Abstract
Background and Purpose-
Previously, murine models Krit1+/−Msh2−/− and Ccm2+/−Trp53−/− showed a reduction or no effect on cerebral cavernous malformation (CCM) burden and favorable effects on lesional hemorrhage by the robust RhoA kinase (Rock) inhibitor fasudil and by simvastatin (a weak pleiotropic inhibitor of Rock). Herein we concurrently investigated treatment of the more aggressive Pdcd10/Ccm3 model with fasudil, simvastatin and higher dose atorvastatin to determined effectiveness of Rock inhibition.
Methods-
The murine models, Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/−, were contemporaneously treated from weaning to 5 months of age with fasudil (100 mg/kg/day in drinking water, n=9), simvastatin (40 mg/kg/day in chow, n=11), atorvastatin (80 mg/kg/day in chow, n=10) or with placebo (n=16). We assessed CCM volume in mouse brains by micro-computed tomography. Lesion burden was calculated as lesion volume normalized to total brain volume. We analyzed chronic hemorrhage in CCM lesions by quantitative intensity of Perls staining in brain sections.
Results-
The Pdcd10+/−Trp53−/−/Msh2−/− models showed a mean CCM lesion burden per mouse reduction from 0.0091 in placebos to 0.0042 (p=0.027) by fasudil, and to 0.0047 (p=0.025) by atorvastatin treatment, but was not changed significantly by simvastatin. Hemorrhage intensity per brain was commensurately decreased by Rock inhibition.
Conclusions-
These results support the exploration of proof of concept effect of high dose atorvastatin on human CCM disease for potential therapeutic testing.
Keywords: Atorvastatin Calcium; Fasudil; Hemangioma, Cavernous, Central Nervous System; Hydroxymethylglutaryl-CoA Reductase Inhibitors; rho-Associated Kinases;; Simvastatin; Therapeutics
Keywords: Animal Models of Human Disease, Basic Science Research, Translational Studies, Genetically Altered and Transgenic Models, Pharmacology, Cerebrovascular Malformations
Cerebral cavernous malformation (CCM), a disease frequently leading to hemorrhagic stroke and seizures, has 3 familial forms: KRIT/CCM1, CCM2 and PDCD10/CCM3. In murine models, as in humans, Pdcd10/Ccm3 heterozygosity is more aggressive in forming CCM lesions than Krit1/Ccm1 or Ccm2 heterozygosity.1 Pdcd10+/− mice bred in sensitized backgrounds with Trp53−/− or Msh2−/− harbored more lesions attributed to the Knudsonian 2-hit mechanism than Pdcd10+/− mice not bred in sensitized backgrounds since these genetic sensitizers enhance somatic mutations.1, 2
Acute models of CCM disease have been used to evaluate possible therapies, including the anti-oxidant tempol,3 the VEGF receptor inhibitor SU5416 semaxanib,4 TGF-β and β-catenin inhibitor sulindac metabolites,5 δ-notch activators recombinant DLL46 and Sorafenib,7 anti-MEK5 BIX021898 and anti-ERK5 XMD17–109.8 More clinically relevant studies would be expected by using chronic models which more closely resemble the human disease. We have shown in a previous report,9 that similar to the human disease, but in contrast to murine acute models, lesions in chronic murine models are distributed throughout the brain, with associated hemorrhage, B- and T- cell infiltration and disruption of junctional proteins. We previously reported that lesion burden was decreased in chronic models by the Rock inhibitor fasudil in Krit1+/−Msh2−/− mice10 and by B cell depletion in Pdcd10+/− and Pdcd10+/−Trp53−/− models.11
The small GTPase Rho effector, Rho-associated protein kinase (Rock), is a regulator of cellular contraction, cell division, and gene expression, as well as other functions. CCM therapies include targeting against Rho or the upstream effector proteases, including disintegrins and metalloproteinases.8 Rock can be inhibited specifically with fasudil, or by statins with pleotropic effects.12 Previously, we showed that fasudil, but not simvastatin, decreased lesion burden in the Krit1+/−Msh2−/− model, with no effect in the Ccm2+/−Trp53−/− model with any of these Rock inhibitors.10
Herein we assessed the effect of higher dose and more potent atorvastatin in the Krit1+/−Msh2−/− model. We concurrently investigated treatment of the more aggressive Pdcd10 models with fasudil and simvastatin, and higher dose and potency atorvastatin, on lesion burden and hemorrhage, and determined the effect of these Rock inhibitors on animal survival and the prevalence of endothelial cells and leukocytes with Rock activity within CCM lesions.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Murine Models
The Duke University Institutional Animal Care and Use Committee approved the animal procedures. The Pdcd10+/−, Pdcd10+/−Trp53−/−, Pdcd10+/−Msh2−/− and Krit1+/−Msh2−/− models for CCM disease were developed as previously reported.1, 2 The experiments included 53 Pdcd10+/−Trp53−/− (45 males, 8 females), 6 Pdcd10+/−Msh2−/− (5 males, 1 female), 88 Pdcd10+/− (50 males, 38 females), 55 Krit1+/−Msh2−/− (34 males, 21 females) animals assigned to groups after weaning.
