Introduction
Eosinophilic esophagitis (EoE) is a chronic immune/antigen-mediated disorder characterized histologically by eosinophilic-predominant esophageal inflammation and clinically by esophageal dysfunction. The condition is diagnosed when there are at least 15 eosinophils per high-power field (eos/hpf) on esophageal biopsies following the exclusion of alternative etiologies of esophageal eosinophilia.1–4 EoE contributes significantly to esophageal morbidity and represents the second-leading cause of esophagitis.5,6
Genetic linkage studies, animal models, and the frequency of comorbid allergic disorders link the pathogenesis of EoE with atopy.7,8 Most patients with EoE are sensitive to environmental triggers including aeroallergens in addition to food allergens.1,9,10 In addition, elimination and elemental diets can resolve esophageal eosinophilia and symptoms,11–13 diagnosis of EoE varies by season,14–16 esophageal eosinophilia differs by climate zone,17 and EoE histologic activity fluctuates by season in some children.18 However, limited data support the latter supposition,18,19 and few data20–22 report the impact of seasonal aeroallergen exposure on EoE-related histologic activity in an adult cohort. Addressing this knowledge gap may better clarify the natural history of EoE, provide a novel therapeutic target, and ultimately improve remission rates and ameliorate the burden imparted by this disease.6,21,23,24
Therefore, the aim of this study was to assess the impact of seasonal aeroallergen exposure on EoE disease activity in a cohort comprised of adults and children. We also aimed to assess for differences between the cohort of patients with and without seasonal exacerbations of disease activity.
Methods
We conducted a retrospective cohort study at the University of North Carolina (UNC) Hospitals utilizing the UNC EoE Clinicopathologic database. This database has previously been described,15,25 and consists of all patients with EoE diagnosed per consensus guidelines treated at UNC Hospitals from 2002 – 2017.1,2 Of note, the patients in this study had non-response to a trial of proton pump inhibitor (PPI) therapy, as they were included in our database prior to the 2017 European guidelines and 2018 international consensus removing this diagnostic requirement.3,4 Patients with eosinophilic infiltration of additional segments of the gastrointestinal tract were excluded. The UNC institutional review board approved this study.
For inclusion in this study, we identified a sub-cohort of patients who were in histologic remission (<15 eos/hpf)10,26 after successful treatment, and then had evidence of seasonal exacerbation(s) with increased histologic activity. We defined a seasonal exacerbation of EoE-related histologic activity as an increase from less than to greater than 15 eos/hpf on esophageal biopsy and with at least a concomitant doubling in eosinophil counts between two consecutive biopsies obtained in different seasons that occurred without a change in EoE treatment.18 For patients with an undetectable eosinophil count at baseline, a seasonal exacerbation was defined as an increase an increase from 0 eos/hpf to > 15 eos/hpf on follow-up esophageal biopsy. We identified each occurrence of seasonal disease exacerbation over the length of available follow-up time. Each flare of disease activity was demarcated by an index (“pre-flare”) and follow-up (“flare”) biopsy. For this study, March to May delineated spring, June to August delineated summer, September to November delineated fall, and December to February delineated winter.16
To minimize confounding, data were abstracted from treatment intervals when subjects remained on a stable therapeutic regimen. Additionally, to help minimize measurement and misclassification biases, patients were excluded if any change in therapeutic regimen was made within 8 weeks of each pre-flare endoscopy, or if more than 24 months passed between consecutive endoscopic procedures. Patients returning for endoscopy with dilatation of a known or suspected stricture were not considered to have a change in treatment. At our center, the typical clinical protocol was to repeat an upper endoscopy to assess for endoscopic and histologic responses following 6 – 8 weeks of dietary or pharmacologic treatment. Additionally, in the correct clinical scenarios, repeat endoscopy was temporarily deferred when a patient reported worsening atopic symptoms. Following histologic and clinical remission of disease activity on a stable treatment regimen, asymptomatic patients undergo surveillance endoscopy at the discretion of the treating provider. Subsequent procedures were typically performed for stricture dilation, recurrent symptoms, changes to treatment, or to ensure ongoing endoscopic or histologic response.
