Abstract
Growing evidence supports an important role for the intrauterine environment in shaping fetal development and subsequent child health and disease risk. The fetal brain is particularly plastic, whereby even subtle changes in structure and function produced by in utero conditions can have long-term implications. Based on the consideration that conditions related to energy substrate and likelihood of survival to reproductive age are particularly salient drivers of fetal programming, maternal nutrition and stress represent the most commonly, but independently, studied factors in this context. However, the effects of maternal nutrition and stress are context dependent and may be moderated by one another. Studies examining the effects of the bidirectional nutrition-stress interplay in pregnancy on fetal programming of brain development are beginning to emerge in the literature. This review incorporates all currently available animal and human studies of this interplay and provides a synthesis and critical discussion of findings. Nine of the 10 studies included here assessed nutrition–stress interactions and offspring neurodevelopmental or brain development outcomes. Despite significant heterogeneity in study design and methodology, two broad patterns of results emerge to suggest that the effects of prenatal stress on various aspects of brain development may be mitigated by 1) higher fat diets or increased intake and/or status of specific dietary fats and 2) higher dietary intake or supplementation of targeted nutrients. The limitations of these studies are discussed, and recommendations are provided for future research to expand on this important area of fetal programming of brain development.
Keywords: Brain development, Fetal programming, Neurodevelopment, Nutrition, Pregnancy, Stress
The concept of fetal programming describes the process whereby the fetus senses, receives, and responds to (or is acted on by) the intrauterine environment. During sensitive periods of development, fetal programming can produce structural and functional changes in cells, tissues, and organ systems that may independently, or through interactions with subsequent developmental processes and environments, confer critical long-term consequences for future health and disease susceptibility (1–5). The developing brain is particularly plastic and thus sensitive to fetal programming effects because the vast majority of differentiation of major brain structures occurs during prenatal life, orchestrated by a cascade of bidirectional interactions occurring between the maternal and fetal compartments and the external environment (6,7). Given the brain’s protracted period of development (from embryonic life through birth and extending to adolescence), even small or subtle alterations in brain structure and function during embryonic and fetal life can become progressively and substantially magnified over time, exerting long-term implications for brain anatomy and connectivity and, consequently, mental as well as physiological health (8,9). These effects of the prenatal environment may be further modified by the early postnatal environment (10,11) and may differ as a function of offspring sex (9,12,13).
Maternal stress and nutrition during pregnancy are two of the most commonly studied factors in the context of fetal programming of brain development; however, the vast majority of these studies have considered only their independent effects (14). During fetal development, adequate energy and protein supply, essential fatty acids, and various key micro-nutrients are required to supply the necessary substrates for fetal tissue synthesis within the central nervous system and as cofactors in biochemical processes that coordinate normal brain development (15,16). In animal studies, the offspring of undernourished rat dams showed impaired neurogenesis and neuronal functionality, disorganization of feeding pathways, altered glucose sensing, and leptin and insulin resistance (17). In humans, extreme cases of nutritional deprivation during pregnancy, such as in times of famine, have provided insight into the protracted impact of malnutrition on brain development (18). For example, middle-aged offspring of mothers exposed to undernutrition during the Dutch and Chinese famines were found to exhibit reduced cognitive capabilities (19,20). Specific nutrient deficiencies or dietary imbalances may also produce adverse neurodevelopmental effects, such as neural tube defects (21), language delay (22,23), reduced cognitive abilities (24), and mental and neurodevelopmental disorders (25,26). Various human studies have linked exposure of pregnant women to a range of different stressors with higher risk for neurodevelopmental disorders, affective disorders, and reduced cognitive ability in their children (27–29). Experimental animal and observational human pregnancy studies have also demonstrated that maternal anxiety and/or depression may alter offspring brain anatomy (30,31) and predict increased risk for offspring cognitive impairment (32), neurodevelopmental disorders (33–35), and mental illness (35). Furthermore, higher levels of maternal stress hormones (e.g., cortisol) (12,36) and other biological stress mediators (e.g., interleukin-6) (37) across gestation are associated with alterations in offspring brain structure, connectivity, and behavioral problems. It is notable that these separate but equally important prenatal factors that have the potential to alter the trajectory of fetal brain development have, until recently, been almost entirely studied in isolation.
EVIDENCE FOR A BIDIRECTIONAL RELATIONSHIP BETWEEN NUTRITION AND STRESS
Evidence from studies of nonpregnant humans strongly supports the presence of a bidirectional relationship between nutrition and stress (14,38–40). For example, psychological stress can affect nutritional status by influencing hunger and/or satiety, the amount of and the type of foods consumed (41), digestive processes (42), and metabolic response to ingested food (43–45). On one hand, stress typically induces a preference for a high-fat and high-sucrose (HFS) diet, which can dampen the cortisol stress response, giving rise to a state of emotional eating (46,47) as well as susceptibility for weight gain and metabolic dysfunction (40,47). On the other hand, the interaction of stress and high-fat meal consumption has been demonstrated to induce a proinflammatory response in women(48), which in the context of pregnancy could increase the susceptibility of the offspring for neurodevelopmental disorders owing to exposure of the developing fetal brain to excessive cytokine levels (8,49). It is also recognized that specific nutrients play a critical role in modulating mood, stress, and development of psychological disorders. Polyunsaturated fatty acids (PUFAs) have received particular attention in this regard owing to their multiple roles in brain function, including modification of membrane fluidity, membrane enzyme activity, the number and affinity of receptors, the function of neuronal membrane ionic channels, and the production of neurotransmitters and ionic peptides (50). In particular, the long-chain omega-3 (n-3) docosahexanoic acid (DHA) has a high concentration in phospholipids of neural cells (51), and its incorporation in the brain occurs almost exclusively in prenatal and early postnatal life (52). The proportion of DHA in brain cell membranes modulates neurotransmission and neuroinflammation, which are key processes in cognition and mood (53,54).
Deficiencies in various micronutrients, including B-complex vitamins, vitamin D, zinc, iron, chromium, and iodine, have also been associated with stress-related disorders, such as depression, anxiety, and other neuropsychiatric disorders (55). The mechanisms underlying the associations of these disorders with micronutrients typically involve interactions with stress hormones and stress-related elevations in proinflammatory cytokines and neurotransmitters. For example, iron is required for myelination and neurotransmitter synthesis during neurodevelopment, but its bioavailability to the fetus during pregnancy may be affected by maternal stress levels, mediated by hepcidin (56). Hepcidin is a critical iron-regulatory hormone that responds to body iron status and inflammation to alter intestinal iron absorption and its distribution across the body’s tissues. On exposure to stressors (e.g., infection, inflammation, and, potentially, psychosocial stress), hepcidin action is modulated to decrease iron availability to invading pathogens, which concomitantly restricts iron supply to red blood cell precursors, contributing to the development of anemia (57).
