Abstract
Background/Aims
Mother-to-child transmission (MTCT) is a common transmission mode of hepatitis B virus (HBV). It has been shown that the infection may occur in some infants despite the use of immunoprophylaxis, and many studies have demonstrated the efficacy of antivirals such as lamivudine to reduce such events.
Materials and Methods
A meta-analysis was conducted concerning the efficacy and safety of lamivudine during pregnancy, in the prevention of vertical transmission of HBV infection. Studies were identified by searching various databases up to January 2016 for variations of the following phrase: “lamivudine AND (pregnancy or pregnant) AND (HBV or hepatitis).” Subjects who had received lamivudine were included in the case group, and those who had not were included in the control group.
Results
Our search identified a total number of 881 citations, of which 25 studies (with a total number of 2,667 pregnant women) were included in the meta-analysis. The analysis showed a significant difference between the seropositive HBsAg infants from the case and control groups (RR= 16.97, 95% confidence interval 8.36–34.45), which is the most critical factor in determining the MTCT of HBV. No significant difference was reported between the prevalence of side effects in the case and control groups.
Conclusion
This meta-analysis strongly suggests the use of lamivudine in the prevention of HBV vertical transmission in carrier pregnant women with the HBV DNA levels greater than 106 copies/mL. And for women with the HBV viral loads lower than 106 copies/mL, we suggest clinicians to examine the use of lamivudine on a case-to-case basis, noting that lamivudine seems to be a safe drug for the mother and the fetus.
Keywords: Lamivudine, hepatitis B, pregnancy, mother-to-child transmission, anti-retroviral agents, fetomaternal infection
INTRODUCTION
Hepatitis B virus (HBV) infection is a major global health issue. It is estimated that more than 240 million individuals are chronically infected with HBV worldwide (1). Mother-to-child transmission (MTCT), which usually occurs perinatally and in rare cases in utero, is a common mode of HBV’s transmission, especially in endemic countries (2,3).
Chronic hepatitis B (CHB) arises in up to 90% of infants who were infected perinatally, and it may eventually lead to serious complications that may happen as soon as adolescence, such as liver failure, cirrhosis, and hepatocellular carcinoma (3).
Immunoprophylaxis with hepatitis B immunoglobulin (HBIG) administered within 12 hours of birth and a series of three doses of hepatitis B vaccination can prevent perinatal HBV transmission in 90%–95% of infants born to HBsAg-positive mothers. However, it has been shown that the infection may occur in 5%–10% of infants despite the use of immunoprophylaxis (3–5); the main risk factors of prevention failure are the maternal high viral load and the HBeAg positivity (5–7). Therefore, the administration of oral antiviral drugs to decrease the maternal serum HBV DNA levels is expected to reduce the rate of perinatal HBV infection in newborns (8).
Many studies have shown the efficacy of antiviral therapy to reduce the MTCT of HBV infection in pregnant women with the high viral load (8,9). Lamivudine is a nucleoside analog antiviral drug with a record of safe use in pregnancy that effectively decreased the MTCT of HBV in several clinical trials with no increased adverse outcomes (10,11).
In this study, we conducted a systematic review and meta-analysis of clinical trials and cohort studies up to January 2016 to evaluate lamivudine’s efficacy and safety on the prevention of MTCT of HBV. Three meta-analyses have already been conducted on this subject (9–11) of which two are outdated (2010 and 2011) considering the vast amount of studies performed after their publication, and the third meta-analysis is conducted about the efficacy of all used antivirals, not exclusively lamivudine.
MATERIALS AND METHODS
Search strategy and study selection
An online literature search was performed to identify relevant English articles pertaining the efficacy of lamivudine in the prevention of vertical transmission of Hepatitis B from mother-to-child. Publications and abstracts up to January 2016 were searched and obtained from the following databases: PubMed, EMBASE, Scopus, Web of Science, The Cochrane Library, Irandoc, IRCT, Iran medex, ClinicalTrials.gov, African Index Medicus, European Union Clinical Trials Registry, Google Scholar, Proquest, Biosis Citation Index, HSRProj (Health Services Research Projects in Progress), Open Grey, AIHW, CogPrints, OAIster (WorldCat), Science.gov, and OpenDoar.
Different variations of the following phrase were searched mainly in the abstracts: “lamivudine AND (pregnancy or pregnant) AND (HBV or hepatitis)”. Moreover, cited articles in the selected studies were manually examined to prevent any omission of related studies.
Relevant articles that had one or more of the following criteria were excluded from this analysis: animal studies, case reports, co-infection of HBV and HIV, combination therapy with other antivirals, review articles, and letters to editors.
Data extraction
Titles and abstracts of all potentially relevant articles were reviewed independently by two authors (P.KH. and R.J), and relevant articles were included in the analysis; disagreements were reconciled by consensus or by a third reviewer (M.G.).
