Abstract
Background:
Establishing sustained reoxygenation/reperfusion ensures not only the recovery, but may initiate a reperfusion injury in which oxidative stress plays a major role. This study offers the mechanism and this mechanism-specific therapeutic strategy against excessive release of reactive oxygen species (ROS) associated with reperfusion-driven recovery of mitochondrial metabolism.
Aims and methods:
In neonatal mice subjected to cerebral hypoxia-ischaemia (HI) and reperfusion, we examined conformational changes and activity of mitochondrial complex I with and without post-HI administration of S-nitrosating agent, MitoSNO. Assessment of mitochondrial ROS production, oxidative brain damage, neuropathological and neurofunctional outcomes were used to define neuroprotective strength of MitoSNO. A specificity of reperfusion-driven mitochondrial ROS production to conformational changes in complex I was examined in-vitro.
Results:
HI deactivated complex I, changing its conformation from active form (A) into the catalytically dormant, de-active form (D). Reperfusion rapidly converted the D-form into the A-form and increased ROS generation. Administration of MitoSNO at the onset of reperfusion, decelerated D→A transition of complex I, attenuated oxidative stress, and significantly improved neurological recovery. In cultured neurons, after simulated ischaemia-reperfusion injury, MitoSNO significantly reduced ROS generation and neuronal mortality. In isolated mitochondria subjected to anoxia-reoxygenation, MitoSNO restricted ROS release during D→A transitions.
Conclusion:
Rapid D→A conformation in response to reperfusion reactivates complex I. This is essential not only for metabolic recovery, but also contributes to excessive release of mitochondrial ROS and reperfusion injury. We propose that the initiation of reperfusion should be followed by pharmacologically-controlled gradual reactivation of complex I.
Keywords: Hypoxia/ischaemia, Mitochondrial complex I, Nitrosation, Ischaemia/reperfusion damage
1. Introduction
With an incidence of 1–2 per 1000 full-term birth, perinatal asphyxia remains the major cause of permanent neurological disability in survived children [1]. The mechanisms of hypoxia-ischaemia-reperfusion brain injury (HI) in asphyxiated and successfully resuscitated infants remain unclear. This precludes the development of therapeutic strategies addressing adverse metabolic changes in the immediate post-HI state.
During reperfusion, reactivation of mitochondrial metabolism is paramount for bioenergetics recovery and survival. However, mitochondria can also contribute to reperfusion-driven elevation of ROS [2–5]. Mitochondrial complex I (C-I) has been proposed as the major site of ROS production in mitochondria [6–9]. Elevated ROS production at the level of C-I requires succinate-supported reverse electron transfer (RET) and catalytically competent C-I. While, accumulation and preferential mitochondrial oxidation of succinate in the post-ischemic brains has been shown [10,34], the mechanism of C-I inhibition and reactivation during ischaemia and reperfusion and their role in the ROS production remains unclear.
In hypoxia, mitochondrial C-I undergoes conformational change from the active (A) to the de-active (D) form [10–12]. The D-form is characterized by exposure of a functionally critical SH-group of the mitochondria-encoded ND3 subunit [13]. Upon re-oxygenation, C-I reactivates by converting the D-form back to the A-form. This reversible A/D/A (normoxia/hypoxia/reoxygenation) transition has never been observed in cerebral tissue. We hypothesized that A→D transition during HI-insult accounts for inhibition of C-I-dependent mitochondrial respiration reported in our model [14,15]. Upon reperfusion, the D to the A conversion reactivates C-I. While this is important for eventual bioenergetics recovery, it also drives succinate-supported elevation of mitochondrial ROS release. Preferential oxidation of succinate in reperfusion regenerates energy via forward electron transfer (FET) using complexes II-III-IV and does not require C-I. Therefore, C-I reactivation (D→A transition) could be inhibited by mitochondria-targeted nitrosating agent, MitoSNO. This will limit RET-dependent elevation of ROS release without affecting a recovery of mitochondrial energy metabolism.
2. Materials and methods
2.1. Unilateral cerebral hypoxia-ischaemia injury
Studies were approved by the Columbia University Institutional Animal Care and Use Committee (IACUC) in accordance with the ARRIVE guidelines [16]. Three-day-old C57BL/6J mice with their dams were purchased from Jackson Laboratories (Bar Harbor). At 10 days of age (p10), mice were subjected to HI-insult (ligation of the right carotid artery + hypoxia) as described [17].
2.2. Treatment protocol and study groups
Immediately after HI, mice were administered with either MitoSNO (500 ng/kg) or vehicle (inactive MitoSNO metabolite, Mito-N-acetyl penicillamine (MitoNAP)) intranasally, 1 μl aliquots (10 μl total), over 40 min. At 60 min of reperfusion, the level of MitoNAP was measured in olfactory bulbs and brain hemispheres in one group of mice. Other groups of mice were used to determine changes in C-I activity associated with the D→A transition, mitochondrial O2 consumption and H2O2 production rate (Fig. 1A). At 24 h of reperfusion, two cohorts of animals were used for assessment of cerebral infarct volumes and extent of oxidative injury. At ten days after HI, in a separate cohort of mice, a long-term outcome was examined by assessment of sensorimotor deficit (wire-holding, bridge-crossing, and rota-rod tests) [18] and the extent of the ipsilateral hemisphere atrophy [19].
