Abstract
Background:
Few studies have examined the relationship between lifestyle activity engagement and cognitive trajectories among individuals who were cognitively normal at baseline.
Objective:
To examine the relationship of current engagement in lifestyle activities to prior cognitive performance among individuals who were cognitively normal at baseline, and whether this relationship differed for individuals who subsequently developed Mild Cognitive Impairment (MCI), or by APOE-4 genotype, age, and level of cognitive reserve.
Methods:
Participants (N=189) were primarily middle-aged (M=56.6 years) at baseline and have been prospectively followed with annual assessments (M follow-up=14.3 years). Engagement in physical, cognitive, and social activities was measured by the CHAMPS activity questionnaire. Longitudinal cognitive performance was measured by a global composite score.
Results:
Among individuals who progressed to MCI (n=27), higher lifestyle activity engagement was associated with less decline in prior cognitive performance. In contrast, among individuals who remained cognitively normal, lifestyle activity engagement was not associated with prior cognitive trajectories. These effects were largely independent of APOE-4 genotype, age, and cognitive reserve.
Conclusions:
Greater engagement in lifestyle activities may modify the rate of cognitive decline among those who develop symptoms of MCI, but these findings need to be confirmed in prospective studies.
Keywords: lifestyle factors, cognitive, physical, social, Mild Cognitive Impairment, cognitive reserve
Introduction
With dementia prevalence expected to triple by 2050,1 and limited treatment options, it is important to identify lifestyle factors that may alter risk of cognitive decline. As summarized by a number of recent reports,1–3 the existing literature provides encouraging evidence that engagement in physical, cognitive, and social lifestyle activities may modify patterns of cognitive change over time.
Prior longitudinal observational and epidemiological studies suggest that higher levels of engagement in lifestyle activities are associated with a reduced risk of incident Mild Cognitive Impairment (MCI)4–8 and dementia.5,8,9 Although some studies among individuals who were non-demented at baseline have found that engagement in lifestyle activities is associated with reduced rates of cognitive decline,5,8–15 findings have been inconsistent.16–18
Only a small number of observational studies have examined the association between lifestyle activities and longitudinal cognitive trajectories among individuals who were cognitively normal at baseline.5,7,19–22 To our knowledge, the relationship of physical, cognitive, and social activities to longitudinal cognitive trajectories has not been examined within the same group of initially cognitively normal individuals. It also remains unclear if the associations between lifestyle activities and cognitive trajectories differ among individuals who have remained cognitively normal over time versus those who progress to MCI, and whether these relationships are modified by other factors that may affect cognitive decline.
This study examined the relationship between current engagement in physical, cognitive, and social activities and prior cognitive trajectories in a well-characterized cohort of individuals who were cognitively normal and primarily middle-aged at baseline, allowing us to extend prior studies in number of ways. First, we examined these lifestyle factors within a cohort that has been followed annually for a mean of 14 years, a longer period of time than most prior studies. This long period of follow-up also made it feasible to test whether the association between engagement in lifestyle activities and prior cognitive trajectories differed for individuals who were initially cognitively normal but subsequently progressed to MCI (relative to individuals who remained cognitive normal). Second, we examined whether the associations between lifestyle activities and cognitive trajectories were modified by APOE-4 genetic status, age, or level of cognitive reserve (CR). We hypothesized that individuals with higher engagement in lifestyle activities would have reduced rates of decline in prior cognitive trajectories, and that these effects might be stronger among individuals at greater risk for cognitive decline.
Methods
Study Design and Participant Selection
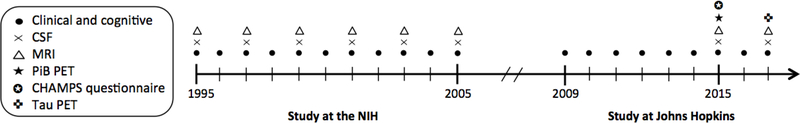
Data were derived from the BIOCARD study, an ongoing longitudinal prospective cohort study designed to identify variables among cognitively normal individuals that predict subsequent development of mild to moderate symptoms of Alzheimer’s disease (AD).23 The study was initiated in 1995 at the National Institutes of Health (NIH). At baseline, following a comprehensive evaluation, 349 cognitively normal individuals were enrolled after providing written informed consent. By design, approximately 75% of the cohort had a first degree relative with dementia of the Alzheimer type. The study was stopped in 2005 for administrative reasons, and re-established in 2009 by a team from Johns Hopkins University (JHU). In 2015, the collection of additional measures, including a questionnaire about engagement in lifestyle activities, was initiated (see Figure 1 for a study timeline; for additional details, see Supplemental Digital Content 1). This study was approved by the JHU Institutional Review Board.
Figure 1.
Timeline showing the design of the BIOCARD study, and types of data collected each year from 1995–2017.
