Abstract
Background/Aims
Herb-induced liver injury (HILI) can lead to chronic liver injury, liver transplantation, or even death. This study aimed to identify the predictors of poor HILI outcomes, especially chronic HILI.
Materials and Methods
Clinical data of 488 patients with HILI were retrospectively analyzed from a Chinese center between January 2010 and January 2014. Logistic regression and C-statistic were used to identify risk factors and prognostic models for HILI outcomes.
Results
In all patients, 69 (14.1%) developed chronic HILI, and 20 (4.1%) died due to liver injury or underwent liver transplantation. To predict the fatal HILI prognosis, the model for end-stage liver disease (MELD) with a C-statistic of 0.981 (95%CI 0.968–0.995) was better than Hy’s law (C-statistic 0.569; 95%CI 0.449–0.689). The latency, course of peak alanine aminotransferase decreasing ≥50% after discontinuation of herb application, peak triglyceride value, and platelet count at liver injury onset were identified as independent risk factors for chronicity with the adjusted odds ratios of 1.268 (95% confidence interval [CI] 1.034–1.554), 2.303 (95%CI 1.588–3.340), 0.580 (95%CI 0.343–0.978), and 0.183 (95%CI 0.091–0.368), respectively. A prognostic model for chronic HILI based on these four factors yielded the best prediction with a C-statistic of 0.812 (95%CI 0.755–0.868), compared with MELD (C-statistic 0.506; 95%CI 0.431–0.581) and Hy’s law (C-statistic 0.418; 95%CI 0.343–0.492).
Conclusion
Model for end-stage liver disease can be used to predict the fatal prognosis of HILI. A long latency, slow recovery, and low triglyceride value and platelet counts are important determinants for chronic HILI.
Keywords: Drug-induced liver injury, herbal hepatotoxicity, mortality, chronicity, prognosis
INTRODUCTION
Herb-induced liver injury (HILI) has increasingly been reported and attracted the attention of physicians, pharmaceutical companies, and regulatory agencies over the past years (1,2). In general, HILI is self-limited after withdrawing implicated herbs or herbal products; however, in some cases, chronic liver injury, liver transplantation, or even death is observed (3,4). In a previous case collection about the outcomes of HILI, 70 (12.4%) of 563 patients developed chronic HILI; 29 (5.2%) underwent liver transplantation or died from liver injury (3). Until now, only a few studies tried to survey clinical characteristics of HILI patients with poor outcomes and define risk factors for poor HILI prognosis (5). Thus, it is still challenging to predict the HILI prognosis and guide its treatment (2,5).
In this study, we retrospectively analyzed the clinical data of 488 patients diagnosed with HILI to identify the risk factors and prognostic models for severe HILI cases, especially development of chronic HILI.
MATERIALS AND METHODS
All patients admitted to our hospital (Beijing, China) due to liver injury between January 2010 and January 2014 were included. HILI was diagnosed based on the following criteria (2,5,6); (i) recent onset of a rise in alanine aminotransferase (ALT)≥5×upper limit of normal (ULN) or alkaline phosphatase (ALP)≥2×ULN, or a rise in total serum bilirubin (TB)≥2 mg/dL associated with ALT≥3×ULN; (ii) exclusion of viral hepatitis, autoimmune liver disease, alcoholic liver disease, metabolic disorders, bile duct obstruction, and other nontoxic causes of liver diseases by laboratory tests (anti-hepatitis A virus IgM, hepatitis B surface antigen, anti-hepatitis B core IgM, anti-hepatitis C virus, hepatitis C virus RNA, and anti-hepatitis E virus IgM, anti-hepatitis E virus IgG, anti-nuclear antibody, anti-smooth muscle antibody, anti-mitochondrial antibody, ceruloplasmin, and α-1-antitrypsin), radiological examinations, and/or liver biopsy; (iii) exclusion of pre-existing liver diseases; (iv) a medical history with only exposure to herbs or herbal products within the previous 6 months before the onset of an abnormal liver test; (v) a Roussel Uclaf Causality Assessment Method (RUCAM) score ≥3 points. All data of patients diagnosed with HILI were extracted from our hospital records, including a detailed history of liver injury events and exposure to the implicated herb(s). Missing or unclear information was clarified with physicians.
All HILI cases were divided into three groups: (i) healed HILI, including patients who had persistent normal liver function after implicated herb(s) discontinuation; (ii) chronic HILI, including patients with sustained liver-related laboratory, radiologic, or histologic abnormalities at 6 months after the HILI onset (5); (iii) fatal HILI, including patients who underwent liver transplantation or died from HILI. HILI was classified as hepatocellular, cholestatic, or mixed pattern, based on the determination of the ratio R of ALT (as a multiple of the ULN) to ALP (as a multiple of the ULN) (7). The model for end-stage liver disease (MELD) score was calculated as follows: 9.6*ln [creatinine (mg/dL)] + 3.8*ln [bilirubin (mg/dL)]+11.2*ln (international normalized ratio) + 6.4 (8). The positivity for Hy’s law was defined as either ALT or AST≥3×ULN, and TB≥2×ULN (2). The definition of re-challenge is for the hepatocellular pattern ALT level before re-exposure was <5×ULN and after re-exposure increased ≥2×ALT level before re-exposure, while for cholestatic or mixed pattern ALP level before re-exposure was <2×ULN and after re-exposure increased ≥2×ALP level before re-exposure (6).
Statistics analysis
Continuous variables were described with mean±standard deviation (SD) and median values including the 25th and 75th percentile values, and they were analyzed with the one-way analysis of variance for normally distributed values or the Kruskal-Wallis test for skewed distributions to calculate significant differences. Frequency and percentages were used to describe categorical variables, and a chi-squared test was used for comparison. To predict the fatal prognosis of HILI, MELD and Hy’s law were compared, and receiver operating characteristics (ROC) curve in the form of C-statistic with 95% confidence interval (CI) was used to describe their predictive abilities. To identify the predictors of chronicity, a multivariate logistic regression analysis including variables based on a univariate analysis with p<0.10 and clinical judgment was conducted. The results were reported as adjusted odds ratio (OR) with 95% CI. Based on the multivariate logistic regression analysis, a model for chronic HILI was developed and compared with MELD and Hy’s law. The C-statistic with 95% CI was used to assess the discriminative accuracy of these models for chronic HILI. A p<0.05 was recognized as statistically significant. All statistical analyses were performed using the SPSS (Statistical Package for the Social Sciences) version 16.0 software (SPSS Inc.; Chicago, IL, USA).
RESULTS
Demographic features
From January 2010 to January 2014, a total of 61,516 patients were hospitalized due to liver injury, but 49,724 cases fulfilled the clinical biochemistry criteria for HILI; a total of 40,683 patients were diagnosed with viral hepatitis, alcoholic hepatitis, autoimmune liver diseases, metabolic disorders, or other liver diseases; 7,787 cases were combined with pre-existing liver diseases; the liver injury in 759 patients was caused by synthetic drug(s) or a combination of synthetic drug(s) and herb(s); all of them were not eligible for this study. In 495 patients only exposed to herb(s) before the onset of abnormalities in liver function, 52 (10.5%) cases were considered high probable according to RUCAM, 370 (74.8%) were probable, 66 (13.3%) were possible, 7 (1.4%) were unlikely, and no cases were excluded. The diagnostic criteria of HILI are presented in Figure 1.