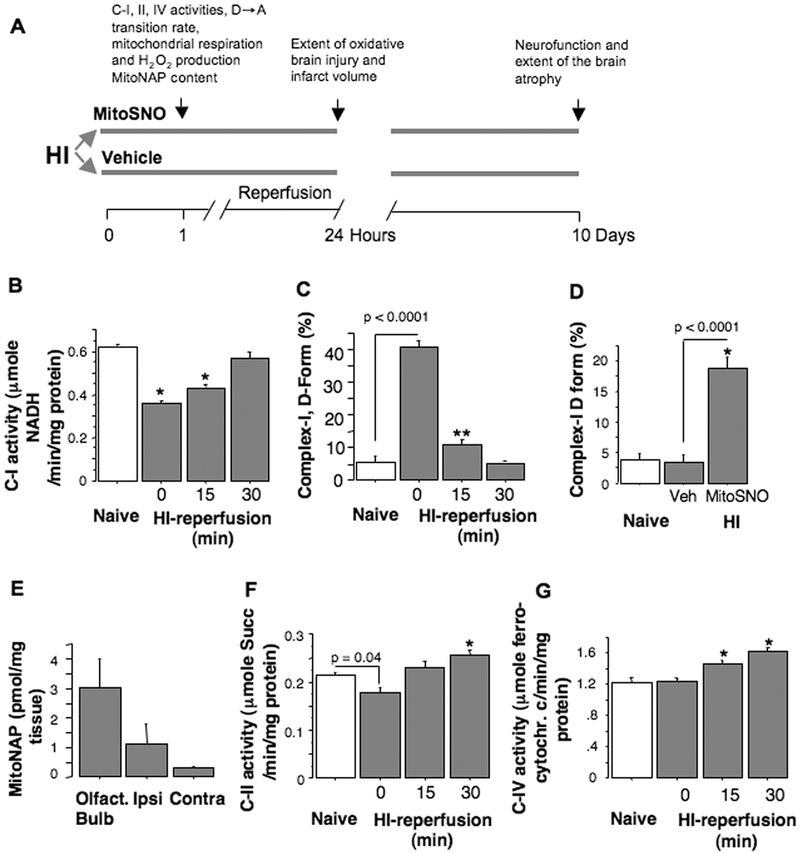
Fig. 1.
A – Illustration of the study design. B, C - C-I activity and the D-form content in naïve (n = 6) and HI-brains at the end of HI-insult (0 min of reperfusion, n = 4), at 15 (n = 4) and 30 min of reperfusion (n = 6). D – The D-form content in naïve (n = 4) and HI-mice treated with vehicle (veh, n = 6) or with MitoSNO (n = 6). E - MitoSNO inactive metabolite (MitoNAP) in different regions of the brain following intranasal administration of MitoSNO to HI-mice (n = 5). F and G – Activities of C-II and C-IV in naïve (n = 6) and HI-mouse brains (n = 4–6).
2.3. Measurement of the cerebral infarct volumes and atrophy
Brains were coronally sectioned and stained with 2% triphenyltetrazolium chloride (TTC). Infarct volumes were calculated using digital images (Adobe Photoshop 4.0.1 and NIH Image J 1.62) and expressed as the percentage of the ipsilateral hemisphere [20]. Brain atrophy was measured using 12 Nissl stained coronal slices (40 nm) per brain, sectioned every 500 nm and expressed as % of the contralateral hemisphere [19].
2.4. Extraction and LC-MS/MS of MitoNAP
For extraction of MitoNAP, 50 mg of brain tissue was incubated in 250 μl 95% acetonitrile, 0.1% formic acid with internal standards (100 pmol d15-MitoNAP). Following protein precipitation and centrifugation, the pellet was re-suspended, centrifuged and transferred to mass spec-trometry vials (TrueView™ LCMS Certified, Waters). MS/MS analysis (Waters Xevo TQ-S): source spray voltage, 3.3 kV; cone voltage, 53 V; ion source temperature, 150 °C; collision energy, 4 V. For LC-MS/MS analyses samples (2 μl) were injected into a 15 μl flow-through needle and RP-UPLC. A standard curve with known amounts of MitoNAP: MitoNAP, 521 > 183; d15-MitoNAP, 536 > 191 was used for quantification using the MassLynx 4.1 software.
2.5. Measurements of mitochondrial complexes activities
Brain mitochondria from naive p10 mice were isolated as in [21]. The activities of C-I were determined as oxidation of NADH (ε340 nm = 6.22 mM−1 × cm−1) in 1 ml of standard SET medium supplemented with 15 μM cytochrome c, 25 μg/ml alamethicin, 2 mM MgCl2 and 10–25 μg protein/ml mitochondria. Oxidation of ferrocytochrome c by C-IV and succinate:Q1 reductase activity of C-II were measured as [21].
The effect of MitoSNO on mitochondrial respiration and H2O2 emission was assessed in isolated brain mitochondria upon completion of post-HI treatment with MitoSNO or vehicle. Mitochondrial respiration (malate 5 mM + glutamate 10 mM) was measured using Clark-type electrode (Hansatech, UK) as in [20]. Mitochondrial H2O2 release rate was measured as in [20].