Abbreviations: CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PET, positron emission tomography; NIH, National Institutes of Health
Clinical and Cognitive Assessments
Detailed cognitive and clinical assessments and consensus diagnoses were completed annually at the NIH and continue to be completed annually at JHU (see Supplemental Digital Content 1 for details). Participants received consensus diagnoses by the JHU BIOCARD Clinical Core, following the National Institute on Aging/Alzheimer’s Association working group recommendations for the diagnosis of MCI24 and dementia due to AD.25 For individuals with evidence of cognitive impairment, the age at which the clinical symptoms began was estimated.
The main outcome variable in these analyses was a global cognitive composite score based on four measures previously identified to be the best combination of cognitive predictors of time to progress from normal cognition to clinical symptom onset of MCI.23 These measures have been administered annually since baseline, and include: Paired Associates immediate recall,26 Logical Memory delayed recall,26 Boston Naming,27 and Digit-Symbol Substitution.28 To calculate the composite, individual measures were z-transformed and then averaged, with the requirement that at least 2/4 scores were present at a given time point.
Lifestyle Activities Assessment
Engagement in lifestyle activities was assessed with the CHAMPS activity questionnaire,29 which measures self-reported frequency and duration of engagement in 40 physical, cognitive, and social activities ‘during a typical week in the past month’. Physical activities were divided into low and moderate-to-high intensity activities according to their Metabolic Equivalent of Task (MET) values; non-physical activities were categorized as either cognitive or social activities based on previous literature (see Supplemental Digital Content 2 for additional details).
Low intensity physical, high intensity physical, cognitive, and social activities were each quantified based on frequency of engagement (times/week), reflecting the sum of all relevant item frequencies within an activity category. Using the formula provided by Stewart et al.,29 low and high intensity physical activities were also quantified based on estimated caloric expenditure per week, calculated as the product of an activity’s self-reported duration, intensity (using MET values adjusted for older adults), and participant body weight, and then summed across all relevant items. We also created a seventh measure reflecting total engagement in all activities to capture the variety of activity engagement, independent of frequency and category.10 For this measure, 1 point was given for each activity endorsed. The lifestyle questionnaire was administered starting in 2015. The analyses reported here were a retrospective evaluation of cognitive changes from visits prior to and including the time that the participants completed the questionnaire. These analyses include 189 participants. However, collection is ongoing so that additional data will ultimately be available (see Supplemental Digital Content 3 for details regarding participant exclusion).
Cognitive Reserve Composite Score
The proxy for CR was a composite score based on three measures collected at baseline: National Adult Reading Test scores,30 WAIS-R vocabulary scores,28 and years of education. To calculate the composite, these measures were z-transformed and then averaged.31
APOE Genotype Coding
APOE genotypes were determined by restriction endonuclease digestion of polymerase chain reaction amplified genomic DNA (Athena Diagnostics, Worcester, MA). Genotype was coded dichotomously (APOE-4 carriers = 1, non-carriers = 0). Analyses including APOE excluded three APOE ε2/ε4 carriers, given these alleles have contrasting effects on dementia risk.32,33
Statistical Analyses
Group differences in sample characteristics were tested by t-tests, Wilcoxon rank sum tests, or chi-square tests as appropriate.
Cross-sectional analyses.
Linear regression models tested the relationship of current engagement in lifestyle activities, referred to as ‘lifestyle variables’, with demographic and genetic variables. Separate models were run for each lifestyle variable, which served as the dependent variable. Model predictors included age at lifestyle questionnaire administration, sex, diagnosis, baseline CR, and APOE-4 genetic status. Diagnosis was coded dichotomously based on diagnosis on the date of completion of the lifestyle questionnaire (remained normal = 0; progressed to MCI = 1).
Longitudinal analyses.
Linear mixed regression models tested the relationship between current engagement in lifestyle activities and prior cognitive trajectories; models included linear effects of time and were specified with random intercepts and slopes. The dependent variable was the cognitive composite score, including baseline measures and all available scores up to and including the visit at which the lifestyle questionnaire was completed. The primary models, run in all participants, included the following predictors: baseline age, sex, education (years), diagnosis, lifestyle variable, time (years from baseline), and the interaction (cross-product) of each predictor with time. For these models, the lifestyle variable x time interactions, reflecting differences in slopes of cognitive trajectories by level of activity engagement, were of primary interest.
These models were then re-run to test whether other variables modify the observed associations. Separate sets of models were run for each of the following variables, with additional model predictors shown in parentheses: diagnosis (diagnosis x lifestyle variable, diagnosis x lifestyle variable x time); level of baseline CR (baseline CR, baseline CR x time, baseline CR x lifestyle variable, baseline CR x lifestyle variable x time); APOE-4 genetic status (APOE, APOE x time, APOE x lifestyle variable, APOE x lifestyle variable x time); and age (baseline age x lifestyle variable, baseline age x lifestyle variable x time). Terms for education were removed from models that included terms for baseline CR. For these models, the three-way interactions were of primary interest.