Following assembly of the patient cohort, data were extracted from patient medical records using a standardized data collection form. Data abstracted for this study included demographics, symptoms, endoscopic findings, and outcomes (symptomatic global worsening [yes/no]; endoscopic severity [EoE Endoscopic Reference Score (EREF scoring system)27 and histologic change [absolute peak eosinophil count] before and after the seasonal exacerbation of EoE). As this was a retrospective cohort study and validated patient reported outcome measures were not uniformly applied in clinical settings, specific symptoms and the patient’s global perception of symptoms, as documented in the chart, were coded using dichotomous variables indicating their presence or absence [yes/no per patient perception of disease activity at time of patient follow-up].26 Comorbid atopic diseases were recorded as per diagnosis in the medical record. The total EREFS score was calculated by adding the severity scores of the individual components of the EREFS score (edema 0–1, rings 0–3, exudates 0–2, furrows 0–2, strictures 0–1) as noted in the endoscopy report. The aforementioned outcome data were collected prior and subsequent to each flare of disease activity. In addition, we assessed report of medication compliance in the medical record. Good adherence signified complete adherence to EoE-specific therapy; partial adherence signified compliance to 50% or more of EoE-specific therapy; poor compliance signified adherence to 50% or less of EoE-specific therapy.
For analysis, descriptive statistics including the mean, standard deviation, and the shape of the distribution were calculated for all continuous variables. Frequencies were tabulated for categorical variables. Bivariable statistics assessed the relationship between pre and post-flare symptomatic, endoscopic, and histologic outcomes. Since we analyzed repeated measures, McNemar’s test was used for dichotomous variables [symptomatic responses] and paired t-tests for continuous variables [endoscopic and histologic responses]. Fisher’s exact test was used to compare non-repeated categorical measures. Additional non-parametric testing did not alter the conclusions of this study. All analyses were performed using Stata 14.2 (Stata Corp, College Station, TX).
Results
Baseline characteristics
The overall cohort included 782 patients with an incident diagnosis of EoE at UNC, 444 of which (57%) were adults at the time of diagnosis. Among these 782 patients, 13 (2%) met criteria for a seasonal exacerbation. These 13 patients had been followed at our institution for a mean length of 3.5 years (Table 1). When considering adult versus child status at diagnosis, 10 of 444 adults (2%) and 3 of 338 children (1%) had a seasonal exacerbation. Individual endoscopic findings were available in all patients for the pre- and post-post flare periods. However, an EREFS score was not available in 3 (23%) patients, as they were treated before adoption of this scoring system. At the time of presumptive flare, good compliance was recorded in 11 (85%) patients and partial compliance in 2 patients (15%).
Table 1.
Patient demographics, season of histologic flare, and treatment at time of documented flare (N = 13)
| Age at diagnosis (mean years ± SD) | 32.3 ± 15.4 |
| Children < 18 years (N, %)† | 3 (23) |
| Age at first seasonal flare (mean years ± SD) | 36.2 ± 14.5 |
| Symptom length preceding diagnosis (mean years ± SD) | 11.9 ± 11.3 |
| Length of follow-up (mean years ± SD) | 3.5 ± 1.6 |
| % Male | 85 |
| % White | 86 |
| Any atopic disease diagnosis (n, %) | 11 (85) |
| Allergic rhinitis | 11 (85) |
| Food allergy(s) | 8 (61) |
| Eczema | 3 (23) |
| Asthma | 2 (15) |
| Indication for repeat endoscopy when flare found (n, %) | |
| Routine surveillance or dilatation of stricture | 9 (69) |
| Flare of underlying atopic disease | 1 (8) |
| Worsening or persistent esophageal symptoms | 3 (23) |
| Season of histologic flare per patient (n, %) | |
| Spring (March - May) | 2 (14) |
| Summer (June - August) | 6 (43) |
| Fall (September - November) | 4 (29) |
| Winter (December - February) | 2 (14) |
| Patients with 1 or more histologic flares recorded | 1 (8) |
| Treatment at time of flare | |
| Topical swallowed steroids | 9 (69) |
| 6-mercaptopurine | 1 (8) |
| Food elimination diet | 4 (31) |
| Topical swallowed steroids + food elimination diet | 2 (15) |
Children: Less than 18 years old at appropriate time
