Abstract
It is unclear whether patients with Hashimoto thyroiditis (HT) are predisposed to develop thyroid nodules and/or thyroid cancer. The objective of our study was therefore to assess the prevalence of thyroid nodules and/or cancer in patients with HT and to look for possible prognostic factors. A retrospective survey of 904 children/adolescents with HT (709 females, 195 males) regularly followed in nine Italian centers of pediatric endocrinology was performed. Median period of follow-up was 4.5 years (1.2 to 12.8 years). We evaluated free T4, TSH, thyroid peroxidase antibody (TPOAb), thyroglobulin antibodies, and thyroid ultrasound yearly. One hundred seventy-four nodules were detected, with an annual incidence rate of 3.5%. Ten nodules were malignant (8 papillary and 2 papillary follicular variant), giving a 5.7% prevalence of cancer among patients with nodules. The severity of hypoechogenity at ultrasound, TPOAb, and free T4 serum concentrations were predictive for the appearance of new nodules. Furthermore, a positive correlation was observed between TPOAb titer and the development of thyroid cancer. In conclusion, HT seems to influence the development of thyroid nodules, but not cancer in children and adolescents.
Keywords: Hashimoto thyroiditis, thyroid nodules, cancer, children, adolescents, follow-up
It is unclear whether patients with Hashimoto thyroiditis (HT) are predisposed to the development of thyroid nodules and/or thyroid cancer. Different approaches have led to discordant findings in adult subjects. A clear association between HT and differentiated thyroid cancer (DTC), mainly papillary, has been reported in surgical series [1–6]. However, this association was not found in patients with nodular pathology without suspicion of malignancy who underwent fine needle biopsy (FNA) and cytological studies [7–13] Because the surgically treated patients are those considered at higher risk of malignancy, the association between HT and DTC based on surgical pathology may be secondary to selection bias.
Nodular pathology and cancer are rarely observed in children and adolescents compared with the adult population. In a survey of 5179 healthy children performed in 1975, Rallison et al. [14] reported a frequency of nodules of 1.8%. However, in this study the presence of thyroid nodules was assessed by the poorly sensitive method of palpation. Furthermore, the prevalence of autoimmunity has increased in recent decades and so it may no longer be appropriate to make comparisons to a study performed more than 40 years ago. More recent studies that use ultrasound imaging, reported a prevalence of 0.2% to 5.1% [15–17].
Thyroid nodules in children carry a greater risk of malignancy compared with adults [18]. However, there are few published data on the frequency of the development of thyroid cancer in children and adolescents with thyroid nodules. Niedziela [19] reviewed 18 studies published between 1960 and 2004 and reported a frequency of 9.2% to 50% based on surgical specimens. More recently, studies based on cytological/histological evaluation reported a frequency of more than 16% [20–22], which is greater than that seen in adult patients (2.3%) [23, 24].
Nethertheless, thyroid cancer is a rare condition in children and adolecents with an average age-adjusted rate of 0.59 per 100,000 cases [25]. A higher prevalence of up to 36 to 48 per 100,000 can be found in children exposed to radiation, such as in Fukushima after the Fukushima Daiichi nuclear power plant accident [26]. Whether the presence of HT influences the development of nodules and/or cancer in children is even more debated. The data are of poor quality, usually based on retrospective cross-sectional studies and the diagnosis is commonly made on clinical grounds. An Italian multicenter study on 365 children with HT reported the occurrence of thyroid nodularity and cancer in 31% and 3% of the patients, respectively [24]. Kambalapalli et al. [27] found an equal incidence of thyroid nodules and DTC in thyroid peroxidase antibody (TPOAb)-positive and -negative patients.
HT is a frequent condition in children and adolescents, and therefore, a better understanding of the risk for developing cancer is of great importance. This retrospective study was designed to investigate the role of HT on the development of thyroid nodules and cancer in a large group of children and adolescents followed-up for up to 12 years.
1. Subjects and Methods
A. Study Protocol
Between December 2003 and July 2016, 3754 patients with HT were regularly followed up in nine Italian centers of pediatric endocrinology. From this cohort, we included in this study 904 children/adolescents (709 females and 195 males) for whom free T4, TSH, TPOAb, thyroglobulin antibody (TGAb) measurement, thyroid ultrasound, and auxological data were available from the time of diagnosis and during the follow-up period. Median follow up was 4.5 years (1.2 to 12.8 years). The diagnosis of HT was based on the presence of TPOAb and/or TGAb, and/or the typical ultrasound pattern. Thyroid function (free T4 and TSH) and auxological data were obtained at diagnosis and yearly during follow-up. Thyroid ultrasound was performed at diagnosis and every other year in the subjects without nodularity, and yearly in those with nodules. Hypothyroidism was defined by a TSH level >10 mU/L and free T4 within or below the normal range, whereas subclinical hypothyroidism was defined by a TSH between 5 and 10 mU/L and free T4 in the normal range.