Randomized Assignment and Treatment Groups
The National Institute of Neurological Disorders and Stroke guidelines for objectivity in preclinical research were followed for all groups, including randomization, blinding of outcome assessment, appropriate sample-size estimation based on the primary outcome, and prespecified data analyses.13 Mice receiving treatments were contemporaneously raised with placebo controls. Pdcd10+/−Trp53−/− or Pdcd10+/−Msh2−/− mice were randomized at weaning into 4 groups to receive fasudil (100 mg/kg/day in the drinking water), simvastatin (40 mg/kg/day in the chow), atorvastatin (80 mg/kg/day in the chow) or placebo with the same drug-free diet and drinking water until 4 months of age. Treatment was carried out until at least 100 days of age in all groups, unless there was attrition or compassionate sacrifice because of illness before then. Varying dates of completion of treatment were influenced by signs of poor health (Supplemental Methods in the online-only Data Supplement). Duration of treatment (range/mean/median) were not significantly different between the treatment groups (Table VI in the online-only Data Supplement). Survival lifetables were compared for up 100 days and all attritions were catalogued and compared (Table IV in the online-only Data Supplement). Pdcd10+/− (without sensitizing background) mice were similarly assigned to receive the same regimen of fasudil, simvastatin, atorvastatin or placebo until 5 months of age. Krit1+/−Msh2−/− mice were assigned to receive the same regimen of atorvastatin or placebo until 5 months of age. Duration of treatment (range/mean/median) were not significantly different between the treatment groups (Table VI in the online-only Data Supplement). Both simvastatin and atorvastatin, but not fasudil, significantly reduced the mean body weights of the combined Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− mice from 2–4 months of age (Table I in the online-only Data Supplement). Only simvastatin, but not fasudil or atorvastatin, significantly reduced the mean body weights of Pdcd10+/− mice from 2–5 months of age (Table II in the online-only Data Supplement). Atorvastatin significantly reduced the mean body weights of Krit1+/−Msh2−/− mice at 2, 3 and 5, but not 4, months of age (Table III in the online-only Data Supplement).
Lesion Burden Analysis
At the conclusion of drug administration, the brain of each mouse was removed and assessed for lesion burden by micro-computed tomography, with volumes of CCM lesions and total brain determined as previously reported.14 After imaging, brains were soaked in formalin, sliced into 1-mm thick coronal slices that were examined microscopically. Those slices determined to have possible CCM lesions and anatomically correlated with the micro-computed tomography were photographed. All slices were processed and embedded in paraffin. Those blocks identified to contain putative lesions were cut (5-μm) and sections were stained with hematoxylin and eosin (H&E). Sections were confirmed to be multicavernous lesions with at least 1 cavern > 100 μm by 2 observers (RS and TM) with adjudication by a third observer (IAA), as described previously.10 All assessors were blinded to the treatment assignment.
H&E brain sections from mice that either died or were euthanized due to a non-CCM related pathology are summated in Tables IV and V in the online-only Data Supplement. Tumors on sections were confirmed by neuropathologist co-author, PP.