All continuous variables except time were standardized before regression model fitting. Analyses were run in R.34
Results
At baseline, all participants were cognitively normal and primarily middle-aged. Participant characteristics are shown Table 1, subdivided by diagnosis at the time of completion of the lifestyle questionnaire. Baseline characteristics for the entire BIOCARD cohort and participants included in the analyses are shown in Supplemental Digital Content 3.
Table 1.
Characteristics for participants included in the analyses, shown for all participants and stratified by diagnosis (remain normal; progressed to MCI). Values reflect means (standard deviations) unless otherwise indicated.
| All | Remained normal | Progressed to MCI | |
|---|---|---|---|
| N | 189 | 162 | 27 |
| Age at baseline | 56.6 (8.5) | 55.9 (8.0) | 60.7 (10.3) ** |
| Age at lifestyle activities assessment | 70.9 (8.8) | 70.2 (8.6) | 75.4 (8.8) ** |
| Years of follow-up (baseline to lifestyle activities assessment) | 14.3 (3.0) | 14.3 (2.9) | 14.8 (3.2) |
| Number of cognitive assessments over time | 9.8 (3.0) | 9.8 (3.1) | 9.8 (2.8) |
| Female sex, % | 60.8% | 62.3% | 51.9% |
| Race/ethnicity, white, % | 97.4% | 98.8% | 88.9% |
| APOE ɛ4 carriers, % | 35.4% | 35.2% | 37.0% |
| Years of education | 17.4 (2.2) | 17.5 (2.1) | 17.2 (2.7) |
| CR composite score at baseline | 0.0 (1.0) | 0.2 (0.9) | −1.1 (0.9) ** |
| Cognitive composite score at baseline | −0.15 (0.6) | −0.08 (0.6) | −0.58 (0.5) ** |
| Cognitive composite score at lifestyle activities assessment | 0.08 (0.7) | 0.21 (0.7) | −0.75 (0.7) ** |
| Low intensity physical activities, caloric expenditure/week | 1508.8 (1169.2) | 1505.2 (1183.8) | 1530.6 (1098.5) |
| High intensity physical activities, caloric expenditure/week | 2557.8 (2354.2) | 2621.9 (2456.3) | 2170.9 (1587.3) |
| Low intensity physical activities, frequency/week | 11.3 (7.9) | 11.2 (8.1) | 12.0 (6.4) |
| High intensity physical activities, frequency/week | 9.3 (7.1) | 9.3 (7.4) | 8.7 (5.5) |
| Cognitive activities, frequency/week | 22.0 (12.1) | 22.7 (12.7) | 17.4 (6.6) * |
| Social activities, frequency/week | 6.8 (5.2) | 6.8 (5.3) | 6.5 (4.9) |
| Total engagement in all activities, number of activities/week | 13.4 (3.9) | 13.5 (3.9) | 12.7 (3.6) |
Asterisks indicate significant differences between diagnostic groups (remained normal vs. progressed to MCI);
p < .05;
p < .01.
On average, the lifestyle questionnaire was completed 14.3 years after baseline and participants had 9.8 cognitive assessments over time. Of the 189 participants, 27 progressed from normal cognition to MCI at some point during follow-up; on average, their estimated age of onset of symptoms, which preceded the date of diagnosis, was 7.0 (SD = 2.4) years prior to the completion of the lifestyle questionnaire. Participants who progressed to MCI were older, had lower baseline CR composite scores, and lower cognitive composite scores both at baseline and at the visit at which the lifestyle questionnaire was completed. Correlations among the lifestyle variables are shown in Supplemental Digital Content 4.
Cross-Sectional Relationships of Lifestyle Activities to Demographic and Genetic Variables
The full results of the cross-sectional models are shown in Supplemental Digital Content 5. Briefly, engagement in lifestyle activities did not differ by diagnosis or APOE-4 genetic status. Younger participants tended to report higher levels of engagement in lifestyle activities. Males and participants with higher baseline CR reported a higher frequency of engagement in cognitive activities.
Longitudinal Relationships of Current Lifestyle Activity Engagement to Prior Cognitive Trajectories
In the linear mixed effects models (Table 2), there were significant main effects of time (reflecting practice-related improvements in prior cognitive performance), education (reflecting better cognitive performance with higher education), sex (reflecting lower cognitive performance among men), and diagnosis (reflecting lower cognitive performance among the participants who progressed to MCI). Significant age x time interactions also indicated less practice-related improvements in prior cognitive performance with increasing age. Importantly, the significant lifestyle variable x time interaction for total engagement in all activities indicated more practice-related improvements in prior cognitive performance in participants who endorsed a greater overall number of activities.
Table 2.
