Abstract
Objective:
Umbilical hernias are a common diagnosis in young children. To our knowledge, no formal practice guideline exists to guide timing of operative repair for asymptomatic pediatric umbilical hernias, which often resolve spontaneously. To evaluate and better understand variations in practice patterns, we analyzed ambulatory surgery claims data from three demographically diverse states to assess the relationship between age at umbilical hernia repair and patient, hospital and geographic characteristics.
Study Design:
We performed a cross-sectional descriptive study of uncomplicated hernia repairs performed as a single procedure in 2012–2014, using the State Ambulatory Surgery and Services databases (SASD) for Wisconsin, New York and Florida. Age and demographic characteristics of umbilical hernia repair patients are described.
Results:
The SASD analysis included 6551 patients. Across three states, 8.2% of hernia repairs were performed in children <2 years, 18.7% in children 2–3 years and 73.0% in children ≥4 years, but there was significant variability (p<0.001) in practice patterns by state. In regression analysis, race, Medicaid insurance and rural residence were predictive of early repair, with African-American patients less likely to have a repair before age 2 (OR 0.62, p=0.046) and rural children (OR 1.53, p=0.009) and Medicaid patients (OR 2.01, p<0.001) more likely to do so. State of residence predicted early repair even when holding these variables constant.
Conclusion:
The age of pediatric umbilical hernia repair varies widely. As hernias may resolve over time and can be safely monitored with watchful waiting, formal guidelines are needed to support delayed repair and prevent unnecessary operations.
BACKGROUND
Umbilical hernias are extremely common, with an incidence of 15–23% in newborns (1, 2). Despite their prevalence, the natural history of asymptomatic umbilical hernias has not been well described. The majority of existing literature focuses on incidence and outcomes of incarcerated or gangrenous umbilical hernias, which are rare, occurring in less than 0.07% of umbilical hernia patients (3). Spontaneous hernia closure occurs in up to 90 percent of cases identified in newborns (4) and watchful waiting does not significantly increase the risk of complications from unrepaired pediatric umbilical hernias (4–10).
To our knowledge, no formal practice guidelines currently exist to define the appropriate age for repair of uncomplicated umbilical hernias in children. The few published cohort studies that address appropriate age for repair propose minimum age recommendations ranging from over 2 years to 10–12 years (5,6,11–16), but no increased risk of complications has been described by waiting until patients are at least 4 years old (4). A review of the websites of 63 American children’s hospitals that provided age-based recommendations for umbilical hernia repair found that 44% recommended repair at age 3 or older, 29% at age 4 or older and 21% at age 5 or older (4). Only 6% recommended repair at age 2 and none recommended repair at younger ages. The American College of Surgeons has released a patient education document suggesting that parents wait until age 5 to allow for the chance of spontaneous closure (17).
Although umbilical hernia repair is a comparatively minor operation involving minimal tissue disruption, the risks of any operation are relatively high for young children. Concern about the risk of neurocognitive effects of anesthesia has been growing, particularly for very young patients. A 2015 editorial published in the New England Journal of Medicine described the possible effects on children of anesthetic neurotoxicity, proposing that the risks of anesthetics for the developing brain should be carefully considered before their use on children aged 2 or younger (18). Also, the Federal Drug Administration (FDA) issued a 2016 statement proposing that whenever medically appropriate, surgery requiring prolonged general anesthesia or more than one anesthetic should be postponed in children under age 3 (19). Umbilical hernia repairs do not commonly require prolonged anesthesia; however, an additional procedure needed by a young pediatric patient after an elective hernia repair could put the child at risk of receiving multiple anesthetics. Data describing the risks of anesthesia in young children are found in meta-analyses of human and animal studies, which show long-term effects including academic performance deficits, behavioral problems, cognitive and language delays, and learning disabilities, although these results may be confounded by patient selection bias (20–29). The risks of acute surgical or anesthetic complications are also higher in young children. One study of children undergoing elective surgical procedures found that the likelihood of an adverse respiratory event declined 8% with each year of age (30). Anesthesia research has also described the long-term sequelae for young children of brain hypoperfusion and intraoperative hypotension, such as encephalopathy or seizures (31–34).