Figure 1.
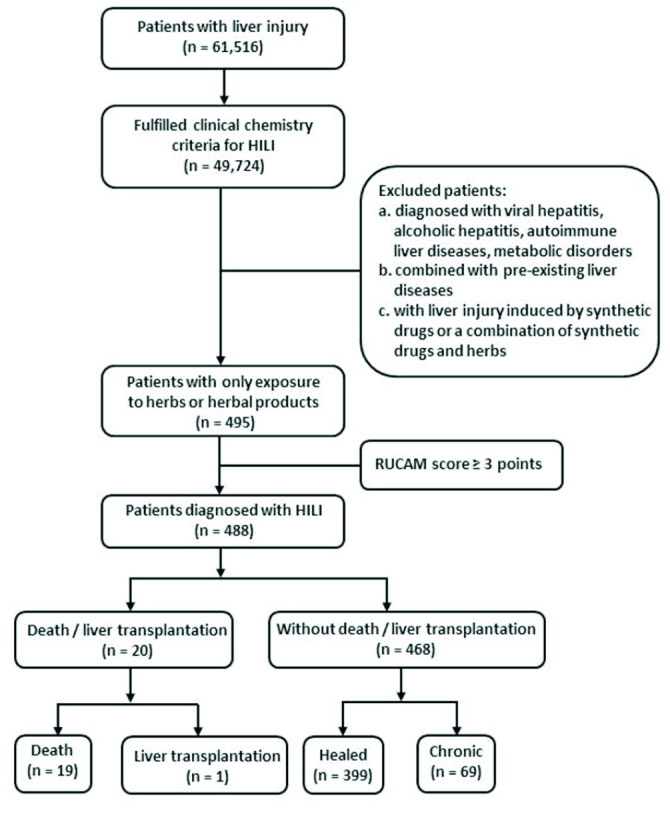
The diagnosis and prognosis of patients with HILI
HILI: Herb-induced liver injury
Clinical characteristics
Out of 488 patients who fulfilled the diagnostic criteria of HILI, 420 (86.1%) cases were classified as hepatocellular, 31 (6.3%) as cholestatic, and 37 (7.6%) as a mixed pattern of liver injury; clinical characteristics are summarized in Table 1. The mean age was 45 years (range, 15–89), with 480 (98.4%) adults (≥18 years). With respect to the time from the beginning of herb application to the onset of liver injury, 27 (5.5%) cases had a short latency (<5 days), and 90 (18.4%) had a long latency (>90 days); all clinical characteristics are listed in Table 2. After withdrawal of the suspected herbs, 399 (81.8%) patients achieved sustained normalization of liver biochemistry, 69 (14.1%) developed chronic HILI, 37 (7.6%) cirrhosis, 1 (0.2%) patient underwent liver transplantation, and 19 (3.9%) died due to their liver injury.
Table 1.
Characteristics of the 488 patients with HILI: comparisons according to the liver injury patterns
| Characteristics | Total (n=488) | Hepatocellular (n=420) | Cholestatic (n=31) | Mixed (n=37) | p |
|---|---|---|---|---|---|
| Age (y), mean±SD | 45±13 | 43±13 | 54±16 | 51±13 | <.001 |
| Proportion of patients aged 65 years or older, % | 6.4 | 4.3 | 19.4 | 18.9 | <.001 |
| Female, % | 71.5 | 71.0 | 61.3 | 86.5 | 0.057 |
| Alcohol use*, % | 12.1 | 13.1 | 12.9 | 0.0 | 0.030 |
| Allergy history, % | 15.8 | 15.2 | 22.6 | 16.2 | 0.486 |
| Latency (d), median (25th, 75th) | 30 (15, 63) | 30 (15, 60) | 60 (30, 1100) | 30 (10, 75) | 0.010 |
| Course of peak ALT decreasing ≥50% after herbal intake discontinuation, median (25th, 75th) | 7 (5, 11) | 7 (5, 8) | 18 (10, 30) | 12 (6, 21) | <.001 |
| Positive re-challenge, % | 7.2 | 7.6 | 0.0 | 8.1 | 0.313 |
| Fever, % | 6.8 | 6.9 | 3.2 | 8.1 | 0.788 |
| Skin rashes, % | 2.9 | 2.9 | 0.0 | 5.4 | 0.455 |
| Biochemistries, mean±SD | |||||
| Peak ALT (U/L) | 935±672 | 1050±649 | 118±122 | 311±263 | <.001 |
| Peak ALP (U/L) | 186±115 | 168±72 | 341±263 | 264±178 | <.001 |
| Peak TB (mg/dL) | 10.7±9.2 | 10.6±9.0 | 12.2±11.1 | 10.7±9.9 | 0.740 |
| Lowest ALB (g/L) | 37±5 | 37±5 | 31±5 | 35±6 | <.001 |
| Peak Scr (μmol/L) | 77.6±34.8 | 75.7±21.6 | 97.7±99.8 | 82.0±47.5 | 0.362 |
| Peak TG (mmol/L) | 2.08±1.25 | 2.08±1.21 | 1.90±1.46 | 2.20±1.54 | 0.724 |
| Peak TC (mmol/L) | 3.89±1.78 | 3.72±1.30 | 4.92±4.02 | 4.94±2.71 | 0.012 |
| Peak INR | 1.19±0.48 | 1.18±0.49 | 1.25±0.40 | 1.21±0.45 | 0.729 |
| Blood routine at onset, mean±SD | |||||
| White blood cell count (×109/L) | 5.76±2.28 | 5.73±2.27 | 5.19±1.86 | 6.46±2.69 | 0.115 |
| Eosinophil count (×109/L) | 0.24±0.29 | 0.23±0.27 | 0.24±0.17 | 0.26±0.50 | 0.819 |
| Platelet count (×109/L) | 209±84 | 210±80 | 179±104 | 218±104 | 0.242 |
| MELD score, mean±SD | 13.2±7.2 | 13.1±7.0 | 14.7±8.0 | 13.0±8.7 | 0.485 |
| Hy’s law, % | 66.8 | 74.0 | 6.5 | 35.1 | <.001 |
| Cirrhosis, % | 7.6 | 5.0 | 35.5 | 13.5 | <.001 |
| Prognosis, % | <.001 | ||||
| Healed | 81.8 | 85.7 | 45.2 | 67.6 | |
| Chronic | 14.1 | 10.7 | 45.2 | 27.0 | |
| Fatal | 4.1 | 3.6 | 9.7 | 5.4 | |
The patients had a drinking history (alcohol intake of >2 drinks per day for women and >3 drinks per day for men) but did not drink a month before the liver injury
ALT: alanine transaminase; ALP: alkaline phosphatase; TB: total bilirubin; ALB: albumin; Scr: serum creatinine; TG: triglyceride; TC: total cholesterol; INR: international normalized ratio; MELD: model for end-stage liver disease
Table 2.