Blank sections from brains harboring multicavernous CCM lesions were processed for quantitative assessment of non-heme iron deposition by Perls Prussian blue through methods previously described.2, 10, 11 There is no unit for the non-heme iron measurements because this value is the integrated sum of pixel values each having a unitless value between 0 and 255 depending on the intensity of the Perls blue stain. Five similar sections randomly selected from each of the 4 treatment groups of combined Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− mice were assessed for Rock activity by staining for phosphorylated myosin light chain (pMLC) and for inflammation by staining for B and T lymphocytes as previously described.2, 10, 11 The number of leukocytes and endothelial cells in CCM lesions stained or not stained, as well as the total number of B and T lymphocytes per lesion, were assessed by 2 observers (RS and JK, SPP, SBL or DZ).
Primary and secondary outcomes are defined with statistical methods in the online-only Data Supplement under Methods section.
Results
Fasudil and Atorvastatin Decreased Lesion Burden and Lesion Hemorrhage in Pdcd10 and Krit1 Models Bred in Sensitized Backgrounds
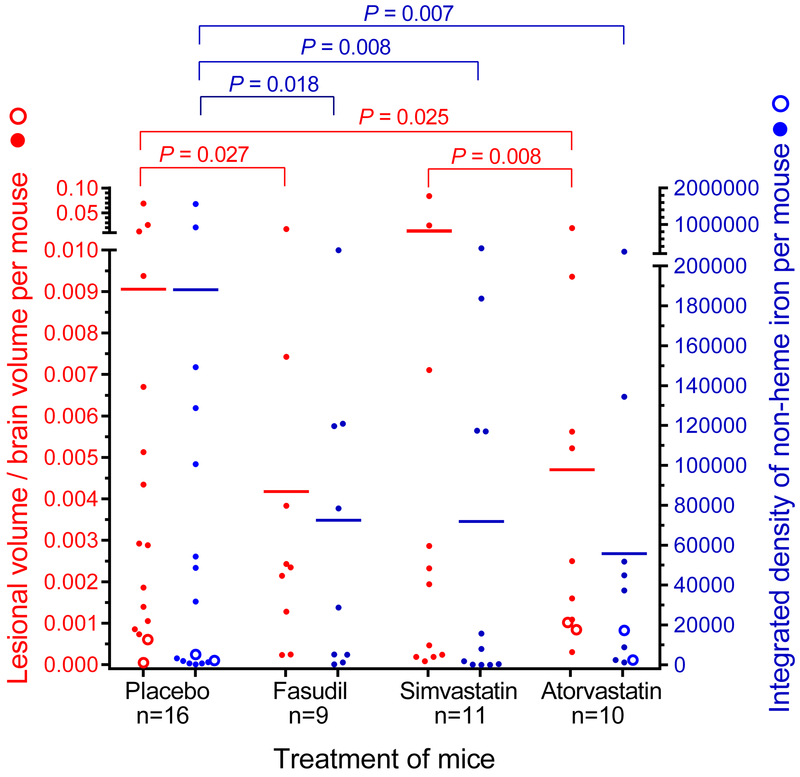
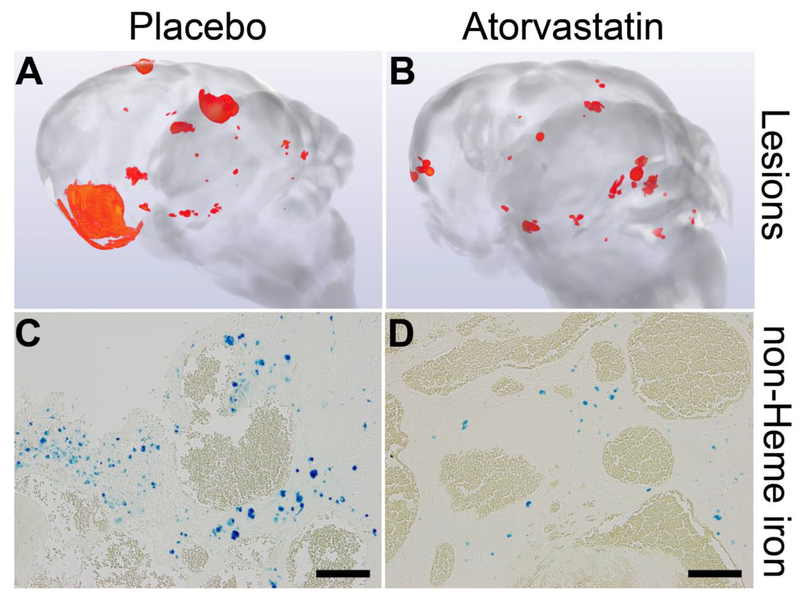
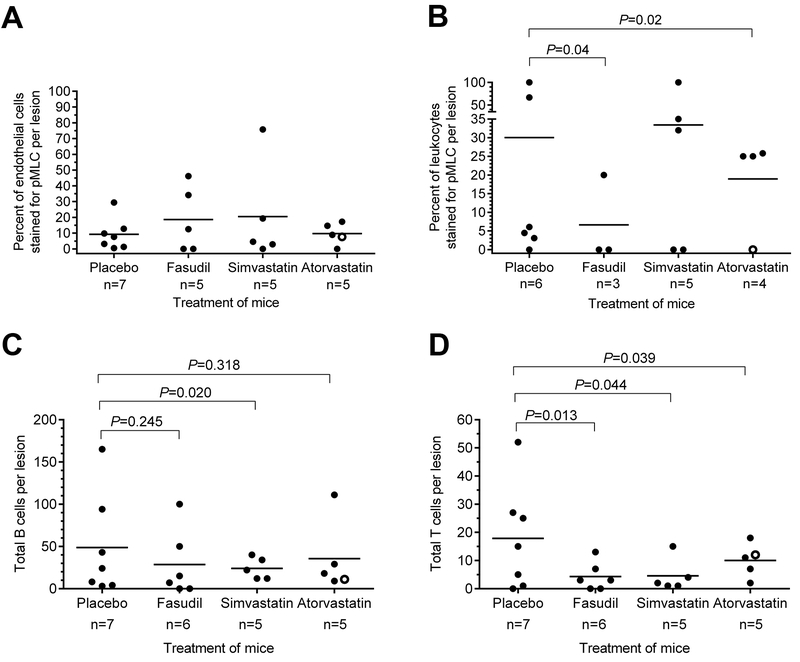
Mean lesion burden (±SEM) in Pdcd10+/− models bred in sensitized backgrounds with Trp53−/− or Msh2−/− was halved by fasudil treatment (0.0042±0.0018, n=9, P=0.03) and by atorvastatin treatment (0.0047±0.0019, n= 10, P=0.03) compared with placebo controls (0.0091±0.0043, n=16), but was not affected by simvastatin treatment (0.0113±0.0076, n=11), with a significant difference (P=0.008) between those treated with simvastatin and those treated with atorvastatin (Figure 1). Mean non-heme iron deposition in lesions per mouse was halved by fasudil treatment (72490±32171, n=9, P=0.02) and by simvastatin treatment (71934±33742, n=11, P=0.008) and was decreased by 70% with atorvastatin treatment (55709±25698, n=10, P=0.007) compared with placebos (188138±107423, n=16). Similar results were obtained when only the Pdcd10+/−Trp53−/− model was analyzed separately, except that there was a trend for decreased lesion burden in mice treated with atorvastatin (Figure I in the online-only Data Supplement). Representative images show decreased lesion burden and iron deposition in atorvastatin-treated mice compared with placebos (Figure 2). Since much lower numbers of female from Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− mice survived to weaning age than males (age of onset of treatment), they could not be included in the study in similar proportion as males, hence results from females were not possible to evaluate (Table VII in the online-only Data Supplement). Rock inhibition did not decrease Rock activity in lesion endothelium from 5 mice in each group (Figure 3A). However, the mean prevalence of lesion leukocytes stained with pMLC from 5 mice in each group was significantly decreased by treatment with fasudil (P=0.04) or atorvastatin (P=0.02) compared with placebos (Figure 3B). Within CCM lesions from 5 mice in each group, there were significantly less B lymphocytes in mice treated with simvastatin, but not with fasudil or atorvastatin (Figure 3C), and significantly fewer T lymphocytes in mice treated with any of the 3 Rock inhibitors compared with placebos (Figure 3D).
Figure 1.
Rock inhibition diminishes lesion burden (left axis, red) and lesion hemorrhage (right axis, blue) in combined Pdcd10+/−Trp53−/− (solid circles) and Pdcd10+/−Msh2−/− (hollow circles) models. Treatment with fasudil and atorvastatin, but not simvastatin, decreased lesion burden compared to contemporaneously raised placebos. Compared with placebos, treatment of mice with any of 3 Rock inhibitors decreased non-heme iron deposition in lesions. The 2-sided Conover 2-sample test was used to assess for significance.