The same two authors performed data extraction, and in case of any disagreements, reconciliation was made by consensus or by a third reviewer. Data were extracted from full texts of the English studies, and also from the abstract of non-English studies as well as the abstract of studies that had not been published as a finished project yet. To obtain further data, we tried to contact corresponding authors of all the studies who did not have a full text in English or did not have a full text at all.
The following data of case and control groups were extracted and indexed in an Excel sheet from each eligible study: name of the first author, year of publication, type of study, country, blinding method, sample size, number of newborns, mother’s mean age, intervention on mothers, intervention on newborns, maternal serum HBV DNA level before intervention and before delivery, ALT changes, HBsAg-positive newborns and infants, and adverse events in newborns (still birth, premature rupture of membrane, low birth weight, cerebral palsy, and apparent deformities).
Infancy was defined as the age greater than 28 days.
Pregnant women who had received lamivudine were included in the case group, and those who had not received lamivudine were included in the control group. Table 1 demonstrates the interventions performed on mothers and newborns of both groups.
Table 1.
General information of included studies
| First author, year | Accessed article | Country | Sample Size (Mothers) (N) | Blinding | Mothers’ mean (± SD) / median age (Years) | Intervention on mothers | Intervention on newborns | Duration of treatment in cases | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||
| Case group | Control group | Case group | Control Group | Case group | Control Group | Case group | Control Group | Start | Discontinuation | ||||
| Ayres, 2013 | Full-text | Australia | 21 | 5 | No Blinding | N/A | N/A | 100mg LMV qd | None | N/A | N/A | 32nd GW | 2 weeks postpartum |
| Erturk, 2014 | Full-text | Turkey | 8 | No control | N/A | 26.5 | No control | 100mg LMV qd | No control | N/A | N/A | 28th – 32nd GW | After 8–12 weeks |
| Jackson, 2015 | Full-text | Ireland | 34 | 9 | N/A | 26 | N/A | 100–150 mg LMV qd | None | HBIG; 0.4 mL/kg + recombinant HBV vaccine | HBIG; 0.4 mL/kg + recombinant HBV vaccine | 32nd GW | Delivery |
| Jiang, 2012 | Abstract | China | 164 | 92 | N/A | N/A | N/A | 100mg LMV qd | None | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | 2nd or 3rd trimester | N/A |
| Kose, 2011 | Full-text | Turkey | 7 | No control | N/A | 26.5 | No control | 100mg LMV qd | No control | N/A | N/A | 32nd GW | After 8 weeks |
| Lawler, 2011 | Conference Abstract | Australia | 44 | 15 | N/A | N/A | N/A | N/A | None | N/A | N/A | 32nd GW | After 50 days |
| Li, 2003 | Full-text | China | 43 | 52 | N/A | N/A | N/A | 100mg LMV qd | None | N/A | N/A | 28th GW | 30days after delivery |
| Min, 2008 | Abstract | China | 15 | No control | N/A | 29 | No control | 100mg LMV qd | No control | N/A | N/A | Before pregnancy | Delivery |
| Pan, 2011 | Conference Abstract | China | 164 | 92 | N/A | 27 | 26 | 100mg LMV qd | None | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | 2nd or 3rd trimester | 4 weeks postpartum |
| Pan, 2014 | Conference Abstract | China & USA | 94 | 89 | N/A | 27.4 | 27.2 | 100mg LMV qd | None | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | 3rd trimester | After 11.63 weeks |
| Su, 2004 | Full-text | China | 38 | 10 | N/A | N/A | N/A | 100mg LMV qd | None | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | Before pregnancy | N/A |
| Uchila, 2015 | Conference Abstract | Australia | 6 | No control | N/A | N/A | No control | 100mg LMV qd | No control | N/A | N/A | 3rd trimester | N/A |
| Van Bang, 2015 | Full-text | Vietnam | 33 | No control | N/A | N/A | No control | 100mg LMV qd | No control | recombinant HBV vaccine (standard 4 doses) | N/A | 32nd GW | 4 weeks postpartum |
| Xu, 2009 | Full-text | China & Philippines | 89 | 61 | Double Blinded | 26 | 25 | 100mg LMV qd | None | 56 cases Received Vaccine + HBIG, 26 cases Received Vaccine only | Vaccine + HBIG | 32nd GW | 4 weeks postpartum |
