Abstract
Background/Objectives
Glutathione (GSH) is the most abundant endogenous antioxidant and a critical regulator of oxidative stress. Maintenance of optimal tissues GSH levels may be an important strategy for prevention of oxidative stress-related diseases. We investigated if oral administration of liposomal GSH is effective at enhancing GSH levels in vivo.
Subjects/Methods
A 1-month pilot clinical study of oral liposomal GSH administration at two doses (500 and 1000 mg GSH per day) was conducted in healthy adults. GSH levels in whole blood, erythrocytes, plasma and peripheral blood mononuclear cells (PBMCs) were assessed in 12 subjects at baseline and after 1, 2 and 4 weeks of GSH administration.
Results
GSH levels were elevated after 1 week with maximum increases of 40% in whole blood, 25% in erythrocytes, 28% in plasma and 100% in PBMCs occurring after 2 weeks (P<0.05). GSH increases were accompanied by reductions in oxidative stress biomarkers including decreases of 35% in plasma 8-isoprostane and 20% in oxidized:reduced GSH ratios (P<0.05). Enhancements in immune function markers were observed with liposomal GSH administration including NK cell cytotoxicity, which was elevated by up to 400% by 2 weeks (P<0.05), and lymphocyte proliferation, which was elevated up to 60% after 2 weeks (P<0.05). Overall, there were no differences observed between dose groups, but statistical power was limited due to the small sample size in this study.
Conclusions
Collectively, these preliminary findings support the effectiveness of daily liposomal GSH administration at elevating stores of GSH and impacting immune function and levels of oxidative stress.
Keywords: glutathione, liposomal glutathione, supplementation, antioxidant, immune function
Introduction
Glutathione (GSH) is the most abundant non-protein thiol in cells and has an array of critical functions which include detoxifying drugs, protecting macromolecules from oxidative damage and maintaining immune functions 1–7. GSH is synthesized from cysteine (Cys), glutamic acid and glycine with Cys most often being the rate limiting substrate 8, 9. As a result, GSH levels can be depleted when Cys levels are limited such as during periods of fasting 10, 11. GSH depletion has numerous detrimental effects including impaired immune function 6 and increased susceptibility to xenobiotics 12 and oxidants 13. Maintenance of optimal tissue levels of GSH is thought to be an important factor for maintaining health and low GSH levels have been associated with increased risks for diseases including cancer, cardiovascular diseases, arthritis and diabetes 14–16.
GSH enhancement represents a potentially important approach in the treatment and prevention of disorders associated with GSH-depletion. Studies linking dietary GSH intake with increased blood levels and reduced risk for cancer 17, 18 support the use of orally administered GSH for this purpose. Studies in laboratory animals have demonstrated that oral GSH is bioavailable and effective at enhancing blood and tissue GSH levels 19–24 and can protect against aging-related impairments in immune function 25, influenza infections 26, and cancer 27–30. In a recent clinical trial, we demonstrated that daily oral supplementation of GSH was effective at enhancing GSH levels in oral buccal cells and a variety of intra- and extra-cellular blood compartments 31.
Liposomes have been used as an effective means of drug delivery allowing for more efficient absorption and delivery of both hydrophilic and lipophilic substances and greater protection against oxidation and degradation. Since GSH is subject to destruction in the acid environment of the stomach, we proposed that oral liposomal GSH might be an effective means of GSH delivery in vivo. While liposomal GSH preparations are commercially available, there have been few clinical reports on their effectiveness and no data on their ability to enhance body GSH stores. Thus, our current objectives were to conduct a pilot study to determine the short-term (1 mo) effects of daily oral supplementation with liposomal GSH on the levels of GSH in different intracellular and extracellular blood compartments in healthy adults. In addition, effects on specific immune functions and biomarkers of oxidative stress were assessed.
Subjects and Methods
Study Protocol
The study (ClinicalTrials.gov identifier: NCT02278822) was approved by the Institutional Review Board of the Penn State College of Medicine in accordance with the Helsinki Declaration of 1975 as revised in 1983. Subjects were recruited from the local Hershey/Harrisburg, PA area using fliers, online announcements, and word of mouth. Interested individuals were prescreened by telephone and eligible subjects were asked to visit the Clinical Research Center at the Penn State Cancer Institute, Hershey, PA. After providing informed consent, subjects were further screened for eligibility based upon the following criteria: healthy non-smokers, 50–80 years of age, no antioxidant supplementation for ≥ 1 month. Eligible subjects were randomly assigned to one of two treatment groups with equal probability: Low dose (500 mg, p.o.) or high dose (1000 mg p.o.) liposomal GSH (Tri-Fortify™ Orange [phosphatidylcholine liposome GSH] provided by Researched Nutritionals, Los Olivos, CA). Liposomal GSH was provided in 8 oz tubes and individual daily doses were prepared by subjects using a 5.6 ml spoon (low dose, 1 spoonful; high dose, two spoonfuls). Tubes were returned and weighted at the completion of the study to assess compliance. Questionnaire data were collected on demographics, occupation, lifestyle habits, medical history, and medication, supplements and alcohol use. Supplementation continued for 1 month and blood and urine were collected at baseline and 1, 2 and 4 weeks.