Figure 2.
Representative images generated from micro-computed tomography showing lesions (red) in brains (A, B) and from Perls staining of non-heme iron deposition (blue) depicting hemorrhage (C, D) in combined Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− models. Placebo mice had a greater lesion burden (A) and lesional hemorrhage (C) compared to those treated with atorvastatin (B, D). Scale bars are 100 μm.
Figure 3.
Rock activity and inflammation within lesions of combined Pdcd10+/−Trp53−/− (solid circles) and Pdcd10+/−Msh2−/− (hollow circles) models treated by Rock inhibitors. (A) Rock inhibition did not affect the prevalence of lesion endothelial cells stained with phosphorylate myosin light chain (pMLC) compared with placebos. (B) The prevalence of lesion leukocytes stained with pMLC was decreased by 80% in mice treated with fasudil and was halved in those treated with atorvastatin compared with placebos. In contrast, simvastatin did not affect the prevalence of lesion leukocytes with Rock activity compared with placebos. (C) The total number of B lymphocytes per lesion was halved by simvastatin treatment, but unaffected by fasudil and atorvastatin, compared with placebos. (D) The total number of T lymphocytes per lesion was significantly decreased by fasudil, simvastatin and atorvastatin treatments compared with placebos. The number of lesions analyzed is designated by n, from 5 mice per group. The 2-sided Conover 2-sample test was used to assess for significant differences.
The decreased lesion burden previously observed with fasudil (but not simvastatin) treatment in the Krit1+/−Msh2−/− model10 was recapitulated with atorvastatin treatment (Figure II in the online-only Data Supplement). Mean lesion burden in Krit1+/−Msh2−/− mice was significantly decreased (P=0.03) from 52.8±21.4 × 10−6 in placebos (n=19) to 2.6±1.0 × 10−6 in mice treated with atorvastatin (n=16). Mean non-heme iron in this model was completely eliminated by atorvastatin treatment (0.0±0.0, n=16, P=0.03) compared with placebos (68514±67776, n=19). These effects were reproduced when males and females were analyzed separately (Table VIII in the online-only Data Supplement), with only the decreased lesion burden in males treated with atorvastatin reaching significance (P=0.02).
In contrast, both lesion burden and non-heme iron deposition were unaffected with any of the three Rock inhibitor treatments in the Pdcd10+/− model, without sensitized background (Figure III in the online-only Data Supplement), including no differences observed between the sexes (Table IX in the online-only Data Supplement).
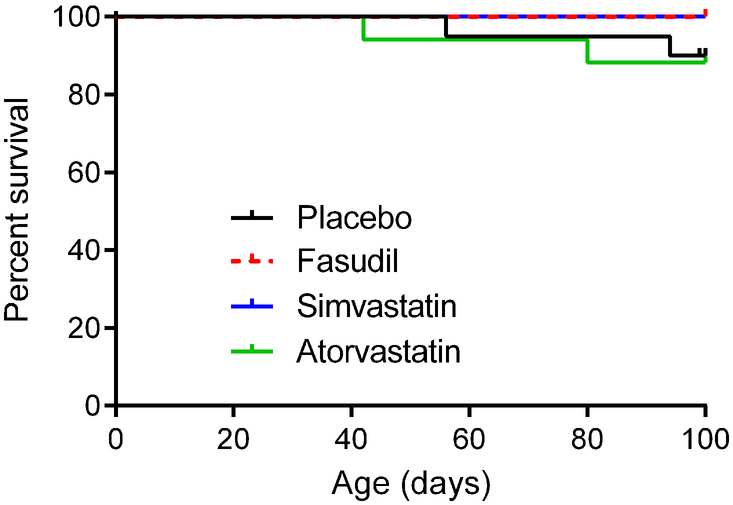
Survival of the Pdcd10 and Krit1 Models was not Affected by Rock Inhibition
Rock inhibition did not significantly affect survival after weaning in the combined Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− (Figure 4) and Krit1+/−Msh2−/− (Figure IV in the online-only Data Supplement) models. Male Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− mice showed similar results for attrition, while female Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− mice could not analyzed for attrition due to the small numbers of animals treated. Atorvastatin treatment did not affect survival in Krit1+/−Msh2−/− mice when males and females were analyzed separately. Brain hemorrhage did not increase in association with animal attrition with fasudil, simvastatin or atorvastatin treatment in either genotype (Tables IV and V in the online-only Data Supplement). In Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− mice, several different comparisons between statin-treated mice and control did not yield any statistical differences for attrition and brain hemorrhage (Table IV in the online-only Data Supplement).
Figure 4.
Attrition in combined Pdcd10+/−Trp53−/− and Pdcd10+/−Msh2−/− models. Kaplan-Meier plots show no significant effect of fasudil (n=9), simvastatin (n=13) or atorvastatin (n=17) treatment on survival compared with placebos (n=20) from weaning to the earliest age for the end of treatment (100 days of age). Although end of treatment ranged between 100 and 126 days of age, we compared survival curves up to 100 days, as all mice in the placebo and treatment groups were electively euthanized per intention to treat after at least 100 days. All animal attritions are reported and compared in Table IV in the online-only Data Supplement. There were neither overall significant differences (P=0.467), nor any individual significant differences between Rock inhibitor treatment and placebo groups (all P>0.24). When the entire 26-day range for ages of end of treatment were included in the analysis, there were neither overall significant differences (P=0.259), nor any individual significant differences between Rock inhibitor treatment and placebo groups (all P>0.10). The log-rank (Mantel-Cox) test was used to assess for significant differences.