Results of the linear mixed effects models examining the association between engagement in lifestyle activities and prior cognitive trajectories. Values reflect standardized coefficients (95% confidence intervals), with the exception of the time variable and the discrete variables.
| Model predictor | Low intensity physical, frequency | High intensity physical, frequency | Cognitive, frequency | Social, frequency | Total engagement, number |
|---|---|---|---|---|---|
| Baseline age | −0.045 (−0.16, 0.07) | −0.063 (−0.18, 0.05) | −0.045 (−0.16, 0.07) | −0.042 (−0.16, 0.07) | −0.066 (−0.18, 0.05) |
| Sex, male | −0.397 (−0.64, −0.15) ** | −0.382 (−0.62, −0.14) ** | −0.407 (−0.66, −0.15) ** | −0.409 (−0.65, −0.16) *** | −0.39 (−0.63, −0.15) ** |
| Education | 0.137 (0.02, 0.26) * | 0.152 (0.03, 0.27) * | 0.142 (0.02, 0.26) * | 0.142 (0.02, 0.26) * | 0.148 (0.03, 0.27) * |
| Diagnosis | −0.762 (−1.10, −0.43) *** | −0.758 (−1.09, −0.43) *** | −0.759 (−1.10, −0.42) *** | −0.763 (−1.10, −0.43) *** | −0.769 (−1.10, −0.44) *** |
| Lifestyle variable | 0.029 (−0.08, 0.41) | −0.136 (−0.25, −0.02) * | 0.002 (−0.12, 0.12) | −0.036 (−0.15, 0.08) | −0.131 (−0.25, −0.02) * |
| Time | 0.034 (0.02, 0.04) *** | 0.034 (0.02, 0.04) *** | 0.034 (0.03, 0.04) *** | 0.033 (0.02, 0.04) *** | 0.034 (0.02, 0.04) *** |
| Baseline age x time | −0.019 (−0.03, −0.01) *** | −0.018 (−0.03, −0.01) *** | −0.018 (−0.03, −0.01) *** | −0.019 (−0.03, −0.01) *** | −0.017 (−0.03, −0.01) *** |
| Sex x time | −0.003 (−0.02, 0.01) | −0.004 (−0.02, 0.01) | −0.006 (−0.02, 0.01) | −0.003 (−0.02, 0.01) | −0.004 (−0.02, 0.01) |
| Education x time | 0.003 (−0.004, 0.01) | 0.003 (−0.01, 0.01) | 0.002 (−0.01, 0.01) | 0.003 (−0.004, 0.01) | 0.003 (−0.01, 0.01) |
| Diagnosis x time | −0.019 (−0.04, 0.002) # | −0.019 (−0.04, 0.001) # | −0.016 (−0.04, 0.004) | −0.018 (−0.04, 0.002) # | −0.018 (−0.04, 0.002) # |
| Lifestyle variable x time | < 0.001 (−0.01, 0.01) | 0.005 (−0.002, 0.01) | 0.006 (−0.001, 0.01) | 0.003 (−0.004, 0.01) | 0.008 (0.001, 0.02) * |
p ≤ .10;
p ≤ .05;
p ≤ .01,
p ≤ .001
Notes: The pattern of results for the physical activity caloric expenditure measures was comparable to the results for the measures of frequency of engagement in physical activities (results not shown). With the exception of time, all continuous variables were standardized to a mean of 0 and a standard deviation of 1.0 before model fitting. Coefficients for time reflect the effect per year, whereas coefficients for the remaining continuous variables reflect the effect of the variable per standard deviation unit. Coefficients for categorical variables reflect the comparison of variable categories. (Using the results for the total engagement variable as an example, the main effect of education on cognitive trajectories means that baseline cognition would increase by 0.148 standard deviations per unit increase in education. The lifestyle variable x time interaction reflects a 0.008 increase in the annual rate of change in cognition, for each standard deviation unit increase in the lifestyle variable.)