For those with an exacerbation, the mean age at EoE diagnosis was 32.3 years (range: 1.2 – 59.1 years), and most patients were male (85%) and white (86%) (Table 1). The mean age of patients under 18 years old at the time of diagnosis was 11.2 years (range: 1.2 – 17.0 years). At the time of the first documented seasonal flare of disease activity, the mean age was 36.2 years. For patients under 18 years old at the time of seasonal flare, the mean age was 13.4 years (range: 9.7 – 17.4 years). At the time of the first flare, only two (15%) patients were less than 18 years old. A diagnosis of any atopic disease was present in 11 subjects (85%). The most prevalent atopic condition was allergic rhinitis (85%), which was followed by a history of food allergy (61%), and atopic dermatitis (23%). A diagnosis of allergic rhinitis was made or confirmed by an allergist in 6 (55%). The remaining 5 (45%) patients with allergic rhinitis self reported the diagnosis. For the 3 patients with food allergies, 2 diagnoses were captured via patient report; the remaining patient saw an allergist at UNC. Most patients were maintained on a topical corticosteroid at the time of initial seasonal flare of disease activity (69%) with a smaller percentage receiving a food elimination diet (31%). There were two patients (15%) managed with both a topical corticosteroid and a food elimination diet. Among patients treated with topical corticosteroids, four patients were on swallowed fluticasone via metered dose inhaler (mean daily dose: 1820 mcg) and six were on oral viscous budesonide (mean daily dose: 2.3 mg). Food elimination diets included six-food elimination diet (2 patients), dairy and wheat elimination (1 patient), honey, soy and nut elimination (1 patient). Formal allergy testing had been conducted in four (31%) patients, three patients with skin prick testing and one with serum radioallergosorbent testing; three had documented sensitization to environmental triggers. The first patient was found to be sensitized to weeds; the second patient was found to be sensitized to ragweed, multiple trees, molds, feathers, and dust mites; the third patient was found to be sensitized to trees, grasses, cats, and weeds. For patients treated specifically for allergic rhinitis with anti-histamines and/or nasal corticosteroids, no allergic rhinitis medicinal regimen differed between the flare and pre-flare endoscopy.
Comparison of patients with and without seasonal exacerbations of disease activity
When compared to the larger EoE cohort treated at UNC, patients with a seasonal exacerbation of disease activity did not differ by age at diagnosis (32.3 years vs. 26.4 years; p = 0.16), race (85% white vs. 80% white; p =0.52), gender (85% male vs. 67% male; p = 0.24) (Table 2), or time to diagnosis following symptom initiation (9.9 vs. 7.3 years; p = 0.36). The group of patients with seasonal exacerbation of disease activity also did not significantly differ from the larger cohort by presenting symptoms or baseline peak eosinophil counts.
Table 2.
Comparison of patients with and without seasonal flares of eosinophilic esophagitis histologic activity
| Documented flare (n = 13) | No documented flare (n = 769) | P | |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis (mean years ± SD) | 32.3 ± 15.4 | 26.4 ± 18.5 | 0.16 |
| Gender (n, %) | 11 (85) | 515 (67) | 0.24 |
| Race (n, %) | 11 (85) | 612 (80) | 0.52 |
| Any atopic disease (n, %) | 11 (85) | 316 (43) | 0.01 |
| Asthma | 2 (15) | 180 (23) | 0.75 |
| Food allergy | 8 (61) | 199 (29) | 0.03 |
| Symptoms (n, %)† | |||
| Dysphagia | 12 (19) | 544 (71) | 0.13 |
| Heartburn | 9 (69) | 285 (38) | 0.78 |
| Chest pain | 9 (69) | 73 (10) | 0.07 |
| Abdominal pain | 1 (8) | 160 (21) | 0.34 |
| Endoscopy (n, %)† | |||
| Normal | 0 (0) | 94 (12) | 0.39 |
| Rings | 12 (92) | 344 (45) | 0.001 |
| Stricture | 8 (62) | 165 (22) | 0.002 |
| Furrows | 10 (77) | 454 (59) | 0.26 |
| Exudates | 8 (62) | 280 (37) | 0.08 |
| Histology | |||
| Mean peak eos/HPF ± SD‡ | 70.2 ± 18.6 | 78.6 ± 72.2 | 0.73 |
Symptom and endoscopic comparisons are for baseline findings
Mean peak eos/HPF ± SD: mean peak eosinophils per high-power field
Individual endoscopic findings did differ in those with seasonal flares. For instance, rings (92% vs. 35%; p =0.001), furrows (77% vs. 59%; p = 0.26), and strictures (62% vs. 22%; p = 0.002) were more frequently seen at diagnosis in those with seasonal exacerbations of disease activity. Furthermore, patients meeting inclusion for this study more frequently received a diagnosis of food allergy(s) (61% vs. 29%; p = 0.03) as well as an atopic disease diagnosis overall (85% vs. 43%; p = 0.01). No differences were seen for asthma diagnoses (15% vs. 24%; p = 0.75).