Thus, operative repair of umbilical hernia in young children with a high likelihood of spontaneous closure may expose patients to unnecessary anesthetic and operative risk (20–22,35). Unnecessary operations may also place a burden on healthcare systems with limited resources, as well as financial pressure on parents, insurers, and society. In order to evaluate and better understand the variation in age of umbilical hernia repair, we used all-payer databases in three demographically diverse states (Florida, New York, and Wisconsin) to determine the ages at which umbilical hernia repairs are performed and assess their relationship to patient, hospital and geographic characteristics.
METHODS
We performed a cross-sectional descriptive study using an all-payer database of three states (Wisconsin, New York, and Florida) over the years 2012–2014. The data source was the State Ambulatory Surgery and Services Database (SASD) of the Healthcare Cost and Utilization Project (HCUP), by the Agency for Healthcare Research and Quality (36). The SASD database contains de-identified patient data for ambulatory surgery and outpatient services. In the absence of a national outpatient-procedure claims database, we chose these states to represent large-population states with both urban and rural areas from geographically diverse areas of the country (Midwest, Northeast, and South). Furthermore, the data variables provided to the SASD by these specific states are comprehensive and include all payers and ambulatory surgery procedures. All analyses are in compliance with the HCUP Data Use Agreement.
We included children aged 17 years or younger who underwent an umbilical hernia repair, as defined by International Classification of Diseases, Ninth Revision (ICD-9) procedure codes 53.49 and 53.41 (Wisconsin) or current procedural terminology (CPT) codes 49580, 49582, 49585, and 49587 (Florida and New York). All associated procedure and CPT codes for each child’s encounter date were examined. Because umbilical hernias can be repaired as a component of another procedure (such as laparoscopy) or while the patient was under a general anesthetic for other reasons, children undergoing multiple procedures on the same day were excluded. Children with complicated umbilical hernias were also excluded, specifically those with diagnosis codes of “umbilical hernia with obstruction or gangrene” (551.1, 552.1) or CPT codes “repair of umbilical hernia, incarcerated or strangulated” (49582 and 49587). After applying our exclusion criteria, only single procedure, uncomplicated pediatric umbilical hernia repairs were included in our analysis.
Demographic data including patient age, gender, race and rural residence were abstracted. Age was categorized into clinically relevant groups: children less than two years old, two to three years old, and four years old or older. In order to characterize any chronic conditions of children undergoing hernia repair, we used the chronic condition indicator (CCI) included in the HCUP databases for the years 2012–13, which classified the conditions by body system (37). These data are excluded from the logistic regression analysis because they are not available for 2014. A variable on type of insurance was created based on the expected primary payer for the claim, specifically private insurance, Medicaid and other, which includes self-pay, Medicare and other government programs.
Hospitals were divided into high and low-volume centers to help identify whether practice patterns differed between surgeons who routinely perform umbilical hernia repairs in children, such as pediatric surgeons at children’s hospitals, and those who perform them as part of an all-age general surgical practice. A high-volume center was defined as a hospital performing 100 or more pediatric umbilical hernia repairs in the period 2012–14 and a low volume center as one that performed fewer.
Analyses
We conducted descriptive analyses, including Pearson χ2 tests to identify significant relationships between age of repair and characteristics of the patient or facility. We calculated the rates of umbilical hernia repair per 1000 children using 2013 state population data by single years of age (38). We also used logistic regression to examine the predictors of early umbilical hernia repair. Analysis was performed using Stata version 15 (39). Independent variables were state (Wisconsin, Florida, and New York), year (2012, 2013, and 2014), demographic characteristics (gender, race/ethnicity, and rural residence), payer type and the hospital’s volume of umbilical hernia repairs. P-value of less than 0.05 was considered statistically significant.
RESULTS
The SASD databases for Wisconsin, Florida, and New York contained 9,269 children who underwent umbilical hernia repair from 2012–2014. Of these, 2,571 children (28%) were excluded for having multiple procedures on the day of umbilical hernia repair. An additional 147 children (2.2%) were excluded for having an umbilical hernia with incarceration or acute complication. The remaining 6,551 children who underwent a single procedure, uncomplicated umbilical hernia repair were included in our analysis (Figure 1).