In addition to HT, 58 patients also had celiac disease, 12 had type 1 diabetes mellitus (T1DM), 31 had vitiligo, 22 had alopecia, and 2 had Addison disease. Two females had T1DM and celiac disease, 3 females had celiac disease and vitiligo, 1 male had T1DM and alopecia, and 3 males and 1 female had vitiligo and alopecia. Sixteen patients had Turner syndrome (1 also associated with celiac disease) and 10 (7 females and 3 males) had Down syndrome. A positive family history for autoimmune disease was present in 355 (39.26%, 272 females) of patients. Consent has been obtained from each patient or subject after full explanation of the purpose and nature of all procedures used. Formal ethical approval by an ethical committee is not required in accordance with the Italian regulation for noninterventional (observational) retrospective studies concerning human beings (AIFA Guidelines for Observational Studies, see www.AIFA.gov). All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
B. Thyroid Ultrasound and Thyroid Score
Ultrasound was performed in each center using a 9 to 13 MHz transducer. The volume of the thyroid gland is the sum of the volume of each lobe, calculated using the formula for a prolate ellipse: volume = 0.5 × (L × D × W) were L is the length (L), (D) is the anterior-posterior depth, and (W) is the transverse width of the gland. The presence of goiter was defined as a thyroid volume of more than 2 SD according to the normal values reported by Vitti et al. [28]. A hypoechogenic signal was considered a typical ultrasound pattern for the diagnosis of HT.
Alterations of echogenicity and homogeneity of the parenchyma were further quantified according to a pediatric scoring system [29]. Score 0: normal; score 1: mild parcelled hypoechogenicity; score 2: severe parcelled hypoechogenicity; score 3: mild generalized hypoechogenicity; score 4: severe generalized hypoechogenicity; score 5: near-anechogenicity. We refer to this score system throughout the manuscript as the thyroid score (TS). The presence of cervical adenopathy was also evaluated by ultrasound in all patients.
C. Thyroid Nodules
Criteria for FNA of a thyroid nodule were diameter >1 cm or diameter 0.5 to 1 cm with an echographic pattern suspicious for malignancy (solid nodule, internal calcifications, hypoechoic, irregular margins, taller than wider on transverse view, absence of halo). Cytological findings were evaluated according to the Italian consensus for the classification and reporting of thyroid cytology [30]. The presence of cervical adenopathy was also checked and postoperative staging in patients with thyroid cancer was performed according to the eighth edition of the AJCC/TNM classification system for DTC, based on the staging of the primary tumor (T), lymph nodes (N) and distant metastases (M) [31]. There was no central review of the thyroid ultrasound, FNA, and/or surgical pathology.
D. Assays
From 2006, serum TSH, free T4, TGAb, and TPOAb were measured by chemiluminescent immunometric assays in all participating centers. TSH [32] and free T4 [33] were measured using reagents provided by Roche Diagnostics GmbH (Manheim, Germany); the intra- and interassay coefficient of variation (CV) for TSH were 2.7% and 3.2%, with a sensitivity limit of 0.005 mU/L. For free T4 the intra- and interassay CV were 1.8% and 2.6%, and the limit of sensitivity was 0.08 pg/mL. TGAb [34] and TPOab [35] were measured using Immulite 2000 Anti-TG Ab (DPC, LA, Siemens Medical Solutions-Diagnostics, Malvern, PA). For TGab the intra- and interassay CV were 3.2% and 4.6%, and the limit of sensitivity was of 2.2 U/mL. For TPOAb the intra- and interassay CV were 5.2% and 3.2%, with a sensitivity limit of 5 U/mL.
Prior to this, laboratory measurements were performed using different methods by the participating centers.
E. Statistical Analysis
Data are shown as mean ± SD (range). Differences between groups were analyzed using the Student t test. Time-to-event data (nodules and cancer) was analyzed with the Kaplan-Meier method. Survival curves have been evaluated with the log-rank test. Two different models were considered. In the first, the thyroid score values determined the stratum levels, in the second a list of covariates (TS, TSH, free T4, TPOAb, TGAb, T4 treatment) were used to test the association with the failure time. The level of significance was set at 5%. Comparison between quantitative variables at diagnosis versus last visit was carried out using the nonparametric Wilcoxon test.