Characteristics of seasonal exacerbations of disease activity
Included patients were followed for a total of 3.5 years. There were a total of 14 seasonal flares of disease activity identified over this interval of time. The majority of flares occurred over the summer and fall seasons. Specifically, there were six (43%) and four (29%) events that occurred in the summer and fall, respectively. Only four total flares were identified in the spring and winter months (28% of total events). No individual patient was found to have a flare of disease activity in different seasons. Moreover, only a single patient was noted to have multiple flares of disease activity, and in this case, both exacerbations manifested over summer months. There were 5 (38%) patients for whom follow-up endoscopies were specifically delayed in the fall and spring, which corresponded to seasons in which allergic symptoms were described. When considering environmental allergens in the context of formal allergen testing and season of flare, testing corresponded with the season of flare in 2 of 3 patients.
Outcome responses during time of seasonal flare of disease activity
The indication for physician ordered follow-up endoscopy and esophageal biopsies differed across the cohort. However, the majority of patients (69%) were seen for routine surveillance or for ongoing dilatation of a known esophageal stricture (Table 1). A repeat endoscopy was ordered for a single patient in the setting of an exacerbation of seasonal allergies. There were an additional three patients (23%) evaluated for worsening or persistent esophageal symptoms.
In regards to symptoms, a total of four (31%) subjects endorsed a global worsening of symptoms at the time of follow-up endoscopy. Additionally, five patients (38%) noted an exacerbation of allergic rhinitis. Changes in individual symptoms attributable to the esophagus were otherwise infrequently recorded.
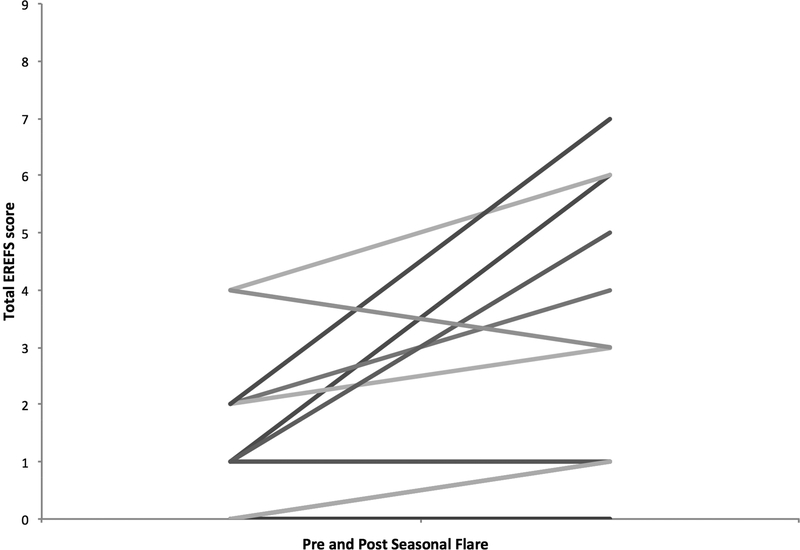
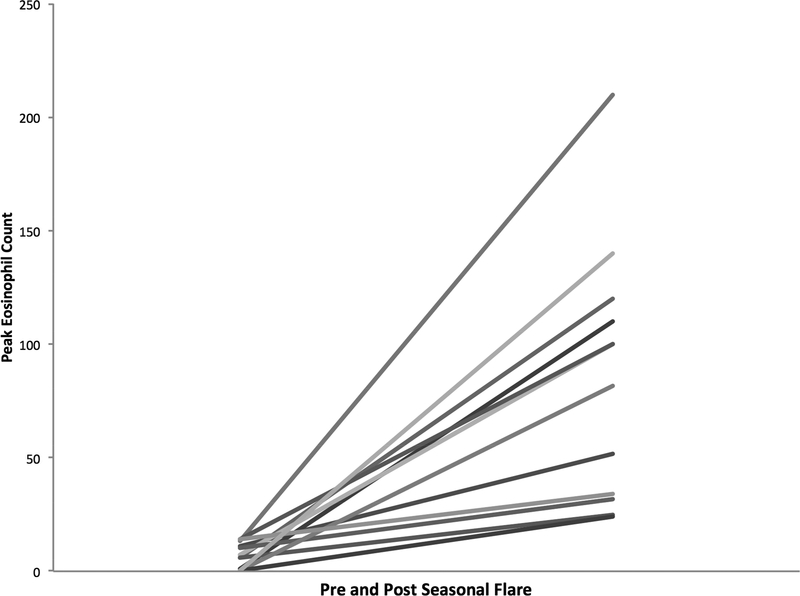
The total EREFS score was found to worsen when compared to the pre-flare endoscopy findings (1.7 vs. 3.7; p = 0.01) (Figure 1). Significant deteriorations were found in the individual findings of edema (0.1 vs. 0.7; p = 0.005) and furrows (0.2 vs. 0.8; p = 0.02) while exudates trended towards a worsening (0.2 vs. 0.6; p = 0.10). For the entire cohort of 13 patients, the mean of the peak eosinophil count increased from 6.8 ± 5.4 eos/hpf to 86.8 ± 54.0 at the time of exacerbation (p < 0.001) (Figure 2).