Characteristics among the HILI cases with short and long latency
| Characteristics | Short Latency (<5 days) (n=27) | Long Latency (>90 days) (n=90) | p |
|---|---|---|---|
| Age (y), mean±SD | 43±16 | 44±15 | 0.650 |
| Proportion of patients aged 65 years or older, % | 7.4 | 8.9 | 1.000 |
| Female, % | 74.1 | 74.4 | 0.969 |
| Alcohol use*, % | 11.1 | 7.8 | 0.695 |
| Allergy history, % | 18.5 | 20.0 | 0.865 |
| Course of peak ALT decreasing ≥50% after herbal intake discontinuation, median (25th, 75th) | 7 (6, 11) | 7 (5, 15) | 0.735 |
| Positive re-challenge, % | 22.2 | 2.2 | 0.002 |
| Fever, % | 14.8 | 3.3 | 0.049 |
| Skin rashes, % | 7.4 | 0.0 | 0.052 |
| Biochemistries, mean±SD | |||
| Peak ALT (U/L) | 859±672 | 682±554 | 0.170 |
| Peak ALP (U/L) | 226±195 | 165±105 | 0.037 |
| Peak TB (mg/dL) | 14.0±10.6 | 9.2±9.4 | 0.027 |
| Lowest ALB (g/L) | 36±4 | 36±7 | 0.616 |
| Peak Scr (μmol/L) | 76±31 | 82±61 | 0.605 |
| Peak TG (mmol/L) | 2.19±1.45 | 1.72±1.33 | 0.115 |
| Peak TC (mmol/L) | 4.21±2.34 | 3.69±1.44 | 0.161 |
| Peak INR | 1.41±1.10 | 1.25±0.60 | 0.478 |
| Blood routine at onset, mean±SD | |||
| White blood cell count (×109/L) | 6.64±3.70 | 5.30±2.25 | 0.084 |
| Eosinophil count (×109/L) | 0.24±0.26 | 0.17±0.12 | 0.225 |
| Platelet count (×109/L) | 213±73 | 185±88 | 0.136 |
| Clinical pattern, % | 0.583 | ||
| Hepatocellular | 85.2 | 75.6 | |
| Cholestatic | 7.4 | 15.6 | |
| Mixed | 7.4 | 8.9 | |
| MELD score, mean±SD | 15.2±8.6 | 12.8±8.1 | 0.190 |
| Hy’s law, % | 70.4 | 53.3 | 0.117 |
| Cirrhosis, % | 3.7 | 21.1 | 0.041 |
| Prognosis, % | 0.032 | ||
| Healed | 74.1 | 64.4 | |
| Chronic | 14.8 | 33.3 | |
| Fatal | 11.1 | 2.2 | |
The patients had a drinking history (alcohol intake of >2 drinks per day for women and >3 drinks per day for men) but did not drink a month before the liver injury
ALT: alanine transaminase; ALP: alkaline phosphatase; TB: total bilirubin; ALB: albumin; Scr: serum creatinine; TG: triglyceride; TC: total cholesterol; INR: international normalized ratio; MELD: model for end-stage liver disease
Death and liver transplantation
The clinical characteristics of the 20 patients with a fatal outcome (death or liver transplantation) as compared to the 468 cases in the healed and chronic groups are summarized in Table 3. The herbs or herbal products associated with death or liver transplantation and their indications are listed in Table 4. Only 3 patients who died were induced by a single implicated herb, such as Fructus psoraleae, Gynura segetum, and Radix polygoni multiflora. Among the 19 lethal cases, 10 (52.6%) died of septic shock, 6 (31.6%) of cerebral edema due to encephalopathy, 2 (10.5%) of hepatorenal syndrome, and 1 (5.3%) of hemorrhagic shock due to upper gastrointestinal bleeding. To predict the fatal prognosis, MELD yielded a C-statistic of 0.981 (95%CI 0.968–0.995), superior to Hy’s law (C-statistic 0.569, 95%CI 0.449–0.689) (Figure 2).
Table 3.
Comparison of characteristics between the death/liver transplantation group and the healed/chronic group
| Characteristic | Death/Liver Transplantation (n=20) | Healed/Chronic (n=468) | p |
|---|---|---|---|
| Age (y), mean±SD | 47±16 | 45±13 | 0.468 |
| Proportion of patients aged 65 years or older, % | 15.0 | 6.0 | 0.127 |
| Females, % | 70.0 | 71.6 | 0.878 |
| Alcohol use*, % | 5.0 | 12.4 | 0.492 |
| Allergy history, % | 10.0 | 16.0 | 0.753 |
| Latency (d), median (25th, 75th) | 20 (11,30) | 30 (15,70) | 0.019 |
| Short latency (<5 days), % | 15.0 | 5.1 | 0.092 |
| Long latency (>90 days), % | 10.0 | 18.8 | 0.554 |
| Course of peak ALT decreasing ≥50% after herbal intake discontinuation, median (25th, 75th) | 8 (5,28) | 7 (5,10) | 0.446 |
| Positive re-challenge, % | 5.0 | 7.3 | 1.000 |
| Fever, % | 10.0 | 6.6 | 0.638 |
| Skin rashes, % | 5.0 | 2.8 | 0.448 |
| Ascites, % | 55.0 | 9.0 | <.001 |
| Encephalopathy, % | 40.0 | 0.9 | <.001 |
| Hyponatremia, % | 35.0 | 4.1 | <.001 |
| Biochemistries, mean±SD | |||
| Peak ALT (U/L) | 752±549 | 943±676 | 0.213 |
| Peak ALP (U/L) | 250±192 | 183±110 | 0.139 |
| Peak TB (mg/dL) | 26.7±7.1 | 9.5±8.6 | <.001 |
| Lowest ALB (g/L) | 29±4 | 37±5 | <.001 |
| Peak Scr (μmol/L) | 104±67 | 76±32 | 0.077 |
| Peak TG (mmol/L) | 0.95±0.89 | 1.59±1.33 | 0.005 |
| Peak TC (mmol/L) | 1.35±1.87 | 3.48±1.76 | <.001 |
| Peak INR | 2.40±1.31 | 0.70±0.47 | <.001 |
| Blood routine at onset, mean±SD | |||
| White blood cell count (×109/L) | 8.20±3.79 | 5.12±2.14 | 0.002 |
| Eosinophil count (×109/L) | 0.15±0.49 | 0.25±1.24 | 0.726 |
| Platelet count (×109/L) | 156±79 | 211±84 | 0.004 |
| Clinical pattern, % | 0.144 | ||
| Hepatocellular | 75.0 | 86.5 | |
| Cholestatic | 15.0 | 6.0 | |
| Mixed | 10.0 | 7.5 | |
| MELD score, mean±SD | 29.6±6.1 | 12.0±6.3 | <.001 |
| Hy’s law, % | 80.0 | 66.2 | 0.201 |
| Cirrhosis, % | 10.0 | 7.5 | 0.658 |
| Plasma exchange, % | 50.0 | 2.8 | <.001 |
| Steroid use, % | 10.0 | 6.6 | 0.638 |
The patients had a drinking history (alcohol intake of >2 drinks per day for women and >3 drinks per day for men) but did not drink a month before the liver injury
ALT: alanine transaminase; ALP: alkaline phosphatase; TB: total bilirubin; ALB: albumin; Scr: serum creatinine; TG: triglyceride; TC: total cholesterol; INR: international normalized ratio; MELD: model for end-stage liver disease
Table 4.