2. Results
The main clinical characteristics, the thyroid function and morphology of the patients at diagnosis and at last visit are reported in Table 1.
Table 1.
Clinical Characteristics, Thyroid Function and Morphology of the Patients at Diagnosis of Hashimoto Thyroiditis and at the Last Visit
| Chronological Age (y) | Height (SD) | BMI (SD) | TSH mU/L | Free T4 pg/mL | TPOAb U/mL | TGAb U/mL | Thyroid Volume (SD) | TS | |
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | 10.6 ± 3.2 | −0.07 ± 1.17 | 0.07 ± 1.17 | 20.0 ± 80.4 | 7.9 ± 6.2 | 865 ± 1671 | 672 ± 1794 | 2.6 ± 3.4 | 2.0 ± 1.1 |
| (1–20.8) | (−4.9 – 3.2) | (−3.7– 3.9) | (0–1000) | (0.46-–67.4) | (0–14.250) | (0–28160) | (−3.4–24.8) | (0–4) | |
| Last visit | 15.1 ± 3.1 | −0.11 ± 1.19 | 0.19 ± 3.49 | 4.5 ± 18.3 | 8.3 ± 6.1 | — | — | 1.8 ± 3.2 | 2.6 ± 1.1 |
| (7.9–23.1) | (−3.9– 3.2) | (−3.8– 2.8) | (0–422) | (0.8–15.8) | (−4.3–23) | (0–4) | |||
| P a | <0.0001 | NS | <0.0001 | <0.0001 |
Data are mean ±SD (range).
Normal values: TSH 0.3–3.69 mU/L; free T4 9–17.7 pg/mL; TPOAb < 35 U/mL; TGAb < 45 U/mL.
Abbreviation: NS, not significant.
Wilcoxon test.
A. Thyroid Function and Morphology
At diagnosis, TSH ranged from 0 to 1000 mU/L and free T4 from 0.46 to 67.4 pg/mL. Five patients had hyperthyroidism (0.55%), 697 were euthyroid (77.10%), 58 had subclinical hypothyroidism (6.42%), and 144 had hypothyroidism (15.93%).
All patients with hypothyroidism commenced levothyroxine treatment immediately, whereas those with subclinical hypothyroidism were followed up and treatment started when TSH concentrations exceeded 10 mU/L according to the current recommendations [36]. At the last visit 397 patients (43.92%) were on levothyroxine. In these patients, TSH and thyroid volume decreased substantially during the follow-up, presumably as a result of treatment, whereas the TS increased, indicating worsening of the echographic pattern.
At diagnosis, thyroid ultrasound revealed the presence of thyroid nodules in 77 children (8.52%), with associated cervical adenopathy in 19 (24.6%); thyroid cancer was already present in 3 of 77 (0.33%) children. At the last visit, the frequency of thyroid nodules had increased substantially (174 children, 19.2%; P < 0.0001) compared with baseline, as well as the frequency of cervical adenopathy (21 children, 12.1%; P < 0.05) and of thyroid cancer within nodules (10 children, 5.7%; P < 0.0001).
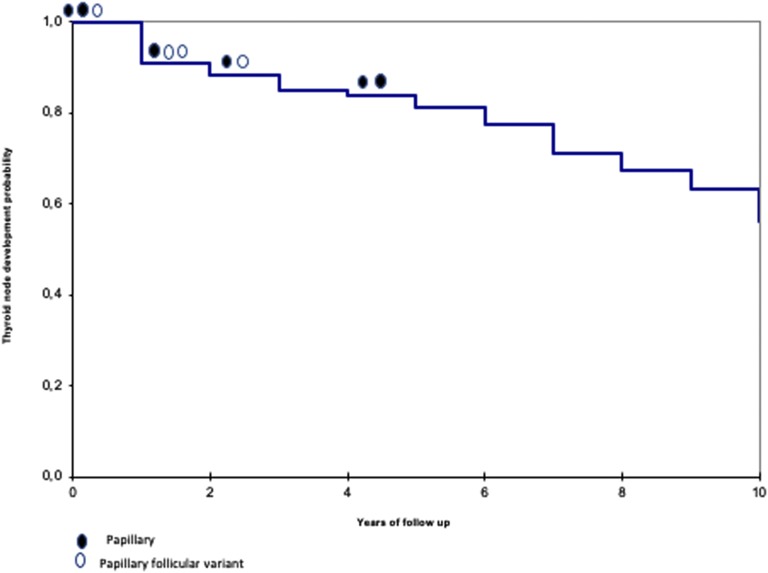
The size of the nodules remained stable during follow-up, with slight volume changes in both directions, independent from levothyroxine treatment. The incidence of thyroid nodularity over 10-year follow-up is shown in Figure 1, with a calculated annual rate of 3.5%. The probability of having a nodule is 9.3% at diagnosis, increasing to 43.9% after 10 years.