Figure 1:
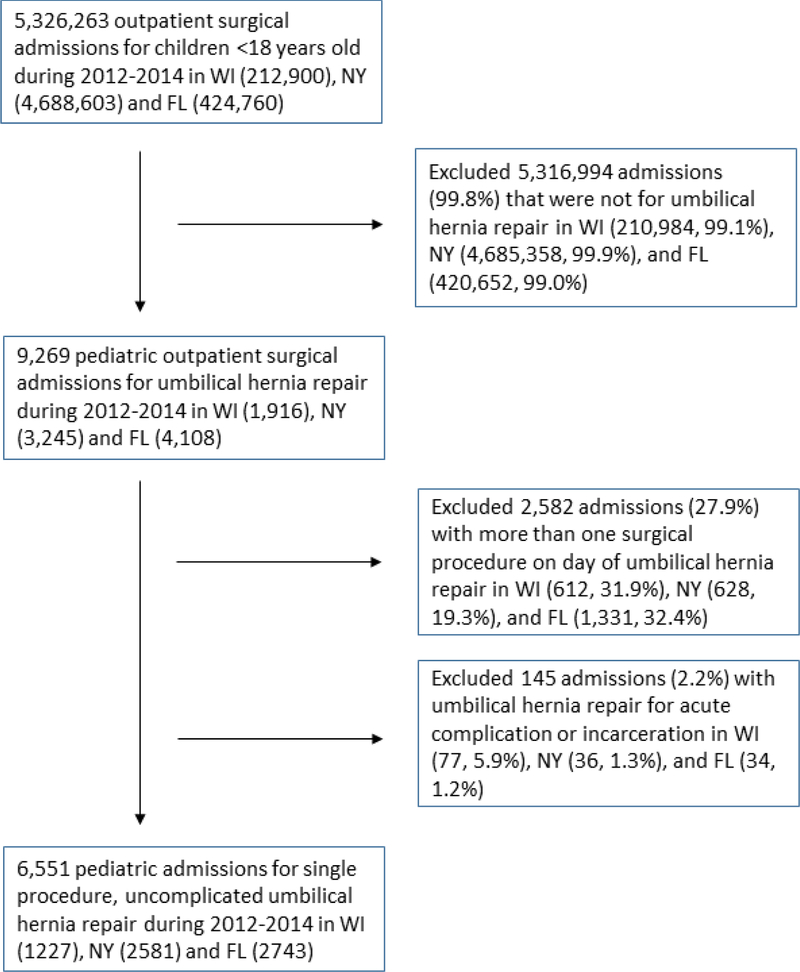
Study design and excluded patients, SASD (2012–2014)
Patient age at the time of umbilical hernia repair is shown in Table 1. Early umbilical hernia repairs (under age 2) were performed in 538 cases (8.2% of pediatric repairs). The percentage of repairs performed at this young age varied significantly across the three states studied, from 13.9% in Wisconsin to 5.4% in New York (p<.001). Figure 2 shows the rate of uncomplicated umbilical hernia repairs per 1000 children for each state, demonstrating that the rate of early repair is much higher in Wisconsin (with a peak at 2 years) than in New York and Florida (with peaks at 5 years). The overall rate of pediatric uncomplicated umbilical hernia repairs in Wisconsin (0.94 repairs per 1000 children under 18 years) was also significantly higher than the rates in Florida (0.68) and New York (0.61) (p<.001 for both).
Table 1:
Age of umbilical hernia repair and chronic conditions of patients undergoing repair by age
| Total (N=6551) |
Wisconsin (N= 1227) |
Florida (N= 2743) |
New York (N= 2581) |
p value* | |
|---|---|---|---|---|---|
| Age†: | <0.001 | ||||
| <2 years old | 538 (8.2%) | 171 (13.9%) | 228 (8.3%) | 139 (5.4%) | |
| 2–3 years old | 1227 (18.7%) | 402 (32.8%) | 388 (14.2%) | 437 (16.9%) | |
| ≥4 years old | 4786 (73.1%) | 654 (53.3%) | 2127 (77.5%) | 2005 (77.7%) | |
| Chronic conditions‡: | |||||
| Any system | 1090 (24.3%) | 235 (31.3%) | 466 (24.0%) | 389 (21.9%) | <0.001 |
| <2 years old | 111 (10.2%) | 32 (13.6%) | 52 (11.1%) | 27 (6.9%) | <0.001 |
| 2–3 years old | 223 (20.5%) | 85 (36.2%) | 79 (16.9%) | 59 (15.2%) | |
| ≥4 years old | 756 (69.3%) | 118 (50.2%) | 335 (71.9%) | 303 (77.9%) | |
| Respiratory | 482 (10.8%) | 93 (12.3%) | 189 (9.7%) | 200 (11.2%) | 0.1026 |
| <2 years old | 30 (6.2%) | -- | -- | -- | |
| 2–3 years old | 77 (16.0%) | -- | -- | -- | |
| ≥4 years old | 375 (77.8%) | -- | -- | -- | |
Pearson χ2 tests of independence were performed. P-values ≤ 0.05 are significant.