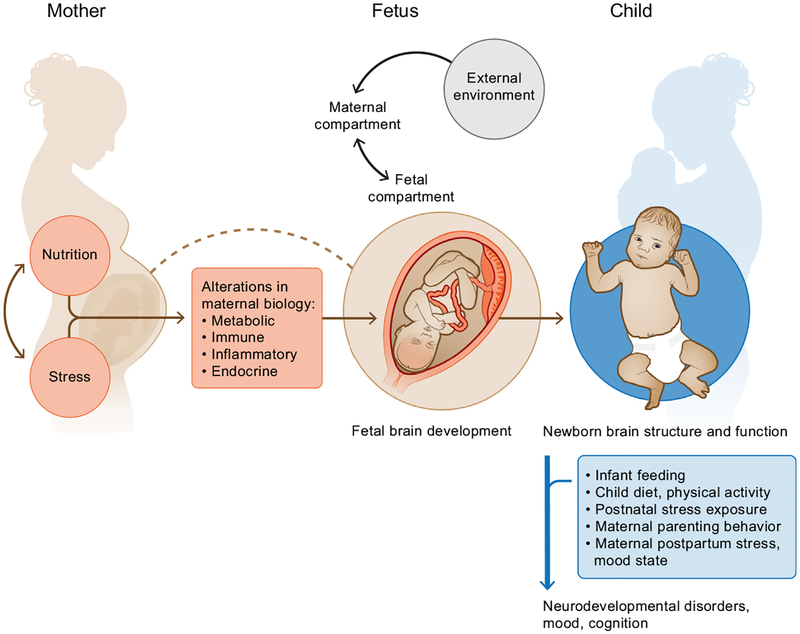
Given the overlapping outcomes in offspring brain development described separately in studies of prenatal nutrition and prenatal stress exposures and the evidence for nutrition–stress interactions in the nonpregnant state and during pregnancy (14,58–62), these prenatal processes warrant concurrent examination in the context of fetal programming. Some perspectives articles in recent years have outlined arguments for this notion (14,59,61), and subsequently several experimental animal and observational human studies have empirically addressed this issue with respect to various brain development–related outcomes in offspring. Alterations in metabolic, endocrine, and inflammatory processes likely represent mechanistic pathways underlying these associations (Figure 1) (14,63,64). The goal of this article is to review and critically discuss the currently available literature from animal and human studies on the topic of prenatal nutrition–stress interactions with implications for fetal programming of brain development. We highlight limitations of study design and methodology in the existing studies on this topic, which limit our ability to draw firm conclusions, and provide guidance for future studies to develop and improve this field of research.
Figure 1.
Interactive effects of prenatal nutrition and stress on fetal programming of offspring brain and neurodevelopmental outcomes.
CHARACTERISTICS OF INCLUDED STUDIES
A detailed description of our literature search methodology is included in the Supplement. We identified and included in this review 10 studies that directly addressed some aspect of prenatal or perinatal nutrition–stress interactions with implications for fetal programming of neurodevelopmental outcomes. The studies included seven experimental studies conducted in rodents (mice or rats) (65–71) and three observational studies in humans (72–74). An overview of the characteristics, methodology, and findings from these studies is presented in Table 1.
Table 1.
Overview of Characteristics, Methodology, and Results From Studies of Prenatal Nutrition-Stress Interactions and Outcomes Related to Offspring Brain Development
| Reference and Study Population | Results | Perinatal Stress | Perinatal Nutrition | Offspring Brain Development Outcomes With Behavioral Implications | Stress × Diet Interaction Conclusions | Notes/Limitations |
|---|---|---|---|---|---|---|
| Animal Studies | ||||||
| Borsonelo et al., 2011 (65). Pregnant Wistar rats (n = 42) and male adult offspring (n = 49). | Main effects:
Interaction effects:
|
PNS group: Between gestational days 14 and 20, dams exposed to 3 daily sessions of physical restraint and bright light. Prenatal control group (non-PNS): Throughout gestation, pregnant dams left undisturbed with normal light/dark cycle. |
Time frame delivered: Preconception, throughout pregnancy, and lactation. Assignment: PNS and non-PNS group divided into 3 diet subgroups. Diet groups: a) Regular, b) fish oil supplemented, c) coconut fat supplemented. |
Offspring sex: Males only. Age at assessment: 90 days. Outcomes assessed:
|
Coconut fat and fish oil diets equally ameliorated corticosterone response to a stress test among adult offspring of non-PNS rats. Conflicting main effects of fish oil diet on depressive behavior may be attributed to differential action on serotonergic vs. noradrenergic systems. |
Only male offspring included. |
| Huang et al., 2015 (66). Pregnant Sprague Dawley rats (n = 32). | Main effects:
Interactive effects (HFD+LPS):
|
PNS group (LPS): Gestational days 8, 10, and 12, dams received intraperitoneal injection of LPS (0.4 mg/kg). Prenatal control group: No LPS in pregnancy. |
Time frame delivered: Throughout pregnancy and lactation. Assignment: LPS and control group divided into 2 diet subgroups. Diet groups: a) HFD (normal diet plus added 10% lard oil, 5% cholesterin, 1.5% bile salt, 10 egg yolks/kg, 0.14 kg milk powder/kg, some sugar and trace elements), b) regular diet. |
Offspring sex: Males and females; analysis not stratified by sex. Age at assessment: 90 days. Outcomes assessed:
|
Unexpectedly, interaction of perinatal LPS+HFD in rats exerted a beneficial effect on offspring hippocampal development. This may represent a predictive adaptive response to prenatal inflammation. | Biological stress exposure (LPS)-not directly assessing psychological stress. Did not stratify analysis by offspring sex. |
| Naninck et al. 2017 (71). Newborn male C57BL/6 mice. | Main effects:
Interactive effects:
|
Postnatal stress group: Postnatal days 2–9, pups had limited nesting and bedding material. Postnatal control group: Pups had standard nesting and bedding material. |
Time frame delivered: Postnatal days 2–9 to lactating dams. Assignment: Mothers of pups in each postnatal stress or control group were divided into 2 diet subgroups. Postnatal diet groups: a) Regular diet supplemented with 1-CMAM, b) regular diet. |
Offspring sex: Males only. Age at assessment: 9–265 days postnatal. Outcomes assessed:
|
Replenishing peripheral and central methionine levels through 1-CMAM supplementation may prevent early-life stress impairments in cognitive function. These effects may be mediated by prevention of stress-induced hyperactivation of HPA axis, rather than direct effects on hippocampal volume, neurogenesis, or epigenetic alterations. | Male mice only. Stress and diet interventions introduced in postnatal period, and therefore prenatal programming effects were not assessed. |
| Paternain et al., 2013 (70). Pregnant Wistar rats (n = 22). | Main effects:
Interactive effects:
|
PNS group: Gestational days 14–22, dams underwent variable stress paradigm consisting of restraint, wet bedding, forced swim, noise, overnight light. Prenatal control group (non-PNS): No stress during pregnancy. |
Time frame delivered: Postnatal weeks 8–18 (pups only). Assignment: Pups of mothers from PNS and non-PNS groups divided into 2 diet subgroups. Diet groups: a) HFS diet (20% protein, 35% carbohydrate, of which 17% was sucrose, and 45% fat), b) regular diet. |
Offspring sex: Females only. Age at assessment: Adults—age not specified. Outcomes assessed: Expression and methylation of hypothalamic genes related to a) reward system (dopamine active transporter [Slc6a3]); b) food intake (proopiomelanocortin [Pomc], neuropeptide Y [Npy]); c) HPA axis function (corticotropin-releasing hormone [Crh], glucocorticoid receptor [Gr]). |
PNS ameliorates hypermethylation effects of poor postnatal diet in a key gene regulating food intake (Pomc). | Female offspring only included—gene expression could be affected by estrous cycle stage. HFS diet was given postnatally only. |
| Rincel et al., 2016 (69). Pregnant Wistar rats (n = 76). | Main effects:
Interactive effects:
|
Assignment: Pups from each prenatal diet group were divided into stress or nonstress subgroups. Postnatal stress group: On postnatal days 2–14, pups had daily maternal separation sessions of 180 min. Postnatal nonstress group: Pups remained undisturbed with dams. |