Figure 1.
Kaplan-Meier survival curve.
Cervical adenopathy by ultrasound at diagnosis of HT was observed at ultrasound in 183 subjects. Three children had already developed thyroid cancer at the time of the diagnosis of HT, yielding a frequency of 1.64% of cancer among patients with cervical adenopathy.
FNA was performed in 97 suspicious nodules. Three tumors (2 papillary and 1 papillary follicular variant) were identified at the time of diagnosis of HT by routine ultrasound. An additionalr 7 cases (3 papillary follicular variant and 4 papillary) were identified during follow-up. Specifically, 1 papillary and 2 papillary follicular variant were diagnosed after 1 year of follow- up, 1 papillary and 1 papillary follicular variant after 2 years, and 2 papillary after 4 years (Fig. 1). Altogether, 10 cases of cancers (5 females and 5 males) were detected. The clinical characteristics of these patients, ultrasound findings, morphological and cytological characteristics of the cancer, and TNM classification are reported in Table 2. All patients had hard, firm, and enlarged cervical lymph nodes, frequently with ultrasound abnormalities, at the time of diagnosis. Uninodularity was present in 6 patients. At ultrasound, the diameters ranged between 0.8 and 3 cm, with only one nodule <1 cm diameter. The nodules detected during follow-up showed progressive growth despite treatment with levothyroxine. Six patients were euthyroid, 3 had subclinical hypothyroidism, and 1 was hypothyroid. Among these patients, HT was diagnosed between 7.9 and 14 years of age.
Table 2.
Clinical Characteristics of the Patients with Cancer and Tumor Characteristics
| N | Gender (F/M) | Positive Family History (Y/N) | Age at Diagnosis of HT (Y) | Age at Diagnosis of the Nodules (Y) | Age at Diagnosis of the Cancer (Y) | Clinical Data at Diagnosis of Cancer |
Biochemical Data at Diagnosis | Ultrasound Data |
FNAB | Histology | TNM Classification | Additional Pathologies | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cervical Adenopathy (Y/N) | Signs of Local Compression (Y/N) | A: Euthyroidism; B: Subclinical hypothyroidism; C: Hypothyroidism; | Thyroid Nodularity: U: Uninodular; M: Multinodular | Largest Diameter of the Nodules at Diagnosis (cm) | Growth of the Nodule | ||||||||||
| A: None | |||||||||||||||
| B: While in Therapy; | |||||||||||||||
| C: Without Therapy | |||||||||||||||
| D: Appearance of the Nodule During Therapy | |||||||||||||||
| 1 | F | N | 14 | 14 | 14 | Y | N | A | U | 1.1 | A | TIR 5 | PTC | pT1bN1a M0 | Von Willebrand disease |
| 2 | F | N | 12.2 | 16.8 | 16.8 | Y | N | B | U | 1.3 | D | TIR 3b | PTC | pT1bN1a M0 | |
| 3 | F | Y | 12.5 | 12.5 | 12.5 | Y | Y | A | M | 2.1 | A | TIR 4 | PTCfollicular variant | pT3N1b M0 | |
| 4 | M | N | 12.8 | 12.8 | 13.9 | Y | N | C | U | 2.2 | A | TIR 4 | PTCfollicular variant | pT1bN1a M0 | |
| 5 | M | N | 12 | 13.9 | 16.1 | Y | N | B | M | 1.5 | D | TIR 4 | PTC | pT3aN1a M0 | Addison disease |
| 6 | F | Y | 12,3 | 12,3 | 13,4 | Y | Y | A | M | 3.0 | A | TIR 5 | PTCfollicular variant | pT3aN1a M0 | |
| 7 | M | N | 7.9 | 7.9 | 7,9 | Y | Y | A | U | 1.5 | A | TIR 5 | PTC | pT3aN1a M0 | |
| 8 | F | Y | 10.6 | 10.6 | 12.6 | Y | N | B | U | 1.0 | C | TIR 4 | PTCfollicular variant | pT2N1a M0 | |
| 9 | M | N | 11 | 11 | 11,6 | Y | N | A | U | 0,8 | D | TIR 5 | PTC | pT3N1aM0 | |
| 10 | F | Y | 11.7 | 11.7 | 13.6 | Y | N | A | M | 2.4 | B | TIR 3b | PTC | pT2N1a M0 | |
According to cytology, four cases were TIR 5, three cases TIR 4 and two were TIR 3b [TIR (1 to 5) is the acronym used by the Italian thyroid cytology classification system]. According to the TNM classification, three patients were pT1bN1aM0, two pT2N1aM0, one pT3N1bM0, three pT3aN1aM0, and one pT3N1aM0. All patients had cervical metastases and none had distal ones. All patients with a normal FNA were subsequently followed up; none developed cancer.