Subjects
A total of 12 subjects were enrolled and received intervention from 11/13/2014 to 04/28/2015 and none withdrew or were withdrawn (Figure 1). There were no significant differences in study subject characteristics between treatment arms at baseline (Table 1). Compliance was assessed by daily diary entries and by difference in tube weights before and after the completion of the study.
Figure 1:
Subject flowchart summary.
Table 1.
Study Subject Characteristics
| Liposomal GSH (500 mg/d) | Liposomal GSH (1000 mg/d) | All | |
|---|---|---|---|
| Number of subjects | 6 | 6 | 12 |
| Age (yr) | |||
| Mean | 60.8 | 59.7 | 60.2 |
| s.d. | 7.39 | 4.46 | 5.85 |
| Range | 51 – 72 | 55 – 67 | 51 – 72 |
| Sex, n (%) | |||
| Female | 5 (83%) | 6 (100%) | 11 (92%) |
| Male | 1 (17%) | 0 (0%) | 1 (8%) |
| BMI (kg/m2) | |||
| Mean: | 26.9 | 29.9 | 28.4 |
| s.d. | 4.07 | 3.93 | 4.12 |
| Range | 21.7 – 32.0 | 24.7 – 34.6 | 21.7 – 34.6 |
Collection and processing of biological samples
Blood was collected between 9:00 am and 1:00 pm into tubes containing sodium heparin and immediately placed on ice and processed in ≤1 hr. Plasma and erythrocytes were obtained by centrifugation and further processed for GSH (see below) or stored at -80°C. Peripheral blood mononuclear cells (PBMCs) were obtained from ~24 ml of whole blood by density gradient centrifugation with Ficoll-Hypaque (Sigma, St Louis, MO) as described previously 31. Viable cells were counted using trypan blue and aliquots of 5 × 106 cells/ml in 95% FBS, 5% DMSO frozen at −80°C and stored in liquid nitrogen.
For GSH and GSSG analyses, whole blood, red cells and PBMCs were deproteinized with metaphosphoric acid (MPA) as described previously 31. Acid insoluble pellets were stored at −80°C until analysis for GSSP. Plasma samples were first reduced with KBH4 prior to deproteinization with MPA32.
Analytical Procedures
Glutathione (GSH)
GSH and glutathione disulfide (GSSG) levels were determined in MPA extracts using a 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB)/GSSG reductase enzymatic recycling procedure as described previously 33. For GSSP, MPA insoluble pellets from whole blood or erythrocytes were reduced with KBH4 at neutral pH and re-acidification with MPA prior to analysis of released GSH as described previously 34. Protein concentrations were measured by the bicinchoninic acid procedure (Pierce, Rockford, IL). Hemoglobin was determined spectrophotometrically using Drabkin’s reagent 35.
8-Isoprostane
Plasma 8-isoprostane levels were measured as a marker of oxidative stress by competitive ELISA (Cayman Chemical, Ann Arbor).
Immune Function Assays:
Lymphocyte proliferation was assessed by measuring the incorporation of 3H-thymidine as described previously 31. In brief, lymphocytes were thawed, washed 3X prior to counting and determination of viability. Cells were plated at 3 dilutions (2×105, 1×105 and 5×104 cells per well) and incubated in RPMI-1640 with 10% FBS for 48 hr at 37°C. After addition of 0 or 2 μg/ml phytohemagglutinin (PHA), cells were incubated for 72 hr prior to addition of 3H-thymidine. Radioactivity was assessed by liquid scintillation counting after 6 hr. All assays were run in triplicate.
NK cell cytotoxicity was assessed using a standard 51Chromium release assay as described previously 31, 36. In brief, lymphocytes were thawed, washed 3X prior to counting and determination of viability. Cells (1×104 cells per well) were incubated in triplicate in complete RPMI-1640 with 10% FBS for 48 hr at 37°C, and then combined with human K562 cells labeled overnight with 200 μCi sodium 51chromate at a 10:1 effector:target cell ratio. After 4 hr at 37°C, cells were analyzed for radioactivity by gamma counting. Results are expressed as percent of target cells lysed calculated as follows: (CPM experimental-CPM spontaneous release)/(CPM maximum – spontaneous) x 100.
Statistics:
Descriptive statistics were provided as means and standard errors of the mean. The normality of data distribution was assessed by using the Kolmogorov-Smirnov goodness-of-fit test. Dosage group differences at baseline were assessed by ANOVA followed by Tukey’s post hoc test or χ2 where ropriate. Comparisons over time were assessed by repeated measures ANOVA followed by post hoc testing where appropriate. Correlations of changes in outcomes with levels at baseline or between measures were evaluated using Pearson (r) correlations.
Results
Compliance and Adverse Effects
Overall, compliance appeared high based on diary entries with <1.5% of scheduled doses missed. Based upon returned tube weights, mean±SD compliance was 109±9.5% in all subjects (108±11.1% for low dose group; 111±8.4% for high dose group). Values >100% could be due to slightly larger doses in some individuals resulting from overfilling of the spoon during dosing.
No serious adverse effects were reported by the study participants in either dose group. All potential adverse events were minor and none were attributed to either treatment group.