| Yang, 2008 | Abstract | China | 20 | 20 | Double Blinded | N/A | N/A | 100mg LMV qd | None | N/A | N/A | 28th GW | Delivery |
| Xiamoing, 2012 | Abstract | China | 57 | 66 | N/A | N/A | N/A | 100mg LMV qd | HBIG 200 IU | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | 20th – 26th GW | Delivery |
| Yi, 2012 | Full-text | China | 72 | No control | N/A | 30.5±3.1 | No control | 100mg LMV qd | No control | HBIG; 200 IU+Three doses of recombinant HBV vaccine (10 mug) | N/A pregnancy | Before or early | N/A |
| Han, 2005 | Abstract | China | 42 | No control | N/A | N/A | No control | 100mg LMV qd | No control | N/A | N/A | 28th GW | N/A |
| Jiang, 2012 | Conference Abstract | China | 100 | 100 | N/A | N/A | N/A | 100mg LMV qd | HBIG 200 IU | N/A | N/A | N/A | N/A |
| Tekin Koruk, 2015 | Full-text | Turkey | 20 | 54 | N/A | 28.1 | 28.7 | 100mg LMV qd | None | HBIG; 200 IU+recombinant HBV vaccine (10 mug) | HBIG; 200 IU+recombinant HBV vaccine (10 mug) | 22.2±8.5 GW | N/A |
| Yu, 2012 | Full-text | China | 94 | 91 | Double Blinded | 26.64±4.17 | 25.78±3.89 | 100mg LMV qd | None | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | 24th – 32nd GW | Delivery |
| Yu, 2014 | Full-text | China | 154 | 100 | N/A | 26.66± 3.48 | 26.11± 3.18 | 100mg LMV qd | HBIG 200 IU | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | HBIG; 200 IU+recombinant HBV vaccine (20 mug) | 8th 32nd GW | Delivery |
| Zhang, 2014 | Full-text | China | 53 | 363 | Open Label | 28.42± 7.12 | 28.97± 4.59 | 100mg LMV qd | None | HBIG; 200 IU+recombinant HBV vaccine (10 mug) | HBIG; 200 IU+recombinant HBV vaccine (10 mug) | 28th – 32nd GW | 4 weeks postpartum |
| Zonneveld, 2003 | Full-text | Netherlands | 8 | 24 | N/A | 20 | 23 | 150mg LMV qd | None | HBIG; 300 IU+recombinant HBV vaccine (20 mug) | HBIG; 300 IU+recombinant HBV vaccine (20 mug) | 34th GW | Delivery |
| Yu, 2011 | Full-text | China | 14 | 30 | N/A | 27.68± 3.65 | 26.33± 3.24 | 100mg LMV qd | None | HBIG & Vaccine | HBIG & Vaccine | 3rd trimester | Delivery |
LMV: Lamivudine; HBIG: Hepatitis B Immunoglobulin; HBV: Hepatitis B Virus; N/A: Not available; GW: Gestational Week
The analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Statistical analysis
Maternal HBV DNA level changes during lamivudine treatment between the case and control groups and the relative risk were combined by the “metan” command. Also, we used the “metaprop” command for the prevalence of abnormal ALT levels in the case group of each study (12). Statistical tests of heterogeneity among studies were carried out using the Q test (P<0.10 indicated a statistically significant heterogeneity) and the I-squared statistics. If heterogeneity was confirmed, the “Fixed Effect Method” was used for a combination of effects, and if it was not confirmed, the “Random Effect Method” was used. In this study, we also used a funnel plot to investigate publication bias and forest plot for showing the study effect and 95% confidence interval (CI). The analyses were performed using the 11th version of Stata software.
RESULTS
A total number of 881 citations were identified via our search in the previously mentioned databases. After processing the studies, which is described in Figure 1, 25 studies (13–37) were included in this meta-analysis. The included studies were published between 2003 and 2015 and enrolled a total number of 2,667 HBV-infected pregnant women of which 1,394 received lamivudine as an adjuvant therapy to the standard care to prevent MTCT of HBV. Table 1 summarizes the characteristics of the studies.
Figure 1.
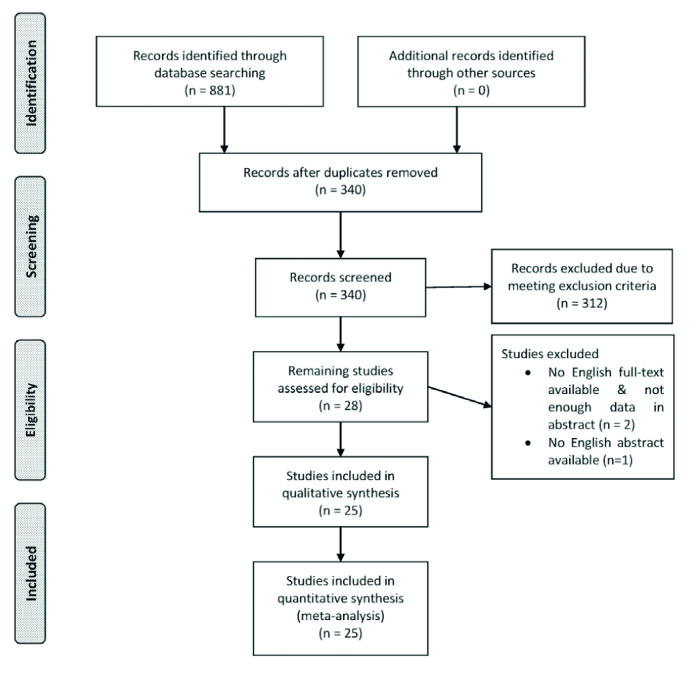
Flow chart of study recruitment and data selection
Infants’ outcome
The prevalence of seropositivity of HBsAg and detectable loads of HBV DNA in newborns and infants are demonstrated in Table 2, Figure 2, and Figure 3. The analysis shows a significant difference between the seropositive HBsAg infants from the case and control groups (relative risk [RR]=16.97, 95% CI 8.36–34.45). It is also apparent that there are significant differences between the two groups regarding the HBsAg in infants and HBV DNA in both infants and newborns.