2.6. Measurement of C-I the A and the D states
The relative content of the A-form of C-I was measured as described [11,12,22]. In brief, the initial NADH-oxidase activity was measured in the alkaline (pH 8.8) SET medium in the presence of 5.5 mM MgCl2 before and after C-I activation with a pulse of 20 μM NADH. Because in the presence of divalent cations and at alkaline pH, the rate of C-I reactivation (D→A transition) is slower than the rate of the reaction [12,22], this C-I activity was attributed only to the A-form. Then a total (A+D) C-I activity was estimated in the same manner, but in neutral media (pH 7.0) and in the absence of Mg2+.
2.7. Preparation of submitochondrial particles (SMP) and measurement of superoxide generation
SMP prepared from brain mitochondria of p10 mice [23] were diluted (5 mg/ml) in buffer (250 mM sucrose, 50 mM Tris-PO4 (pH 7.5), 0.2 mM EDTA, 2 mg/ml BSA, malonate (2 mM) and oligomycin (0.5 μg/mg protein SMP)) and incubated at 30 °C for 30 min. This treatment near-completely deactivates C-I. Then SMP were supplemented with 100 μM NAD+, 1% ethanol and distributed into two tubes. 0.1 mg/ml alcohol dehydrogenase was added to one tube to activate C-I (15 min at 25 °C) as described [24].
The superoxide generation supported by succinate (20 mM) was measured in 70–100 μg/ml SMP as described [25]. SOD (100 U/ml)-sensitive reduction, i.e. superoxide generation was 80–90% decreased by 0.2 μM FCCP or 1 μM rotenone, indicating potential-dependent reaction in C-I.
2.8. Measurement of oxidative stress
Oxidative injury was defined by detecting markers of lipids (4-hydroxy-nonenal, 4-HNE) and proteins (3-nitrotyrosine, 3-NT) oxidation in the ipsilateral cortex using immunohistochemistry and western blot analysis [20]. 4-HNE+ cells were quantified using Image J and expressed as % of 4-NHE+ cells. Three sections (0 mm, −0.5 and −1.2 mm to bregma), containing images (6 per mouse) of penumbra (residual presence of microtubule-associated protein, MAP2) and necrotic core (a total loss of MAP2) were examined and mean % of 4HNE+ cells per total cell count (Dapi-positive) was used for analysis.
2.9. Cell culture studies
HI-injury in vitro was simulated by subjecting HT22 murine hippocampal neurons to oxygen-glucose deprivation (OGD) as described [26]. Following four hours of OGD, cells were reperfused with normoxic media supplemented either with 0.5 μM of MitoSNO or vehicle (MitoNAP). At 30 min of reperfusion, cells were incubated with super-oxide-sensing probe, MitoSOX (30 min) followed by confocal microscopy of MitoSOX fluorescence. Cellular viability was assessed after 12 h of OGD. At six hours of reperfusion, OGD-cells treated with either MitoSNO (0.5 μM for the initial 60 min of reperfusion) or vehicle (0.5 μM of MitoNAP) were loaded with 2 μM of calcein AM and calcein fluorescence was measured. The viability was expressed as percentage of naïve controls.
2.10. Measurement of mitochondrial H2O2 release during A-D-A transition of C-I
Intact p10 mouse brain mitochondria were isolated [27], and their respiration and H2O2 release were measured using a respirometer (Oroboros) equipped with fluorescence setup (525 nm LED-excitation, a photodiode (Hamamatsu Photonics)) as sensor, and emission filter 580 nm (Rosco Laboratories). The calibration was performed by adding H2O2 (100–200 nM) to the chamber. Mitochondrial (0.14–0.18 mg of protein) state 3 respiration was initiated with 200 μM ADP in 2 ml of KCl respiration buffer containing 2 mM malate and 5 mM pyruvate or 5 mM succinate, 10 μM Amplex UltraRed (Invitrogen) and 4 U/ml horseradish peroxidase. Oxygen concentration in the respirometer was changed by continuous purge of humidified nitrogen into gas headspace (1 ml). Deactivation of C-I was carried out by pre-incubation of mitochondria in the anaerobic respiration buffer for 20 min at 37 °C without substrates.
2.11. Statistical analysis
All data are mean ± SEM. One-way ANOVA or Student’s t-test (where appropriate) were employed to determine statistically significant differences between naïve, vehicle-treated and MitoSNO-treated groups. Fisher’s post-hoc analysis was used to determine p-value. To analyze temporal differences in the same indices we used ANOVA for repeated measures.
3. Results
3.1. C-I conformation in HI and reperfusion
HI-insult deactivated C-I, by 8-fold increasing the D-form content compared to controls (Fig. 1B, C). By 30 min of reperfusion, C-I activity and the D-form level normalized, except in the mice treated with MitoSNO (Fig. 1B–D). The presence of inactive MitoSNO metabolite (MitoNAP) in the olfactory bulbs > ipsilateral > contralateral hemispheres of the MitoSNO-treated HI-mice confirmed the delivery of MitoSNO to their brains (Fig. 1E). Immediate post-HI activity of C-II was slightly (~ 15%) decreased and the activity of C-IV was unchanged. At 30 min of reperfusion, C-II and C-IV activities exceeded that of naïve counterparts (Fig. 1F, G).