Effects of oral liposomal GSH supplementation on body GSH stores
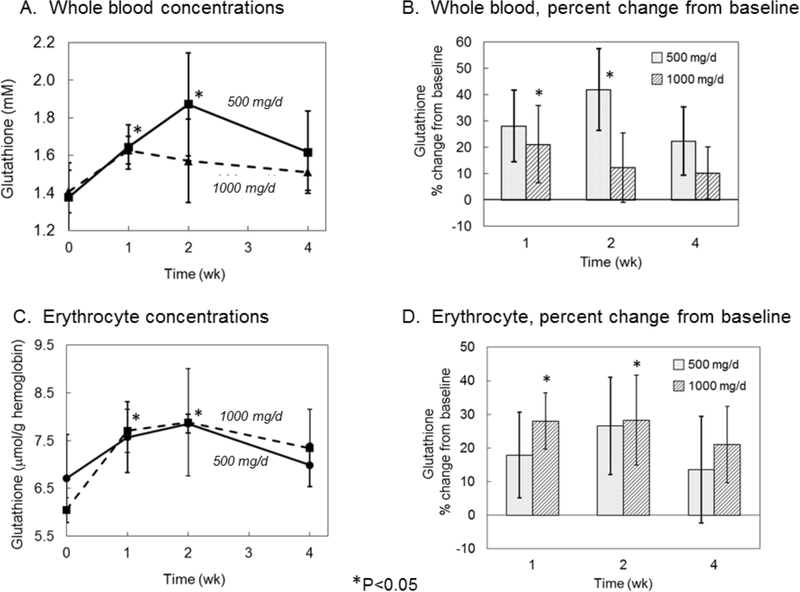
In whole blood, a trend of higher GSH was observed in both groups with liposomal GSH administration (Figure 2A). The largest increase (>40%) occurred in the 500 mg/d dose group at two weeks (Figure 2B) (P<0.05). GSH levels in blood reflect primarily those of erythrocytes due to the relatively low GSH levels in plasma and low abundance of other cell types. Thus, as expected, GSH profiles in erythrocytes were similar to those observed in whole blood with a trend of higher levels in both groups with liposomal GSH administration (Figure 2C). The largest increase (~28%) occurred in the high dose group after 1 and 2 weeks and in the low dose group after 2 weeks (P<0.05) (Figure 2D).
Figure 2:
Effect of liposomal glutathione supplementation on whole blood glutathione concentrations. Subjects were randomized to 500 or 1000 mg/d liposomal GSH for 4 weeks. Blood was collected at baseline and after 1, 2 and 4 weeks and free and protein-bound GSH was determined in whole blood (A & B) and in erythrocytes (C & D) as described in text. Whole blood levels are expressed as mmol/L (A) or percent change from baseline (B). Erythrocyte GSH levels are expressed as μmol/g hemoglobin (C) or percent change from baseline (D). Bars are mean ± SE. *Significantly different from baseline by repeated measures ANOVA, P<0.05.
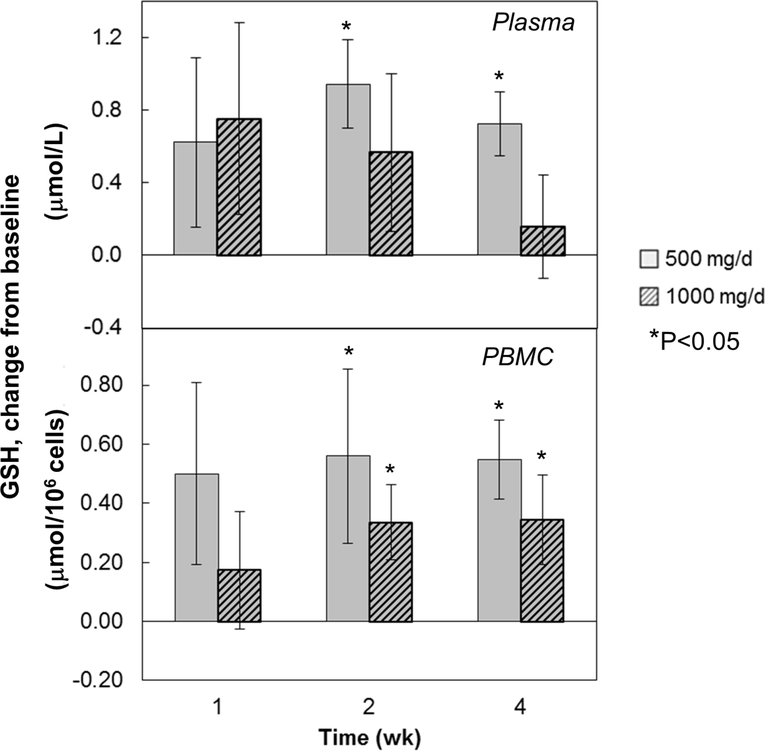
In plasma, GSH levels showed an increased trend above baseline in both groups after liposomal GSH administration; however, results were only significant after 2 and 4 weeks for the 500 mg dose group (Figure 3, upper panel). The largest increase (~25%) occurred in the low dose group after 2 weeks.