Time frame delivered: Throughout pregnancy and lactation. Diet groups: a) High-fat diet (40% energy from fat), b) regular diet (12% energy from fat). |
Offspring sex: Males and females; analysis not stratified by sex. Age at assessment: 11 days to 8 mo postnatal. Outcomes assessed:
|
Prenatal HFD exerted protective effect by ameliorating stress-induced endophenotypes in adulthood (anxiety, social behavior, spatial memory, HPA axis response to stress) and preventing adverse neurodevelopmental gene expression alterations in response to early-life stress. |
Stress introduced postnatally only and therefore not concurrent with prenatal diet intervention. Did not stratify analysis by offspring sex. |
| Schulz et al., 2014 (68). Pregnant Sprague Dawley rats (n = 24). | Main effects:
Interactive effects:
|
PNS group: Gestational days 14–21, dams underwent a variable stress paradigm 2–3 times/day (restraint, forced swim, social stress, overnight fast, exposure to loud radio static, transport on a noisy cart). Prenatal control group (non-PNS): No stress during pregnancy. |
Time frame delivered: Throughout pregnancy and lactation. Assignment: PNS and non-PNS group divided into 2 diet subgroups. Diet groups: a) Choline supplemented diet (5 g/kg of choline chloride), b) regular diet (1 g/kg of choline chloride). |
Offspring sex: Males and females; analysis stratified by sex. Age at assessment: Postnatal days 79–106. Outcomes assessed: Anxiety-related behavior among adult offspring in a) open field test (exploration of field center; age 79 days), b) elevated zero maze (frequency, duration of visits to open arms; age 98 days), c) social interaction tests (duration of sniffing other rats; age 106 days). |
Sex-specific effects detected: In female offspring, perinatal choline supplementation ameliorates anxiogenic effects of PNS. In male offspring, prenatal choline mitigates deficits in social interaction behaviors induced by PNS. | |
| Yajima et al., 2013 (67). Pregnant C57BL/6J Jms Slc mice. | Main effects:
Interactive effects:
|
PNS group: From late pregnancy and throughout lactation, dams exposed to 24-hour constant light exposure. Prenatal control group (non-PNS): Throughout pregnancy and lactation, dams had regular dark/light cycle (10/14 hours). |
Time frame delivered: Late pregnancy and through lactation. Assignment: PNS and non-PNS groups divided into 2 diet subgroups. Diet groups: a) 0.2% lutein-supplemented diet, b) soybean oil supplemented diet (control). |
Offspring sex: Males and females; analysis not stratified by sex. Age at assessment: 15 days and 63 days postnatal. Outcomes assessed:
|
Perinatal lutein supplementation ameliorates anxiogenic effects of PNS in adult offspring subjected to stress. An unexpected finding was that in offspring without PNS, perinatal lutein reduced 5HTT levels in the adult brain in response to stress. |
Did not stratify analysis by offspring sex. |
| Human Studies | ||||||
| Barker et al., 2013 (74). N = 6979 mother-offspring dyads participating in ALSPAC in United Kingdom. | Main effects: Prenatal depression associated with:
Mediation effects:
|
PNS predictor: Depressive symptoms. Assessment tool: Edinburgh Postnatal Depression Scale. Time of assessment: 32 weeks’ gestation and multiple times postnatally (8 weeks, 8 mo, 21 mo, 33 mo). |
Prenatal diet predictor: Healthy or unhealthy diet patterns. Assessment tool: Food frequency questionnaire plus factor analysis. Times of assessment: 32 weeks’ gestation and at postnatal age 4 years. |
Offspring sex: Males and females; analysis not stratified by sex. Age at assessment: 8 years. Outcomes assessed: Cognitive function assessment tool: Verbal and IQ components of WISC-III. |
Not assessed. | Mediation analysis using only latent path models; moderation effects of nutrition on prenatal depression not assessed. |
| Brunst et al., 2014 (73). N = 255 mother-offspring dyads, primarily minorities (21% black, 42% Hispanic) from urban setting in U.S. | Main effects:
Interactive effects:
|
PNS predictor: Maternal report of NLEs occurring in past 6 months. Assessment tool: Crisis in Family Systems Revised survey. Time of assessment: On enrollment (first and second trimesters). |
Prenatal diet predictor: Ratio of n-3:n-6 PUFAs in diet. Assessment tool: Food frequency questionnaire reflecting 3 months before pregnancy. Time of assessment: On enrollment (first and second trimesters). |
Offspring sex: Males and females; infant sex included as covariate. Age at assessment: 6 months. Outcomes assessed: Subscales of infant temperament—Extraversion, Orienting and Regulation, Negative Affectivity. Assessment tool: IBQ-R. |
Among blacks, higher ratio of n-3:n-6 in preconception diet attenuated effect of increasing maternal NLEs on infant orienting and regulation score. | Reporting bias in maternal self-report of child behavior in IBQ-R. Recall bias in reporting of past dietary intake and stressful life events. |
| Lipton et al., 2017 (72). N = 137 mother-offspring dyads from an urban setting in U.S. | Main effects:
Interactive effects:
|
As above for Brunst et al. (73) | Prenatal diet predictor: Antioxidant intake from diet plus supplements; vitamins A, C, and E, magnesium, zinc, selenium, (β-carotene. Assessment tool: Food frequency questionnaire reflecting 3 months before pregnancy. Time of assessment: On enrollment (first and second trimesters). |
Offspring sex: Males and females; infant sex included as covariate. Age at assessment: 30 months. Outcomes assessed: Subscales of infant temperament—Surgency/Extraversion, Effortful Control, Negative Affectivity. Assessment tool: Early Childhood Behavior Questionnaire-Very Short. |
Poor preconception intake of antioxidants exacerbates effect of PNS on child negative affectivity. | Reporting bias in maternal self-report of child behavior in IBQ-R. Recall bias in reporting of past dietary intake and negative life events. |
ALSPAC, Avon Longitudinal Study of Parents and Children; 1-CMAM, 1-carbon metabolism–associated micronutrients; GFAP, glial fibrillary acidic protein; HFD, high-fat diet; HFS, high-fat and high-sucrose; HPA, hypothalamic-pituitary-adrenal; IBQ-R, Infant Behavior Questionnaire–Revised; IL-6, interleukin-6; LPS, lipopolysaccharide; mRNA, messenger RNA; NLEs, negative life events; PNS, prenatal stress group; PUFAs, polyunsaturated fatty acids; SYP, synaptophysin; TNFa, tumor necrosis factor alpha; WISC-III, Wechsler Intelligence Scale for Children-III.
Among the animal studies, there were some notable variations in the timing of the introduction of stress and nutrition manipulations, which are summarized in Table 2. We included one study with a concurrent postnatal diet and stress intervention (71) because the early postnatal period (days 1–10) in rats approximately corresponds to the third trimester in human brain development (75).
Table 2.