Rock inhibition did not significantly affect survival after weaning in non-sensitized Pdcd10+/− mice. Only 1 of 22 placebo, 1 of 23 fasudil-, 0 of 21 simvastatin- and 1 of 22 atorvastatin-treated mice did not survive until the end of treatment. Attrition of these animals were not attributed to any known causes, with no hemorrhage or tumors observed within their brains.
Discussion
As observed with the human familial genotypes, Pdcd10+/− models have a more aggressive phenotype than Krit1+/− and Ccm2+/− models.1 Pdcd10 is part of the STRIPAK signaling pathway, thought to be distinct from the RhoA signaling pathway involving the other CCM gene products, but there is conflicting data on whether the two pathways converge.15 In support of a role for Pdcd10 in RhoA signaling, lesion burden was blunted by fasudil, but not simvastatin, in the Pdcd10+/− model bred in sensitized backgrounds, with exceptionally high lesion burdens (Figure 1). This recapitulated previous results in the Krit1+/−Msh2−/− mice.10 In the same studies, hemorrhage, as measured by non-heme iron deposition, was decreased by simvastatin, as well as by fasudil, in both of these models. These findings suggest that in both of these models bred in sensitized backgrounds, fasudil inhibits both lesion genesis and hemorrhage, while simvastatin at the dose used, only inhibits lesion hemorrhage. Atorvastatin, with a greater therapeutic intensity than simvastatin, decreased both lesion burden and hemorrhage in the combined Pdcd10+/−Trp53−/−/Msh2−/− models (Figure 1), as well as in the Krit1+/−Msh2−/− model (Figure IV in the online-only Data Supplement).
A modest increase in survival by fasudil, but not with simvastatin, was previously shown in the Krit1+/−Msh2−/− model.10 However, atorvastatin (Figure II in the online-only Data Supplement) did not affect survival in this model. Neither fasudil, simvastatin nor atorvastatin affected survival in the aggressive Pdcd10+/−Trp53−/−/Msh2−/− models (Figure 4). Hemorrhage, as related to attrition, was not increased by any of these therapies in both of the models.
There was a reduction of pMLC immunopositivity in lesional leukocytes, consistent with ROCK inhibition. An impact of the three drugs on lesional inflammatory cell infiltration was noted, consistent with reported anti-inflammatory effects ROCK inhibitors and statins.16 But we did not observe a reduction of pMLC immunostaining of lesional ECs, while a reduction of EC immunopositivity had been noted with fasudil in Krit1+/−Msh2−/− mice.17 This difference may be attributable to the significantly greater baseline ROCK activity in Pdcd10 mutated endothelium.1
Fasudil and statins had no effect on lesion burden in the less aggressive models, Ccm2+/−Trp53−/−10, and Pdcd10+/− without sensitized backgrounds (Figure III in the online-only Data Supplement). While simvastatin decreased lesion hemorrhage in the Ccm2+/−Trp53−/− model, neither fasudil, simvastatin nor atorvastatin affected lesion hemorrhage in the Pdcd10+/− mice. Lesion burden in these non-sensitized models was exceptionally low, even in placebo treated animals, and this may explain the lack of therapeutic benefit.
Overall, these studies indicate that both fasudil and atorvastatin are effective in reducing lesion burden and hemorrhage in the more aggressive forms of CCM disease. In 1995, parenteral fasudil was approved in Japan for a short course of therapy for the prevention of cerebral vasospasm after aneurysmal subarachnoid hemorrhage,18 but lacks preclinical safety studies for approval in the United States for any condition. Fasudil toxicity includes subcutaneous hemorrhage, subarachnoid hemorrhage, nausea, pyrexia, kidney failure and hypotension, and it has not been used chronically for any condition.18–22 In contrast, atorvastatin is commonly used in clinical practice. Human statin dose varies between approximately 0.1–1 mg/kg bodyweight, while most studies in rodents have used doses of 1–100 (or up to 500) mg/kg.23 Per United States Food and Drug Administration guidelines, an atorvastatin mouse dose of 80 mg/kg/day is equivalent to a “human starting dose” of 44.8 mg/day [Human starting dose = Mouse dose X 0.08 (Scaling factor) X 70 kg (Adult weight)].24, 25 A plasma area under curve (0–24 hours) profile for atorvastatin indicates that a human adult dose of 96 mg/day replicates the mouse dose of 80 mg/kg/day.26 Per other empiric evidence, atorvastatin dose of 100 mg/kg/day or greater is needed to decrease disease activity in collagen induced arthritis murine model,27, 28 while a human dose of 40 mg/day decreases the disease activity score (DAS28) in arthritis patients.29, 30 In the present study, we have shown a Rock inhibitory effect in mouse leukocytes with atorvastatin 80 mg/kg/day, and this was recapitulated in humans at 40–80 mg/day.31, 32 Hence the atorvastatin dose of 80 mg/kg/day in our preclinical experiments is within an equivalent human dose range of 40–80 mg/day, commonly used in clinical practice and shown to achieve a robust ROCK inhibition effect. Therapy of CCM disease with atorvastatin has already been proposed for testing in the Atorvastatin Treatment of Cavernous Angiomas with Symptomatic Hemorrhage Exploratory Proof of Concept (AT CASH EPOC) Trial (NCT02603328), a phase I/IIA randomized, placebo-controlled, double-blinded, single-site clinical trial. Data depicted herein served as pre-clinical justification for approval and funding of this trial (NIH/NINDS R01 NS107887).