Figure 3:
Effect of liposomal glutathione supplementation on plasma and PBMC glutathione concentrations. Subjects were randomized to 500 or 1000 mg/d liposomal GSH for 4 weeks. Blood was collected at baseline and after 1, 2 and 4 weeks and PBMCs were isolated and analyzed for GSH as described in text. Results are expressed as % changes from baseline in μmol/L of plasma (top panel) or μmol/106 PBMCs (bottom panel). For plasma, baseline values ranged from 2.2 to 10.9 μmol/L (mean±SE: 4.57±0.62) for all subjects [low dose group: 2.2 to 10.9 (mean±SE: 4.63±1.29); high dose group: 3.94 to 5.15 (mean±SE: 4.51±0.17)]. For PBMCs, baseline values ranged from 0.23 to 1.34 μmol/106 cells (mean±SE: 0.89±0.11) for all subjects [low dose group: 0.23 to 1.34 (mean±SE: 0.77±0.19); high dose group: 0.67 to 1.34 (mean±SE: 1.01±0.11)]. Bars are mean ± SE. *Significantly different from baseline by repeated measures ANOVA, P<0.05.
In PBMCs, GSH levels tended to be increased above baseline in both groups after liposomal GSH administration, however, results were significant after 2 and 4 weeks only for both dose groups (Figure 3, lower panel). The largest increase (nearly 2-fold) occurred in the low dose group after 2 weeks
Effects of oral liposomal GSH supplementation on oxidative stress biomarkers
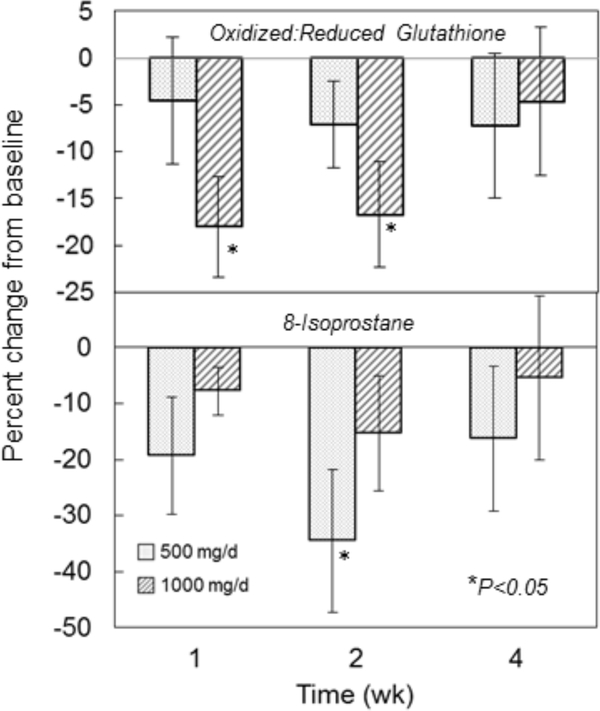
In general, the ratios of oxidized (GSSG + GSSP) to reduced GSH were lower after liposomal GSH administration (Figure 4, top panel; Supplemental Figure 1) with the largest decreases (18–20%) observed in the high dose group after 1 and 2 weeks (P<0.05). When dose groups were combined, significant reductions of ~14% were observed after 1 and 2 weeks, respectively (P<0.05).
Figure 4:
Effect of liposomal glutathione supplementation on blood biomarkers of oxidative stress. Subjects were randomized to 500 or 1000 mg/d liposomal GSH for 4 weeks. Blood was collected at baseline and after 1, 2 and 4 weeks. Top panel: GSH and its major oxidized forms GSSG and GSSP were determined in whole blood as described in text. Baseline ratios ranged from 0.24 to 0.42 (mean±SE: 0.31±0.02) for all subjects [low dose group: 0.24 to 0.34 (mean±SE: 0.30±0.02); high dose group: 0.25 to 0.42 (mean±SE: 0.32±0.02)]. Bottom panel: plasma 8-isoprostane levels were measured by ELISA as described in text. Results are expressed as % changes in pg/ml from baseline. Baseline values ranged from 63.1 to 1170 pg/ml (mean±SE: 214±90.6) for all subjects [low dose group: 78.6 to 1170 (mean±SE: 331±123); high dose group: 63.1 to 181 (mean±SE: 97.7±12.8)]. Results are expressed as % of baseline and symbols and bars are mean ± SE. *Significantly different from baseline by repeated measures ANOVA, P<0.05.
Plasma 8-isoprostane levels tended to decrease after liposomal glutathione administration (Figure 4, bottom panel). The largest decrease (35%) was observed in the 500 mg/d group after 2 weeks (P<0.05).
In order to determine if effects differed by baseline GSH, changes in GSH after 1, 2 and 4 weeks were correlated with GSH levels at baseline. Baseline GSH levels in red cells ranged from 4.66 to 10.9 μmol/g hemoglobin (mean ± SE: 6.38±0.47). There were strong inverse correlations between baseline GSH and changes in GSH levels at the different time points (r=0.6–0.8).