Distribution of Timing of Nutrition and Stress Interventions Across Prenatal and Early Postnatal Periods Among Animal Studies
| Reference | Prenatal Nutrition and Stress Intervention | Prenatal Nutrition and Postnatal Stress Intervention | Prenatal Stress and Postnatal Nutrition Intervention | Postnatal Nutrition and Stress Intervention | Notes |
|---|---|---|---|---|---|
| Borsonelo et al., 2011 (65) | X | ||||
| Huang et al., 2015 (66) | X | ||||
| Naninck et al. 2017 (71) | X | ||||
| Paternain et al., 2013 (70) | X | Offspring assigned to diet intervention after weaning | |||
| Rincel et al., 2016 (69) | X | Offspring pups assigned to stress intervention | |||
| Schulz et al., 2014 (68) | X | ||||
| Yajima et al., 2013 (67) | X |
Among the animal studies that induced stress prenatally, there were few similarities in the content of the stress paradigms used. Two studies used a variable stress paradigm that included partial overlap in the components (e.g., physical restraint, forced swimming, and loud noise exposure) (68,70). Other studies used only one or two prenatal or postnatal stress components, such as constant light exposure (65,67), wet bedding (71), or maternal-pup separation (69), whereas another study administered lipopolysaccharide as a biological stress exposure (66). The perinatal dietary assignments were also highly variable across studies. Two studies examined the effects of a perinatal high-fat diet (HFD) (66,69), a third study administered a test diet high in both fat and sucrose (HFS diet)(70), and a fourth study compared different types of dietary fat compositions with a regular chow rodent diet (65). The remaining three studies supplemented specific micronutrients to a regular diet (67,68,71). We note that the compositions of HFDs between studies varied in terms of quantity and sources of dietary fats, whereas the composition of the regular diet was not described in several studies.
Lastly, there was considerable variation across animal studies with respect to offspring follow-up assessments of brain and behavioral outcomes. Most of the studies evaluated behavioral or cognitive outcomes in the adult offspring, and some studies additionally studied various genetic, epigenetic, or brain morphology outcomes that have implications for neuro-developmental phenotypes. Two studies measured biomarkers of metabolism (glucose, insulin, leptin, satiety hormones) in the offspring (69,70) and one study measured inflammatory cytokines (interleukin-6 and tumor necrosis factor alpha) in the stressed dams (66) to investigate the role of metabolic and in-flammatory pathways in mediating the effects of nutrition–stress interactions on brain outcomes. With regard to sex specificity, only one study stratified its analyses by offspring sex (68), two studies examined outcomes in only male offspring (65,71), and one study examined outcomes in only female offspring (70).
The literature on this topic in humans is particularly sparse; two of the studies originated from the same parent-child cohort (72,73), and the third study did not directly assess prenatal nutrition–stress interaction effects but employed a pathway analysis approach to examine mediating effects of maternal diet on prenatal stress (74). Each of these studies concurrently evaluated some aspect of prenatal nutrition and stress exposure and/or state as predictors of child neurodevelopmental outcomes, but the assessment was reliant on maternal self-report and administered at only a single but variable time point during pregnancy. The cohort described in the studies by Brunst et al. (73) and Lipton et al. (72) administered a food frequency questionnaire on enrollment to reflect dietary intake 3 months before pregnancy, whereas Barker et al. (74) assessed prenatal dietary intake in the third trimester. Brunst et al. (73) and Lipton et al. (72) used maternal experience of recent negative life events as the psychological predictor of interest but did not measure affective state. However, these studies did consider infant sex and race/ethnicity as covariates in their analysis.
SUMMARY OF RESULTS FROM THE INCLUDED STUDIES
For each study included in this review, Table 1 presents a description of the main effects of prenatal nutrition and/or stress and the results of effects of nutrition–stress interactions on offspring brain development. Among the nine studies that directly assessed a prenatal nutrition–stress interaction, each study reported a significant result for an interactive effect on at least one outcome related to offspring brain development. Despite variation in study design, predictor variables, and method and timing of outcome assessments, two broad patterns of results for effects of prenatal nutrition–stress interactions appear to emerge, as follows:
The quantity and/or quality of dietary fat in the prenatal diet interacts with prenatal or perinatal stress exposure to exert protective effects on offspring brain development. Contrary to expectations, evidence from three animal studies suggests that a prenatal HFD or fat-supplemented diet may exert a protective effect on various aspects of offspring brain development and affective response to a postnatal stress test (65,66,69), although there is inconsistent evidence as to whether this beneficial effect of dietary fat is enhanced or diminished by the presence of a prenatal stress exposure. Specifically, Huang et al. (66) and Rincel et al. (69) noted that the offspring of prenatally stressed rats had improved brain development outcomes if fed an HFD throughout pregnancy and lactation compared with a regular diet. Borsonelo et al. (65) reported that two types of fat-supplemented diets (one high in long-chain [LC] PUFAs and the other high in saturated fat) exerted more favorable effects than the regular diet by ameliorating corticosterone levels in the offspring (males only) when subjected to experimental stress tests, but only in the offspring not exposed to prenatal stress. Finally, in a human study, the results of Brunst et al. (73) suggested that the fatty acid profile of the prenatal diet may interact with prenatal stress exposure to affect a child’s neurodevelopment. A low n-3:n-6 ratio in the prenatal diet combined with high prenatal stress resulted in a lower score for orientation and regulation at age 6 months, a measure of infant attention and concentration, but only among the children of black women.
Prenatal or perinatal dietary supplementation with antioxidant or 1-carbon metabolism–associated nutrients may ameliorate the anxiogenic effects of perinatal stress in the adult offspring. Evidence from three animal studies indicate protective effects of targeted nutrient supplementation protocols on behavioral and brain development outcomes following prenatal or perinatal stress exposure (67,68,71). Two of these studies examined the effects of specific individual nutrients added to the regular maternal diet: 1) the water-soluble nutrient choline (68), which plays critical roles during fetal development in the biosynthesis of cell membranes, neurotransmitters, and nucleic acids as well as cellular signaling and replication (76,77), and 2) the phyto-nutrient lutein (67), an antioxidant compound from the carotenoid family that is particularly concentrated in the infant brain (78) and thought to play important roles in developing neural connectivity and cognitive function (79). Meanwhile, Naninck et al. (71) administered a multinutrient supplement containing folate, vitamins B6 and B12, zinc, methionine, betaine, and choline (male offspring only), all of which are involved in the 1-carbon metabolism cycle, a biochemical process critical for cellular replication and biosynthesis of proteins, phospholipids, and neurotransmitters. These studies reported beneficial effects of the nutrient supplementation in reducing the anxiogenic (67,68) and memory impairment (71) effects of prenatal or early postnatal stress as well as improvements in hypothalamic-pituitary-adrenal (HPA) axis function (71). Furthermore, prenatal dietary insufficiency of key antioxidant micro-nutrients in a human prenatal cohort was found to exacerbate the effects of prenatal stress on offspring affective behavior (72), thus supporting the interactive effects observed in the experimental animal studies.
The results of one animal study examining a perinatal nutrition–stress interaction do not fit with either of the above patterns of results. Paternain et al. (70) delivered an early postnatal HFS diet, which differs from the standard HFD whereby the carbohydrate content (including sugars) is kept constant. In contrast to the animal studies described above in which the fat content of the diet alone was manipulated, this study found that the HFS diet exerted negative effects on expression and methylation of genes related to brain function (Slc6a3 and Pomc) in the offspring (females only studied), and, unexpectedly, these adverse outcomes were ameliorated by prenatal exposure to stress despite worsening glucose homeostasis in the combined HFS diet/prenatal stress group. The single study that did not apply statistical analysis to test effects of nutrition–stress interactions on offspring brain development outcomes, but rather conducted a mediation analysis, also reported significant results (74). The pathway analysis model demonstrated that a broadly “unhealthy” prenatal and postnatal dietary pattern mediated the adverse effects of prenatal maternal depression on child cognitive function at 8 years of age.