Figure 1. Pre and Post Seasonal Flare Total EREFS† Score.
†EREFS Score: Edema Rings Exudates Furrows Stricture
Figure 2. Pre and Post Seasonal Flare Mean Peak Eosinophil Counts per High-power field.
Discussion
Though aeroallergen exposure has been implicated in the pathogenesis of EoE, few data exist exploring the role of aeroallergens on the histologic activity of adult EoE patients. In this study, we aimed to determine the association of seasonal aeroallergen exposure on EoE disease activity in a cohort predominantly composed of adults. Though seasonal exacerbations of EoE were rare, we found that aeroallergen exposure was associated with significant deteriorations in endoscopic and histologic findings.
Multiple studies illustrate that EoE is an immune and allergen-mediated disorder, with clear associations between EoE and other atopic disorders.15,28,29 Moreover, different centers noted a seasonal variation in the diagnosis of EoE,15,16,30–32 and the prevalence of esophageal eosinophilia has been found to vary according to climate zone.17 Additionally, food bolus impactions associate with seasonal aeroallergens in adults,33 EoE patients demonstrate a high rate of sensitization to foods and aeroallergens,34,35 and the degree of esophageal eosinophilia varies in children by season.18 Few studies purposefully examined the association between EoE histologic activity and seasonal exposure to aeroallergens.18 One paper considering this association analyzed a retrospective cohort including a subset of 32 of 1,180 pediatric subjects with a confirmed seasonal variation in esophageal eosinophilia, a proportion (~3%) quite similar to what we have found.18 Most of these patients were boys (84%) and all had allergic rhinitis. Most patients also had asthma (75%), and the majority of exacerbations were found to occur in the spring months. This study excluded adults. Other available data pertinent to this issue derive from an early case report published in 200321 as well as a retrospective cohort study.20 For the latter study, which predominantly focused on the association between seasonal allergen levels and season of EoE diagnosis, a comparison was made between mean eosinophil counts and season of diagnosis. However, this was done for the entire cohort rather than a sub-population ostensibly sensitive to environmental triggers. The mechanism of seasonal worsening is not known, but options could include swallowing of aeroallergens or cross-reactivity of pollen and certain foods that are not traditionally removed in empiric elimination diets. The role of skin testing to identify such allergens is also not known, and could be the focus of a future study.