Age data are presented for the period 2012–2014
Because of limited availability, data on chronic conditions are presented for the period 2012–2013
Note that cell sizes of 10 cases or fewer were excluded from table as required by the data use agreement for the HCUP state databases.
Figure 2:
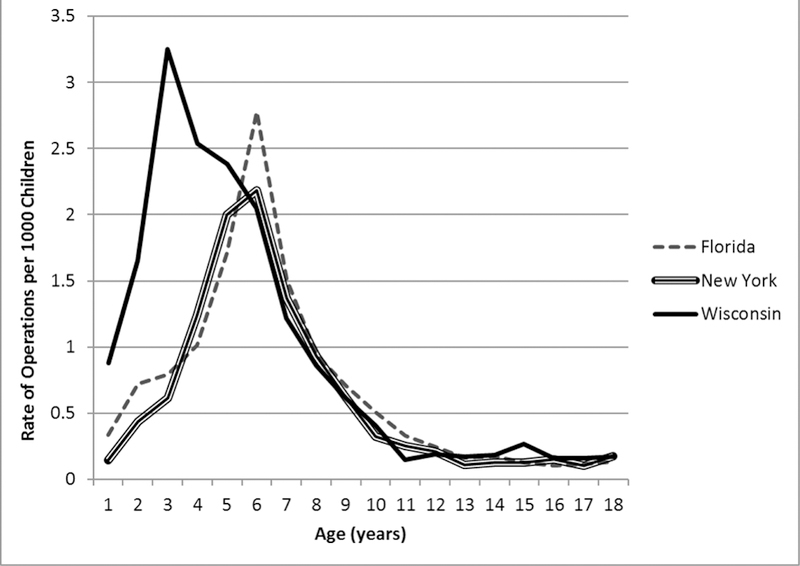
Rates of uncomplicated umbilical hernia repair by age
Chronic conditions were present in 24.3% of patients undergoing hernia repair, including 10.2% of children under 2 and 30.7% of children under 4. The percentage of under-2 repairs in patients with chronic conditions varied significantly across the three states, with the percentage in Wisconsin (13.6%) twice as high as New York (6.9%) (p<0.001). For 22.2 % of the patients with chronic respiratory conditions, repairs were performed before age 4. These conditions are most likely to be associated with anesthetic complications (40).
Table 2 describes the demographic, insurance and hospital characteristics for umbilical hernia repairs by age. Early repairs (under age 2) are performed on girls and boys at similar rates, and the differences between high- and low-volume centers are also small (8.4% in high volume and 7.9% in low volume centers). However, white children were more likely to have an early repair (10.2%) than those in other racial and ethnic groups. Pediatric patients living in rural areas and those with Medicaid insurance were also more likely to have early repairs.
Table 2:
Demographic, insurance and hospital characteristics by age at time of umbilical hernia repair
| Age at time of repair | <2 years | 2–3 years | ≥4 years | p value* |
|---|---|---|---|---|
| TOTAL | 538 (8.2%) | 1227 (18.7%) | 4786 (73.1%) | |
| Gender: | 0.005 | |||
| Female | 284 (8.0%) | 714 (20.2%) | 2543 (71.8%) | |
| Male | 254 (8.4%) | 513 (17.0%) | 2243 (74.5%) | |
| Race/Ethnicity: | <0.001 | |||
| White | 192 (10.2%) | 316 (16.8%) | 1372 (73.0%) | |
| Black | 241 (7.1%) | 661 (19.6%) | 2472 (73.2%) | |
| Hispanic | 59 (8.4%) | 148 (21.1%) | 493 (70.4%) | |
| Other | 32 (6.3%) | 85 (16.8%) | 388 (76.8%) | |
| Location of residence: | <0.001 | |||
| Urban | 479 (7.8%) | 1169 (19.0%) | 4491 (73.2%) | |
| Rural | 59 (14.5%) | 57 (14.0%) | 291 (71.5%) | |
| Payer type: | <0.001 | |||
| Private insurance | 162 (6.4%) | 430 (16.9%) | 1952 (76.7%) | |
| Medicaid | 357 (9.8%) | 735 (20.1%) | 2567 (70.2%) | |
| Other | 19 (5.6%) | 61 (17.8%) | 262 (76.6%) | |
| Volume of Procedures: | 0.001 | |||
| High Volume Center | 368 (8.4%) | 875 (19.9%) | 3153 (71.7%) | |
| Low Volume Center | 170 (7.9%) | 352 (16.3%) | 1633 (75.8%) | |
Pearson χ2 tests of independence were performed. P-values < 0.05 are significant.