Effects of oral GSH on immune function markers in blood
Lymphocyte Proliferation
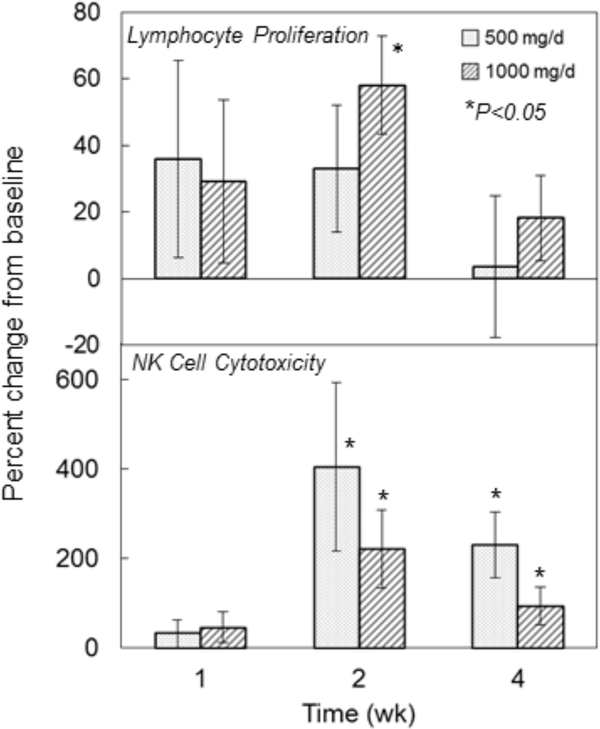
Proliferative capacity tended to be increased 1–2 weeks after liposomal GSH administration in both groups, however, this was only significant in the high dose group after 2 weeks where an increase of 60% was observed (P<0.05) (Figure 5, top panel).
Figure 5:
Effect of liposomal glutathione supplementation on lymphocyte proliferation and NK cell cytotoxicity. Subjects were randomized to 500 or 1000 mg/d liposomal GSH for 4 weeks. Blood was collected at baseline and after 1, 2 and 4 weeks and PBMCs were isolated. Top panel: Lymphocyte proliferation was assessed by measuring 3H-thymidine incorporation after incubation with PHA as described in the text. Results are expressed as % changes in CPM from baseline. Baseline values ranged from 20021 to 88197 CPM (mean±SE: 42105±6736) for all subjects [low dose group: 22270 to 40295 (mean±SE: 32596±1945); high dose group: 20021 to 88197 (mean±SE: 51613±8830)]. Bottom panel: NK cytotoxicity was assessed using 51Cr labeled human K562 cells as the target and measuring the percent of target cells lysed after incubation with lymphocytes for 4 hr at 37°C. Results are expressed as % changes in the extent of cell lysis from baseline. Baseline values ranged from 0.41 to 9.08% lysis (mean±SE: 4.04±0.82) for all subjects [low dose group: 0.41 to 7.73 (mean±SE: 3.89±0.89); high dose group: 1.41 to 9.08 (mean±SE: 4.19±0.83)]. Bars are mean ± SE. *Significantly different from baseline by repeated measures ANOVA, P<0.05.
Natural Killer Cell Cytotoxicity
Increases in mean % lysis values were observed in both dose groups after 2 and 4 weeks with the largest increases of 400% and 210% occurring after 2 weeks in the low dose and high dose groups, respectively (P<0.05) (Figure 5, bottom panel).
Discussion
The results of this pilot study demonstrate for the first time increased body stores of GSH after oral administration of liposomal GSH humans. Liposomal GSH appeared to be effective at two doses (500 and 1000 mg/d) and effects were seen as early as 1 week. In addition, liposomal GSH had positive effects on several GSH-related parameters including decreases in biomarkers of oxidative stress and enhancements in immune functions. Finally, liposomal GSH was highly tolerated and its administration was not associated with any signs of adverse effects. Although small in size, the results from this study provide support for the potential use of oral liposomal GSH as an intervention strategy for enhancing tissue GSH levels for use in disease therapy or prevention. In addition, they provide a rationale for additional larger placebo-controlled trials in both healthy and diseased individuals aimed at assessing the potential therapeutic efficacy of liposomal GSH. The results are consistent with previous findings where oral supplementation with non-liposomal glutathione was effective at enhancing body stores of GSH in laboratory animals and humans 19–24, 31. While a direct comparison between forms of GSH has not been made, liposomal GSH effects were often greater that previously observed for non-liposomal GSH 30. However, future side-by-side comparison studies will be required to establish the relative effectiveness of these GSH forms.
To gain a more comprehensive assessment of supplementation on body GSH stores, we measured GSH levels in different blood compartments. For most measures, GSH increases were time-dependent with maximal increases of up to 40% in whole blood, 25% in red cells, 28% in plasma and 200% in PBMCs occurring within 2 weeks. The strong inverse correlation of GSH changes with baseline levels suggest that supplementation has its greatest effect in subjects with the lowest baseline GSH levels. Increases occurred in all compartments suggesting a systemic effect and that other less-accessible tissues may be impacted in a similar manner.
While there was a trend of increasing GSH at all time points and doses and in all cell types examined, not all changes were statistically significant; likely a result of the small sample size (n=6/group) in this pilot study. The lack of significant differences between dose groups may also be attributed, in part, to the limited power. While unlikely, an alternative explanation for the lack of a dose-response could be a saturation effect leading to optimal effects at the lower 500 mg dose. Additional larger-scale studies will be necessary to investigate these possibilities and establish the optimal dose of liposomal GSH.