Table 2.
Seropositivity of HBsAg and detectable loads of HBV DNA in newborns and infants
| Case groups | Control groups | Relative Risk Comparison | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| No of Studies | Total Events | Total Samples | Risk | 95% CI | No of Studies | Total Events | Total Samples | Risk | 95% CI | RR | 95% CI | ||||
|
|
|
|
|||||||||||||
| L | U | L | U | L | U | ||||||||||
| HBsAg Positive Newborns | 24 | 159 | 1380 | 11.52% | 9.88% | 13.33% | 16 | 278 | 1168 | 23.80% | 21.38% | 26.35% | 2.07*** | 1.73 | 2.47 |
| HBsAg Positive Infants | 18 | 8 | 1001 | 0.80% | 0.35% | 1.57% | 13 | 134 | 988 | 13.56% | 11.49% | 15.86% | 16.97*** | 8.36 | 34.45 |
| HBV DNA Positive Newborns | 13 | 15 | 740 | 2.03% | 1.14% | 3.32% | 7 | 83 | 423 | 19.62% | 15.94% | 23.73% | 9.68*** | 5.66 | 16.56 |
| HBV DNA Positive Infants | 11 | 7 | 611 | 1.15% | 0.46% | 2.35% | 7 | 47 | 381 | 12.34% | 9.21% | 16.06% | 10.77*** | 4.92 | 23.58 |
p<0.0001;
RR: Relative Risk
Figure 2.
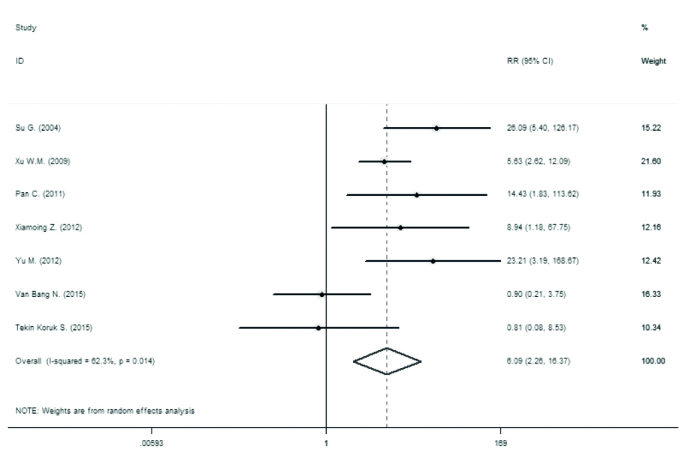
Forest plot for prevalence of detectable loads of HBV DNA in newborns
Figure 3.
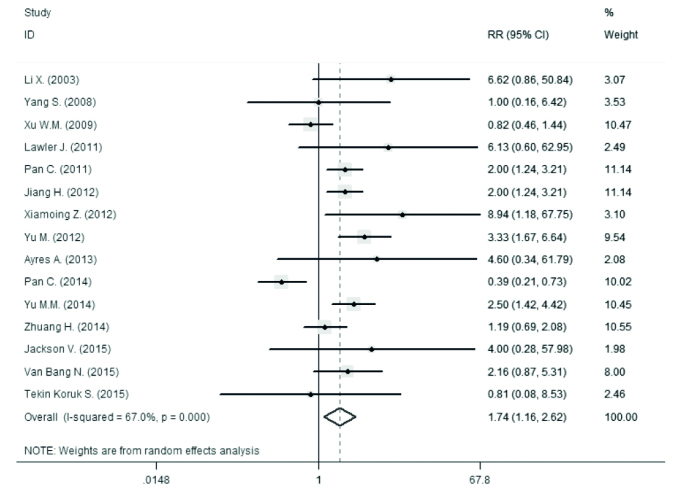
Forest plot for prevalence of HBS Ag positivity in newborns
A comparison of prevalence of side effects in the newborns was performed, which shows no significant difference between the two groups (p>0.1) (Table 3).
Table 3.