Longitudinal Analyses Testing Whether Other Variables Modify the Association Between Current Lifestyle Activity Engagement and Prior Cognitive Trajectories
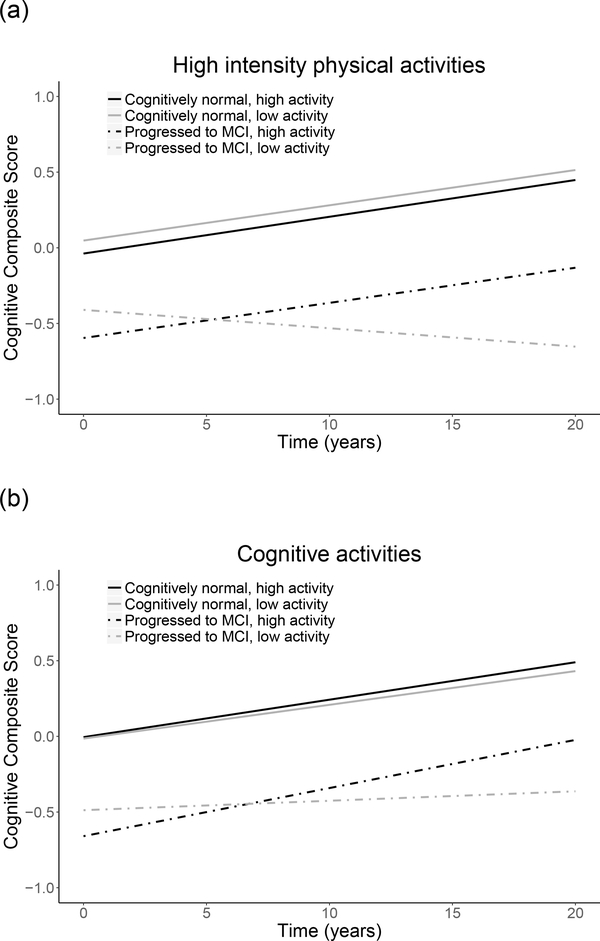
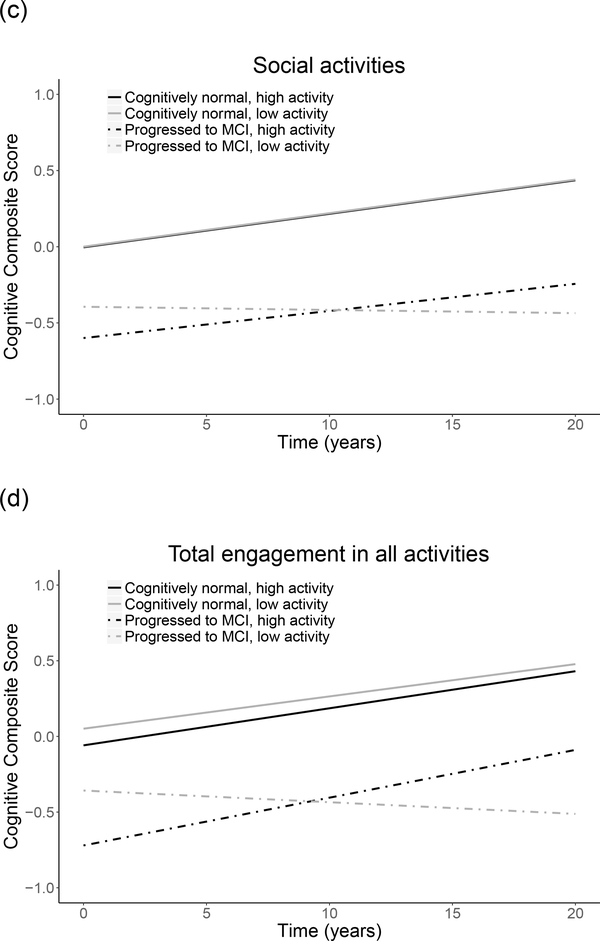
Associations between engagement in lifestyle activities and prior cognitive trajectories differed by diagnosis, as indicated by significant diagnosis x lifestyle variable x time interactions for frequency of engagement in low and high intensity physical activities, cognitive activities, and social activities, as well as total engagement in all activities (Table 3, top). In post hoc models run separately by diagnostic group, among participants who progressed to MCI, significant lifestyle variable x time interactions indicated that higher engagement in physical, cognitive, and social activities, as well as total engagement in all activities, were associated with less decline in prior cognitive performance (Table 3, bottom; Figure 2). The pattern of results was similar when excluding the 5 non-white participants, and when number of years impaired was included as an additional covariate, except that the interaction for low intensity physical activities was no longer significant (results not shown). The pattern of results was also similar when variables reflecting baseline vascular risk (i.e., hypertension, diabetes) were included as additional covariates (see Supplemental Digital Content 6). Additionally, higher frequency of engagement in cognitive and social activities was significantly associated with an older age of symptom onset (r = 0.18, p = .003 and r = 0.19, p = .002, respectively). In contrast, among cognitively normal participants, lifestyle activity engagement did not modify prior cognitive trajectories (all p > .30; results not shown).
Table 3.