Concern has been raised about potentially increased brain hemorrhage by statins based on high dose administration in zebrafish embryos,33–35 although it is unclear that this is relevant in mammals. A clinical database at our institution includes 24 human subjects with CCMs enrolled in Institutional Review Board approved biomarker studies, who are receiving various statins for routine cardiovascular indications unrelated to their CCM disease (atorvastatin 10–80 mg; simvastatin 10–60 mg; lovastatin 80 mg). Among those patients followed prospectively for 247 lesion-years (counting T2-weighted lesions > 4mm), there was only 1 documented symptomatic hemorrhage (SH) (0.4 SH per 100 lesion-years). This bleed rate is substantially lower than 173 bleeds during 1,604 lesion-years (counting T2-weighted lesions > 4 mm) of follow-up of statin naïve cases in the same database (10.7 SH per 100 lesion-years). While this data does not control for dose, patient demographics or CCM features, it does not raise any concern about hemorrhagic propensity in patients receiving statins, nor in comparison to widely reported CCM bleed rates.36 And there were no cases of CCM hemorrhage after initiation of simvastatin therapy (20–40 mg tablet taken daily by mouth. Month 1: 20 mg; Months 2 and 3: 40 mg) in a Phase I clinical trial (trials.gov# NCT01764451) of CCM familial patients in New Mexico (Personal Communication by trial Principal Investigator Leslie Morrison) nor any reported case in the published literature (Search was performed on August 20th 2018 using MEDLINE portal for keywords “cerebral cavernous malformation”, “angioma” or “cavernoma” and “statin” or “atorvastatin” and “hemorrhage” or “bleeding”).
Statins have been widely used and well tolerated in neurosurgical patients, including for the prevention of ischemic deficits after subarachnoid hemorrhage.37 Statins were recently reported to portend better outcome after intracerebral hemorrhage in man.38 In a meta-analysis of clinical trials of stroke prevention, including 83,205 patients, there was no evidence of increased hemorrhagic risk with long term statin use.39 However, two clinical trials of stroke prevention (but not all others) did signal a potentially increased hemorrhage risk with statin in secondary analyses.39 A recent report associated statin use with cerebral microbleeds in patients presenting with intracerebral hemorrhage,40 but did not examine causation nor relevance to CCMs. Considering all evidence class and level, American Stroke Association guidelines on management and prevention of intracerebral hemorrhage include no restrictions on the use of statins in patients with hemorrhagic stroke.41 Altogether the cautious enthusiasm generated by our data, this information, and some concerns about other potentially harmful pleiotropic effects, create a veritable equipoise, motivating a need for specific and careful study of statins in hemorrhagic CCM.34
In all of the CCM murine models tested, body weight was decreased by simvastatin or atorvastatin therapy, but was unaffected with the specific Rock inhibitor fasudil. This suggests that the pleiotropic effect of statins reduces body weights through an alternative mechanism than Rock inhibition. Another group showed that simvastatin reduced body weight in older mice when treatment started at one year of age, without altering caloric consumption, suggesting that this may affect energy utilization in these animals.42 Our study examined quantified differences in lesional bleeding, but was not designed nor powered to detect differences in fatal brain hemorrhages or other causes of attrition due to various pharmacotherapies. The absence of significant differences reported herein does not exclude potential harmful effects of the respective drugs. In particular, potential harmful effects in the non-sensitized models may have been missed in view of the low lesion burdens in these models. However, it is reassuring that none of the drugs increased the very low level of attrition in the non-sensitized mice. Hence, the greater attrition in the more aggressive sensitized models was most likely related to their aggressive disease rather than pharmacologic treatment. Implications of these observations in human use are uncertain, and will need to be examined, along with other side effects, in the course of trials in CCM patients.
Although only male animals reached statistical significance in subgroup analysis by sex, lesion burden was diminished by similar extents in both sex subgroups of Krit1+/−Msh2−/− mice treated with atorvastatin. Lack of statistical significance in female animals is most likely due to the low number of female mice in this model, which was noted previously in CCM models treated with fasudil.10 The biologic basis for male preponderance among mice bred with these models is presented in the Supplemental Methods. The study was also not designed to examine potential differences in sensitizer (Trp53 versus Msh2) in the sensitized Pdcd10 models.
Taken together, these preclinical findings show that atorvastatin is as effective as the specific Rock inhibitor fasudil and more effective than simvastatin in reducing CCM lesion in murine models of CCM disease at the doses indicated. Moreover, all three of these Rock inhibitors decrease hemorrhage as measured by non-heme iron deposition in the lesions. Among outcome parameters in human subjects with CCM disease, hemorrhage with neurological symptoms is the strongest.43 Hence, the AT-CASH-EPOC trial, is enrolling CCM patients with symptomatic hemorrhage, with the primary outcome as a reduction of lesion hemorrhage as measured by quantitative susceptibility mapping magnetic resonance imaging. In parallel, an ongoing trial readiness44 for subjects with the same symptoms will recruit subjects for the AT-CASH-EPOC trial and for other clinical trials.
Summary
Both Rock inhibitors, fasudil and atorvastatin, decreased lesion burden in aggressive Pdcd10 models. Both of these treatments, along with simvastatin, reduced lesion hemorrhage. These preclinical results support the testing of atorvastatin therapy in a clinical trial involving human subjects with CCM disease for potential therapeutic testing.
Supplementary Material
Acknowledgments
Sources of Funding
This work was partially supported by grants from the National Institutes of Health, including from National Institute of Neurological Disorders and Stroke R01 NS077957 and P01 NS092521 to Drs. Awad and Marchuk, the National Center for Advancing Translational Sciences UL1 TR000430 and the University of Chicago Medicine Comprehensive Cancer Center Support Grant P30 CA14599, and by the Immunohistochemistry, Integrated Microscopy, and PaleoCT core facilities’ expertise and services. The funding sources had no input on data analysis or interpretation of results.