3.2. Modification of C-I conformation with MitoSNO limits the severity of reperfusion-driven oxidative injury
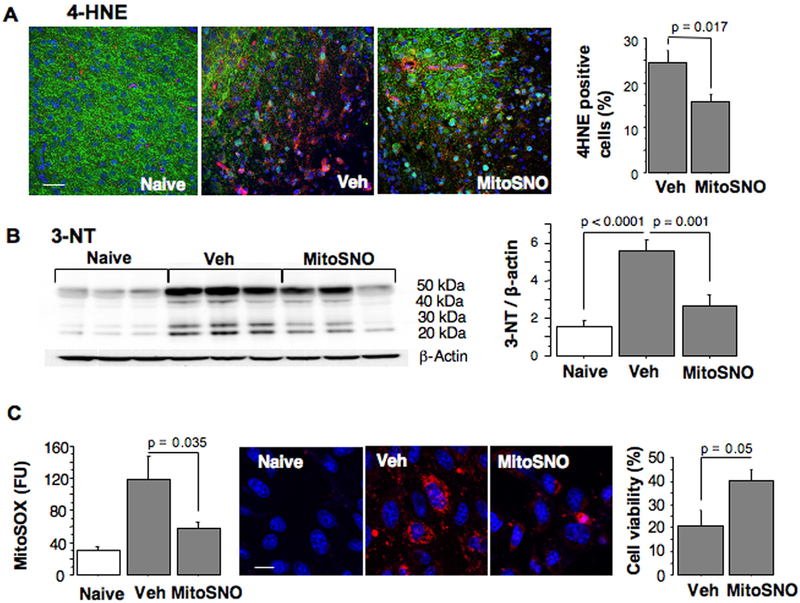
Compared to the vehicle-treated littermates, MitoSNO-treated mice demonstrated significantly decreased presence of cerebral markers for oxidative damage, 4-HNE and 3-NT (Fig. 2A, B). Following oxygen-glucose deprivation, HT22 neurons exposed to MitoSNO at the initiation of reperfusion, exhibited significantly decreased mitochondrial ROS production and exhibited better viability compared to vehicle-treated cells (Fig. 2C).
Fig. 2.
A – Immunostaining and statistical analysis for 4-HNE positive cells (red) in the HI-cortex of mice treated with vehicle (n = 6) or MitoSNO (n = 6) and image of the cortex of naïve mouse. Counterstaining, MAP-2 (green), Dapi (blue). B – Immunoblot and statistical analysis of 3-NT expression in the HI-brains of vehicle-treated and MitoSNO-treated (n = 6) and naive mice (n = 6). C – left and middle; MitoSOX fluorescence (red) at 60 min of reperfusion following OGD-insult in hippocampal murine neurons in the presence (n = 5) or absence (n = 6) of 0.5 μM of MitoSNO, compared to naïve cells (n = 7). Right; Cellular viability of OGD-cells treated with MitoSNO or vehicle for the initial 60 min of reperfusion (n = 5).
3.3. Administration of MitoSNO at the initiation of reperfusion attenuates HI-brain injury
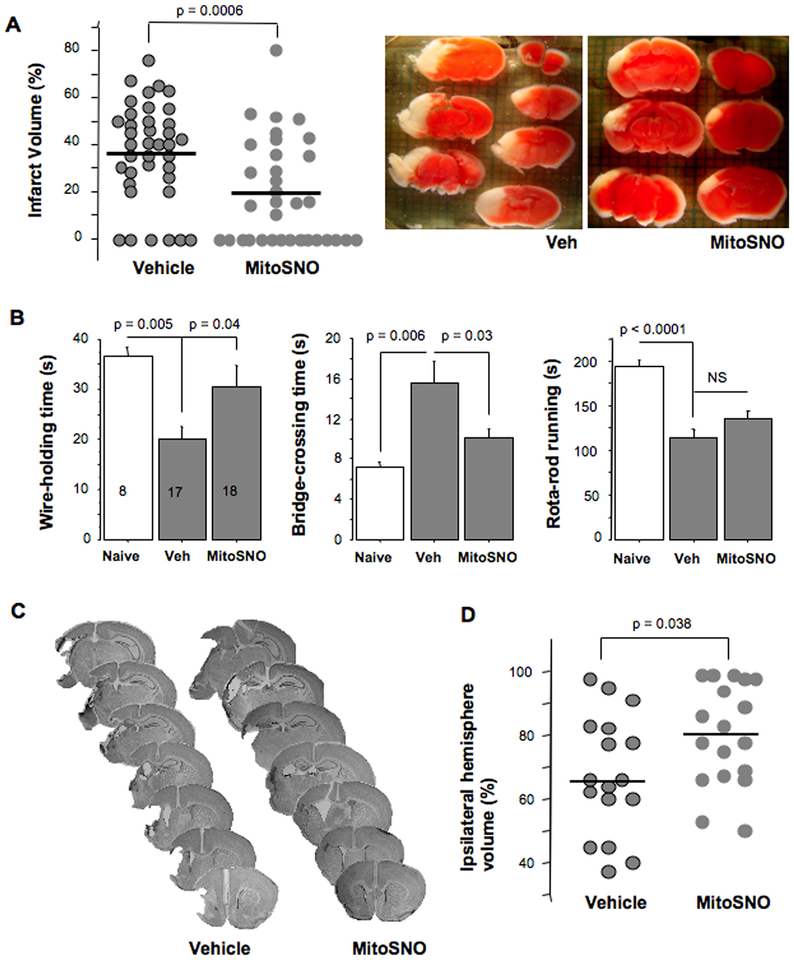
At 24 h of reperfusion, direct infarct volume was significantly decreased in MitoSNO-treated mice compared to vehicle-treated litter-mates (Fig. 3A). Importantly, post-treatment with MitoSNO administered at the onset of reperfusion, exerted a long-term neuroprotection evidenced by a better-preserved cerebral volume and sensorimotor performance (longer wire-holding and shorter bridge-crossing times) compared to vehicle-treated mice (Fig. 3C, D).