Results of the linear mixed effects models examining the impact of diagnosis on the association between engagement in lifestyle activities and prior cognitive trajectories for all subjects (top) and individuals who progressed to MCI (bottom). Values reflect standardized coefficients (95% confidence intervals), with the exception of the time variable and the discrete variables. Models were adjusted for baseline age, sex, education, lifestyle variable, time, and each predictor’s interaction with time.
| Model predictor | Low intensity physical, frequency | High intensity physical, frequency | Cognitive, frequency | Social, frequency | Total engagement, number |
|---|---|---|---|---|---|
| All participants | |||||
| Diagnosis x lifestyle variable x time | 0.02 (0.000, 0.3) * | 0.03 (0.02, 0.05) *** | 0.03 (0.003, 0.05) * | 0.02 (0.004, 0.04) * | 0.02 (0.009, 0.4) ** |
| MCI only | |||||
| Lifestyle variable | −0.02 (−0.32, 0.30) | −0.23 (−0.53, 0.07) | −0.26 (−0.71, 0.19) | −0.20 (−0.48, 0.08) | −0.24 (−0.48, −0.002) # |
| Lifestyle variable x time | 0.03 (0.004, 0.05) * | 0.04 (0.02, 0.06) *** | 0.04 (0.006, 0.06) * | 0.02 (0.001, 0.04) # | 0.03 (0.01, 0.04) ** |
p ≤ .10;
p ≤ .05;
p ≤ .01,
p ≤ .001
Note: With the exception of time, all continuous variables were standardized to a mean of 0 and a standard deviation of 1.0 before model fitting. Coefficients for time reflect the effect per year, whereas coefficients for the remaining continuous variables reflect the effect of the variable per standard deviation unit. Coefficients for categorical variables reflect the comparison of variable categories.
Figure 2.
Relationship of engagement in lifestyle activities to prior cognitive trajectories, shown separately by low and high activity levels (median split) and diagnosis (remained cognitively normal; progressed to MCI). Figures depict frequency of engagement in (a) high intensity physical, (b) cognitive, and (c) social activities, and (e) total engagement in all activities (number). Level of activity engagement was modeled as a continuous variable; it was dichotomized for illustration purposes only.
Associations between current lifestyle activity engagement and prior cognitive trajectories were not modified by APOE or baseline age (all p > .28). Associations between current lifestyle activity engagement and prior cognitive trajectories were also not modified by baseline CR, with the exception of frequency of engagement in social activities (CR x lifestyle variable x time, beta (SE) = −0.009 (0.003), p = .01). Post hoc models indicated higher engagement in social activities was associated with more practice-related improvements in prior cognitive performance among participants with low CR scores (i.e., below the median, beta (SE) = 0.018 (0.008), p = .03), but not high CR scores (i.e., above median, p = .57).
Follow-up models among participants who have remained cognitively normal.
Additional follow-up models examined the above-mentioned three-way interactions among the subset of participants who have remained cognitively normal over time. The relationships between engagement in lifestyle activities and prior cognitive trajectories were not modified by baseline CR, APOE-4 status, or age (all p > .11).
Discussion
This study examined the relationship of current engagement in lifestyle activities with prior cognitive trajectories in a cohort of individuals who were cognitively normal and primarily middle-age at baseline. In the primary analyses, only total number of activities endorsed (but not the individual lifestyle activities) was associated with better cognitive trajectories. However, the relationship between engagement in lifestyle activities and prior longitudinal cognitive trajectories differed by diagnostic outcome. Among individuals who progressed to MCI over the course of follow-up, higher engagement in lifestyle activities was associated with less decline in prior cognitive performance. In addition, higher frequency of engagement in cognitive and social activities was associated with an older age of onset of clinical symptoms of MCI.
In contrast, among individuals who remained cognitively normal over time, engagement in lifestyle activities was not associated with prior cognitive trajectories, and the association between engagement in lifestyle activities and prior cognitive trajectories was not modified by CR, APOE-4 status, or age. This may reflect the fact that individuals who remained cognitively normal demonstrated practice-related improvements in cognitive performance over time, suggesting that any differential impact of lifestyle activities may be difficult to discern.
Taken together, these findings raise the possibility that lifestyle activities provide resilience that allows individuals with preclinical disease to remain asymptomatic for longer periods of time by enhancing their capacity to tolerate accumulating AD pathology,35,36 though biomarker studies (e.g., including PET or MRI) are needed to explicitly test this hypothesis. By comparison, there appears to be less of an impact of lifestyle activities on cognitive changes due to normal aging, at least among individuals who were primarily middle aged at baseline.
Because lifestyle activities were not assessed at baseline, we cannot rule out the possibility of reverse causation. For example, those who progressed to MCI may have declined in their levels of engagement in lifestyle activities as cognitive problems were developing (see37,38 for a discussion), or had difficultly recalling their levels of lifestyle activity engagement, rather than activities being protective. Although this explanation seems less likely given that diagnostic status was unrelated to cross-sectional measures of lifestyle activity engagement, it cannot be ruled out. The causal relationship between changes in cognition and changes in lifestyle activity engagement is an issue for any study that assesses activity engagement among non-demented or mixed diagnosis groups, which includes a large number of studies on this topic. These findings therefore require replication by future studies among cognitively normal individuals that include baseline measures of lifestyle activity engagement, measures of cognition over time, and longitudinal clinical outcomes.
