, BMI and BSA. (D) IEQ: height, weight and age. Males: open circles and females: closed circles.
Discussion
Obesity is defined as an excess of body fat mass. Currently, BMI is the most widely accepted metric for assessing obesity. However, BMI fails to distinguish between fat and lean mass. As a result, the accuracy of BMI in detecting body adiposity at the individual level has been brought into question. Meta-analysis studies demonstrated approximately half of individuals with excess fat were classified as non-obese by BMI.20,21 As shown in these studies, %BF can be measured by a bioelectrical impedance analysis, which accurately determines fat composition, similarly to DXA as performed in NHANES.
In assessing NAIDS in subjects from NHANES, we found that scores were increased in males more than females, possibly resulting from higher BSA scores in males compared to females and the greater influence of BSA than BMI on NAIDS (25 and 10 maximum points, respectively). Some cases showed a large disparity in BSA and BMI, resulting in a high NAIDS in one of these categories and a low score in the other. In both cases, NAIDS remained relatively high, but the body composition of these extreme cases differed dramatically. Individuals with high BSA and low BMI scores tended to be tall with little body fat, while those with low BSA and high BMI scores were short with elevated body fat. These disparities highlight the striking anthropometric differences that may be masked in using BMI and BSA without %BF assessment in potential pancreas donor evaluation.
Existing dogma asserts a strong relationship among islet mass, pancreas weight, and body size.6–10 For all comparisons between islet mass (IEQ) and various body metrics performed in this study, there were statistically significant correlations with p < 0.05. However, such significance plainly indicates a relationship between any of two variables, as all or nothing. In translating from bench to bedside, we propose that it is important to further validate the extent of variability that is explained by a given relationship, which is best indicated by coefficient of determination (r2). In practice, even with highly scored donor pancreata with 80–100 points of NAIDS, only 40–50% cases have been successful in islet isolations.11 It may be possible that the ultimate outcome of isolation is predetermined by intrinsic islet mass in the donor pancreas.5
It is noteworthy that the NAIDS preference for obese donors is based on the long-term experience and observations of transplant surgeons. It appears that this notion somewhat matched with the existing paradigm of the close association between beta-cell mass and/or islet numbers and obesity.6–8,22–27 An alternative explanation for differences in islet counts between lean and obese donors could be attributed to changes in the extracellular matrix (ECM). It is recognized that islet isolation from young donors results in lower recovery with difficulty in separating islets from exocrine tissues by collagenase digestion, regardless of BMI unlike adult donors.28–31 In fact, donors under 20 years of age are listed as an unfavorable factor under NAIDS, although it has been demonstrated that islets from young donors exhibit superior function in insulin secretion.32,33 It is suggested that there are unique ECM components/structure in the pancreas of the young.31 In terms of the ECM components, Collagen IV has been recognized as a major type of collagens, however, Hughes et al. showed abundant expression of Collagen VI in the islet-exocrine interface34, which is resistant to digestion by bacterial collagenase unlike collagens I-V.35 Taken together, it may be a testable hypothesis that the higher yield of islets from obese donors would result from altered ECM structure/composition that facilitates liberating islets from exocrine tissues, not necessarily that obese people have more islets than lean people.
In summary, correlations between BMI, BSA, pancreas weight, and beta-cell mass/islet number as measured by IEQ were low. Although BMI is often used as a clinical indicator of obesity, BMI only reliably predicted adiposity among individuals at the highest BMI levels. The largest variation in %BF from this study occurred in non-obese individuals, which should be more closely monitored, since BMI is commonly used in clinical practice as well as comparative studies in which control subjects are matched based on BMI along with age and sex.
Funding Statement
The study is supported by National Institutes of Health, DK-020595 to the University of Chicago Diabetes Research and Training Center (Animal Models Core), DK-072473, AG-042151, and a gift from the Kovler Family Foundation to M.H;Kovler Family Foundation;
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Abbreviations
BMI
BSA
References
- 1.Wang X, Zielinski MC, Misawa R, Wen P, Wang T-Y, Wang C-Z, Witkowski P, Hara M.. Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PloS One. 2013;8(1):e55501. doi: 10.1371/journal.pone.0055501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J, et al. Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One. 2013;8:e67454. doi: 10.1371/journal.pone.0067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poudel A, Fowler JL, Zielinski MC, Kilimnik G., Hara M.. Stereological analyses of the whole human pancreas. Sci Rep. 2016;6:34049. doi: 10.1038/srep34049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poudel A, Savari O, Striegel DA, Periwal V, Taxy J, Millis JM, Witkowski P, Atkinson MA, Hara M.. Beta-cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine. 2015;49:693–702. doi: 10.1007/s12020-015-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olehnik SK, Fowler JL, Avramovich G, Hara M.. Quantitative analysis of intra-and inter-individual variability of human beta-cell mass. Sci Rep. 2017;7:16398. doi: 10.1038/s41598-017-16300-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC.. Beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4): 32–42.doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 9.Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in beta-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab. 2013;98:3724–3730. doi: 10.1210/jc.2013-1373. [DOI] [PubMed] [Google Scholar]