Fig. 3.
A – Actual and mean values of cerebral infarct volumes at 24 h of reperfusion and representative images of TTC-stained brains from HI-mice treated with vehicle or MitoSNO. B – Sensorimotor performance tested at ten days after HI-insult in mice treated with vehicle (Veh, n = 17) or MitoSNO (n = 18) compared to Naives (n = 8). C – Representative images of Nissl-stained coronal sections obtained at ten days following HI-insult and D – Actual and mean values of residual volume of the ipsilateral hemisphere (% of the contralateral hemisphere).
3.4. D→A conformation elevates succinate-supported mitochondrial ROS production
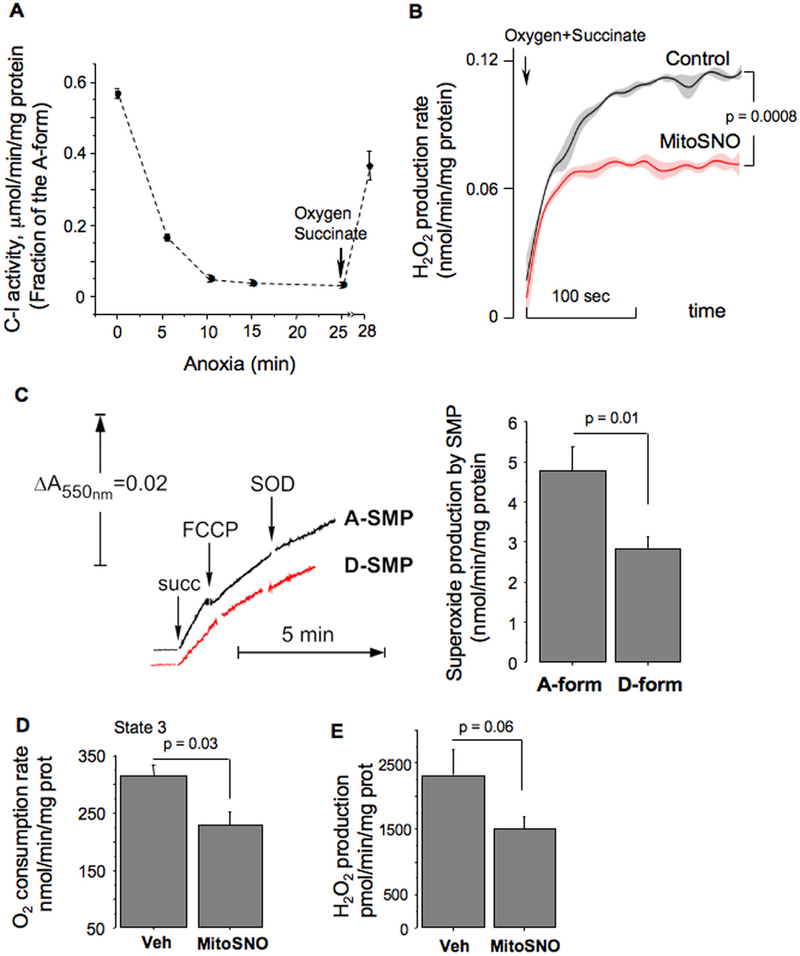
In isolated mitochondria, hypoxia deactivated C-I via conformational change from the A to the D form (Fig. 4A). Re-oxygenation reactivated C-I (D→A conversion) (Fig. 4A) and accelerated mitochondrial H2O2 release (Fig. 4B, Control). In the presence of MitoSNO, the same mitochondria significantly decelerated H2O2 generation (Fig. 4B, MitoSNO). To further validate the role of C-I in this process, we used sub-mitochondrial particles (SMP), where ROS production originates only from the respiratory chain. SMP demonstrated significantly reduced superoxide production rate, if C-I was in the D-form, compared to the C-I being in the A-form (Fig. 4C). Ex-vivo, brain mitochondria isolated after MitoSNO-treatment exhibited decreased phosphorylating respiration and H2O2 emission rates, compared to vehicle-treated counterparts (Fig. 4D, E).
Fig. 4.