In our study, seasonal exacerbations of EoE were rare and primarily documented in adult patients. However, our stringent definition of flare may have underestimated this phenomenon, especially in patients who had not achieved histologic remission. Included patients were more also likely to have active follow-up at our center, which supports under detection of this phenomenon. Of interest, nearly every patient with a documented flare carried a diagnosis of allergic rhinitis and fall and summer flares were most common. As exposure to aeroallergens is thought to temporally align with increased phenotypic manifestations of the disease, this implicates summer exposure to grasses or fall exposure to weeds or mold in our region.19,31
Interestingly, these patients rarely reported symptomatic esophageal complaints. One reason for this is that many had esophageal strictures and were in a dilation program, which typically will improve symptoms without an effect on inflammation.36,37 However, the total EREFS score did significantly worsen from baseline, with all findings becoming more severe in appearance. This was consistent with the esophageal eosinophil counts also increasing significantly at the time of exacerbation. This worsening of endoscopic and histologic disease activity has important ramifications, as it is known that poor disease control correlates with the fibrostenotic complications of the condition.5,38,39 It is conceivable that asymptomatic disease activity may contribute to worse outcomes in this subset of patients with EoE. A prior study documented no association between season and clinical EoE activity.32 As in inflammatory bowel disease,40 it is conceivable that increased histologic activity precedes endoscopic and clinical changes. In this manner, it is conceivable that symptoms of allergic rhinitis would not be captured simultaneously with increased EoE histologic activity. This could be confirmed in a prospective setting. Though not assessed in this study, it is also plausible that seasonal exposure to aeroallergens precludes or challenges disease control in patients who have not obtained histologic remission. Additionally, a minority of subjects noted worsening symptoms of allergic rhinitis when a flare was captured.
There are limitations for this study. It utilized a retrospective design, and data were abstracted from the electronic medical record, making misclassification bias conceivable. This would potentially underestimate the number of subjects with seasonal exacerbations of EoE, especially in patients who have not achieved histologic remission. Because validated patient reported outcome measures were not utilized, the symptom data should be interpreted with caution. The number of patients with seasonal exacerbations was small, limiting the power to detect significant differences in outcomes. However, our stringent inclusion criteria also limited selection biases by assessing all EoE patients treated at our institution. Compliance may also be overestimated as a consequence of recall bias and social desirability bias. Inaccurate reporting of compliance may account for some presumptive season flares. Lastly, we have minimal sensitization data, so we cannot ascribe our patient outcomes to particular aeroallergens. There are also a number of strengths of this study, including comprehensive data collection, a homogenous cohort, and strict and uniformly applied inclusion criteria in a large and established EoE database.
In conclusion, seasonal exacerbations of EoE were uncommon in a predominantly adult cohort of EoE patients, though this was seen in approximately 1 of 50 EoE cases. Additionally, our stringent definition of flare may have underestimated this phenomenon, especially in patients who have not achieved histologic remission. Of interest, most patients with a documented flare carried a diagnosis of allergic rhinitis, and summer and fall flares were most common, implicating summer exposure to grasses or fall exposure to weeds or molds in our region. These data support a role of aeroallergens in the pathogenesis of EoE. Aeroallergens may modulate EoE disease activity in accordance with the state of the esophageal barrier and the degree of exposure. Clinicians should consider aeroallergens as a potential cause of disease exacerbation and possibly poor disease control.
Acknowledgments
Conflict of Interest: Dr. Dellon is a consultant for Adare, Alivio, Banner, Enumeral, Receptos/Celegene, Regeneron, and Shire, receives funding from Adare, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, and has received an educational grant from Banner. The other authors report no conflicts of interest.
Funding source: NIH Awards T32 DK007634 (CCR) and R01 DK101856 (ESD).
Abbreviations/Acronym:
- EoE
Eosinophilic esophagitis
- eos/hpf
eosinophils per high-power field
- UNC
University of North Carolina
- PPI
proton pump inhibitor
- EREF scoring system
EoE Endoscopic Reference Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–92. [DOI] [PubMed] [Google Scholar]
- 3.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018;September 6 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellon ES, Kim HP, Sperry SLW, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(4)577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol. 2015;110(5):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology. 2015;148(6):1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Shea K, Aceves S, Dellon E, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154(2):333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhawt M, Aceves SS, Spergel JM, Rothenberg ME. The management of eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2013;1(4):332–340. [DOI] [PubMed] [Google Scholar]