- 10.Campbell-Thompson ML, Kaddis JS, Wasserfall C, Haller MJ, Pugliese A, Schatz DA, Shuster JJ, Atkinson MA. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59:217–221. doi: 10.1210/jc.2013-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LJ, Kin T, O’Gorman D, Shapiro AJ, Naziruddin B, Takita M, Levy MF, Posselt AM, Szot GL, Savari O, Barbaro B. A multicenter study: north american islet donor score in donor pancreas selection for human islet isolation for transplantation. Cell Transplant. 2016;25:1515–1523. doi: 10.3727/096368916x691141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Gorman D, Kin T, Murdoch T, Richer B, McGhee-Wilson D, Ryan E, Shapiro AM, Lakey JR. The standardization of pancreatic donors for islet isolations. Transplantation. 2005;80:801–806. doi: 10.1016/j.transproceed.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 13.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Core Team R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2013. [Google Scholar]
- 15.Shapiro AM, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro AM. Strategies toward single-donor islets of Langerhans transplantation. Curr Opin Organ Transplant. 2011;16:627–631. doi: 10.1097/MOT.0b013e32834cfb84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LJ, Kaufman DB. Digital image analysis to assess quantity and morphological quality of isolated pancreatic islets. Cell Transplant. 2016;25:1219–1225. doi: 10.3727/096368915X689947. [DOI] [PubMed] [Google Scholar]
- 18.AACE/ACE Obesity Task Force 1998. AACE/ACE position statement on the prevention, diagnosis, and treatment of obesity. Endocr Pract. 4:297–350. [Google Scholar]
- 19.World Health Organization 1995. Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 854:1–452. [PubMed] [Google Scholar]
- 20.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond). 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 22.Lakey JR, Warnock GL, Rajotte RV, Suarez-Almazor ME, Ao Z, Shapiro AJ, Kneteman NM. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61:1047–1053. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Toso C, Oberholzer J, Ris F, Ris F, Triponez F, Bucher PA, Demirag A, Andereggen EM, Buehler LH, Cretin N, Fournier B, Majno P. Factors affecting human islet of Langerhans isolation yields. Transplant Proc. 2002;34:826–827. doi: 10.1016/s0041-1345(01)02925-6. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto I, Sawada T, Nakano M, Sakai T, Liu B, Ansite JD, Zhang HJ, Kandaswamy R, Sutherland DE, Hering BJ. Improvement in islet yield from obese donors for human islet transplants. Transplantation. 2004;78:880–885. doi: 10.1097/01.tp.0000134396.03440.1e. [DOI] [PubMed] [Google Scholar]
- 25.Nano R, Clissi B, Melzi R, Calori G, Maffi P, Antonioli B, Marzorati S, Aldrighetti L, Freschi M, Grochowiecki T, Socci C. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48:906–912. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 26.Takita M, Naziruddin B, Matsumoto S, Noguchi H, Shimoda M, Chujo D, Itoh T, Sugimoto K, Tamura Y, Olsen GS, Onaca N. Body mass index reflects islet isolation outcome in islet autotransplantation for patients with chronic pancreatitis. Cell Transplant. 2011;20:313–322. doi: 10.3727/096368910x514611. [DOI] [PubMed] [Google Scholar]
- 27.Hilling DE, Bouwman E, Terpstra OT, Marang-Van De Mheen PJ. Effects of donor-, pancreas-, and isolation-related variables on human islet isolation outcome: a systematic review. Cell Transplant. 2014;23:921–928. doi: 10.3727/096368913x666412. [DOI] [PubMed] [Google Scholar]
- 28.Berkova Z, Saudek F, Girman P, Zacharovova K, Kriz J, Fabryova E, Leontovyc I, Koblas T, Kosinova L, Neskudla T, Vavrova E.. Combining donor characteristics with immunohistological data improves the prediction of islet isolation success. J Diabetes Res. 2016;2016:4214328. doi: 10.1155/2016/4214328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandhorst D, Hering BJ, Brandhorst H, Federlin K, Bretzel RG. Influence of donor data and organ procurement on human islet isolation. Transplant Proc. 1994;26:592–593. [PubMed] [Google Scholar]
- 30.Watt PC, Mullen Y, Benhamou PY, Hober C, Nomura Y, Watanabe Y, Kleinman R, Miyamoto M, Passaro EP, Zinner MJ, Brunicardi FC. Donor factors affecting successful isolation of islets from the human pancreas. Transplant Proc. 1994;26:594–595. [PubMed] [Google Scholar]
- 31.Benhamou PY, Watt PC, Mullen Y, Ingles S, Watanabe Y, Nomura Y, Hober C, Miyamoto M, Kenmochi T, Passaro EP. Human islet isolation in 104 consecutive cases. Factors affecting isolation success. Transplantation. 1994;57:1804–1810. doi: 10.1097/00007890-199406270-00021. [DOI] [PubMed] [Google Scholar]
- 32.Meier RP, Sert I, Morel P, Muller YD, Borot S, Badet L, Toso C, Bosco D, Berney T. Islet of Langerhans isolation from pediatric and juvenile donor pancreases. Transpl Int. 2014;27:949–955. doi: 10.1111/tri.12367. [DOI] [PubMed] [Google Scholar]
- 33.Niclauss N, Bosco D, Morel P, Demuylder-Mischler S, Brault C, Milliat-Guittard L, Colin C, Parnaud G, Muller YD, Giovannoni L, Meier R. Influence of donor age on islet isolation and transplantation outcome. Transplantation. 2011;91:360–366. doi: 10.1097/tp.0b013e31820385e6. [DOI] [PubMed] [Google Scholar]
- 34.Berney T, Johnson PR. Donor pancreata: evolving approaches to organ allocation for whole pancreas versus islet transplantation. Transplantation. 2010;90:238–243. doi: 10.1097/tp.0b013e3181e25a40. [DOI] [PubMed] [Google Scholar]
- 35.Hughes SJ, Clark A, McShane P, Contractor HH, Gray DW, Johnson PR. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81:423–426. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]