B. Kaplan-Meier Survival Analysis
Survival analysis (Table 3) showed that gender, celiac disease, T1DM, and family history for autoimmune disease did not influence the rate of appearance of new thyroid nodules. The incidence of new nodules was positively influenced by the TS (P < 0.001), TPOAb (P < 0.05), and treatment with levothyroxine (P < 0.05). Furthermore, a positive correlation was found between the development of cancer and TPOAb level (P < 0.01).
Table 3.
Thyroid Nodule Development
| Test of Equality Over Strata Log Rank Ttest | Stratified Linear Rank Tests Univariate χ2 for the Wilcoxon Test | |
|---|---|---|
| Gender | P = 0.3365 | |
| Celiac disease | P = 0.9941 | |
| T1DM | P = 0.6152 | |
| Family history | P = 0.1371 | |
| TS | P < 0.001 | |
| TSH | P = 0.8931 | |
| Free T4 | P = 0.0431 | |
| TPOAb | P = 0.0034 | |
| TGAb | P = 0.6279 |
Comparison of survival curves and rank tests for the associations with covariates.
3. Discussion
The aim of this study was to investigate the influence of HT on the development of thyroid nodules and/or thyroid cancer in a large cohort of children and adolescents. Our findings clearly show that HT influences the development of thyroid nodular pathology but not of cancer. These findings are comparable to those reported in adult subjects [37].
At the end of the follow-up period, the frequency of nodularity among the 904 patients with HT was 19.2%, a figure substantially higher than that reported in children without HT (0.2% to 5.1%) [15, 16]. This frequency is lower than that previously reported in another Italian study (31.3%) [24], but similar to the studies of Skarpa et al. [38] and Keskin et al. [39] who reported a prevalence of thyroid nodules in children and adolescent with HT of 14% and 13%, respectively.
In our study, the frequency of cancer within the thyroid nodules (5.7%) was similar to that reported by Rago et al. (6.1%) [21] in a large cohort of adolescents and adult patients, and by Keskin et al. (5.1%) [39] in a selected cohort of children and adolescents with HT. However, in other studies the risk of thyroid cancer within thyroid nodules was higher, being 9.6% in the cohort of Corrias et al. [24] and 9.4% in the study by Skarpa et al. [38].
In the current study, only 10 of 904 subjects (1.1%) developed thyroid cancer. The prevalence of cancer in pediatric patients affected by HT has generally been reported to be low, ranging from 0.67% in the study by Keskin et al. [39] to 3% in the study by Corrias et al. [24]. However, the reported prevalence is variable, and has been found to be as high as 58% of subjects with HT in a large Chinese cohort [40] Ethnic and /or environmental differences might account for these discrepancies. Other possibilities may also exist, such as the presence in the Chinese children of an immunoglobulin G4-positive HT, a condition strongly associated with papillary thyroid carcinoma (PTC) [41, 42], or an overexpression of the oncogenic RET/PTC rearrangement, a specific genetic alteration in patients with PTC that may also be present in HT [43]. Last, methodological issues as well as patient selection may also account for these differences.
The probability of having a thyroid nodule was 9.3% at diagnosis and increased to 43.9% after 10 years of follow-up, resulting in a calculated annual incidence of 3.5%. We found no influence of gender, celiac disease, T1DM, and family history of autoimmune disease on the onset of new nodules, whereas a positive influence was observed for the TS, TPOAb, and treatment with levothyroxine. Because the TS, TPOAb, and treatment are related to the morphological and functional impairment of the gland, we hypothesize that the inflammatory state might favor the onset of nodules but not the development of cancer.
High TSH levels have been correlated with an increased frequency of papillary cancer in adult patients with HT [44], but this correlation was not found in our cohort. This may be because of the small number of patients with cancer in our study. Similar to previous findings [20, 39, 45], all patients with cancer in our study had cervical lymphadenopathy. However, cervical lymphadenopathy is frequent in children with or without HT and was also present in a substantial number of noncancer patients with HT of our study. The presence of isolated or multiple nodules or thyroid function at diagnosis are also not predictive of cancer risk. All but one tumor had a diameter >1 cm, confirming that this threshold is a valid indication to FNA. Our data also confirm that FNA should always be performed for nodules <1 cm diameter when there is a suspicious ultrasound appearance or in the presence of risk factors such as extrathyroidal invasion, nodal metastasis, iodine deficiency, previous exposure to radiation, in cancer survivors, and in patients with genetic syndromes or with a family history of thyroid cancer [18, 46].