Herbal preparations associated with death, liver transplantation, and chronic HILI
| Indication | Herbal Preparation | |
|---|---|---|
| Death/liver transplantation (n=20) | n=7 Skin diseases n=2 Bone and joint diseases Health care Endocrine system diseases Urinary diseases n=1 Gastrointestinal diseases Neurological diseases Gynecological disease Cardiovascular diseases Respiratory diseases |
n=10 Herbal decoction with unknown constituents. n=1 Fructus psoraleae; Herbal decoction (Radix codonopsis, Radix ophiopogonis, Pericarpium citri reticulatae, Radix achyranthis bidentatae, Radix glycyrrhizae, Poria, Radix astragali seu hedysari, Semen arecae, Rhizoma alismatis, Rhizoma coptidis, Rhizoma ligustici chuanxiong, Radix paeoniae alba, Caulis polygoni multiflora, Fructus jujubae); Gynura segetum; Radix polygoni multiflora; Dan-lu-tong-du tablet (Radix salviae miltiorrhizae, Colla corni cervi, Radix astragali seu hedysari, Rhizoma corydalis, Cortex eucommiae); Gu-kang capsule (Radix musae, Oxalis corniculata, Fructus psoraleae, Radix dipsaci, Radix notoginseng); Long-dan-xie-gan pill (Radix gentianae, Radix bupleuri, Radix scutellariae, Fructus gardeniae, Rhizoma alismatis, Caulis akebiae, Semen plantaginis, Radix angelicae sinensis, Radix rehmanniae recens, Radix glycyrrhizae); Shi-du-qing capsule (Radix rehmanniae recens, Radix angelicae sinensis, Radix salviae miltiorrhizae, Periostracum cicadae, Radix sophorae flavescentis, Cortex dictamni, Radix glycyrrhizae, Radix scutellariae, Rhizoma smilacis glabrae); Shu-gan granule (Radix angelicae sinensis, Radix paeoniae alba, Radix bupleuri, Rhizoma cyperi, Rhizoma atractylodis macrocephalae, Poria, Cortex moutan radicis, Herba menthae, Radix glycyrrhizae) + Bai-ban granule with unknown constituents. |
| Chronic (n=69) | n=15 Skin diseases n=10 Cardiovascular diseases n=8 Endocrine system diseases n=6 Bone and joint diseases Health care Gastrointestinal diseases n=4 Urinary diseases n=3 Gynecological disease n=2 Neurological diseases Breast diseases Hair loss Grey hair n=1 Respiratory diseases Indication Bone and joint disease combined with hair loss Cardiovascular disease combined with urinary disease |
n=29 Herbal decoction with unknown constituents. n=3 Radix polygoni multiflori. n=2 Dan-lu-tong-du tablet (Radix salviae miltiorrhizae, Colla corni cervi, Radix astragali seu hedysari, Rhizoma corydalis, Cortex eucommiae); Yan-shou pill (Radix polygoni multiflori); Chinese patent medicine with unknown constituents. n=1 Tripterygium wilfordii; Semen cassiae; Herbal decoction (Radix polygoni multiflori, Fructus lycii, Stigma croci); Herbal decoction (Radix polygoni multiflori, Radix notoginseng, Rhizoma gastrodiae, Radix astragali seu hedysari); Herbal decoction (Radix polygoni multiflori, Poria, Pteridium aquilinum, Black soya bean); Herbal Preparation Herbal decoction (Radix astragali seu hedysari, Rhizoma atractylodis macrocephalae, Radix saposhnikoviae, Ramulus cinnamomi, Radix paeoniae alba, Poria cocos, Rhizoma pinelliae, Radix angelicae sinensis, Radix puerariae, Semen armeniacae amarum, Cortex magnoliae officinalis, Radix codonopsis, Massa medicata fermentata, Radix salviae miltiorrhizae, Radix glycyrrhizae, Rhizoma zingiberis recens, Fructus jujubae); Herbal decoction (Radix polygoni multiflori, Radix ginseng, Radix astragali seu hedysari, Herba hedyotis, Herba scutellariae barbatae, Herba lobeliae chinensis, Herba lysimachiae, Radix bupleuri, Herba artemisiae scopariae, Fructus meliae toosendan, Radix aconiti lateralis preparata, Herba epimedii); Herbal decoction containing Rhizoma alismatis, Radix trichosanthis, and other unknown herbs; Herbal decoction containing Radix polygoni multiflori, Radix salviae miltiorrhizae, Semen cassia, Fructus crataegi, and other unknown herbs. Ci-gu-yin-xie capsule with unknown constituents; Qu-xuan-ling capsule with unknown constituents; Yang-xue-qing-nao granule (Radix angelica sinensis, Rhizoma ligustici chuanxiong, Radix paeoniae alba, Radix rehmanniae preparata, Ramulus uncariae cum uncis, Caulis spatholobi, Spica prunellae, Semen cassiae, Concha margaritifera, Rhizoma corydalis, Herba asari); Ke-yin pill (Rhizoma smilacis glabrae, Cortex dictamni, Rhizoma menispermi, Rhizoma bistortae); San-qi-yin-xing tea (Radix notoginseng, Folium ginkgo); Wan-mei-sha-ji tea (Fructus hippophae, Fructus momordicae, Citric acid, Fructooligosaccharides, other unknown ingredients); Shen-qi-jian-wei granule (Radix codonopsis, Radix angelicae sinensis, Fructus crataegi, Radix astragali seu hedysari, Poria, Radix glycyrrhizae, Rhizoma atractylodis macrocephalae, Ramulus cinnamomi, Pericarpium citri reticulatae, Caulis perillae, Radix paeoniae alba, Os sepiellae seu sepiae, Radix inulae, Herba taraxaci); Xin-yuan capsule (Radix polygoni multiflori, Radix salviae Miltiorrhizae, Radix rehmanniae recens, other unknown ingredients); Niu-huang-jiang-ya pill (Artificial bezoar, Cornu saigae tataricae, Baicalin, Pearl powder, Semen cassia, Rhizoma ligustici chuanxiong, Borneolum syntheticum, Radix paeoniae alba, Radix curcumae, Radix et