For several parameters, the impact of liposomal GSH administration appeared to be lower at 4 wk then at earlier time points. A decrease in effectiveness is not expected based on previous studies. One possible explanation may be a drop in the self-administered dose occurring toward the end of the study. The test substance is a viscous liquid, which was measured out by participants in a deep-welled spoon (~1 teaspoon). In the laboratory, this dosing procedure was found to be precise (CV=8.2%). However, it remains possible that subjects could have had a tendency to deliver lower doses when reaching the end of the study. Unfortunately, there was no way to determine if this may have occurred in the current study.
While the majority of glutathione in cells is in the reduced form, GSH oxidation can occur resulting in the formation of GSSG or GSSP. The levels of both GSSG and GSSP are greater during periods of oxidative stress and the ratio of GSSG and/or GSSP to GSH have often been used as biomarkers of oxidative stress 34. Thus, the decreases observed in oxidized:reduced GSH as a result of oral liposomal GSH supplementation are likely indicative of a general systemic reduction in oxidative stress levels. This is supported by a concomitant decrease in levels of the lipid peroxidation biomarker 8-isporostane. These differences were observed even though all subjects were healthy non-smokers and, hence, not likely experiencing high levels of oxidative stress in general. Even greater effects of might be expected in individuals exposed to higher levels of oxidants such as tobacco smokers.
GSH plays an important role in maintenance of numerous immune functions including lymphocyte proliferation and NK cell activity 37–39. We found that liposomal GSH administration resulted in increases in both of these activities; lymphocyte proliferation was enhanced up to 60% and as early as 1 wk and NK cell cytotoxicity was increased up to 400% and as early as 2 wk. Further, the time course of these effects on immune function coincided with the observed increases in PBMC GSH content. While the mechanisms are not known, these finding are consistent with previous in vitro studies of GSH on lymphocyte proliferation and NK cell activity 40–43. These results are also consistent with earlier clinical findings with non-liposomal GSH 31 and observed correlations of GSH content with NK cell activity 44.
Overall, these results provide promising rationale for the potential use of liposomal GSH to enhance antioxidant capacity and immune functions. There have been few previous reports of clinical trials with other antioxidants in healthy individuals. In fact, most have shown minimal or no effects on oxidative stress biomarkers and/or immune functions 45–51. As noted above, the small size of this pilot study limits the overall representativeness of the findings to other healthy or diseased populations. Additionally, the study design did not use a placebo control. While, based on our previous study we would not expect significant changes to occur in the measured study outcomes 31, future placebo-controlled randomized trials with liposomal GSH will be required to confirm the specificity of its intervention effects.
Supplementary Material
Acknowledgements:
We acknowledge the support of the staff of the Pennsylvania State University College of Medicine Clinical Research Center and Investigational Pharmacy. This work was supported by Research Nutritionals, LLC. Liposomal glutathione preparations (Tri-Fortify™ Orange) were provided by Researched Nutritionals, LLC (Los Olivos, CA). Immunological analyses were supported in part by the Penn State Cancer Institute through the Clinical Correlative Immunology Laboratory (TS, JH). The project described was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL1TR002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial Support: This work was supported by Research Nutritionals, LLC. Liposomal glutathione preparations (Tri-Fortify™ Orange) were provided by Researched Nutritionals, LLC (Los Olivos, CA).
Footnotes
Conflict of Interest:
RS and JPR received research funding for this study from Researched Nutritionals, LLC. Researched Nutritionals, LLC is a nutraceutical company that provides liposomal glutathione (Tri-Fortify™ Orange) to health care professionals. Other than providing research funding and liposomal GSH, Researched Nutritionals, LLC did not play a role in the design of the study, collection and analysis of the data and decision to publish. There were no personal financial interests between any of the authors with Researched Nutritionals, LLC.
References
- 1.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. Faseb J 1999; 13(10): 1169–1183. [PubMed] [Google Scholar]
- 2.Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983; 52: 711–760. [DOI] [PubMed] [Google Scholar]
- 3.Vina J Glutathione: Metabolism and Physiological Functions, CRC Press: Boca Raton, 1990. [Google Scholar]