To examine the predictors of early repair while holding potentially confounding variables constant, we performed a logistic regression model predicting a repair being performed when the patient is younger than 2 years old (see table 3). No significant differences were seen in the incidence of early hernia repair based on year of operation, gender of patient, or hospital volume. The last finding indicates that the likelihood of early repair is similar for general surgeons at community hospitals and for pediatric specialists at children’s hospitals. In the logistic regression, patient race remains a significant predictor of early hernia repair. Relative to white children, African-American patients are less likely to have an umbilical hernia repaired early (odds ratio 0.62, p<0.001). Other racial and ethnic groups were not significantly different from whites. Rural residence continues to be associated with early repair (odds ratio 1.53, p=0.009), even holding constant the effect of surgical volume. Patients with Medicaid insurance were more likely to have umbilical hernias repaired early (odds ratio 2.01, p<0.001). Holding constant differences in racial composition, rural population and other characteristics, living in the state of Wisconsin is positively associated with early repair (compared to living in Florida), while living in New York is negatively associated with early repair. This suggests that practice patterns in each state are related to factors not included in our model or in the HCUP state outpatient data, such as cultural expectations, physician training, and individual practice patterns.
Table 3:
Logistic regression predicting early repair
| Odds ratio | S.E. | z | p value | |
|---|---|---|---|---|
| Year: | ||||
| 2012 (reference) | ||||
| 2013 | 0.95 | 0.11 | −0.47 | 0.640 |
| 2014 | 0.91 | 0.10 | −0.87 | 0.386 |
| Gender: | ||||
| Male (reference) | ||||
| Female | 0.96 | 0.09 | −0.43 | 0.665 |
| Race/ethnicity: | ||||
| White (reference) | ||||
| African-American | 0.62 | 0.07 | −4.08 | <0.001 |
| Hispanic | 0.76 | 0.13 | −1.65 | 0.098 |
| Other | 0.71 | 0.15 | −1.66 | 0.097 |
| Rural residence: | ||||
| Urban (reference) | ||||
| Rural | 1.53 | 0.25 | 2.63 | 0.009 |
| Payer type: | ||||
| Private (reference) Medicaid |
2.01 | 0.22 | 6.35 | <0.001 |
| Other | 1.04 | 0.27 | 0.14 | 0.886 |
| Hospital Type: | ||||
| Low Volume (reference) | ||||
| High Volume Center | 1.13 | 0.12 | 1.13 | 0.260 |
| State: | ||||
| Florida (reference) | ||||
| New York | 0.68 | 0.08 | −3.25 | 0.001 |
| Wisconsin | 1.81 | 0.21 | 5.01 | <0.001 |
| Constant | 0.07 | 0.01 | −15.48 | |
Bold text indicates effects are statistically significant at p < 0.05.
DISCUSSION
This study is the first to describe the widely variable practice patterns for the timing of uncomplicated pediatric umbilical hernia repair. Our findings demonstrate that elective umbilical hernia repairs are routinely performed in very young children.
We compared the timing of repair for patients in three clinically relevant age categories. Umbilical hernias in children younger than 2 years have a high likelihood of spontaneous closure, and elective repair at this age is not supported by the literature or consensus practice (4). We found that across the three states studied in 2012–14, 538 hernia repairs (8.2% of all pediatric repairs) were performed on children in this age group. We found that 1227 hernia repairs (18.7%) were performed on children aged 2–3 years. Older children (≥4 years old) are less likely to have their hernias close spontaneously, and we found 4786 repairs (73.1%) in children in this age group. The number of repairs under age 4 suggests that some umbilical hernia repairs may have been performed unnecessarily, potentially exposing these children to unnecessary operative and anesthetic risks.