The results of this study extend prior literature in a number of ways. First, this is the first study to our knowledge that has examined multiple categories of lifestyle activities in cognitively normal individuals with extensive longitudinal follow-up. Second, prior studies examining the relationship between lifestyle activities and cognitive trajectories among initially cognitively normal individuals have tended to include individuals who were older at baseline (70s-80s) or had relatively short follow-up (≤ 5 years).5,7,19–22
Third, although prior studies have reported a reduced risk of progressing to MCI among those with greater lifestyle activity engagement, few have examined the impact of other factors known to affect MCI risk (i.e., APOE status, level of CR, age). For example, in a prior study among primarily middle-aged, cognitively normal individuals, higher engagement in physical activities was associated with small working memory/executive function performance gains among APOE-4 carriers, but not APOE-4 non-carriers.19 Although we found no interactions between physical activity engagement and APOE in the present study, this discrepancy may be attributed to differences in outcome variables. Additionally, aside from engagement in social activities, baseline CR did not modify the relationship between lifestyle activity engagement and prior cognitive trajectories. These effects were likely driven by the subset of individuals who progressed to MCI, given that baseline CR did not modify prior cognitive trajectories among those who remained cognitively normal. Although some prior studies among non-demented individuals found social activities to reduce the likelihood of cognitive decline,12,14 findings have been inconsistent.16,38 Taken together with the existing literature, the results of the present study suggest that the protective effects of lifestyle activities on longitudinal cognitive trajectories may be most evident in the subset of cognitively normal individuals at greatest risk for cognitive decline, such as those at increased risk of progressing to MCI who likely have higher levels of brain pathology, or those with low levels of CR.
One of the primary findings of this study, that higher current lifestyle activity engagement was associated with less decline in prior cognitive performance among those who progressed to MCI, appears to contrast with our previous findings showing that higher levels of baseline CR were associated with greater cognitive decline after symptom onset among those who progressed to MCI.39 One possible explanation for this difference is that the protective effects of CR and lifestyle activity engagement operate through different mechanisms, with subtly different neural implementations. We hypothesize that baseline CR, measured here by proxy variables that peak around middle age and remain relatively stable thereafter (e.g., education, vocabulary), may be most directly related to neural reserve, such as the development of brain networks with greater efficiency, capacity, and/or flexibility.36 These networks may function normally until a threshold of pathology is reached, at which point cognition begins to decline and symptoms of MCI emerge. As hypothesized by theoretical models of CR,36,39 individuals with higher levels of baseline CR show a later age of MCI symptom onset, followed by greater cognitive decline after symptom onset. By comparison, continued engagement in lifestyle activities may be more closely related to neural compensation, reflected in the ability to use brain networks more flexibly in the face of accumulating pathology,36 and could be one mechanism by which lifestyle activities modify cognitive decline among those who develop symptoms of MCI. Future studies could explicitly test this hypothesis, for example, by comparing neural outcomes (e.g., functional imaging measures) for individuals with equivalent levels of education/vocabulary, equivalent levels of AD pathology (measured by biomarkers), but different levels of lifestyle activity engagement.
The present study has several limitations. Participants were primarily white, highly educated, and have a strong family history of dementia, limiting the generalizability of these findings to the population at large. Additionally, lifestyle activity engagement was not assessed at baseline. Although all participants were cognitively normal at baseline, a subset had progressed to MCI by the time they completed the lifestyle activities questionnaire. Accordingly, we cannot rule out the possibility that those who progressed to MCI had corresponding declines in, or more difficulty recalling, their levels of activity engagement (as discussed above). Additionally, although we examined the potential impact of a subset of vascular risks, these analyses did not comprehensively examine other potential risk factors (e.g., obesity, smoking) that may also impact rates of cognitive decline and/or levels of engagement in lifestyle activities. Future prospective studies are needed to examine larger groups of initially cognitively normal individuals who progress to MCI, with lifestyle activity measures collected prior to development of cognitive impairment.
A recent consensus report on the current state of evidence regarding the effectiveness of interventions for preventing cognitive decline and dementia indicated that physical and cognitive interventions might be effective for delaying or slowing age-related cognitive decline.2 In contrast, there was insufficient evidence to indicate that such interventions prevent or delay the development of MCI or dementia, and insufficient evidence for drawing conclusions about social engagement interventions. The report noted, however, that most interventions have very limited follow-up, which reduces their ability to detect effects on longer-term clinical outcomes (but see40). This study’s results raise the possibility that interventions among middle-aged, cognitively normal adults may be more effective among subgroups at the greatest risk of progression to MCI. Consistent with this, the report concluded, “targeting interventions to high-risk populations may increase the likelihood of detecting a beneficial effect of an intervention and provide a more accurate assessment of its efficacy” (p. 65). Future interventions could test this by comparing whether lifestyle intervention efficacy varies by baseline risk factors such as AD biomarker positivity, though extensive longitudinal follow-up would likely be required.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (grant numbers U19-AG033655, P50-AG005146).