rhizoma nardostachyos, Cornu bubali powder); Niu-huang-qing-xin pill (Calculus bovis, Indication Herbal Preparation Radix angelicae sinensis, Rhizoma ligustici chuanxiong, Radix glycyrrhizae, Rhizoma dioscoreae, Radix scutellariae, Semen armeniacae amarum, Semen sojae germinatum, Fructus jujubae, Rhizoma atractylodis macrocephalae, Poria, Radix platycodonis, Radix saposhnikoviae, Radix bupleuri, Colla corii asini, Rhizoma zingiberis, Radix paeoniae alba, Radix ginseng, Massa medicata fermentata, Cortex cinnamomi, Radix ophiopogonis, Radix ampelopsis, Pollen typhae, Moschus, Borneolum syntheticum, Cornu bubali powder, Cornu saigae tataricae, Cinnabaris, Realgar); Bai-xiao pill (Radix et rhizoma rhei, Faeces togopteri, Rhizoma cyperi, Semen pharbitidis, Rhizoma sparganii, Semen arecae, Rhizoma curcumae, Fructus gleditsiae abnormalis, Fructus crataegi, Massa medicata fermentata, Radix glycyrrhizae, Fructus hordei germinatus, Fructus aurantii immaturus, Cortex magnoliae officinalis); Pi-fu-bing-xue-du tablet (Radix rubiae, Semen persicae, Herba schizonepetae, Periostracum serpentis, Radix paeoniae rubra, Radix angelicae sinensis, Rhizoma imperatae, Fructus kochiae, Fructus xanthii, Radix rehmanniae recens, Fructus forsythiae, Flos lonicerae, Herba violae, Rhizoma smilacis glabrae, Cortex phellodendri, Spina gleditsiae, Radix platycodonis, Herba leonuri, Semen armeniacae amarum, Radix saposhnikoviae, Poria, Radix paeoniae alba, Periostracum cicadae, Fructus arctii, Cortex moutan radicis, Cortex dictamni, Radix rehmanniae preparata, Radix et rhizoma rhei, Caulis lonicerae, Radix arnebiae, Rhizoma bolbostemmatis, Rhizoma ligustici chuanxiong, Radix glycyrrhizae, Radix angelicae dahuricae, Radix semiaquilegiae, Cercis chinensis, Caulis spatholobi, Herba spirodelae, Flos carthami); Luo-zhen capsule (Folium apocyni veneti, Radix astragali seu hedysari, Radix salviae miltiorrhizae, Rhizoma ligustici chuanxiong, Radix paeoniae alba, Radix curcumae, Semen cassiae, Radix et rhizoma nardostachyos, Herba menthae, Margarita, Cornu saigae tataricae, Radix scutellariae, Calculus bovis, Borneolum syntheticum); Ku-gua-huang-jing capsule (Radix ginseng, Fructus lycii, Semen euryales, Rhizoma polygonati, Flos chrysanthemi, Fructus mori, Rhizoma polygonati odorati, Radix puerariae, Radix astragali seu hedysari, other unknown ingredients); Jin-gang-teng capsule (Smilax scobinicaulis); E-jiao-bai-zhi oral liquid (Exocarpium benincasae, Colla corii asini, Radix angelicae dahuricae, Endothelium corneum gigeriae galli, Fructus gardeniae, Radix puerariae, Bulbus lilii, Fructus crataegi, Semen persicae, Rhizoma polygonati, Arillus longan); Jing-fu-kang granule (Rhizoma et radix notopterygii, Rhizoma ligustici chuanxiong, Radix puerariae, Radix gentianae macrophyllae, Radix clematidis, Rhizoma atractylodis, Radix salviae miltiorrhizae, Radix paeoniae alba, Lumbricus, Flos carthami, Olibanum, Radix astragali seu hedysari, Radix codonopsis, Radix rehmanniae recens, Concha haliotidis, Ophicalcitum, Cortex phellodendri, Semen vaccariae, Semen persicae, Myrrha, Eupolyphaga seu steleophaga) + Yang-xue-sheng-fa capsule (Radix rehmanniae preparata, Radix angelica sinensis, Rhizoma et radix notopterygii, Fructus chaenomelis, Rhizoma ligustici chuanxiong, Radix paeoniae alba, Semen cuscutae, Rhizoma gastrodiae, Radix polygoni multiflori preparata). Lei-gong-teng tablet (Tripterygium wilfordii) + Herbal decoction with unknown constituents; Yang-xue-sheng-fa capsule + Herbal decoction (Radix rehmanniae preparata, Rhizoma dioscoreae, Fructus corni, Fructus lycii, Radix angelicae sinensis, Radix paeoniae alba, Fructus ligustri lucidi, Fructus mori, Semen sesami nigri, Fructus jujubae, Radix polygoni multiflora, Radix glycyrrhizae, Pericarpium citri reticulatae, Fructus hordei germinates, Fructus crataegi massa, Medicata fermentata, Radix dipsaci, Cortex eucommiae); Ru-kang pill (Concha ostreae, Olibanum, Fructus trichosanthis, Sargassum, Radix astragali seu hedysari, Myrrha, Radix asparagi, Spica prunellae, Rhizoma sparganii, Radix scrophulariae, Rhizoma atractylodis macrocephalae, Bulbus fritillariae thunbergii, Rhizoma curcumae, Radix salviae miltiorrhizae, Endothelium corneum gigeriae galli) + Xiao-yao pill (Radix bupleuri, Radix angelicae sinensis, Radix paeoniae alba, Rhizoma atractylodis macrocephalae, Poria, Radix glycyrrhizae, Herba menthae, Rhizoma zingiberis recens) + Herbal decoction with unknown constituents; Shen-song-yang-xin capsule (Radix ginseng, Radix ophiopogonis, Fructus corni, Radix salviae miltiorrhizae, Semen ziziphi spinosae, Herba taxilli, Radix paeoniae rubra, Eupolyphaga seu steleophaga, Radix et rhizoma nardostachyos, Rhizoma coptidis, Fructus schisandrae chinensis, Os draconis) + Shen-fu-kang capsule (Rhizoma smilacis glabrae, Flos sophorae, Rhizoma imperatae, Herba leonuri, Herba pogostemonis) + Herbal decoction with unknown constituents. Bai-xuan-kang for external usage (Radix sophorae flavescentis, Cortex phellodendri, Cortex dictamni, Cortex pseudolaricis, Fructus kochiae, Saussurea involucrata). |
Figure 2.