Only Schulz et al. (68) stratified their analysis of effects of nutrition–stress interactions on brain development outcomes by offspring sex. This study found that prenatal dietary choline supplementation ameliorated the prenatal stress–induced anxiogenic behavior in the female adult offspring under experimental stress conditions (elevated maze test), whereas the anxiogenic effects of the prenatal choline supplement in male offspring was apparent only under test conditions of social interaction (duration of sniffing). Although the human studies included child sex as a covariate in analyses (72,73), we cannot infer from the presentation of their results whether the significant effects of the prenatal nutrition–stress interactions may have been influenced by child sex.
DISCUSSION
It is evident from the studies included in this review that the currently available literature examining the effects of prenatal nutrition–stress interactions on offspring brain development is limited in size and highly heterogeneous in nature. Significant heterogeneity was noted in study design, prenatal nutrition component of interest, characterization of prenatal stress, brain development outcomes assessed, timing of prenatal exposures and of postnatal follow-up, and consideration of sex differences. Despite these variations, all studies indicate that some degree of an effect of prenatal nutrition–stress interaction on fetal programming exists for brain development, highlighting the importance of considering such interactions in future studies. However, the current evidence is insufficient to conclude that one particular diet or nutrient could be beneficial to ameliorate the adverse effects of prenatal stress on brain or neurodevelopmental outcomes in the offspring, and replication studies are required.
The mammalian brain is primarily composed of lipid (60% by weight), consisting of a unique profile of essential LC PUFAs, which cannot be endogenously synthesized (16). The fat-soluble vitamins A, D, E, and K also play key roles in neural patterning and differentiation, cell signaling, growth factor signaling, neuronal and glial population dynamics, and biosynthesis of sphingolipids in brain cellular membranes (80), and these vitamins require dietary fat for their absorption. Thus, adequate maternal intake of dietary fat throughout pregnancy is critical to support normal neurological development of the fetus. DHA, an n-3 LC PUFA, is the fatty acid of highest concentration in the fetal and neonatal brain (81), and decreased DHA is seen in the brain of animals fed an n-3-deficient diet during development, accompanied by alterations in neurotransmitter metabolism and membrane-associated enzyme and receptor activities (82).
However, studies researching the potential detrimental effects of a prenatal HFD in animals on fetal programming of the developing brain are based on the premise that a rodent HFD resembles a junk food diet in humans, which induces a disturbed metabolic milieu indicative of the obese phenotype (83). Indeed, various animal studies have reported behavioral disorders and adverse brain development outcomes in the offspring prenatally exposed to the HFD (84–86). Suggested mechanisms include neuroinflammation, oxidative stress, dysregulated insulin, glucose and leptin signaling, dysregulated serotonergic or dopaminergic systems, and perturbations to synaptic plasticity (86,87). However, most animal studies employing HFD models do not adequately describe the nutritional composition or the dietary sources of fat in either the control or the experimental diets. Standard rodent HFDs typically contain a combination of added saturated, monounsaturated, and polyunsaturated fats, with a total fat content that can be far higher (up to 60% energy from fat) than would ever normally be seen in a junk food–rich human diet (35%–40% fat with a concomitant high dietary intake of sugar and refined carbohydrates) (83). Thus, extrapolation of results from animal diet models and interpretation in the context of human diets should be performed with caution (83).
In the study by Borsonelo et al. (65), both types of fat-supplemented diets (high saturated fat vs. high LC PUFAs) ameliorated the biological stress response (plasma corticosterone) compared with standard diet in offspring subjected to a stress test, but only among offspring not exposed to prenatal stress. Thus, we conclude that the prenatal fat-supplemented diets appear to maintain HPA axis integrity under stressful conditions, possibly through an optimal supply of essential fatty acids during brain development, but these protective effects are not sufficient to override the adverse fetal programming effects of prenatal stress on neurodevelopment. Meanwhile, in the human study by Brunst et al. (73), the lower neurodevelopmental scores detected among children born to black women with a combination of high stress and low dietary n-3:n-6 ratio may be indicative of increased physiological stress susceptibility among this subgroup, arising from a disproportionately high exposure to chronic life stress and social disadvantage, the fetal programming effects of which may be exacerbated by an n-3-insufficient diet (88,89).
The apparent beneficial effect of an HFD in mitigating the adverse effects of prenatal stress exposure on offspring brain development reported in two of the animal studies (66,69) appears to contradict the evidence from prenatal HFD studies that did not manipulate prenatal stress exposure (84–86). The findings are also surprising given that adverse main effects of the HFD alone were reported for brain morphology, gene expression, and markers of inflammation. Huang et al. (66) suggested that their unexpected results may reflect a predictive adaptive response, whereby the prenatal stress condition (lipopolysaccharide injections) primes the developing fetus to adapt to a proinflammatory nutritional environment for long-term protective effects on the brain. Conversely, Rincel et al. (69) did not identify any differences in metabolic hormones (insulin, leptin, peptide YY, glucagon-like peptide-1) among stressed pups fed either the HFD or the standard diet, suggesting that the protective effect of the HFD could not be explained by metabolic alterations. The authors suggested that their unexpected findings may be explained by the longer time nursing among HFD-fed dams after a stress session of maternal-pup separation. This may reflect a dampening of the stress response in the pups owing to the higher fat content of the milk (90) and/or increased comforting response in the dams through longer lactation, which in turn may improve the quality of maternal care to help override the negative impact of the postnatal stress exposure on offspring neurodevelopment. However, we suggest that the beneficial effects could also reflect a direct response to the fat-sufficient content of the experimental diet (40% energy from fat) compared with the control diet, which is arguably fat deficient (12% energy from fat), potentially compromising normal brain development in the offspring. Meanwhile, the results of the study by Paternain et al. (70) demonstrated the effects of nutrition–stress interaction on brain development from an HFS diet, a more accurate model of a junk food diet in humans. Indeed, Paternain et al. (70) observed that the perinatal HFS diet increased serum levels of glucose, insulin, and leptin and increased body weight and fat in the adult offspring, reflecting the metabolic alterations associated with diet-induced obesity. Furthermore, the prenatal stress condition exacerbated the effects of the HFS diet on offspring body weight, fatness, and leptin and postprandial glucose clearance on a glucose tolerance test. These metabolic effects of prenatal stress may be explained by the finding that hyperactivity of the HPA axis in late gestation is mediated by increased hepatic glucocorticoid receptor expression, which drives gluconeogenesis via increased expression and activity of the enzyme phosphoenolpyruvate carboxykinase, leading to glucose intolerance and insulin resistance in adulthood (91,92).