The prevalence of side effects and relative risk comparison
| Case | Control | Relative Risk Comparison | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| No of Studies | Total Events | Total Samples | Risk | 95% CI | No of Studies | Total Events | Total Samples | Risk | 95% CI | RR | 95% CI | ||||
|
|
|
|
|||||||||||||
| L | U | L | U | L | U | ||||||||||
| Still Birth | 18 | 2 | 1180 | 0.17% | 0.02% | 0.61% | 13 | 0 | 1149 | 0.00% | 0.00% | 0.32% | 0.34 | 0.04 | 3.29 |
| Low Birth Weight | 16 | 3 | 1130 | 0.27% | 0.05% | 0.77% | 11 | 5 | 756 | 0.66% | 0.22% | 1.54% | 2.49 | 0.6 | 10.39 |
| Cerebral Palsy | 17 | 0 | 1166 | 0.00% | 0.00% | 0.32% | 12 | 0 | 1119 | 0.00% | 0.00% | 0.33% | 1.04 | 0.07 | 16.64 |
| Apparent deformities | 17 | 3 | 1166 | 0.26% | 0.05% | 0.75% | 12 | 5 | 1119 | 0.45% | 0.15% | 1.04% | 1.74 | 0.42 | 7.25 |
RR: Relative Risk
Mothers’ outcome
The analyses of changes in the maternal serum HBV DNA levels are shown in Figure 4 and Table 4. The efficacy of lamivudine in decreasing HBV DNA levels is calculated as −6.694 log10 IU/mL (95% CI, −7.836 to −5.552).
Figure 4.
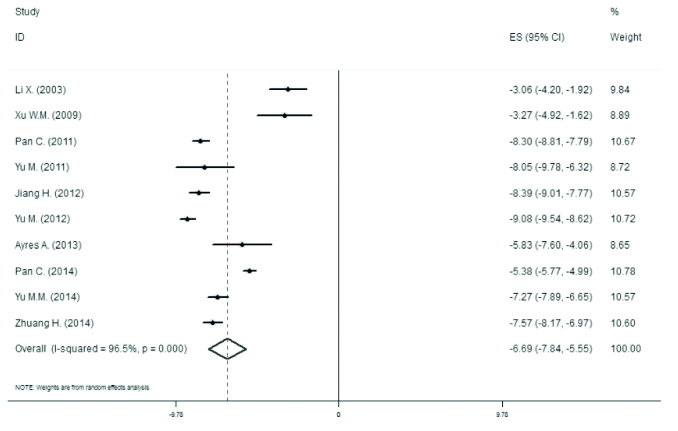
Forest plot for changes of maternal serum levels of HBV DNA during lamivudine treatment
Table 4.
Pooled and study effect for changes in maternal serum levels of HBV DNA
| Author (Year) | Effect | 95 % Confidence Interval | |
|---|---|---|---|
|
| |||
| Lower | Upper | ||
| Li X. (2003) | −3.06 | −4.203 | −1.917 |
| Xu W.M. (2009) | −3.27 | −4.92 | −1.62 |
| Pan C. (2011) | −8.3 | −8.814 | −7.786 |
| Yu M. (2011) | −8.05 | −9.784 | −6.316 |
| Jiang H. (2012) | −8.39 | −9.014 | −7.766 |
| Yu M. (2012) | −9.08 | −9.54 | −8.62 |
| Ayres A. (2013) | −5.83 | −7.597 | −4.063 |
| Pan C. (2014) | −5.38 | −5.766 | −4.994 |
| Yu M.M. (2014) | −7.27 | −7.89 | −6.65 |
| Zhuang H. (2014) | −7.57 | −8.166 | −6.974 |
| Pooled | −6.694 | −7.836 | −5.552 |
The meta-analysis combination for changes in the maternal serum ALT levels during lamivudine treatment is reported in Figure 5. It has been shown that ALT was decreased −84.52 IU/L (95% CI, −163.949 to −5.091) during lamivudine treatment. In this forest plot, the ALT normalization considered was taken as the end-point.
Figure 5.
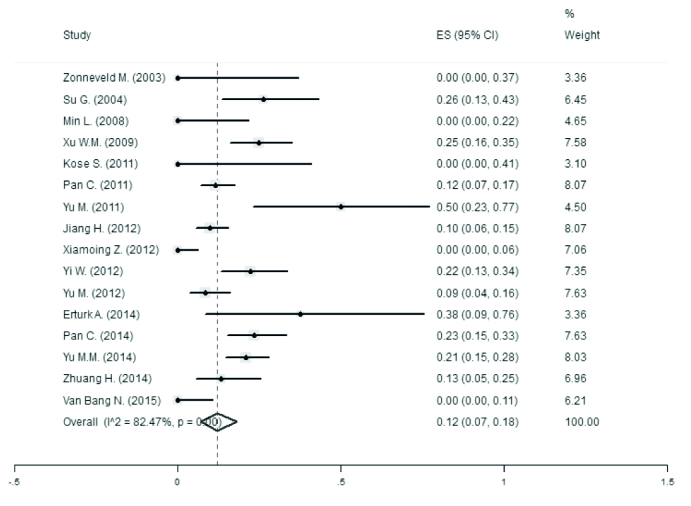
Forest plot for changes of maternal serum ALT levels during lamivudine treatment, with ALT normalization considered as the end-point
Table 5 contains the data regarding the prevalence of abnormal ALT levels. The ALT abnormality prevalence overlay was 12% (95% CI, 7% to 18%) during lamivudine treatment.