The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Rostislav Brichko); (2) the Clinical Core (Marilyn Albert, Anja Soldan, Corinne Pettigrew, Rebecca Gottesman, Ned Sacktor, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Daniel D’Agostino, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Richard O’Brien); (5) the Informatics Core (Roberta Scherer, David Shade, Ann Ervin, Jennifer Jones, Hamadou Coulibaly, April Patterson); (6) the Biostatistics Core (Mei-Cheng Wang, Daisy Zhu, Jiangxia Wang); and (7) the Neuropathology Core (Juan Troncoso, Olga Pletnikova, Gay Rudow, Karen Fisher). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs. John Csernansky, David Holtzman, David Knopman, Walter Kukull, and Kevin Grimm, and Drs. John Hsiao and Laurie Ryan, who provide oversight on behalf of the National Institute on Aging. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs. Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principle investigator: Dr. Trey Sunderland). The authors are particularly indebted to Dr. Karen Putnam, who has provided ongoing documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Source of Funding: This work was supported by the National Institutes of Health (grant numbers U19-AG033655, P50-AG005146).
Footnotes
Conflicts of Interest: Dr. Marilyn Albert is an advisor to Eli Lilly. For the remaining authors, none were declared.
References
- 1.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 2.In: Downey A, Stroud C, Landis S, Leshner AI, eds. Preventing Cognitive Decline and Dementia: A Way Forward. Washington (DC)2017. [PubMed] [Google Scholar]
- 3.Medicine Io. Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 4.Krell-Roesch J, Vemuri P, Pink A, et al. Association Between Mentally Stimulating Activities in Late Life and the Outcome of Incident Mild Cognitive Impairment, With an Analysis of the APOE epsilon4 Genotype. JAMA Neurol. 2017;74(3):332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. [DOI] [PubMed] [Google Scholar]
- 6.Roberts RO, Cha RH, Mielke MM, et al. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84(18):1854–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sattler C, Toro P, Schonknecht P, Schroder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012;196(1):90–95. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911–1920. [DOI] [PubMed] [Google Scholar]
- 9.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. [DOI] [PubMed] [Google Scholar]
- 10.Carlson MC, Parisi JM, Xia J, et al. Lifestyle activities and memory: variety may be the spice of life. The women’s health and aging study II. J Int Neuropsychol Soc. 2012;18(2):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. [DOI] [PubMed] [Google Scholar]
- 12.James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social activity and cognitive decline in old age. J Int Neuropsychol Soc. 2011;17(6):998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vemuri P, Lesnick TG, Przybelski SA, et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol. 2014;71(8):1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HX, Jin Y, Hendrie HC, et al. Late life leisure activities and risk of cognitive decline. J Gerontol A Biol Sci Med Sci. 2013;68(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. [DOI] [PubMed] [Google Scholar]
- 16.Brown CL, Gibbons LE, Kennison RF, et al. Social activity and cognitive functioning over time: a coordinated analysis of four longitudinal studies. J Aging Res. 2012;2012:287438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14(2):245–263. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. [DOI] [PubMed] [Google Scholar]
- 19.Pizzie R, Hindman H, Roe CM, et al. Physical activity and cognitive trajectories in cognitively normal adults: the adult children study. Alzheimer Dis Assoc Disord. 2014;28(1):50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology. 2010;75(11):990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81(4):314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late-life cognitive activity on cognitive health. Neurology. 2012;78(15):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert M, Soldan A, Gottesman R, et al. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr Alzheimer Res. 2014;11(8):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler D WMS-R : Wechsler Memory Scale--Revised : manual. San Antonio: Psychological Corp. : Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 27.Kaplan E, Goodglass H, Weintraub S, Goodglass H. Boston naming test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 28.Wechsler D WAIS-R, Wechsler Adult Intelligence Scale- Revised, Manual. Psychological Corporation; 1981. [Google Scholar]
- 29.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. [DOI] [PubMed] [Google Scholar]
- 30.Nelson H The National Adult Reading Test (NART): Test manual. Windsor, UK: Nfer-Nelson; 1982. [Google Scholar]
- 31.Pettigrew C, Soldan A, Li S, et al. Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Cogn Neurosci. 2013;4(3–4):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–184. [DOI] [PubMed] [Google Scholar]
- 33.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 34.R: A language and environment for statistical computing [computer program]. Version 3.2.2. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 35.Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosma H, van Boxtel MP, Ponds RW, et al. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: a reciprocal association? Z Gerontol Geriatr. 2002;35(6):575–581. [DOI] [PubMed] [Google Scholar]
- 38.Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26(2):144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soldan A, Pettigrew C, Cai Q, et al. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging. 2017;60:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards JD, Xu H, Clark DO, Guey LT, Ross LA, Unverzagt FW. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3(4):603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.