For the second pattern of results described in this review (i.e., the interactive effects of prenatal or perinatal stress with supplementation of specific nutrients targeting antioxidant and/or 1-carbon metabolic pathways), each study reported some beneficial effects of the nutritional intervention in alleviating the adverse neurodevelopmental effects (anxiety, cognition, impaired HPA axis function) induced by prenatal stress exposure (67,68,71). The mechanisms by which perinatal choline mitigates the effects of prenatal stress are not fully understood but might be attributed to increased hippocampal neurogenesis (93–95) and/or increased levels of brain neurotrophic factors (93,96,97). However, Naninck et al. (71) did not observe any increase in hippocampal neurogenesis following early postnatal supplementation of 1-carbon metabolism–associated nutrients, including choline, although this may be attributed to the relatively short duration and late initiation of supplementation in the peri-natal neurodevelopmental time span. Nevertheless, this study did report a protective effect of the supplement against a peri-natal stress–induced increase in plasma corticosterone levels and cognitive deficits in the offspring. The underlying mechanism appears to be repletion of central and peripheral methionine levels, which were seen to decrease after stress exposure in the supplemented group. Meanwhile, in the study by Yajima et al.(67), the antianxiogenic effect of prenatal lutein supplementation administered concurrently with prenatal stress exposure may be attributed to its anti-inflammatory and antioxidant properties (79,98). However, the results of this study should be interpreted with caution, as the method employed to induce prenatal stress was constant light exposure with the aim of disturbing the circadian rhythm (99), which is associated with dysregulated neurotransmitter activities (100,101), but the beneficial effects of lutein in this context may not necessarily extend to other forms of psychological prenatal stress. Lastly, in a human study, adequate dietary intakes of the antioxidant minerals zinc and selenium emerged as potentially important for protection against the adverse effects of prenatal stress on child neurodevelopment (72). Although beneficial effects of antioxidant supplementation have been reported in cases of prenatal carcinogen (102), nicotine (103), or alcohol (104) exposure, such effects have yet to be systematically tested through experimental animal and human studies in the context of prenatal psychological stress.
LIMITATIONS OF EXISTING STUDIES AND FUTURE RESEARCH DIRECTIONS
The existing evidence for prenatal nutrition–stress interactions’ influence on brain development is limited by the number of available studies and heterogeneity in study design and methodological approaches. There is a compelling need for further research on this topic, including replication of the findings of existing studies, but future research could significantly benefit from a more streamlined approach.
Animal studies require standardization in the paradigms used to induce prenatal stress as well as gestational timing and duration of stress exposure, and future studies should investigate sex-specific effects. The nutritional composition of the experimental and control diets should also be adequately described or referenced, and researchers should carefully consider the most appropriate fat content and composition of a rodent HFD to address their research question.
In relation to human studies, existing mother-child cohorts with available data on child neurodevelopmental outcomes, as well as some characterization of prenatal nutrition and stress, should be explored in depth to identify potential nutritional and stress exposures of key interest. Future prospective, observational human studies can be improved through use of more advanced methods to characterize prenatal stress exposure (e.g., ambulatory assessment methodology to directly study stress occurrence and the biological response in ecologically valid settings) and dietary intakes [e.g., diet diaries incorporating food photography (105), validated and automated 24-hour dietary recalls such as the Automated Self-Administered 24-hour tool (106)], objectively assessing structural and functional neurodevelopment via brain magnetic resonance imaging scans, and assessing neurodevelopmental outcomes during the neonatal period to test the effects of prenatal exposures before postnatal factors can modify effects.
CONCLUSIONS
The effects of prenatal psychosocial stress and nutrition are context dependent, simultaneously influencing one another at various physiological and behavioral levels, and thus should not be considered in isolation. Through identification of the interplay of prenatal stress and nutritional factors that are important for offspring brain development, we might develop new clinical intervention pathways for improved maternal health, well-being, and social support that may lead to long-term health benefits for the child. Furthermore, carefully designed animal and human studies are required to identify the primary nutritional factors that should be targeted in the context of prenatal stress to optimize intervention strategies that could ameliorate the adverse effects on child neurodevelopment.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the U.S. Department of Health and Human Services National Institutes of Health Grant Nos. R01 MH-105538 (to PDW, CB), AG-050455 (to PDW), MD-010738 (to PDW, SE), UG3 OD-023349 (to CB, PDW), and ERC-STG 678073 (to SE).
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Entringer S, Buss C, Swanson JM, Cooper DM, Wing DA, Waffarn F, et al. (2012): Fetal programming of body composition, obesity, and metabolic function: The role of intrauterine stress and stress biology. J Nutr Metab 2012:632548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwa PD, Buss C, Entringer S, Swanson JM (2009): Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA (2004): Living with the past: Evolution, development, and patterns of disease. Science 305:1733–1736. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Low FM, Buklijas T, Hanson MA, Beedle AS (2011): How evolutionary principles improve the understanding of human health and disease. Evol Appl 4:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson M, Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD (2011): Developmental plasticity and developmental origins of noncommunicable disease: Theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol 106:272–280. [DOI] [PubMed] [Google Scholar]
- 6.Tau GZ, Peterson BS (2010): Normal development of brain circuits. Neuropsychopharmacology 35:147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, et al. (2017): Intergenerational transmission of maternal childhood maltreatment exposure: Implications for fetal brain development. J Am Acad Child Adolesc Psychiatry 56:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buss C, Entringer S, Wadhwa PD (2012): Fetal programming of brain development: Intrauterine stress and susceptibility to psychopathology. Sci Signal 5:pt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. (2010): Early life programming and neuro-developmental disorders. Biol Psychiatry 68:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, et al. (2007): Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci 27:2592–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tully LA, Arseneault L, Caspi A, Moffitt TE, Morgan J (2004): Does maternal warmth moderate the effects of birth weight on twins’ attention-deficit/hyperactivity disorder (ADHD) symptoms and low IQ? J Consult Clin Psychol 72:218–226. [DOI] [PubMed] [Google Scholar]
- 12.Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, et al. (2017): Prenatal maternal cortisol has sex-specific associations with child brain network properties. Cerebral Cortex 27:5230–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller BR, Bale TL (2008): Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 28:9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay KL, Buss C, Wadhwa PD, Entringer S (2017): The interplay between maternal nutrition and stress during pregnancy: Issues and considerations. Ann Nutr Metab 70:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgieff MK (2007): Nutrition and the developing brain: Nutrient priorities and measurement. Am J Clin Nutr 85:614S–620S. [DOI] [PubMed] [Google Scholar]
- 16.Crawford MA (1993): The role of essential fatty acids in neural development: Implications for perinatal nutrition. Am J Clin Nutr 57:703S–710S. [DOI] [PubMed] [Google Scholar]
- 17.Breton C (2013): The hypothalamus-adipose axis is a key target of developmental programming by maternal nutritional manipulation. J Endocrinol 216:R19–R31. [DOI] [PubMed] [Google Scholar]