Table 5.
Pooled and study effect for prevalence of abnormal ALT levels
| Author (Year) | Effect | 95 % Confidence Interval | |
|---|---|---|---|
|
| |||
| Lower | Upper | ||
| Zonneveld M.(2003) | 0.00% | 0.00% | 37.00% |
| Su G.(2004) | 26.00% | 13.00% | 43.00% |
| Min L.(2008) | 0.00% | 0.00% | 22.00% |
| Xu W.M.(2009) | 25.00% | 16.00% | 35.00% |
| Kose S.(2011) | 0.00% | 0.00% | 41.00% |
| Pan C.(2011) | 12.00% | 7.00% | 17.00% |
| Yu M.(2011) | 50.00% | 23.00% | 77.00% |
| Jiang H.(2012) | 10.00% | 6.00% | 15.00% |
| Xiamoing Z.(2012) | 0.00% | 0.00% | 6.00% |
| Yi W.(2012) | 22.00% | 13.00% | 34.00% |
| Yu M.(2012) | 9.00% | 4.00% | 16.00% |
| Erturk A.(2014) | 38.00% | 9.00% | 76.00% |
| Pan C.(2014) | 23.00% | 15.00% | 33.00% |
| Yu M.M.(2014) | 21.00% | 15.00% | 28.00% |
| Zhuang H.(2014) | 13.00% | 5.00% | 25.00% |
| Total | 12% | 7% | 18% |
DISCUSSION
This meta-analysis included 25 RCTs and cohort studies published either in full text or as posters up to January 2016. Our review showed that the prenatal use of lamivudine is an effective way to lower the serum viral load of HBV and thus lower the risk of its vertical transmission to the newborn. Also, our analysis shows that lamivudine’s efficacy is superior to either no treatment or the HBIg treatment. Moreover, our results showed that lamivudine is a safe drug to use in the third-trimester of pregnancy, as no significant higher rates of adverse events were observed with the use of lamivudine, including stillbirth, low birth weight, CP, and fetal deformities.
Three meta-analyses have already been conducted on the efficacy of lamivudine on the prevention of MTCT of HBV. In the studies performed by Shi et al. (10) and Han et al. (11), only 10 and 15 studies were included, respectively; that is because the majority of the studies on this subject were performed after publication of the mentioned reviews. Moreover, in a recent meta-analysis conducted by Brown and colleagues (9), all antivirals used in pregnant women with HBV were studied, and the focus was not on lamivudine, which is the most used drug from this category. Regarding lamivudine’s safety, the findings of all three reviews were consistent with our results. However, respecting the efficacy, the review by Shi et al. (10) showed no significant difference between the HBIG and lamivudine, while the other two reviews did; this inconsistency was most probably due to the insufficient number of included studies.
The MTCT can be designated by either serum HBsAg/HBeAg or the HBV DNA of newborns or infants; among these, HBsAg is believed to be more reliable and is more widely used. A reason is that the HBV DNA levels may be undetectable despite a positive HBsAg, especially in asymptomatic carriers (21,23,25). Thus, it is recommended to follow the infants of the HBV-carrier mothers for 6–12 months serially.
The AASLD guideline on the CHB management states that lamivudine’s pregnancy safety category is C. (38) Since our review showed no significantly higher incidence of the analyzed pregnancy complications (i.e., stillbirth, low birth weight, cerebral palsy, apparent deformities) among mothers who received lamivudine in comparison to those who did not, it can be assumed that lamivudine is a safe drug for HBV-carrier mothers in the third-trimester. Although, it should be noted that the vast majority of included studies used lamivudine in the third-trimester, which has the lowest risk of adverse events on the fetus. Thus, it cannot be concluded that lamivudine’s use is safe throughout the pregnancy.
Our study had some potential limitations, of which the most important one was a limited access to data from non-English literature. Not only three related studies were excluded from this analysis as one of them did not have an English abstract, and the other two did not include vital data, necessary for this analysis (including the rate of MTCT), but also five of the included studies were published in non-English languages, and we just had access to their abstracts; although the data required for analysis were included in their abstracts, we cannot say for sure what we may have missed in the full text. To control this limitation, we tried to contact the corresponding authors of the mentioned studies to seek more data (as well as authors of unpublished works that were presented as conference posters). Another important limitation of our study was not analyzing maternal seropositivity of HBsAg and HBeAg, as most of the included studies did not mention these variables. In addition to maternal HBV DNA levels, seropositivity of the mentioned antigens may be valuable in determining treatment strategies for pregnant HBV carriers; we suggest researchers to examine this theory further.