A – Changes in the A/D ratio in intact mitochondria during anoxia and reoxygenation (n = 3 for each time point). B – The rate of succinate-supported H2O2 production by control (black) mitochondria and mitochondria pretreated with MitoSNO (red, MitoSNO) during reoxygenation. Four traces were analyzed to obtain mean ± SEM. C – Representative tracing of superoxide generation by succinate-supported SMP with the A-form of C-I (Black) or the D-form of C-I (Red) with statistical analysis. D – Phosphorylating respiration rate (state 3) at 60 min of reperfusion in mitochondria isolated from HI-mice treated with vehicle (n = 4) or MitoSNO (n = 4). Substrate, Malate-Glutamate. E – Succinate-supported H2O2 emission rate in the brain mitochondria isolated at 60 min of reperfusion from the ipsilateral hemisphere of HI-mice treated with vehicle (n = 8) or MitoSNO (n = 10).
4. Discussion
Oxidative damage caused by mitochondria-originating ROS following ischaemia-reperfusion injury in the mature [2] and immature [20] brain has been reported. However, because the mechanism of reperfusion-initiated excessive mitochondrial ROS generation is unclear, no mitochondria-targeted anti-oxidative strategies applicable at the onset of reperfusion exist. In isolated mitochondria, succinate-supported respiration accounts for the highest rate of ROS generation [6,9,20,28–32]. In these experiments, C-I has been identified as the primary site, and RET as the mechanism of ROS production [9,28,33]. Theoretically, in the HI-brain RET-dependent acceleration of mitochondrial ROS may occur, if 1) succinate is accumulated 2) upon reperfusion, mitochondria preferentially oxidize succinate and 3) C-I is enzymatically competent. In models of cardiac and cerebral ischaemiareperfusion injury in mature rodent, a critical role of succinate accumulation for RET-dependent ROS release has been shown [28]. We and others have reported a dramatic accumulation of succinate in the developing brain during HI [34,35]. At the initiation of reperfusion, mitochondria in the developing brain prefer oxidation of succinate [34]. Thus, excessive accumulation and preferential oxidation of succinate, favoring activation of RET upon reperfusion in-vivo have been reported. It has also been shown that C-I dependent respiration is compromised during neonatal HI and reactivates upon reperfusion [14,15,20]. However, how HI deactivates and reperfusion reactivates CI remained unknown. Our study is the first to show that HI-insult causes conformational transition from the A into the D-form which affects the activity of C-I. The enzyme in the D-form is unable to support RET [10,12], this explains C-I limited capacity to generate ROS during ischaemia [33,36]. Our study also shows that upon reperfusion C-I rapidly reactivates via conformation change of the D-from back into the A-form which supports RET, leading to elevation of ROS release in C-I. In contrast to C-I, HI has very little effect on the activity of C-II and C IV. This implies that at the initiation of reperfusion, succinate-fueled respiratory chain is fully capable of forward electron transfer (FET) via complexes II, III and IV, serving for bioenergetics recovery and support of mitochondrial membrane potential. Thus, reperfusion initiates bioenergetics recovery (FET) and excessive ROS release via RET. Experiments with isolated mitochondria and SMP supported mechanistic significance of A→D→A transitions of C-I during hypoxia-reoxygenation in succinate-supported elevation of superoxide production during D→A transition. Thus, our study proposes the molecular mechanism for reperfusion-initiated oxidative stress and reperfusion time-specific therapeutic strategy: S-nitrosation of the C-I D-form with mitochondria-targeted MitoSNO. Indeed, immediate post-HI administration of MitoSNO preserved C-I in the D-form and exerted robust neuroprotection evidenced by superior long-term neurofunctional and neuroanatomical outcomes compared to the vehicle-treated littermates. This suggests, that the D→A transition in response to reperfusion contributes to reperfusion injury. The molecular basis of ischemic deactivation of C-I is the conformational change of the flexible loop of ND3 subunit exposing Cys39 residue in the D-form [13,37]. This thiol is accessible only in the D-form of C-I and can be specifically modified by nitrosothiols, like MitoSNO [24,33]. This strategy of transient preservation of C-I in the D-form was successfully implemented for cardioprotection against reperfusion injury [33,38].
In conclusion, during perinatal HI C-I is mostly in dormant, D-form. At the initial stage of reperfusion, C-I reactivates via conformation from the dormant into the active form. This accelerates RET- dependent ROS release in mitochondria and initiates oxidative stress (Fig. 5). This mechanism is specific only to the early reperfusion stage, when mitochondrial recovery depends on preferential oxidation of succinate [12,34]. Therefore, the strategy addressing this mechanism of reperfusion injury by transient preservation of C-I in the D-form, should be initiated with achievement of effective re-circulation. Because C-I activity is not required in succinate-supported mitochondrial respiration, this strategy is will not restrict the initiation of mitochondrial metabolic recovery.