- 10.Wolf WA, Cotton CC, Green DJ, et al. Evaluation of Histologic Cutpoints for Treatment Response in Eosinophilic Esophagitis. J Gastroenterol Hepatol Res. 2015;4(10):1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98(4):777–782. [DOI] [PubMed] [Google Scholar]
- 12.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–1102. [DOI] [PubMed] [Google Scholar]
- 13.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; Food reintroduction identifies causative factors. Gastroenterology. 2012;142(7):1451–1459. [DOI] [PubMed] [Google Scholar]
- 14.Elitsur Y, Aswani R, Lund V, Dementieva Y. Seasonal distribution and eosinophilic esophagitis: The experience in children living in rural communities. J Clin Gastroenterol. 2013;47(3):287–288. [DOI] [PubMed] [Google Scholar]
- 15.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, Endoscopic, and Histologic Findings Distinguish Eosinophilic Esophagitis From Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2009;7(12):1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen ET, Shah ND, Hoffman K, Sonnenberg A, Genta RM, Dellon ES. Seasonal variation in detection of oesophageal eosinophilia and eosinophilic oesophagitis. Medline. 2015:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107(5):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ram G, Lee J, Ott M, et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy, Asthma Immunol. 2015;115(3):224–228. doi: 10.1016/j.anai.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Wolf WA, Jerath MR, Dellon ES. De-novo onset of eosinophilic esophagitis after large volume allergen exposures. J Gastrointest Liver Dis. 2013;22(2):205–208. [PMC free article] [PubMed] [Google Scholar]
- 20.Moawad FJ, Veerappan GR, Lake JM, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31(4):509–515. [DOI] [PubMed] [Google Scholar]
- 21.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis [1]. J Allergy Clin Immunol. 2003;112(4):796–797. [DOI] [PubMed] [Google Scholar]
- 22.Onbasi K, Sin AZ, Doganavsargil B, Onder GF, Bor S, Sebik F. Eosinophil infiltration of the oesophageal mucosa in patients with pollen allergy during the season. Clin Exp Allergy. 2005;35(11):1423–1431. [DOI] [PubMed] [Google Scholar]
- 23.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147(6):1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellon E Management of refractory eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol. 2017;14(8):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed C, Fan C, Koutlas N, Shaheen N, Dellon E. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46(9):836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed C, Wolf W, Cotton C, et al. Optimal Histologic Cutpoints for Treatment Response in Patients with Eosinophilic Esophagitis: Analysis of Data From a Prospective Cohort Study. Clin Gastroenterol Hepatol. 2018;16(2):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–495. [DOI] [PubMed] [Google Scholar]
- 28.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2010;44(1):22–27. [DOI] [PubMed] [Google Scholar]
- 29.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6(5):531–535. [DOI] [PubMed] [Google Scholar]
- 30.Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104(4):828–833. [DOI] [PubMed] [Google Scholar]
- 31.Fahey L, Robinson G, Weinberger K, Giambrone A, Solomon A. Correlation between Aeroallergen Levels and New Diagnosis of Eosinophilic Esophagitis in NYC. J Pediatr Gastroenterol Nutr. 2017;64(1):22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucendo AJ, Arias A, Redondo-González O, González-Cervera J. Seasonal distribution of initial diagnosis and clinical recrudescence of eosinophilic esophagitis: A systematic review and meta-analysis. Allergy Eur J Allergy Clin Immunol. 2015;70(12):1640–1650. [DOI] [PubMed] [Google Scholar]
- 33.Philpott HL, Nandurkar S, Thien F, et al. Seasonal recurrence of food bolus obstruction in eosinophilic esophagitis. Intern Med J. 2015;45(9):939–943. [DOI] [PubMed] [Google Scholar]
- 34.Van Rhijn BD, Van Ree R, Versteeg SA, et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy Eur J Allergy Clin Immunol. 2013;68(11):1475–1481. [DOI] [PubMed] [Google Scholar]
- 35.Olson AA, Evans MD, Johansson MW, et al. Role of food and aeroallergen sensitization in eosinophilic esophagitis in adults. Ann Allergy, Asthma Immunol. 2016;117(4):387–393.e2. doi: 10.1016/j.anai.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Runge TM, Eluri S, Woosley JT, Shaheen NJ, Dellon ES. Control of inflammation decreases the need for subsequent esophageal dilation in patients with eosinophilic esophagitis. Dis Esophagus. 2017;30(7):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed C, Wolf W, Cotton C, Dellon E. A visual analogue scale and a Likert scale are simple and responsive tools for assessing dysphagia in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;45(11):1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–1236. [DOI] [PubMed] [Google Scholar]
- 39.Warners MJ, Oude Nijhuis RAB, de Wijkerslooth LRH, Smout AJPM, Bredenoord AJ. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am J Gastroenterol. 2018;113(6):836–844. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenstein G, Loftus E, Isaacs K, Regueiro M, Gerson L, Sands B. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol. 2018;113(4):481–517. [DOI] [PubMed] [Google Scholar]