At the moment, the AASLD guideline suggests antiviral treatment (lamivudine, telbivudine, and tenofovir) for pregnant women with the HBV DNA levels greater than 106 copies/mL (200,000 IU/mL), but this is not “strongly” recommended, and it has been stated that the quality/certainty of evidence is low (38). However, our meta-analysis strongly suggests the use of lamivudine for the prevention of MTCT of HBV in carrier pregnant women with HBV DNA levels greater than 106 copies/mL; and for women with HBV, viral loads less than 106 copies/mL, we suggest clinicians to examine the use of lamivudine on a case-by-case basis, keeping in mind that lamivudine seems to be a safe drug both for the mother and the fetus.
Footnotes
Ethics Committee Approval: Ethics committee approval not received for this study as there are no human or animal subjects directly recruited.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - P.K., S.M.A.; Design - P.K., S.M.A., M.G.F., R.J.; Supervision - P.K., S.M.A.; Materials - P.K, M.G.F..; Data Collection and/or Processing - P.K., R.J.; Analysis and/or Interpretation - S.M.A, M.G.F..; Literature Review - P.K., S.M.A.; Writing Manuscript - P.K., R.J.; Critical Review - P.K., S.M.A., M.G.F., R.J.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study supported by Research Centre for Gastroenterology and Liver Disease, Baqiyatallah University of Medical Sciences, Tehran, Iran (grant number 340/13885).
REFERENCES
- 1.World Health Organization. Hepatitis B fact sheet. 2016. [Google Scholar]
- 2.Petrova M, Kamburov V. Breastfeeding and chronic HBV infection: clinical and social implications. World J Gastroenterol. 2010;16:5042–6. doi: 10.3748/wjg.v16.i40.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan L, Owusu-Edusei K, Schillie SF, Murphy TV. Antiviral treatment among pregnant women with chronic hepatitis B. Infect Dis Obstet Gynecol. 2014;2014 doi: 10.1155/2014/546165. 546165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chotiyaputta W, Lok AS. Role of antiviral therapy in the prevention of perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2009;16:91–3. doi: 10.1111/j.1365-2893.2008.01067.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328–36. doi: 10.1136/bmj.38719.435833.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19:e18–25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, Guo Y, Zhou A, et al. Immunoprophylaxis failure against vertical transmission of hepatitis B virus in the Chinese population: a hospital-based study and a meta-analysis. Pediatr Infect Dis J. 2014;33:897–903. doi: 10.1097/INF.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 8.Dusheiko G. Interruption of mother-to-infant transmission of hepatitis B: time to include selective antiviral prophylaxis? Lancet. 2012;379:2019–21. doi: 10.1016/S0140-6736(11)61182-3. [DOI] [PubMed] [Google Scholar]
- 9.Brown RS, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology. 2016;63:319–33. doi: 10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 10.Shi Z, Yang Y, Ma L, Li X, Schreiber A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147–59. doi: 10.1097/AOG.0b013e3181e45951. [DOI] [PubMed] [Google Scholar]
- 11.Han L, Zhang HW, Xie JX, Zhang Q, Wang HY, Cao GW. A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis B virus. World J Gastroenterol. 2011;17:4321–33. doi: 10.3748/wjg.v17.i38.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:2049–3258. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayres A, Yuen L, Jackson K, et al. Short duration of lamivudine for the prevention of hepatitis B virus transmission in pregnancy: lack of potency and selection of resistance mutations. J Viral Hepat. 2014;21:809–17. doi: 10.1111/jvh.12212. [DOI] [PubMed] [Google Scholar]
- 14.Ertürk A, Cüre E, Parlak E, Cüre MC, Çiçek AÇ, Sahin F. Evaluation of the Results of Antiviral Therapy in Pregnant Women with Chronic Hepatitis B. Viral Hepat J. 2014;20 [Google Scholar]
- 15.Jackson V, Ferguson W, Kelleher TB, et al. Lamivudine treatment and outcome in pregnant women with high hepatitis B viral loads. Eur J Clin Microbiol Infect Dis. 2015;34:619–23. doi: 10.1007/s10096-014-2270-0. [DOI] [PubMed] [Google Scholar]
- 16.Jiang HX, Han GR, Wang CM, Ji Y. Maternal-fetal outcomes of lamivudine treatment administered during late pregnancy to highly viremic mothers with HBeAg+ chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2012;20:888–91. doi: 10.3760/cma.j.issn.1007-3418.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Köse S, Türken M, Devrim İ, Taner C. Efficacy and safety of lamivudine treatment in late pregnancy with high HBV DNA: a perspective for mother and infants. J Infect Develop Countries. 2011;5:303–6. doi: 10.3855/jidc.1398. [DOI] [PubMed] [Google Scholar]