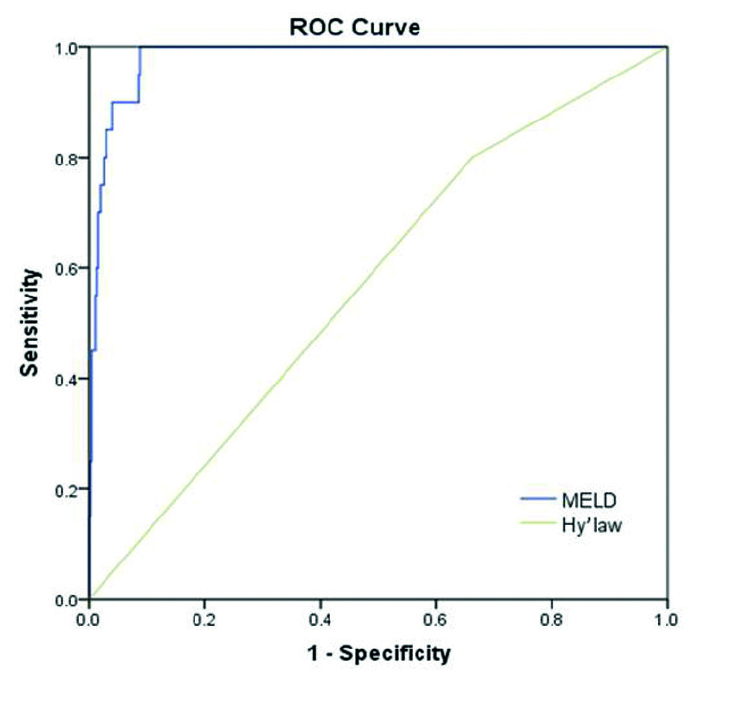
Receiver operating characteristic (ROC) curves to predict the fatal prognosis of HILI: MELD (C-statistic 0.981; 95% CI 0.968–0.995) and Hy’s law (C-statistic 0.569; 95% CI 0.449–0.689)
Chronicity
In the 468 surviving patients, the comparison of clinical features between the healed (n=399) and chronic (n=69) groups is shown in Table 5. The herbs or herbal products associated with chronic HILI and their indications are listed in Table 4. Only 7 chronic patients were induced by a single implicated herb, including Radix polygoni multiflori (n=4), Tripterygium wilfordii (n=1), Semen cassiae (n=1), and Smilax scobinicaulis (n=1). Based on the multivariable logistic regression analysis including age, gender, latency, ascites, peak ALT, lowest albumin (ALB), peak triglyceride (TG), and peak international normalized ratio (INR) values, the course of peak ALT decreasing ≥50% after discontinuation of herbs application (CA50), and platelet count at liver injury onset as covariates, the latency, CA50, peak TG value, and platelet count at liver injury onset predicted chronic HILI with adjusted OR of 1.268 (95%CI 1.034–1.554), 2.303 (95%CI 1.588–3.340), 0.580 (95%CI 0.343–0.978), and 0.183 (95%CI 0.091–0.368), respectively (Table 6).
Table 5.
Comparison of characteristics between the healed and chronic groups
| Characteristic | Healed (n=399) | Chronic (n=69) | p |
|---|---|---|---|
| Age (y), mean±SD | 44±14 | 47±11 | 0.033 |
| Proportion of patients aged 65 years or older, % | 5.8 | 7.2 | 0.586 |
| Females, % | 71.2 | 73.9 | 0.642 |
| Alcohol use*, % | 12.5 | 11.6 | 0.827 |
| Allergy history, % | 16.5 | 13.0 | 0.465 |
| Latency (d), median (25th, 75th) | 30 (15, 60) | 60 (16, 730) | 0.001 |
| Short latency (<5 days), % | 5.0 | 5.8 | 0.768 |
| Long latency (>90 days), % | 14.5 | 43.5 | <.001 |
| Course of peak ALT decreasing ≥50% after herbal intake discontinuation, median (25th, 75th) | 7 (5, 9) | 10 (6, 18) | <.001 |
| Positive re-challenge, % | 6.8 | 10.1 | 0.318 |
| Fever, % | 6.5 | 7.2 | 0.794 |
| Skin rashes, % | 3.0 | 1.4 | 0.702 |
| Ascites, % | 7.0 | 20.3 | <.001 |
| Encephalopathy, % | 0.5 | 2.9 | 0.105 |
| Hyponatremia, % | 3.5 | 7.2 | 0.178 |
| Biochemistries, mean±SD | |||
| Peak ALT (U/L) | 987±679 | 685±604 | 0.001 |
| Peak ALP (U/L) | 184±113 | 176±92 | 0.570 |
| Peak TB (mg/dL) | 9.7±8.7 | 8.3±8.4 | 0.245 |
| Lowest ALB (g/L) | 37±4 | 35±7 | 0.010 |
| Peak Scr (μmol/L) | 75±21 | 82±68 | 0.419 |
| Peak TG (mmol/L) | 1.69±1.35 | 0.99±1.04 | <.001 |
| Peak TC (mmol/L) | 3.48±1.85 | 3.45±1.18 | 0.890 |
| Peak INR | 0.68±0.48 | 0.83±0.38 | 0.006 |
| Blood routine at onset, mean±SD | |||
| White blood cell count (×109/L) | 5.18±2.14 | 4.78±2.11 | 0.151 |
| Eosinophil count (×109/L) | 0.25±1.25 | 0.23±1.20 | 0.908 |
| Platelet count (×109/L) | 221±81 | 154±78 | <.001 |
| Clinical pattern, % | <.001 | ||
| Hepatocellular | 90.2 | 65.2 | |
| Cholestatic | 3.5 | 20.3 | |
| Mixed | 6.3 | 14.5 | |
| MELD score, mean±SD | 12.0±6.3 | 12.1±6.8 | 0.894 |
| Hy’s law, % | 68.7 | 52.2 | 0.007 |
| Cirrhosis, % | 1.8 | 40.6 | <.001 |
| Plasma exchange, % | 2.5 | 4.3 | 0.419 |
| Steroid use, % | 5.3 | 14.5 | 0.015 |
The patients had a drinking history (alcohol intake of >2 drinks per day for women and >3 drinks per day for men) but did not drink a month before the liver injury
ALT: alanine transaminase; ALP: alkaline phosphatase; TB: total bilirubin; ALB: albumin; Scr: serum creatinine; TG: triglyceride; TC: total cholesterol; INR: international normalized ratio; MELD: model for end-stage liver disease
Table 6.
Multivariate logistic regression model predicting chronic HILI
| Variables | Adjusted odds ratio (95% confidence interval) | p |
|---|---|---|
| Ln (latency, d) | 1.268 (1.034–1.554) | 0.022 |
| Ln (CA50, d) | 2.303 (1.588–3.340) | <0.001 |
| Ln (peak TG value, mmol/L) | 0.580 (0.343–0.978) | 0.041 |
| Ln (platelet count at liver injury onset, ×109/L) | 0.183 (0.091–0.368) | <0.001 |
CA50: course of peak ALT decreasing ≥50% after herbal intake discontinuation; TG: triglyceride
Based on the latency, CA50, the peak TG value, and platelet count at the liver injury onset, a risk score for chronicity was calculated using the following equation: 4.502+0.237 Ln (latency)+0.834 Ln (CA50)−0.545 Ln (peak TG value)−1.699 Ln (platelet count at liver injury onset). Using a cutoff point of 0.125, this model had a sensitivity of 0.77 and a specificity of 0.72 to predict chronic HILI with a C-statistic of 0.812 (95%CI 0.755–0.868). A similar calculation for Hy’s law (C-statistic 0.418; 95%CI 0.343–0.492) and MELD (C-statistic 0.506; 95%CI 0.431–0.581) resulted in lower values (Figure 3).