Our results also show that the rate of early repairs (performed on children under age 2) varies significantly across the three states studied; they were more likely in Wisconsin and less likely in New York, even when differences in race and ethnicity, rurality, insurance type and hospital volume were held constant. Other findings indicate that white children, rural residents and those with Medicaid insurance were more likely to undergo hernia repairs early.
Umbilical hernia size was not reflected in this administrative database, so we were unable to assess the relationship between hernia size and the timing of operative repair. Some surgeons have advocated a practice of repairing “giant” umbilical hernias earlier than smaller hernias, though there are no consistent guidelines on what constitutes a “giant” umbilical hernia. One reason for this practice may be because larger defects are less likely to close spontaneously (14,16,43). However, the available literature suggests that there is no correlation between hernia size and risk of complications, and that in fact smaller hernia defects are more likely to become incarcerated (5,6,41,42). Thus, without a risk of complications related to delay, the potential risks of early repair may not be warranted.
Our study has several limitations. No comprehensive national database of outpatient procedures exists, so we determined practice patterns by examining granular, all-payer data for only three states, Florida, New York, and Wisconsin. Our results therefore may not represent national trends. Also, studying retrospective data from administrative databases has inherent limitations. Although the SASD for these three states includes data from all payers, claims databases contain coding errors and have a small amount of missing data. We identified chronic conditions using the HCUP CCI, which is based on ICD-9 diagnoses in claims data. Inaccuracies in this method are well known (44). The SASD database also does not contain comprehensive outpatient data, so we cannot know the total number of umbilical hernias present in the population that were not repaired. We aimed to exclude any patient having two surgical procedures in one day and umbilical hernias with obstruction, incarceration, strangulation, or gangrene, as these conditions should be repaired immediately. These exclusions also required us to rely on the accuracy and completeness of the coding.
CONCLUSION
This study found broad variation in the timing of single, uncomplicated umbilical hernia repairs in pediatric patients. For most children, umbilical hernias often close spontaneously and can be monitored safely through watchful waiting. Evidence-based clinical practice guidelines are needed to help to standardize indications for the appropriate timing of repair of asymptomatic umbilical hernias. Such guidelines could encourage both referring pediatricians and surgical providers to avoid unnecessary operations and operative and anesthetic risks.
Acknowledgments
Funding Source: This research was supported in part by an unrestricted grant from the Cars Curing Kids Foundation. The foundation had no role in study design; collection, analysis or interpretation of data; writing of the report; or the decision to submit the paper for publication.
Abbreviations:
- SASD
State Ambulatory Surgery and Services databases
- ICD-9
International Classification of Diseases, Ninth Revision
- CPT
current procedural terminology
- FDA
Federal Drug Administration
- ASA
American Society of Anesthesia
Footnotes
Financial Disclosure: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Conflict of interest: We have no conflict of interest to disclose.
Clinical Trial Registration: Not applicable
REFERENCES
- 1.Arias E National Vital Statistics Reports 2015;64:63. [PubMed] [Google Scholar]
- 2.Keshtgar AS, Griffiths M. Incarceration of umbilical hernia in children: is the trend increasing? Eur J Pediatr Surg Off J Austrian Assoc Pediatr Surg Al Z Kinderchir 2003. February;13:40–3. [DOI] [PubMed] [Google Scholar]
- 3.Papagrigoriadis S, Browse DJ, Howard ER. Incarceration of umbilical hernias in children: a rare but important complication. Pediatr Surg Int 1998. December;14:231–2. [DOI] [PubMed] [Google Scholar]