- 18.Lawler J, Glass A, Chatterjee U, Wiseman E, Davison S, Manoharan S, et al. Nucleot(s)ide analogues to prevent perinatal transmission of HBV: Lamivudine is effective but Tenofovir may be better. Journal of gastroenterology and hepatology. 2011;26:100. [Google Scholar]
- 19.Li XM, Yang YB, Hou HY, Shi ZJ, Shen HM, Teng BQ, et al. Interruption of HBV intrauterine transmission: A clinical study. World journal of gastroenterology. 2003;9:1501–3. doi: 10.3748/wjg.v9.i7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min L, Ling W, Ping L, Haodong C. Mother-to-infant HBV transmission and infant development in pregnant women during lamivudine therapy: a retrospective investigation of 15 cases [J] Adverse Drug Reactions Journal. 2008;4:007. [Google Scholar]
- 21.Pan C, Han GR, Jiang HX, Ge CY, Xu CL, Zhao W. Efficacy and safety of lamivudine use in highly viremic mothers with HBEAG+ chronic hepatitis B (CHB) in clinical practice. Hepatology (Baltimore, Md) 2011;54:881A–2A. [Google Scholar]
- 22.Pan CQ, Lan B, Chan S, Yi W, Liu M. Tenofovir or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis b virus in real-life practice. Gastroenterology. 2014;1:S-961. [Google Scholar]
- 23.Su GG, Pan KH, Zhao NF, Fang SH, Yang DH, Zhou Y. Efficacy and safety of lamivudine treatment for chronic hepatitis B in pregnancy. World journal of gastroenterology. 2004;10:910–2. doi: 10.3748/wjg.v10.i6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchila R, Kontorinis N, Kong J, Porter M, Dhaliwal S, Cheng W. Does lamivudine have a role in the management of chronic Hepatitis B in pregnant woman with normal ALT? Journal of Gastroenterology and Hepatology (Australia) 2015;30:93. [Google Scholar]
- 25.Van Bang N, Le Thi Lan Anh NT, Van Anh NTQM, Van VT, Mohamed S, Halfon P, et al. Efficacy and Safety of Tenofovir and Lamivudine in Late Pregnancy for Preventing Perinatal Transmission of Hepatitis B Virus in Highly Viremic Mothers. Annals of Clinical and Laboratory Research. 2015 [Google Scholar]
- 26.Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. 16:94–103. doi: 10.1111/j.1365-2893.2008.01056.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Liu M, Wang L. [Effect of high viral hepatitis B virus DNA loads on vertical transmission of hepatitis B virus in late-pregnant women]. Zhonghua fu chan ke za zhi. 2008;43:329–31. [PubMed] [Google Scholar]
- 28.Xiaoming Z. Efficacy and Safety of Lamivudine Treatment on Preventing Hepatitis B Virus Vertical Transmission in Pregnant Women [J] Harbin Medical Journal. 2012;3:003. [Google Scholar]
- 29.Yi W, Liu M, Cai HD. Safety of lamivudine treatment for chronic hepatitis B in early pregnancy. World journal of gastroenterology. 2012;18:6645–50. doi: 10.3748/wjg.v18.i45.6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.HAN Z, CHEN Y, SU X. Clinical study of preventing HBV vertical transmission by lamivudine treatment combined with active-passive immunization for pregnant women. Hebei Med J. 2005;1:012. [Google Scholar]
- 31.Jiang Q, Yu M. A Retrospective Study of the Comparisons Between Telbivudine and Lamivudine on the Efficacy and Safety in Antiviral Treatment of Hepatitis B Virus During Pregnancy. J Hepatol. 2012;56:205–6. doi: 10.1016/S0168-8278(12)60532-7. [DOI] [Google Scholar]
- 32.Tekin Koruk S, Batirel A, Kose S, et al. Evaluation of hepatitis B virus transmission and antiviral therapy among hepatitis B surface antigen-positive pregnant women. J Obstet Gynaecol Res. 2015;41:1870–6. doi: 10.1111/jog.12821. [DOI] [PubMed] [Google Scholar]
- 33.Yu M, Jiang Q, Ji Y, et al. The efficacy and safety of antiviral therapy with lamivudine to stop the vertical transmission of hepatitis B virus. Eur J Clin Microbiol Infect Dis. 2012;31:2211–8. doi: 10.1007/s10096-012-1557-2. [DOI] [PubMed] [Google Scholar]
- 34.Yu MM, Jiang Q, Ji Y, et al. Comparison of telbivudine versus lamivudine in interrupting perinatal transmission of hepatitis B virus. J Clin Virol. 2014;61:55–60. doi: 10.1016/j.jcv.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Pan CQ, Pang QM, Tian RH, Yan ME, Liu X. Telbivudine or Lamivudine Use in Late Pregnancy Safely Reduces Perinatal Transmission of Hepatitis B Virus in Real-Life Practice. Hepatology. 2014;60:468–76. doi: 10.1002/hep.27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zonneveld M, Nunen A, Niesters H, Man R, Schalm S, Janssen H. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294–7. doi: 10.1046/j.1365-2893.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Ji Y, Jiang H, Ju L, Wu K, Kan N. Efficacy of peripartum antiviral treatment for hepatic failure due to hepatitis B virus. Int J Gynecol Obstet. 2011;114:33–6. doi: 10.1016/j.ijgo.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Terrault NA, Bzowej NH, Chang K-M, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]