Figure 3.
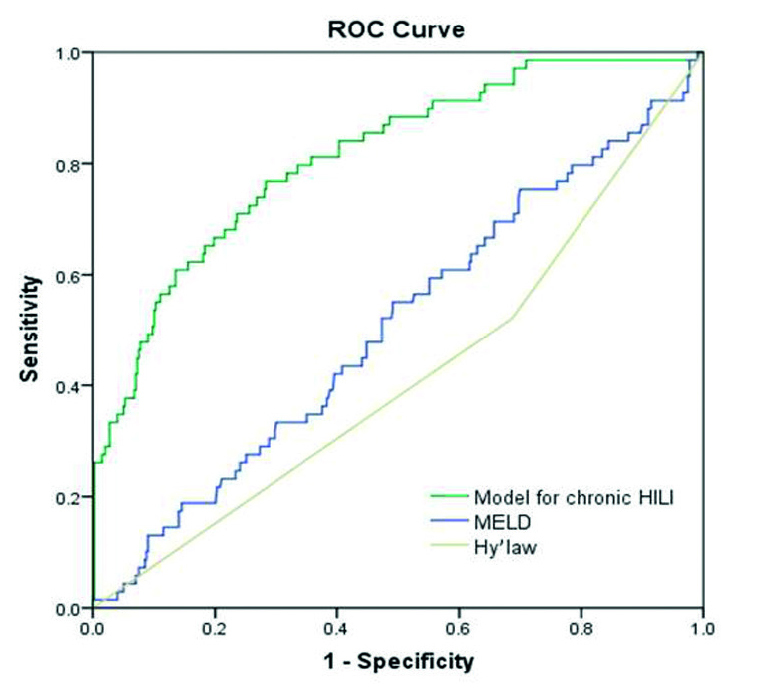
Receiver operating characteristic (ROC) curves to predict the chronic prognosis of HILI: the model for chronic HILI (C-statistic 0.812; 95%CI 0.755–0.868), MELD (C-statistics 0.506; 95%CI 0.431–0.581), and Hy’s law (C-statistics 0.418; 95%CI 0.343–0.492)
DISCUSSION
Herbal medicines have been widely and safely used for various diseases and health care for thousands of years (9). Actually, HILI occurred at a low frequency. In a prospective study from a German Traditional Chinese Medicine (TCM) hospital to analyze the frequency of liver injury induced by TCM, only 0.12% patients developed liver injury with ALT≥5×ULN (10). In this study, only 488 (0.79%) of 61,516 patients hospitalized due to liver injury caused by herbs or herbal products. Among these 488 patients, 18.2% of cases presented poor outcomes mainly for treatment of skin diseases, cardiovascular diseases, and endocrine system diseases.
The diagnosis of HILI is more complicated due to a lack of characteristic biomarkers, compared with liver injury induced by virus, alcohol, and other causes (11). In this study a strict diagnostic criterion of HILI was carried out. First, a biochemistry criterion of HILI was defined to exclude unspecific ALT increases (12). Complete alternative causes of liver injury and pre-existing liver diseases were validly ruled out (13). Detailed medical histories were examined to exclude co-medication with synthetic drugs (3). Moreover, RUCAM was used in this study to assess the causality of herbs and liver injury to further eliminate the substantial background noise of HILI patients (6,10).
Most prognostic scoring systems used in patients with liver injury consider fatal outcome of drug-induced liver injury (DILI); the outcome of HILI has received much less attention (14). Among the prognostic scoring systems, Hy’s law and MELD were shown to predict the short-term outcome like death or liver transplantation in DILI patients (15,16). MELD was initially used to prioritize the liver transplant allocation and predict a short-term outcome for patients with end-stage liver disease (8). Subsequently, MELD was found to be a reliable predictor of fatal prognosis of DILI (17). In this study, MELD with a high C-statistic of 0.981 has been proven again to be a good prognostic model for a fatal prognosis of HILI, but not for development of chronicity with a low C-statistic of 0.506. Hy’s law, a method to predict a drug’s likelihood to induce severe liver injury, stated that in DILI patients, hepatocellular injury and jaundice are associated with a 10% mortality rate (18). Hy’s law was a poor predictor for fatal prognosis and was not at all correlated with chronic HILI in this study. Hy’s law was developed for predicting serious liver injury induced by synthetic drugs, not herbs in clinical practice; it also focused on short-term mortality rather than chronic liver injury (19).
Herbs, like synthetic drugs, can also cause fatal or chronic liver injury in a small percentage of cases (3,5). Some patients with chronic HILI present cirrhosis (20). No model has yet been forwarded to predict a chronic course in HILI, although chronicity likely developed in 10%–14% of HILI cases in cases series (3,21). Patients with chronic HILI, especially cirrhosis, may need a significantly longer therapy both for the liver injury. However, its mechanism is not yet well understood (22). Persistent liver tissue repair reactions likely are not only an essential part of chronic HILI, but also distinguish it from lethal toxic damages. Risk factors for chronic DILI may be related to hepatocyte injury processes, such as the time of drug intake or hepatocellular functional deficits presenting decreased hepatic triglyceride synthesis (22,23). Chronicity carries a poor HILI prognosis, and its development causes lasting health problem. Special attention should be paid to chronic HILI, and a valid prognostic model of chronic HILI development would aid in the early detection of chronicity.
In this study, we have used the multivariable logistic regression analysis and identified that the latency time, course of ALT decreasing, TG value, and platelet count as covariates are significantly correlated with chronic HILI development. Latency, defined as the time from the beginning of application of implicated agents to the onset of liver injury symptoms, was related to clinical severity and an unfavorable outcome (23). In this study, patients with long latency were more likely to develop into chronic HILI or cirrhosis than those with short latency. Chronicity and cirrhosis were more common in HILI cases with cholestatic pattern than other patterns. The bile duct damage needs more time to recover in comparison with hepatocellular injury (24). Patients with a low TG value or platelet count, as signals of liver dysfunction and portal hypertension, also had been proven to indicate a poor outcome of liver injury both in patients (25) and in rats (26).
In this study, C-statistic was used to evaluate the predictive ability of models. A C-statistic of 0.7 has a reasonable clinical utility, while a model with a C-statistic of ≥0.8 has been considered predictive (17). For a fatal outcome, we calculated a C-statistic value of 0.981 for MELD, in agreement with prior publications (16,27). The prognostic model for chronic HILI based on latency, CA50, TG value, and platelet count predicted chronicity with a C-statistic of 0.812, whereas the C-statistic for MELD and Hy’s law were 0.506 and 0.418, respectively. This indicated that the prognostic model for chronic HILI had the best predictive ability.
The conclusions are limited by the fact that all cases were hospitalized in a single center, leading to a low representability for the overall population; this study was a retrospective survey, so missing data could not be supplemented. The prognostic model for chronic HILI was developed based on the data from patients with liver injury induced by herbal medicine, but not for some specific herbs; however, the model still needs validation in multicenter and prospective researches to prove its value for other settings.