- 4.Zens T, Cartmill R, Nichol P, Kohler J. Management of Asymptomatic Pediatric Umbilical Hernias: A Systematic Review. J Pediatr Surg 2017;52:1723–31. [DOI] [PubMed] [Google Scholar]
- 5.Lassaletta L, Fonkalsrud EW, Tovar JA, Dudgeon D, Asch MJ. The management of umbilicial hernias in infancy and childhood. J Pediatr Surg 1975. June;10:405–9. [DOI] [PubMed] [Google Scholar]
- 6.Zendejas B, Kuchena A, Onkendi EO, Lohse CM, Moir CR, Ishitani MB, et al. Fifty-three–year experience with pediatric umbilical hernia repairs. J Pediatr Surg 2011. November;46:2151–6. [DOI] [PubMed] [Google Scholar]
- 7.Ezomike UO, Ituen MA, Ekpemo SC, Eke CB, Eke BC. Profile of paediatric umbilical hernias managed at Federal Medical Centre Umuahia. Niger J Med J Natl Assoc Resid Dr Niger 2012. September;21:350–2. [PubMed] [Google Scholar]
- 8.Chirdan LB, Uba AF, Kidmas AT. Incarcerated umbilical hernia in children. Eur J Pediatr Surg Off J Austrian Assoc Pediatr Surg Al Z Kinderchir 2006. February;16:45–8. [DOI] [PubMed] [Google Scholar]
- 9.Mawera G, Muguti GI. Umbilical hernia in Bulawayo: some observations from a hospital based study. Cent Afr J Med 1994. November;40:319–23. [PubMed] [Google Scholar]
- 10.Ireland A, Gollow I, Gera P. Low risk, but not no risk, of umbilical hernia complications requiring acute surgery in childhood. J Paediatr Child Health 2014. April;50:291–3. [DOI] [PubMed] [Google Scholar]
- 11.Sibley WL, Lynn HB, Harris LE. A twenty-five year study of infantile umbilical hernia. Surgery 1964. March;55:462–8. [PubMed] [Google Scholar]
- 12.Morgan WW, White JJ, Stumbaugh S, Haller JA. Prophylactic umbilical hernia repair in childhood to prevent adult incarceration. Surg Clin North Am 1970. August;50:839–45. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg NA. Infantile umbilical hernia. Surg Gynecol Obstet 1980. February;150:187–92. [PubMed] [Google Scholar]
- 14.Meier DE, OlaOlorun DA, Omodele RA, Nkor SK, Tarpley JL. Incidence of umbilical hernia in African children: redefinition of “normal” and reevaluation of indications for repair. World J Surg 2001. May;25:645–8. [DOI] [PubMed] [Google Scholar]
- 15.Brown RA, Numanoglu A, Rode H. Complicated umbilical hernia in childhood. South Afr J Surg Suid-Afr Tydskr Vir Chir 2006. November;44:136–7. [PubMed] [Google Scholar]
- 16.Walker SH. The natural history of umbilical hernia. A six-year follow up of 314 Negro children with this defect. Clin Pediatr (Phila) 1967. January;6:29–32. [DOI] [PubMed] [Google Scholar]
- 17.American College of Surgeons. Pediatric Umbilical Hernia Repair [Internet] [cited 2018 Mar 8]. Available from: https://www.facs.org/~/media/files/education/patient%20ed/pediatricumbilical.ashx
- 18.Rappaport BA, Suresh S, Hertz S, Evers AS, Orser BA. Anesthetic Neurotoxicity — Clinical Implications of Animal Models. N Engl J Med 2015. February 26;372:796–7. [DOI] [PubMed] [Google Scholar]
- 19.Center for Drug Evaluation and Research. Drug Safety and Availability - FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women [Internet] [cited 2018 May 18]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery
- 20.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 2011. November;128:e1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Xu Z, Miao C-H. Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: an updated systematic review with meta-regression. PloS One 2014;9:e85760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Du L, Du Z, Jiang H, Han D, Li Q. Association between childhood exposure to single general anesthesia and neurodevelopment: a systematic review and meta-analysis of cohort study. J Anesth 2015. October;29:749–57. [DOI] [PubMed] [Google Scholar]
- 23.Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 2012. September;130:e476–485. [DOI] [PubMed] [Google Scholar]
- 24.Ing CH, DiMaggio CJ, Malacova E, Whitehouse AJ, Hegarty MK, Feng T, et al. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology 2014. June;120:1319–32. [DOI] [PubMed] [Google Scholar]