The major limitations of this study are its retrospective nature, the comparison of results obtained by different analytical methods, the multicenter selection of the patients and that there was no central review of the thyroid ultrasound, FNA and/or surgical pathology.
On the other hand, this study’s major strength is the large number of children followed up for a long period of time in the same centers of pediatric endocrinology using similar clinical approaches, which allowed meaningful comparison of the data. The presence of skilled and experienced pediatric ultrasonographers, and the adoption of a common echographic grading score likely resulted in homogeneous ultrasound data.
Overall, the results of our study are reassuring because we found a low incidence of thyroid cancer in our children and adolescents with HT, with no children having distant metastasis. Moreover, considering the slow growth and the relatively favorable prognosis of these tumors [47], we suggest that there is no need for frequent echographic evaluation (more than every other year) in children with HT without any other risk factors. Similarly, measurement of thyroid antibodies, which were used in this study as markers of thyroid inflammation, are not useful in the management of patients after the initial diagnosis.
Even though the relation between HT and thyroid cancer remains an open issue, our data suggest that in children and adolescents, HT is associated with an increased risk of developing thyroid nodules but not of thyroid cancer.
Acknowledgments
We thank Dr. Lan Guyen for revising the English version of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CV
coefficient of variation
- DTC
differentiated thyroid cancer
- FNA
fine-needle aspiration
- HT
Hashimoto thyroiditis
- PTC
papillary thyroid carcinoma
- T1DM
type 1 diabetes mellitus
- TgAb
thyroglobulin antibody
- TPOAb
thyroid peroxidase antibody
- TS
thyroid score
References and Notes
- 1. Cipolla C, Sandonato L, Graceffa G, Fricano S, Torcivia A, Vieni S, Latteri S, Latteri MA. Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg. 2005;71(10):874–878. [PubMed] [Google Scholar]
- 2. Kurukahvecioglu O, Taneri F, Yüksel O, Aydin A, Tezel E, Onuk E. Total thyroidectomy for the treatment of Hashimoto’s thyroiditis coexisting with papillary thyroid carcinoma. Adv Ther. 2007;24(3):510–516. [DOI] [PubMed] [Google Scholar]
- 3. Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204(5):764–-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradly DP, Reddy V, Prinz RA, Gattuso P. Incidental papillary carcinoma in patients treated surgically for benign thyroid diseases. Surgery. 2009;146(6):1099–1104. [DOI] [PubMed] [Google Scholar]
- 5. Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto’s thyroiditis. Eur Surg Res. 2010;45(3–4):333–337. [DOI] [PubMed] [Google Scholar]
- 6. Iliadou PK, Effraimidis G, Konstantinos M, Grigorios P, Mitsakis P, Patakiouta F, Pazaitou-Panayiotou K. Chronic lymphocytic thyroiditis is associated with invasive characteristics of differentiated thyroid carcinoma in children and adolescents. Eur J Endocrinol. 2016;174(2):X1. [DOI] [PubMed] [Google Scholar]
- 7. Fiore E, Rago T, Scutari M, Ugolini C, Proietti A, Di Coscio G, Provenzale MA, Berti P, Grasso L, Mariotti S, Pinchera A, Vitti P. Papillary thyroid cancer, although strongly associated with lymphocytic infiltration on histology, is only weakly predicted by serum thyroid auto-antibodies in patients with nodular thyroid diseases. J Endocrinol Invest. 2009;32(4):344–351. [DOI] [PubMed] [Google Scholar]
- 8. Matesa-Anić D, Matesa N, Dabelić N, Kusić Z. Coexistence of papillary carcinoma and Hashimoto’s thyroiditis. Acta Clin Croat. 2009;48(1):9–12. [PubMed] [Google Scholar]
- 9. Castagna MG, Belardini V, Memmo S, Maino F, Di Santo A, Toti P, Carli AF, Caruso G, Pacini F. Nodules in autoimmune thyroiditis are associated with increased risk of thyroid cancer in surgical series but not in cytological series: evidence for selection bias. J Clin Endocrinol Metab. 2014;99(9):3193–3198. [DOI] [PubMed] [Google Scholar]
- 10. Grani G, Calvanese A, Carbotta G, D’Alessandri M, Nesca A, Bianchini M, Del Sordo M, Vitale M, Fumarola A. Thyroid autoimmunity and risk of malignancy in thyroid nodules submitted to fine-needle aspiration cytology. Head Neck. 2015;37(2):260–264. [DOI] [PubMed] [Google Scholar]
- 11. Anil C, Goksel S, Gursoy A. Hashimoto’s thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid. 2010;20(6):601–606. [DOI] [PubMed] [Google Scholar]
- 12. Mazokopakis EE, Tzortzinis AA, Dalieraki-Ott EI, Tsartsalis AN, Syros PK, Karefilakis CM, Papadomanolaki MG, Starakis IK. Coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. A retrospective study. Hormones (Athens). 2010;9(4):312–317. [DOI] [PubMed] [Google Scholar]