In conclusion, herbal medicine is associated with a very low risk of liver injury. MELD was confirmed as a good predictive model of the HILI mortality; for predicting the HILI chronicity, a long latency, a slow recovery, and low triglyceride value and platelet count make the best fit.
Footnotes
Ethics Committee Approval: The authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013).
Informed Consent: Since the data for this study were analyzed retrospectively and anonymously, no written informed consent was possible or is necessary.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.Z., J.S.; Design - Y.Z., J.S.; Supervision - Y.Z., X.H.X.; Materials - Y.Z., M.N.; Data Collection and/or Processing - M.N., J.B.W., R.L.W., J.Y.L., Y.L.Z., Y.F.Z, T.T.H., S.M.Y., Y.M.G., F.Z.; Analysis and/or Interpretation - Y.Z., Y.Q.M., X.H.X., J.S.; Literature Search - Y.Z.; Writing manuscript - Y.Z., J.S.; Critical Review - X.H.X., J.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This study was financially supported by Specialized Research Fund of National Traditional Chinese Medicine Clinical Research and Base Construction Project (JDZX2015188), National Natural Science Foundation of China (81630100), and National Science and Technology Major Project (2015ZX09501-004-001-008).
REFERENCES
- 1.Teschke R, Andrade RJ. Drug, herb, and dietary supplement hepatotoxicity. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091488. pii: E1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drug-induced Liver Injury Study Group, Chinese Society of Hepatology, Chinese Medical Association. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221–41. doi: 10.1007/s12072-017-9793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Niu M, Chen J, et al. Hepatobiliary and pancreatic: comparison between Chinese herbal medicine and western medicine-induced liver injury of 1985 patients. J Gastroenterol Hepatol. 2016;31:1476–82. doi: 10.1111/jgh.13323. [DOI] [PubMed] [Google Scholar]
- 4.Lee WJ, Kim HW, Lee HY, Son CG. Systematic review on herb-induced liver injury in Korea. Food Chem Toxicol. 2015;84:47–54. doi: 10.1016/j.fct.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Branch of Hepatobiliary Diseases China Association of Chinese Medicine, Branch of Chinese Patent Medicine China Association of Chinese Medicine. Guidelines for the diagnosis and treatment of herb-induced liver injury. Chin J Integr Med. 2018 Mar 15; doi: 10.1007/s11655-018-3000-8. doi: 10.1007/s11655-018-3000-8. [Epub ahead of print] [DOI] [Google Scholar]
- 6.Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17 doi: 10.3390/ijms17010014. pii: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–6. doi: 10.1016/0168-8278(90)90124-A. [DOI] [PubMed] [Google Scholar]
- 8.Kamath PS, Kim WR Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 9.Teschke R. Traditional Chinese medicine induced liver injury. J Clin Transl Hepatol. 2014;2:80–94. doi: 10.14218/JCTH.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melchart D, Hager S, Albrecht S, Dai J, Weidenhammer W, Teschke R. Herbal traditional Chinese medicine and suspected liver injury: a prospective study. World J Hepatol. 2017;9:1141–57. doi: 10.4254/wjh.v9.i29.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teschke R, Larrey D, Melchart D, Danan G. Traditional Chinese medicine (TCM) and herbal hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as microRNAs. Medicines (Basel) 2016;3 doi: 10.3390/medicines3030018. pii: E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–15. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 13.Teschke R, Danan G. Diagnosis and management of drug-Induced liver injury (DILI) in patients with pre-existing liver disease. Drug Saf. 2016;39:729–44. doi: 10.1007/s40264-016-0423-z. [DOI] [PubMed] [Google Scholar]
- 14.Dağ MS, Aydınlı M, Oztürk ZA, et al. Drug- and herb-induced liver injury: a case series from a single center. Turk J Gastroenterol. 2014;25:41–5. doi: 10.5152/tjg.2014.4486. [DOI] [PubMed] [Google Scholar]
- 15.Alempijevic T, Zec S, Milosavljevic T. Drug-induced liver injury: Do we know everything? World J Hepatol. 2017;9:491–502. doi: 10.4254/wjh.v9.i10.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathi C, Pipaliya N, Patel R, Ingle M, Phadke A, Sawant P. Drug induced liver injury at a Tertiary hospital in India: etiology, clinical features and predictors of mortality. Ann Hepatol. 2017;16:442–50. doi: 10.5604/01.3001.0009.8600. [DOI] [PubMed] [Google Scholar]
- 17.Jeong R, Lee YS, Sohn C, Jeon J, Ahn S, Lim KS. Model for end-stage liver disease score as a predictor of short-term outcome in patients with drug-induced liver injury. Scand J Gastroenterol. 2015;50:439–46. doi: 10.3109/00365521.2014.958094. [DOI] [PubMed] [Google Scholar]
- 18.Reuben A. Hy’s law. Hepatology. 2004;39:574–8. doi: 10.1002/hep.20081. [DOI] [PubMed] [Google Scholar]
- 19.Bessone F. Predicting fatalities in serious idiosyncratic drug-induced liver injury-a matter of choosing the best Hy’s law. Transl Gastroenterol Hepatol. 2017;2:112. doi: 10.21037/tgh.2017.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Li YG, Wang Y, et al. Analysis of Clinical Characteristics in 595 Patients with Herb-induced Liver Injury. Chin J Integr Trad West Med. 2016;36:44–8. [PubMed] [Google Scholar]
- 21.Pang L, Yang W, Hou F. Features and outcomes from a retrospective study of 570 hospitalized Chinese patients with drug-induced liver injury. Clin Res Hepatol Gastroenterol. 2018;42:48–56. doi: 10.1016/j.clinre.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Medina-Caliz I, Robles-Diaz M, Garcia-Muñoz B, et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol. 2016;65:532–42. doi: 10.1016/j.jhep.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stine JG, Chalasani N. Chronic liver injury induced by drugs: a systematic review. Liver Int. 2015;35:2343–53. doi: 10.1111/liv.12958. [DOI] [PubMed] [Google Scholar]
- 24.de Vries E, Beuers U. Management of cholestatic disease in 2017. Liver Int. 2017;37:123–9. doi: 10.1111/liv.13306. [DOI] [PubMed] [Google Scholar]
- 25.Lo Re V, 3rd, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13:2360–8. doi: 10.1016/j.cgh.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonomura Y, Kato Y, Hanafusa H, et al. Diagnostic and predictive performance and standardized threshold of traditional biomarkers for drug-induced liver injury in rats. J Appl Toxicol. 2015;35:165–72. doi: 10.1002/jat.3053. [DOI] [PubMed] [Google Scholar]
- 27.Ou P, Chen Y, Li B, et al. Causes, clinical features and outcomes of drug-induced liver injury in hospitalized patients in a Chinese tertiary care hospital. Springerplus. 2015;4:802. doi: 10.1186/s40064-015-1600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