- 18.Szutorisz H, Hurd YL (2016): Feeding the developing brain: The persistent epigenetic effects of early life malnutrition. Biol Psychiatry 80:730–732. [DOI] [PubMed] [Google Scholar]
- 19.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ (2010): Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci U S A 107:16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong H, Xi Y, An Y, Tao L, Zhang X, Yu H, et al. (2017): The correlation between early stages of life exposed to Chinese famine and cognitive decline in adulthood: Nutrition of adulthood plays an important role in the link? Front Aging Neurosci 9:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czeizel EA, Dudás I, Vereczkey A, Bánhidy F (2013): Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 5:4760–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth C, Magnus P, Schjølberg S, Stoltenberg C, Surén P, McKeague IW, et al. (2011): Folic acid supplements in pregnancy and severe language delay in children. JAMA 306:1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH (2012): Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 129:485–493. [DOI] [PubMed] [Google Scholar]
- 24.Ars CL, Nijs IM, Marroun HE, Muetzel R, Schmidt M, Steenweg-de Graaff J, et al. (2016): Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: The Generation R Study [published online ahead of print Jan 22]. Br J Nutr. [DOI] [PubMed] [Google Scholar]
- 25.Eyles DW, Burne THJ, McGrath JJ (2013): Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 34:47–64. [DOI] [PubMed] [Google Scholar]
- 26.Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. (2013): Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 309:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talge NM, Neal C, Glover V; Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health (2007): Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry 48:245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinsella MT, Monk C (2009): Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin Obstet Gynecol 52:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King S, Dancause K, Turcotte-Tremblay A-M, Veru F, Laplante DP (2012): Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res C Embryo Today 96:273–288. [DOI] [PubMed] [Google Scholar]
- 30.Sandman CA, Buss C, Head K, Davis EP (2015): Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry 77:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buss C, Davis EP, Muftuler LT, Head K, Sandman CA (2010): High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9 year-old children. Psycho-neuroendocrinology 35:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buss C, Davis EP, Hobel CJ, Sandman CA (2011): Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress 14:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandman CA, Davis EP, Buss C, Glynn LM (2012): Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology 95:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van den Bergh BR, Mulder EJ, Mennes M, Glover V (2005): Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neurosci Biobehav Rev 29:237–258. [DOI] [PubMed] [Google Scholar]
- 35.O’Donnell KJ, Meaney MJ (2017): Fetal origins of mental health: The developmental origins of health and disease hypothesis. Am J Psychiatry 174:319–328. [DOI] [PubMed] [Google Scholar]
- 36.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA (2012): Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A 109:E1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, et al. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry 83:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam TC, Epel ES (2007): Stress, eating and the reward system. Physiol Behav 91:449–458. [DOI] [PubMed] [Google Scholar]
- 39.Torres SJ, Nowson CA (2007): Relationship between stress, eating behavior, and obesity. Nutrition 23:887–894. [DOI] [PubMed] [Google Scholar]
- 40.Kiecolt-Glaser JK (2010): Stress, food, and inflammation: Psycho-neuroimamunology and nutrition at the cutting edge. Psychosom Med 72:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, et al. (2012): What is eating you? Stress and the drive to eat. Appetite 58:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin J, Levanon D, Chen JD (2004): Inhibitory effects of stress on postprandial gastric myoelectrical activity and vagal tone in healthy subjects. Neurogastroenterol Motil 16:737–744. [DOI] [PubMed] [Google Scholar]
- 43.Stoney CM, West SG, Hughes JW, Lentino LM, Finney ML, Falko J, et al. (2002): Acute psychological stress reduces plasma triglyceride clearance. Psychophysiology 39:80–85. [DOI] [PubMed] [Google Scholar]
- 44.Le Fur C, Romon M, Lebel P, Devos P, Lancry A, Guedon-Moreau L, et al. (1999): Influence of mental stress and circadian cycle on postprandial lipemia. Am J Clin Nutr 70:213–220. [DOI] [PubMed] [Google Scholar]
- 45.Teff KL (2008): Visceral nerves: Vagal and sympathetic innervation. JPEN J Parenter Enteral Nutr 32:569–571. [DOI] [PubMed] [Google Scholar]
- 46.Mikolajczyk RT, El Ansari W, Maxwell AE (2009): Food consumption frequency and perceived stress and depressive symptoms among students in three European countries. Nutr J 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dallman MF (2010): Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser JK, Fagundes CP, Andridge R, Peng J, Malarkey WB, Habash D, et al. (2017): Depression, daily stressors and inflammatory responses to high-fat meals: When stress overrides healthier food choices. Mol Psychiatry 22:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Entringer S, Buss C, Wadhwa PD (2015): Prenatal stress, development, health and disease risk: A psychobiological perspective—2015 Curt Richter Award Winner. Psychoneuroendocrinology 62:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yehuda S (2003): Omega-6/omega-3 ratio and brain-related functions. World Rev Nutr Diet 92:37–56. [DOI] [PubMed] [Google Scholar]
- 51.Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, et al. (2004): Polyunsaturated fatty acids in the central nervous system: Evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev 44:509–538. [DOI] [PubMed] [Google Scholar]
- 52.Cunnane SC, Francescutti V, Brenna JT, Crawford MA (2000): Breast-fed infants achieve a higher rate of brain and whole body docosahexaenoate accumulation than formula-fed infants not consuming dietary docosahexaenoate. Lipids 35:105–111. [DOI] [PubMed] [Google Scholar]
- 53.Spencer SJ, Korosi A, Layé S, Shukitt-Hale B, Barrientos RM (2017): Food for thought: How nutrition impacts cognition and emotion. npj Science of Food 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joffre C, Nadjar A, Lebbadi M, Calon F, Laye S (2014): n-3 LCPUFA improves cognition: The young, the old and the sick. Prostaglandins Leukot Essent Fatty Acids 91:1–20. [DOI] [PubMed] [Google Scholar]
- 55.Rao TS, Asha MR, Ramesh BN, Rao KS (2008): Understanding nutrition, depression and mental illnesses. Indian J Psychiatry 50:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coe CL, Lubach GR, Shirtcliff EA (2007): Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res 61:520. [DOI] [PubMed] [Google Scholar]
- 57.Ganz T, Nemeth E (2012): Hepcidin and iron homeostasis. Biochim Biophys Acta 1823:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marques AH, O’Connor TG, Roth C, Susser E, Bjørke-Monsen A-L (2013): The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci 7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monk C, Georgieff MK, Osterholm EA (2013): Research review: Maternal prenatal distress and poor nutrition—mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry 54:115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoeijmakers L, Lucassen PJ, Korosi A (2014): The interplay of early-life stress, nutrition, and immune activation programs adult hippo-campal structure and function. Front Mol Neurosci 7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lucassen PJ, Naninck EF, van Goudoever JB, Fitzsimons C, Joels M, Korosi A (2013): Perinatal programming of adult hippocampal structure and function: Emerging roles of stress, nutrition and epigenetics. Trends Neurosci 36:621–631. [DOI] [PubMed] [Google Scholar]
- 62.Baskin R, Hill B, Jacka FN, O’Neil A, Skouteris H (2015): The association between diet quality and mental health during the perinatal period. A systematic review. Appetite 91:41–47. [DOI] [PubMed] [Google Scholar]
- 63.Yam KY, Naninck EF, Schmidt MV, Lucassen PJ, Korosi A (2015): Early-life adversity programs emotional functions and the neuroendocrine stress system: The contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress 18:328–342. [DOI] [PubMed] [Google Scholar]