- 4.Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I. S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med 2004; 8(2): 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 2009; 30(1–2): 1–12. e-pub ahead of print 2008/09/18; doi: 10.1016/j.mam.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Droge W, Breitkreutz R. Glutathione and immune function. The Proceedings of the Nutrition Society 2000; 59(4): 595–600. e-pub ahead of print 2000/01/11; [DOI] [PubMed] [Google Scholar]
- 7.Hamilos DL, Zelarney P, Mascali JJ. Lymphocyte proliferation in glutathione-depleted lymphocytes: direct relationship between glutathione availability and the proliferative response. Immunopharmacology 1989; 18(3): 223–235. [DOI] [PubMed] [Google Scholar]
- 8.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 1999; 27(9–10): 922–935. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Forman HJ. Glutathione synthesis and its role in redox signaling. Seminars in cell & developmental biology 2012; 23(7): 722–728. e-pub ahead of print 2012/04/17; doi: 10.1016/j.semcdb.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt BL, Richie JP Jr. Fasting-induced depletion of glutathione in the aging mouse. Biochem Pharmacol 1993; 46(2): 257–263. [DOI] [PubMed] [Google Scholar]
- 11.Jaeschke H, Wendel A. Diurnal fluctuation and pharmacological alteration of mouse organ glutathione content. Biochem Pharmacol 1985; 34(7): 1029–1033. [DOI] [PubMed] [Google Scholar]
- 12.Jollow DJ. Glutathione thresholds in reactive metabolite toxicity. Arch Toxicol Suppl 1980; 3: 95–110. [DOI] [PubMed] [Google Scholar]
- 13.Ellouk-Achard S, Levresse V, Martin C, Pham-Huy C, Dutertre-Catella H, Thevenin M et al. Ex vivo and in vitro models in acetaminophen hepatotoxicity studies. Relationship between glutathione depletion, oxidative stress and disturbances in calcium homeostasis and energy metabolism. Arch Toxicol Suppl 1995; 17: 209–214. [DOI] [PubMed] [Google Scholar]
- 14.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother 2003; 57(3–4): 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuttall SL, Martin U, Sinclair AJ, Kendall MJ. Glutathione: in sickness and in health. Lancet 1998; 351(9103): 645–646. [DOI] [PubMed] [Google Scholar]
- 16.Julius M, Lang CA, Gleiberman L, Harburg E, DiFranceisco W, Schork A. Glutathione and morbidity in a community-based sample of elderly. J Clin Epidemiol 1994; 47(9): 1021–1026. [DOI] [PubMed] [Google Scholar]
- 17.Flagg EW, Coates RJ, Eley JW, Jones DP, Gunter EW, Byers TE et al. Dietary glutathione intake in humans and the relationship between intake and plasma total glutathione level. Nutr Cancer 1994; 21(1): 33–46. [DOI] [PubMed] [Google Scholar]
- 18.Flagg EW, Coates RJ, Jones DP, Byers TE, Greenberg RS, Gridley G et al. Dietary glutathione intake and the risk of oral and pharyngeal cancer. Am J Epidemiol 1994; 139(5): 453–465. [DOI] [PubMed] [Google Scholar]
- 19.Favilli F, Marraccini P, Iantomasi T, Vincenzini MT. Effect of orally administered glutathione on glutathione levels in some organs of rats: role of specific transporters. Br J Nutr 1997; 78(2): 293–300. e-pub ahead of print 1997/08/01; [DOI] [PubMed] [Google Scholar]
- 20.Kariya C, Leitner H, Min E, van Heeckeren C, van Heeckeren A, Day BJ. A role for CFTR in the elevation of glutathione levels in the lung by oral glutathione administration. American journal of physiology. Lung cellular and molecular physiology 2007; 292(6): L1590–1597. e-pub ahead of print 2007/03/21; doi: 10.1152/ajplung.00365.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aw TY, Wierzbicka G, Jones DP. Oral glutathione increases tissue glutathione in vivo. Chem Biol Interact 1991; 80(1): 89–97. [DOI] [PubMed] [Google Scholar]
- 22.Hagen TM, Wierzbicka GT, Sillau AH, Bowman BB, Jones DP. Bioavailability of dietary glutathione: effect on plasma concentration. Am J Physiol 1990; 259(4 Pt 1): G524–529. [DOI] [PubMed] [Google Scholar]
- 23.Vina J, Perez C, Furukawa T, Palacin M, Vina JR. Effect of oral glutathione on hepatic glutathione levels in rats and mice. Br J Nutr 1989; 62(3): 683–691. [DOI] [PubMed] [Google Scholar]
- 24.Hunjan MK, Evered DF. Absorption of glutathione from the gastro-intestinal tract. Biochim Biophys Acta 1985; 815(2): 184–188. e-pub ahead of print 1985/05/14; [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Meydani SN, Blumberg JB. Reversal of age-associated decline in immune responsiveness by dietary glutathione supplementation in mice. Mech Ageing Dev 1987; 38(2): 107–117. [DOI] [PubMed] [Google Scholar]
- 26.Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP. Inhibition of influenza infection by glutathione. Free Radic Biol Med 2003; 34(7): 928–936. e-pub ahead of print 2003/03/26; [DOI] [PubMed] [Google Scholar]
- 27.Novi AM. Regression of aflatoxin B1-induced hepatocellular carcinomas by reduced glutathione. Science 1981; 212(4494): 541–542. [DOI] [PubMed] [Google Scholar]
- 28.Trickler D, Shklar G, Schwartz J. Inhibition of oral carcinogenesis by glutathione. Nutr Cancer 1993; 20(2): 139–144. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JL, Shklar G. Glutathione inhibits experimental oral carcinogenesis, p53 expression, and angiogenesis. Nutr Cancer 1996; 26(2): 229–236. [DOI] [PubMed] [Google Scholar]
- 30.Shklar G, Schwartz J, Trickler D, Cheverie SR. The effectiveness of a mixture of beta-carotene, alpha-tocopherol, glutathione, and ascorbic acid for cancer prevention. Nutr Cancer 1993; 20(2): 145–151. [DOI] [PubMed] [Google Scholar]