- 25.McGowan FX, Davis PJ. Anesthetic-related neurotoxicity in the developing infant: of mice, rats, monkeys and, possibly, humans. Anesth Analg 2008. June;106:1599–602. [DOI] [PubMed] [Google Scholar]
- 26.Stratmann G Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg 2011. November;113:1170–9. [DOI] [PubMed] [Google Scholar]
- 27.Glatz P, Sandin RH, Pedersen NL, Bonamy A-K, Eriksson LI, Granath F. Association of Anesthesia and Surgery During Childhood With Long-term Academic Performance. JAMA Pediatr 2017. January 2;171:e163470. [DOI] [PubMed] [Google Scholar]
- 28.Ing C, Wall MM, DiMaggio CJ, Whitehouse AJO, Hegarty MK, Sun M, et al. Latent Class Analysis of Neurodevelopmental Deficit After Exposure to Anesthesia in Early Childhood. J Neurosurg Anesthesiol 2017. July;29:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun LS, Li G, Miller TLK, Salorio C, Byrne MW, Bellinger DC, et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016. June 7;315:2312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamie C, Habre W, Delhumeau C, Argiroffo CB, Morabia A. Incidence and risk factors of perioperative respiratory adverse events in children undergoing elective surgery. Paediatr Anaesth 2004. March;14:218–24. [DOI] [PubMed] [Google Scholar]
- 31.de Graaff JC, Pasma W, van Buuren S, Duijghuisen JJ, Nafiu OO, Kheterpal S, et al. Reference Values for Noninvasive Blood Pressure in Children during Anesthesia: A Multicentered Retrospective Observational Cohort Study. Anesthesiology 2016. November;125:904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCann ME, Schouten ANJ, Dobija N, Munoz C, Stephenson L, Poussaint TY, et al. Infantile Postoperative Encephalopathy: Perioperative Factors as a Cause for Concern. Pediatrics 2014. March 1;133:e751–7. [DOI] [PubMed] [Google Scholar]
- 33.Vavilala MS, Lee LA, Lam AM. The lower limit of cerebral autoregulation in children during sevoflurane anesthesia. J Neurosurg Anesthesiol 2003. October;15:307–12. [DOI] [PubMed] [Google Scholar]
- 34.Rhondali O, Juhel S, Mathews S, Cellier Q, Desgranges F-P, Mahr A, et al. Impact of sevoflurane anesthesia on brain oxygenation in children younger than 2 years. Paediatr Anaesth 2014. July;24:734–40. [DOI] [PubMed] [Google Scholar]
- 35.Chemaly M, El-Rajab MA, Ziade FM, Naja ZM. Effect of one anesthetic exposure on long-term behavioral changes in children. J Clin Anesth 2014. November;26:551–6. [DOI] [PubMed] [Google Scholar]
- 36.HCUP State Ambulatory Surgery and Services Databases (SASD). Healthcare Cost and Utilization Project (HCUP). 2012–2014 [Internet]. Agency for Healthcare Research and Quality, Rockville, MD: [cited 2018 May 18]. Available from: https://www.hcup-us.ahrq.gov/sasdoverview.jsp [Google Scholar]
- 37.Agency for Healthcare Research and Quality. HCUP-US Tools & Software Page [Internet] [cited 2018 May 18]. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp
- 38.Annie E Casey Foundation. KIDS COUNT Data Center [Internet] [cited 2018 Jun 6]. Available from: https://datacenter.kidscount.org/
- 39.StataCorp. Stata Statistical Software: Release 15 College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 40.von Ungern-Sternberg BS, Boda K, Chambers NA, Rebmann C, Johnson C, Sly PD, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet Lond Engl 2010. September 4;376:773–83. [DOI] [PubMed] [Google Scholar]
- 41.Komlatsè A-NG, Anani M-AK, Azanledji BM, Komlan A, Komla G, Hubert T. Umbilicoplasty in children with huge umbilical hernia. Afr J Paediatr Surg AJPS 2014. September;11:256–60. [DOI] [PubMed] [Google Scholar]
- 42.Vrsansky P, Bourdelat D. Incarcerated umbilical hernia in children. Pediatr Surg Int 1997;12:61–2. [DOI] [PubMed] [Google Scholar]
- 43.Mack NK. The incidence of umbilical herniae in Africans. East Afr Med J 1945. November;22:369–71. [PubMed] [Google Scholar]
- 44.U.S. Department of Health & Human Services Office of the Assistant Secretary for Planning and Evaluation (ASPE). Defining Diagnosis of Chronic Condition [Internet]. 2015. [cited 2018 Aug 29]. Available from: https://aspe.hhs.gov/report/understanding-high-prevalence-low-prevalence-chronic-disease-combinations-databases-and-methods-research/defining-diagnosis-chronic-condition