- 64.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW (2018): Depression and obesity: Evidence of shared biological mechanisms [published online ahead of print Feb 16]. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 65.Borsonelo EC, Suchecki D, Calil HM, Galduroz JC (2011): Supplementation with fish oil and coconut fat prevents prenatal stress-induced changes in early postnatal development. Int J Dev Neurosci 29:521–527. [DOI] [PubMed] [Google Scholar]
- 66.Huang CF, Du JX, Deng W, Cheng XC, Zhang SY, Zhao SJ, et al. (2015): Effect of prenatal exposure to LPS combined with pre- and post-natal high-fat diet on hippocampus in rat offspring. Neuroscience 286:364–370. [DOI] [PubMed] [Google Scholar]
- 67.Yajima M, Matsumoto M, Harada M, Hara H, Yajima T (2013): Effects of constant light during perinatal periods on the behavioral and neuronal development of mice with or without dietary lutein. Biomed Res 34:197–204. [DOI] [PubMed] [Google Scholar]
- 68.Schulz KM, Pearson JN, Gasparrini ME, Brooks KF, Drake-Frazier C, Zajkowski ME, et al. (2014): Dietary choline supplementation to dams during pregnancy and lactation mitigates the effects of in utero stress exposure on adult anxiety-related behaviors. Behav Brain Res 268:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rincel M, Lepinay AL, Delage P, Fioramonti J, Theodorou VS, Laye S, et al. (2016): Maternal high-fat diet prevents developmental programming by early-life stress. Transl Psychiatry 6:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paternain L, de la Garza AL, Batlle MA, Milagro FI, Martinez JA, Campion J (2013): Prenatal stress increases the obesogenic effects of a high-fat-sucrose diet in adult rats in a sex-specific manner. Stress 16:220–232. [DOI] [PubMed] [Google Scholar]
- 71.Naninck EF, Oosterink JE, Yam KY, de Vries LP, Schierbeek H, van Goudoever JB, et al. (2017): Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB J 31:505–518. [DOI] [PubMed] [Google Scholar]
- 72.Lipton LR, Brunst KJ, Kannan S, Ni YM, Ganguri HB, Wright RJ, et al. (2017): Associations among prenatal stress, maternal antioxidant intakes in pregnancy, and child temperament at age 30 months. J Dev Orig Health Dis 8:638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brunst KJ, Enlow MB, Kannan S, Carroll KN, Coull BA, Wright RJ (2014): Effects of prenatal social stress and maternal dietary fatty acid ratio on infant temperament: Does race matter? Epidemiology (Sunnyvale) 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barker ED, Kirkham N, Ng J, Jensen SK (2013): Prenatal maternal depression symptoms and nutrition, and child cognitive function. Br J Psychiatry 203:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clancy B, Finlay BL, Darlington RB, Anand KJS (2007): Extrapolating brain development from experimental species to humans. Neurotoxicology 28:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson AR, Zeisel SH (2011): Dietary choline for brain development In: Preedy VR, Watson RR, Martin CR, editors. Handbook of Behavior, Food and Nutrition. New York: Springer New York, 2089–2104. [Google Scholar]
- 77.Zeisel SH (2006): Choline: Critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 26:229–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ (2014): Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr 59:659–665. [DOI] [PubMed] [Google Scholar]
- 79.Johnson EJ (2014): Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 72:605–612. [DOI] [PubMed] [Google Scholar]
- 80.Sánchez-Hernández D, Anderson GH, Poon AN, Pannia E, Cho CE, Huot PSP, et al. (2016): Maternal fat-soluble vitamins, brain development, and regulation of feeding behavior: An overview of research. Nutr Res 36:1045–1054. [DOI] [PubMed] [Google Scholar]
- 81.Innis SM (2008): Dietary omega 3 fatty acids and the developing brain. Brain Res 1237:35–43. [DOI] [PubMed] [Google Scholar]
- 82.Innis SM (2007): Dietary (n-3) fatty acids and brain development. J Nutr 137:855–859. [DOI] [PubMed] [Google Scholar]
- 83.Lai M, Chandrasekera PC, Barnard ND (2014): You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr Diabetes 4:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sullivan EL, Riper KM, Lockard R, Valleau JC (2015): Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm Behav 76:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sullivan EL, Nousen L, Chamlou K (2014): Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav 123:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edlow AG (2017): Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn 37:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. (2010): Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci 30:3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watters JL, Satia JA, Kupper LL (2008): Correlates of antioxidant nutrients and oxidative DNA damage differ by race in a cross-sectional study of healthy African American and white adults. Nutr Res 28:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salihu HM, Ghaji N, Mbah AK, Alio AP, August EM, Boubakari I (2012): Particulate pollutants and racial/ethnic disparity in feto-infant morbidity outcomes. Matern Child Health J 16:1679–1687. [DOI] [PubMed] [Google Scholar]
- 90.Krolow R, Noschang CG, Arcego D, Andreazza AC, Peres W, Gonçalves CA, et al. (2010): Consumption of a palatable diet by chronically stressed rats prevents effects on anxiety-like behavior but increases oxidative stress in a sex-specific manner. Appetite 55:108–116. [DOI] [PubMed] [Google Scholar]
- 91.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR (1998): Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 101:2174–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozanne SE, Hales CN (2002): Early programming of glucose-insulin metabolism. Trends Endocrinol Metab 13:368–373. [DOI] [PubMed] [Google Scholar]
- 93.Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL (2007): Prenatal choline availability modulates hippocampal neuro-genesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci 25:2473–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, et al. (2008): Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res 1237:110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rajarethnem HJ, Bhat KMR, Jc M, Gopalkrishnan SK, Gopalram RBM, Rai KS (2017): Combined supplementation of choline and docosahexaenoic acid during pregnancy enhances neurodevelopment of fetal hippocampus. Neurol Res Int 2017:8748706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Napoli I, Blusztajn JK, Mellott TJ (2008): Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippo-campus and frontal cortex. Brain Res 1237:124–135. [DOI] [PubMed] [Google Scholar]
- 97.Sandstrom NJ, Loy R, Williams CL (2002): Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res 947:9–16. [DOI] [PubMed] [Google Scholar]
- 98.Santocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G (2007): Lutein, zeaxanthin and astaxanthin protect against DNA damage in SK-N-SH human neuroblastoma cells induced by reactive nitrogen species. J Photochem Photobiol B 88:1–10. [DOI] [PubMed] [Google Scholar]
- 99.Ohta H, Mitchell AC, McMahon DG (2006): Constant light disrupts the developing mouse biological clock. Pediatr Res 60:304. [DOI] [PubMed] [Google Scholar]
- 100.Sandu C, Hicks D, Felder-Schmittbuhl M-P (2011): Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci 34:507–516. [DOI] [PubMed] [Google Scholar]
- 101.Pardini L, Kaeffer B (2006): Feeding and circadian clocks. Reprod Nutr Dev 46:463–480. [DOI] [PubMed] [Google Scholar]
- 102.Kelvin EA, Edwards S, Jedrychowski W, Schleicher RL, Camann D, Tang D, et al. (2009): Modulation of the effect of prenatal PAH exposure on PAH-DNA adducts in cord blood by plasma antioxidants. Cancer Epidemiol Biomarkers Prev 18:2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gallo C, Renzi P, Loizzo S, Loizzo A, Piacente S, Festa M, et al. (2010): Potential therapeutic effects of vitamin E and C on placental oxidative stress induced by nicotine: An in vitro evidence. Open Biochem J 4:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ledig M, Holownia A, Copin JC, Tholey G, Anokhina I (1996): Development of glial cells cultured from prenatally alcohol treated rat brain: Effect of supplementation of the maternal alcohol diet with a grape extract. Neurochem Res 21:313–317. [DOI] [PubMed] [Google Scholar]
- 105.Martin CK, Nicklas T, Gunturk B, Correa JB, Allen HR, Champagne C (2014): Measuring food intake with digital photography. J Hum Nutr Diet 27:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kirkpatrick SI, Subar AF, Douglass D, Zimmerman TP, Thompson FE,Kahle LL, et al. (2014): Performance of the automated self-administered 24-hour recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr 100:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.