- 13. Gul K, Dirikoc A, Kiyak G, Ersoy PE, Ugras NS, Ersoy R, Cakir B. The association between thyroid carcinoma and Hashimoto’s thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010;20(8):873–878. [DOI] [PubMed] [Google Scholar]
- 14. Rallison ML, Dobyns BM, Keating FR Jr, Rall JE, Tyler FH. Thyroid nodularity in children. JAMA. 1975;233(10):1069–1072. [PubMed] [Google Scholar]
- 15. Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, Leoli F, Rago T, Grasso L, Valeriano R, Balestrieri A, Pinchera A. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. J Clin Endocrinol Metab. 1999;84(2):561–566. [DOI] [PubMed] [Google Scholar]
- 16. Niedziela M. Thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2014;28(2):245–277. [DOI] [PubMed] [Google Scholar]
- 17. Avula S, Daneman A, Navarro OM, Moineddin R, Urbach S, Daneman D. Incidental thyroid abnormalities identified on neck US for non-thyroid disorders. Pediatr Radiol. 2010;40(11):1774–1780. [DOI] [PubMed] [Google Scholar]
- 18. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S; American Thyroid Association Guidelines Task Force . Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. 2006;13(2):427–453. [DOI] [PubMed] [Google Scholar]
- 20. Corrias A, Mussa A, Baronio F, Arrigo T, Salerno M, Segni M, Vigone MC, Gastaldi R, Zirilli G, Tuli G, Beccaria L, Iughetti L, Einaudi S, Weber G, De Luca F, Cassio A; Study Group for Thyroid Diseases of Italian Society for Pediatric Endocrinology and Diabetology (SIEDP/ISPED) . Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med. 2010;164(8):714–719. [DOI] [PubMed] [Google Scholar]
- 21. Rago T, Fiore E, Scutari M, Santini F, Di Coscio G, Romani R, Piaggi P, Ugolini C, Basolo F, Miccoli P, Pinchera A, Vitti P. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. 2010;162(4):763–770. [DOI] [PubMed] [Google Scholar]
- 22. Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, Wassner AJ, Smith JR, Marqusee E, Alexander EK, Barletta J, Doubilet PM, Peters HE, Webb S, Modi BP, Paltiel HJ, Kozakewich H, Cibas ES, Moore FD Jr, Shamberger RC, Larsen PR, Huang SA. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013;98(8):3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizzo M, Sindoni A, Talamo Rossi R, Bonaffini O, Panetta S, Scisca C, Altavilla G, Denaro L, Rosanò A, Saraceno G, Trimarchi F, Benvenga S. Annual increase in the frequency of papillary thyroid carcinoma as diagnosed by fine-needle aspiration at a cytology unit in Sicily. Hormones (Athens). 2013;12(1):46–57. [DOI] [PubMed] [Google Scholar]
- 24. Corrias A, Cassio A, Weber G, Mussa A, Wasniewska M, Rapa A, Gastaldi R, Einaudi S, Baronio F, Vigone MC, Messina MF, Bal M, Bona G, de Sanctis C; Study Group for Thyroid Diseases of Italian Society for Pediatric Endocrinology and Diabetology (SIEDP/ISPED) . Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med. 2008;162(6):526–531. [DOI] [PubMed] [Google Scholar]
- 25. Dermody S, Walls A, Harley EH Jr. Pediatric thyroid cancer: an update from the SEER database 2007–2012. Int J Pediatr Otorhinolaryngol. 2016;89:121–126. [DOI] [PubMed] [Google Scholar]
- 26. Ohira T, Takahashi H, Yasumura S, Ohtsuru A, Midorikawa S, Suzuki S, Fukushima T, Shimura H, Ishikawa T, Sakai A, Yamashita S, Tanigawa K, Ohto H, Abe M, Suzuki S; Fukushima Health Management Survey Group . Comparison of childhood thyroid cancer prevalence among 3 areas based on external radiation dose after the Fukushima Daiichi nuclear power plant accident: the Fukushima health management survey. Medicine (Baltimore). 2016;95(35):e4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kambalapalli M, Gupta A, Prasad UR, Francis GL. Ultrasound characteristics of the thyroid in children and adolescents with goiter: a single center experience. Thyroid. 2015;25(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vitti P, Martino E, Aghini-Lombardi F, Rago T, Antonangeli L, Maccherini D, Nanni P, Loviselli A, Balestrieri A, Araneo G.. Thyroid volume measurement by ultrasound in children as a tool for the assessment of mild iodine deficiency. J Clin Endocrinol Metab. 1994;79(2):600–603. [DOI] [PubMed] [Google Scholar]
- 29. Cesaretti G, Saggese G. Accurate ultrasonographic evaluation in autoimmune juvenile thyroiditis with subclinical hypothyroidism: a useful tool to establish the treatment. A retrospective study. Horm Res. 2003;60(Suppl 2): 107. [Google Scholar]
- 30. Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, Palombini L, Papini E, Zini M, Pontecorvi A, Vitti P. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest. 2014;37(6):593–599. [DOI] [PubMed] [Google Scholar]
- 31. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid. 2017;27(6):751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. RRID:AB_2756380.