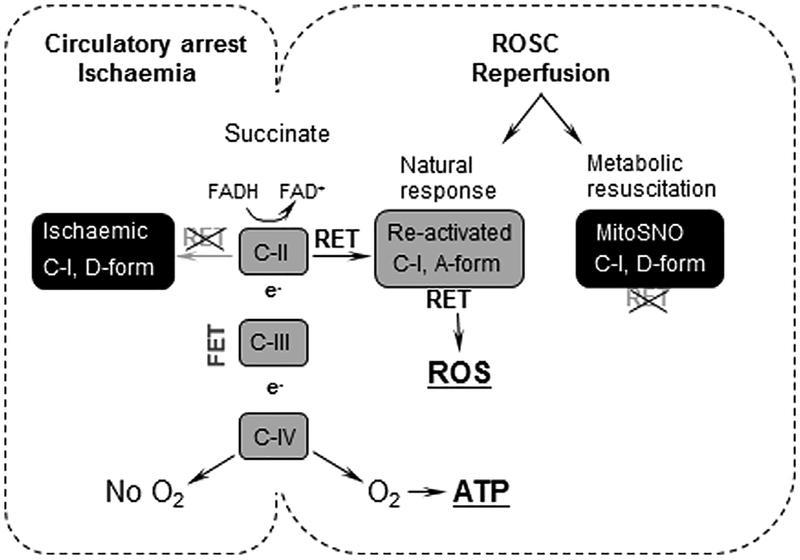
Fig. 5.
Schematic presentation of the proposed mechanism and therapeutic strategy. During circulatory arrest (ischaemia) accumulated succinate does not generate ATP (lack of O2) and ROS (C-I in the D form). Upon ROSC (reperfusion), succinate metabolism produces ATP (FET) and contributes to ROS burst as C-I transitions to the A-form which activates RET. Administration of MitoSNO by preservation of C-I in the D-form restricts RET and limits ROS release.
Acknowledgement
Authors thank Dr. Starkov for valuable comments
Sources of Funding
This study was supported by NIH grant, NS100850 (V.T.), by MRC grant MR/L007339/1 (A.G.) and MC_U105663142 and by a Wellcome Trust Investigator award 110159/Z/15/Z (M.P.M).
Footnotes
Disclosures
None.
Declaration of interest
None.
References
- [1].Volpe JJ, Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy, Ann. Neurol 72 (2012) 156–166. [DOI] [PubMed] [Google Scholar]
- [2].Piantadosi CA, Zhang J, Mitochondrial generation of reactive oxygen species after brain ischemia in the rat, Stroke 27 (1996) 327–331 (discussion 32). [DOI] [PubMed] [Google Scholar]
- [3].Starkov AA, Chinopoulos C, Fiskum G, Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury, Cell Calcium 36 (2004) 257–264. [DOI] [PubMed] [Google Scholar]
- [4].Ten VS, Starkov A, Hypoxic-ischemic injury in the developing brain: the role of reactive oxygen species originating in mitochondria, Neurol.Res.Int 2012 (2012) 542976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Almeida A, Allen KL, Bates TE, Clark JB, Effect of reperfusion following cerebral ischaemia on the activity of the mitochondrial respiratory chain in the gerbil brain, J. Neurochem 65 (1995) 1698–1703. [DOI] [PubMed] [Google Scholar]
- [6].Hinkle PC, Butow RA, Racker E, Chance B, Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavincytochrome beta region of the respiratory chain of beef heart submitochondrial particles, J. Biol. Chem 242 (1967) 5169–5173. [PubMed] [Google Scholar]
- [7].Andreyev AY, Kushnareva YE, Starkov AA, Mitochondrial metabolism of reactive oxygen species, Biochemistry 70 (2005) 200–214. [DOI] [PubMed] [Google Scholar]
- [8].Murphy MP, How mitochondria produce reactive oxygen species, Biochem J 417 (2009) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stepanova A, Kahl A, Konrad C, Ten V, Starkov AS, Galkin A, Reverse electron transfer results in a loss of flavin from mitochondrial complex I: potential mechanism for brain ischemia reperfusion injury, J. Cereb. Blood Flow. Metab 37 (2017) 3649–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kotlyar AB, Vinogradov AD, Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase, Biochim. Biophys. Acta 1019 (1990) 151–158. [DOI] [PubMed] [Google Scholar]
- [11].Gorenkova N, Robinson E, Grieve D, Galkin A, Conformational change of mitochondrial complex I increases ROS sensitivity during ischaemia, Antioxid. Redox Signal 19 (2013) 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Babot M, Birch A, Labarbuta P, Galkin A, Characterisation of the active/de-active transition of mitochondrial complex I, Biochim. Biophys. Acta 1837 (2014) 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Galkin A, Meyer B, Wittig I, Karas M, Schägger H, Vinogradov A, et al. , Identification of the mitochondrial ND3 subunit as a structural component involved in the active/deactive enzyme transition of respiratory complex I, J. Biol. Chem 283 (2008) 20907–20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Puka-Sundvall M, Gajkowska B, Cholewinski M, Blomgren K, Lazarewicz JW, Hagberg H, Subcellular distribution of calcium and ultrastructural changes after cerebral hypoxia-ischemia in immature rats, Brain Res. Dev. Brain Res 125 (2000) 31–41. [DOI] [PubMed] [Google Scholar]
- [15].Matsiukevich D, Randis TM, Utkina-Sosunova I, Polin RA, Ten VS, The state of systemic circulation, collapsed or preserved defines the need for hyperoxic or normoxic resuscitation in neonatal mice with hypoxia-ischemia, Resuscitation 81 (2010) 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research, PLoS Biol 8 (2010) e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mayurasakorn K, Niatsetskaya ZV, Sosunov SA, Williams JJ, Zirpoli H, Vlasakov I, et al. , DHA but Not EPA emulsions preserve neurological and mitochondrial function after brain hypoxia-ischemia in neonatal mice, PLoS One 11 (2016) e0160870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Juliano C, Sosunov S, Niatsetskaya Z, Isler JA, Utkina-Sosunova I, Jang I, et al. , Mild intermittent hypoxemia in neonatal mice causes permanent neurofunctional deficit and white matter hypomyelination, Exp. Neurol 264 (2015) 33–42. [DOI] [PubMed] [Google Scholar]