- 31.Richie JP Jr., Nichenametla S, Neidig W, Calcagnotto A, Haley JS, Schell TD et al. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. European journal of nutrition 2015; 54(2): 251–263. e-pub ahead of print 2014/05/06; doi: 10.1007/s00394-014-0706-z [DOI] [PubMed] [Google Scholar]
- 32.Kleinman WA, Richie JP Jr. Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol 2000; 60(1): 19–29. [DOI] [PubMed] [Google Scholar]
- 33.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 2006; 1(6): 3159–3165. doi: 10.1038/nprot.2006.378 [DOI] [PubMed] [Google Scholar]
- 34.Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J et al. Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic Biol Med 2004; 36(4): 464–470. [DOI] [PubMed] [Google Scholar]
- 35.Drabkin DL. The standardization of hemoglobin measurements. Am J Med Sci 1949; 217: 711–714. [PubMed] [Google Scholar]
- 36.Schell TD, Mylin LM, Georgoff I, Teresky AK, Levine AJ, Tevethia SS. Cytotoxic T-lymphocyte epitope immunodominance in the control of choroid plexus tumors in simian virus 40 large T antigen transgenic mice. Journal of virology 1999; 73(7): 5981–5993. e-pub ahead of print 1999/06/11; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilos DL, Wedner HJ. The role of glutathione in lymphocyte activation. I. Comparison of inhibitory effects of buthionine sulfoximine and 2-cyclohexene-1-one by nuclear size transformation. J Immunol 1985; 135(4): 2740–2747. [PubMed] [Google Scholar]
- 38.Gmunder H, Droge W. Differential effects of glutathione depletion on T cell subsets. Cellular immunology 1991; 138(1): 229–237. e-pub ahead of print 1991/11/01; [DOI] [PubMed] [Google Scholar]
- 39.Morris D, Khurasany M, Nguyen T, Kim J, Guilford F, Mehta R et al. Glutathione and infection. Biochim Biophys Acta 2013; 1830(5): 3329–3349. e-pub ahead of print 2012/10/24; doi: 10.1016/j.bbagen.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 40.Galter D, Mihm S, Droge W. Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur J Biochem 1994; 221(2): 639–648. e-pub ahead of print 1994/04/15; [DOI] [PubMed] [Google Scholar]
- 41.Suthanthiran M, Anderson ME, Sharma VK, Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A 1990; 87(9): 3343–3347. e-pub ahead of print 1990/05/01; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hargrove ME, Wang J, Ting CC. Regulation by glutathione of the activation and differentiation of IL-4-dependent activated killer cells. Cellular immunology 1993; 149(2): 433–443. e-pub ahead of print 1993/07/01; doi: 10.1006/cimm.1993.1168 [DOI] [PubMed] [Google Scholar]
- 43.Liang CM, Lee N, Cattell D, Liang SM. Glutathione regulates interleukin-2 activity on cytotoxic T-cells. J Biol Chem 1989; 264(23): 13519–13523. e-pub ahead of print 1989/08/15; [PubMed] [Google Scholar]
- 44.Vojdani A, Mumper E, Granpeesheh D, Mielke L, Traver D, Bock K et al. Low natural killer cell cytotoxic activity in autism: the role of glutathione, IL-2 and IL-15. Journal of neuroimmunology 2008; 205(1–2): 148–154. e-pub ahead of print 2008/10/22; doi: 10.1016/j.jneuroim.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 45.Huang HY, Helzlsouer KJ, Appel LJ. The effects of vitamin C and vitamin E on oxidative DNA damage: results from a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2000; 9(7): 647–652. [PubMed] [Google Scholar]
- 46.Jacob RA, Aiello GM, Stephensen CB, Blumberg JB, Milbury PE, Wallock LM et al. Moderate antioxidant supplementation has no effect on biomarkers of oxidant damage in healthy men with low fruit and vegetable intakes. J Nutr 2003; 133(3): 740–743. [DOI] [PubMed] [Google Scholar]
- 47.Ottestad I, Vogt G, Retterstol K, Myhrstad MC, Haugen JE, Nilsson A et al. Oxidised fish oil does not influence established markers of oxidative stress in healthy human subjects: a randomised controlled trial. Br J Nutr 2012; 108(2): 315–326. doi: 10.1017/S0007114511005484 [DOI] [PubMed] [Google Scholar]
- 48.Stewart RJ, Askew EW, McDonald CM, Metos J, Jackson WD, Balon TW et al. Antioxidant status of young children: response to an antioxidant supplement. Journal of the American Dietetic Association 2002; 102(11): 1652–1657. [DOI] [PubMed] [Google Scholar]
- 49.Patrignani P, Panara MR, Tacconelli S, Seta F, Bucciarelli T, Ciabattoni G et al. Effects of vitamin E supplementation on F(2)-isoprostane and thromboxane biosynthesis in healthy cigarette smokers. Circulation 2000; 102(5): 539–545. [DOI] [PubMed] [Google Scholar]
- 50.Nemzer BV, Rodriguez LC, Hammond L, Disilvestro R, Hunter JM, Pietrzkowski Z. Acute reduction of serum 8-iso-PGF2-alpha and advanced oxidation protein products in vivo by a polyphenol-rich beverage; a pilot clinical study with phytochemical and in vitro antioxidant characterization. Nutrition journal 2011; 10: 67. doi: 10.1186/1475-2891-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trebble TM, Wootton SA, Miles EA, Mullee M, Arden NK, Ballinger AB et al. Prostaglandin E2 production and T cell function after fish-oil supplementation: response to antioxidant cosupplementation. Am J Clin Nutr 2003; 78(3): 376–382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.