- 33. RRID:AB_2756379.
- 34. RRID:AB_2756378.
- 35. RRID:AB_2756377.
- 36. Monzani A, Prodam F, Rapa A, Moia S, Agarla V, Bellone S, Bona G. Endocrine disorders in childhood and adolescence. Natural history of subclinical hypothyroidism in children and adolescents and potential effects of replacement therapy: a review. Eur J Endocrinol. 2012;168(1):R1–R11. [DOI] [PubMed] [Google Scholar]
- 37. Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98(2):474–482. [DOI] [PubMed] [Google Scholar]
- 38. Skarpa V, Kousta E, Tertipi A, Anyfandakis K, Vakaki M, Dolianiti M, Fotinou A, Papathanasiou A. Epidemiological characteristics of children with autoimmune thyroid disease. Hormones (Athens). 2011;10(3):207–214. [DOI] [PubMed] [Google Scholar]
- 39. Keskin M, Savas-Erdeve S, Aycan Z. Co-existence of thyroid nodule and thyroid cancer in children and adolescents with Hashimoto thyroiditis: a single-center study. Horm Res Paediatr. 2016;85(3):181–187. [DOI] [PubMed] [Google Scholar]
- 40. Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, Huang CP, Shen Q, Li DS, Wu Y. The clinical features of papillary thyroid cancer in Hashimoto’s thyroiditis patients from an area with a high prevalence of Hashimoto’s disease. BMC Cancer. 2012;12(1):610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taşli F, Ozkök G, Argon A, Ersöz D, Yağci A, Uslu A, Erkan N, Salman T, Vardar E. The role of IgG4 (+) plasma cells in the association of Hashimoto’s thyroiditis with papillary carcinoma. APMIS. 2014;122(12):1259–1265. [DOI] [PubMed] [Google Scholar]
- 42. Yu Y, Zhang J, Lu G, Li T, Zhang Y, Yu N, Gao Y, Gao Y, Guo X. Clinical Relationship between IgG4-positive Hashimoto’s thyroiditis and papillary thyroid carcinoma. J Clin Endocrinol Metab. 2016;101(4):1516–1524. [DOI] [PubMed] [Google Scholar]
- 43. Arif S, Blanes A, Diaz-Cano SJ. Hashimoto’s thyroiditis shares features with early papillary thyroid carcinoma. Histopathology. 2002;41(4):357–362. [DOI] [PubMed] [Google Scholar]
- 44. Fiore E, Rago T, Latrofa F, Provenzale MA, Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L, Pinchera A, Vitti P. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocr Relat Cancer. 2011;18(4):429–437. [DOI] [PubMed] [Google Scholar]
- 45. Mussa A, De Andrea M, Motta M, Mormile A, Palestini N, Corrias A. Predictors of malignancy in children with thyroid nodules. J Pediatr. 2015;167(4):886–892. [DOI] [PubMed] [Google Scholar]
- 46. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375(11):1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pazaitou-Panayiotou K, Kaprara A, Boudina M, Georgiou E, Drimonitis A, Vainas I, Raptou E, Galaktidou G. Thyroid carcinoma in children and adolescents: presentation, clinical course, and outcome of therapy in 23 children and adolescents in Northern Greece. Hormones (Athens). 2005;4(4):213–220. [DOI] [PubMed] [Google Scholar]