- [19].Sosunov SA, Ameer X, Niatsetskaya ZV, Utkina-Sosunova I, Ratner VI, Ten VS, Isoflurane anesthesia initiated at the onset of reperfusion attenuates oxidative and hypoxic-ischemic brain injury, PLoS One 10 (2015) e0120456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Niatsetskaya ZV, Sosunov SA, Matsiukevich D, Utkina-Sosunova IV, Ratner VI, Starkov AA, et al. , The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice, J. Neurosci 32 (2012) 3235–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stepanova A, Shurubor Y, Valsecchi F, Manfredi G, Galkin A, Differential susceptibility of mitochondrial complex II to inhibition by oxaloacetate in brain and heart, Biochim. Biophys. Acta 1857 (2016) 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maklashina E, Kotlyar AB, Karliner JS, Cecchini G, Effect of oxygen on activation state of complex I and lack of oxaloacetate inhibition of complex II in Langendorff perfused rat heart, FEBS Lett 556 (2004) 64–68. [DOI] [PubMed] [Google Scholar]
- [23].Kalashnikov DS, Grivennikova VG, Vinogradov AD, Submitochondrial fragments of brain mitochondria: general characteristics and catalytic properties of NADH: ubiquinone oxidoreductase (complex I), Biochemistry 76 (2011) 209–216. [DOI] [PubMed] [Google Scholar]
- [24].Galkin A, Moncada S, S-nitrosation of mitochondrial complex I depends on its structural conformation, J. Biol. Chem 282 (2007) 37448–37453. [DOI] [PubMed] [Google Scholar]
- [25].Galkin A, Brandt U, Superoxide radical formation by pure complex I (NADH: ubiquinone oxidoreductase) from Yarrowia lipolytica, J. Biol. Chem 280 (2005) 30129–30135. [DOI] [PubMed] [Google Scholar]
- [26].Utkina-Sosunova IV ZV, Niatsetskaya SA Sosunov, V.I. Ratner, D. Matsiukevich, V.S. Ten, Nelfinavir inhibits intra-mitochondrial calcium influx and protects brain against hypoxic-ischemic injury in neonatal mice, PLoS One 8 (2013) e62448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S, Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine, J. Cereb. Blood Flow. Metab 7 (1987) 752–758. [DOI] [PubMed] [Google Scholar]
- [28].Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. , Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS, Nature 515 (2014) 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Votyakova TV, Reynolds IJ, ΔΨm-dependent and -independent production of reactive oxygen species by rat brain mitochondria, J. Neurochem 79 (2001) 266–277. [DOI] [PubMed] [Google Scholar]
- [30].Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD, Sites of reactive oxygen species generation by mitochondria oxidizing different substrates, Redox Biol 1 (2013) 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tahara EB, Navarete FD, Kowaltowski AJ, Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation, Free Radic. Biol. Med 46 (2009) 1283–1297. [DOI] [PubMed] [Google Scholar]
- [32].Cino M, Del Maestro RF, Generation of hydrogen peroxide by brain mitochondria: the effect of reoxygenation following postdecapitative ischemia, Arch. Biochem. Biophys 269 (1989) 623–638. [DOI] [PubMed] [Google Scholar]
- [33].Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, et al. , Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I, Nat. Med 19 (2013) 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sahni PV, Zhang J, Sosunov S, Galkin A, Niatsetskaya Z, Starkov A, et al. , Krebs cycle metabolites and preferential succinate oxidation following neonatal hypoxicischemic brain injury in mice, Pediatr. Res (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamel D, Sanchez M, Duhamel F, Roy O, Honore JC, Noueihed B, et al. , G-protein-coupled receptor 91 and succinate are key contributors in neonatal post-cerebral hypoxia-ischemia recovery, Arterioscler. Thromb. Vasc. Biol 34 (2014) 285–293. [DOI] [PubMed] [Google Scholar]
- [36].Murphy MP, Understanding and preventing mitochondrial oxidative damage, Biochem. Soc. Trans 44 (2016) 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blaza JN, Vinothkumar KR, Hirst J, Structure of the deactive state of mammalian respiratory complex I, Structure 26 (2018) 312–319 (e3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Methner C, Chouchani ET, Buonincontri G, Pell VR, Sawiak SJ, Murphy MP, et al. , Mitochondria selective S-nitrosation by mitochondria-targeted S-nitrosothiol protects against post-infarct heart failure in mouse hearts, Eur. J. Heart Fail 16 (2014) 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]