Footnotes
Disclosures
JKL has consultancy for Asahi-Kasei Pharmaceuticals, Inc., Celgene, Pliant and Amgen.
Presented in part at the International Stroke Conference, Los Angeles, CA, January 24-26, 2018
References
- 1.Shenkar R, Shi C, Rebeiz T, Stockton RA, McDonald DA, Mikati AG, et al. Exceptional Aggressiveness of Cerebral Cavernous Malformation Disease Associated with Pdcd10 Mutations. Genet Med. 2015;17:188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald DA, Shenkar R, Shi C, Stockton RA, Akers AL, Kucherlapati MH, et al. A Novel Mouse Model of Cerebral Cavernous Malformations Based on the Two-Hit Mutation Hypothesis Recapitulates the Human Disease. Hum Mol Genet. 2011;20:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson CC, Zhu W, Davis CT, Bowman-Kirigin JA, Chan AC, Ling J, et al. Strategy for Identifying Repurposed Drugs for the Treatment of Cerebral Cavernous Malformation. Circulation. 2015;131:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiStefano PV, Kuebel JM, Sarelius IH, Glading AJ. Krit1 Protein Depletion Modifies Endothelial Cell Behavior Via Increased Vascular Endothelial Growth Factor (Vegf) Signaling. J Biol Chem. 2014;289:33054–33065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravi L, Rudini N, Cuttano R, Giampietro C, Maddaluno L, Ferrarini L, et al. Sulindac Metabolites Decrease Cerebrovascular Malformations in Ccm3-Knockout Mice. Proc Natl Acad Sci U S A. 2015;112:8421–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You C, Sandalcioglu IE, Dammann P, Felbor U, Sure U, Zhu Y. Loss of Ccm3 Impairs Dll4-Notch Signalling: Implication in Endothelial Angiogenesis and in Inherited Cerebral Cavernous Malformations. J Cell Mol Med. 2013;17:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wustehube J, Bartol A, Liebler SS, Brutsch R, Zhu Y, Felbor U, et al. Cerebral Cavernous Malformation Protein Ccm1 Inhibits Sprouting Angiogenesis by Activating Delta-Notch Signaling. Proc Natl Acad Sci U S A. 2010;107:12640–12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Tang AT, Wong WY, Bamezai S, Goddard LM, Shenkar R, et al. Cerebral Cavernous Malformations Arise from Endothelial Gain of Mekk3-Klf2/4 Signalling. Nature. 2016;532:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeineddine HA, Girard R, Saadat L, Shen L, Lightle R, Moore T, et al. Phenotypic Characterization of Murine Models of Cerebral Cavernous Malformations. [published online ahead of print June 26, 2018]. Lab Invest. 2018. http://www.nature.com/articles/s41374-018-0030-y. Accessed October 25, 2018. [DOI] [PMC free article] [PubMed]
- 10.Shenkar R, Shi C, Austin C, Moore T, Lightle R, Cao Y, et al. Rhoa Kinase Inhibition with Fasudil Versus Simvastatin in Murine Models of Cerebral Cavernous Malformations. Stroke. 2017;48:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi C, Shenkar R, Zeineddine HA, Girard R, Fam MD, Austin C, et al. B-Cell Depletion Reduces the Maturation of Cerebral Cavernous Malformations in Murine Models. J Neuroimmune Pharmacol. 2016;11:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Liao JK. Pleiotropic Effects of Statins. - Basic Research and Clinical Perspectives. Circ J. 2010;74:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A Call for Transparent Reporting to Optimize the Predictive Value of Preclinical Research. Nature. 2012;490:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard R, Zeineddine HA, Orsbon C, Tan H, Moore T, Hobson N, et al. Micro-Computed Tomography in Murine Models of Cerebral Cavernous Malformations as a Paradigm for Brain Disease. J Neurosci Methods. 2016;271:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goudreault M, D’Ambrosio LM, Kean MJ, Mullin MJ, Larsen BG, Sanchez A, et al. A Pp2a Phosphatase High Density Interaction Network Identifies a Novel Striatin-Interacting Phosphatase and Kinase Complex Linked to the Cerebral Cavernous Malformation 3 (Ccm3) Protein. Mol Cell Proteomics. 2009;8:157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedi O, Dhawan V, Sharma PL, Kumar P. Pleiotropic Effects of Statins: New Therapeutic Targets in Drug Design. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:695–712. [DOI] [PubMed] [Google Scholar]
- 17.McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH, et al. Fasudil Decreases Lesion Burden in a Murine Model of Cerebral Cavernous Malformation Disease. Stroke. 2012;43:571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao JK, Seto M, Noma K. Rho Kinase (Rock) Inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishihara M, Yamanaka K, Nakajima S, Yamasaki M. Intracranial Hemorrhage after Intra-Arterial Administration of Fasudil for Treatment of Cerebral Vasospasm Following Subarachnoid Hemorrhage: A Serious Adverse Event. Neuroradiology. 2012;54:73–75. [DOI] [PubMed] [Google Scholar]
- 20.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, et al. Efficacy and Safety of Fasudil in Patients with Stable Angina: A Double-Blind, Placebo-Controlled, Phase 2 Trial. J Am Coll Cardiol. 2005;46:1803–1811. [DOI] [PubMed] [Google Scholar]
- 21.Jiang DQ, Xu LC, Jiang LL, Li MX, Wang Y. Fasudil Combined with Methylcobalamin or Lipoic Acid Can Improve the Nerve Conduction Velocity in Patients with Diabetic Peripheral Neuropathy: A Meta-Analysis. Medicine (Baltimore). 2018;97:e11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong MM. Actelion Pharmaceuticals and Cotherix Held Liable for Fighting Off Competition to Tracleer® by Interfering with Rival Asahi’s Development of Fasudil. https://ashmasons.quora.com/Actelion-Pharmaceuticals-and-CoTherix-held-liable-for-fighting-off-competition-to-Tracleer%C2%AE-by-interfering-with-rival. February 2, 2014. Accessed October 24, 2018
- 23.Bjorkhem-Bergman L, Lindh JD, Bergman P. What Is a Relevant Statin Concentration in Cell Experiments Claiming Pleiotropic Effects? Br J Clin Pharmacol. 2011;72:164–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reigner BG, Blesch KS. Estimating the Starting Dose for Entry into Humans: Principles and Practice. Eur J Clin Pharmacol. 2002;57:835–845. [DOI] [PubMed] [Google Scholar]
- 25.USFDA. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Guidance for Industry, https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. 2005. Accessed December 7, 2018. [Google Scholar]
- 26.USFDA. Lipitor® (Atorvastatin Calcium) Tablets, for Oral Use. Drugs@Fda Label https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020702s067s069lbl.pdf. 2017. Accessed December 7, 2018.
- 27.Vandebriel RJ, De Jong HJ, Gremmer ER, Klungel OH, Tervaert JW, Slob W, et al. Statins Accelerate the Onset of Collagen Type Ii-Induced Arthritis in Mice. Arthritis Res Ther. 2012;14:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer G, Chobaz V, Talabot-Ayer D, Taylor S, So A, Gabay C, et al. Assessment of the Efficacy of Different Statins in Murine Collagen-Induced Arthritis. Arthritis Rheum. 2004;50:4051–4059. [DOI] [PubMed] [Google Scholar]
- 29.Sarabi ZS, Saeidi MG, Khodashahi M, Rezaie AE, Hashemzadeh K, Khodashahi R, et al. Evaluation of the Anti-Inflammatory Effects of Atorvastatin on Patients with Rheumatoid Arthritis: A Randomized Clinical Trial. Electron Physician. 2016;8:2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mowla K, Rajai E, Ghorbani A, Dargahi-Malamir M, Bahadoram M, Mohammadi S. Effect of Atorvastatin on the Disease Activity and Severity of Rheumatoid Arthritis: Double-Blind Randomized Controlled Trial. J Clin Diagn Res. 2016;10:OC32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawlings R, Nohria A, Liu PY, Donnelly J, Creager MA, Ganz P, et al. Comparison of Effects of Rosuvastatin (10 Mg) Versus Atorvastatin (40 Mg) on Rho Kinase Activity in Caucasian Men with a Previous Atherosclerotic Event. Am J Cardiol. 2009;103:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nohria A, Prsic A, Liu PY, Okamoto R, Creager MA, Selwyn A, et al. Statins Inhibit Rho Kinase Activity in Patients with Atherosclerosis. Atherosclerosis. 2009;205:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisa-Beygi S, Hatch G, Noble S, Ekker M, Moon TW. The 3-Hydroxy-3-Methylglutaryl-Coa Reductase (Hmgcr) Pathway Regulates Developmental Cerebral-Vascular Stability Via Prenylation-Dependent Signalling Pathway. Dev Biol. 2013;373:258–266. [DOI] [PubMed] [Google Scholar]
- 34.Eisa-Beygi S, Wen XY, Macdonald RL. A Call for Rigorous Study of Statins in Resolution of Cerebral Cavernous Malformation Pathology. Stroke. 2014;45:1859–1861. [DOI] [PubMed] [Google Scholar]
- 35.Eisa-Beygi S Simvastatin and Cerebral Cavernous Malformations (Re: Reinhard Et Al., 2016). J Neurol Sci. 2016;369:391. [DOI] [PubMed] [Google Scholar]
- 36.Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, et al. Untreated Clinical Course of Cerebral Cavernous Malformations: A Prospective, Population-Based Cohort Study. Lancet Neurol. 2012;11:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugawara T, Ayer R, Zhang JH. Role of Statins in Cerebral Vasospasm. Acta Neurochir Suppl. 2008;104:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biffi A, Devan WJ, Anderson CD, Ayres AM, Schwab K, Cortellini L, et al. Statin Use and Outcome after Intracerebral Hemorrhage: Case-Control Study and Meta-Analysis. Neurology. 2011;76:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amarenco P, Labreuche J. Lipid Management in the Prevention of Stroke: Review and Updated Meta-Analysis of Statins for Stroke Prevention. Lancet Neurol. 2009;8:453–463. [DOI] [PubMed] [Google Scholar]
- 40.Haussen DC, Henninger N, Kumar S, Selim M. Statin Use and Microbleeds in Patients with Spontaneous Intracerebral Hemorrhage. Stroke. 2012;43:2677–2681. [DOI] [PubMed] [Google Scholar]
- 41.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 42.Spindler SR, Mote PL, Flegal JM. Combined Statin and Angiotensin-Converting Enzyme (Ace) Inhibitor Treatment Increases the Lifespan of Long-Lived F1 Male Mice. Age (Dordr). 2016;38:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA, Angioma Alliance Scientific Advisory B. Hemorrhage from Cavernous Malformations of the Brain: Definition and Reporting Standards. Angioma Alliance Scientific Advisory Board. Stroke. 2008;39:3222–3230. [DOI] [PubMed] [Google Scholar]
- 44.Polster SP, Cao Y, Carroll T, Flemming K, Girard R, Hanley D, et al. Trial Readiness in Cavernous Angiomas with Symptomatic Hemorrhage (Cash). [published online ahead of print April 11, 2018]. Neurosurgery. 2018. https://academic.oup.com/neurosurgery/advance-article/doi/10.1093/neuros/nyy108/4967785. Accessed October 25, 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.