This medical progress report will outline the epidemiology and healthcare utilization associated with cerebrospinal fluid (CFS) shunt-associated infections in the US, the clinical features of CSF shunt infection, and our evolving understanding of the prevention and treatment of CSF shunt infection. We will describe an emerging paradigm in CSF shunt infection under active investigation.
Epidemiology of CSF Shunt Infections
CSF shunt placement has been the mainstay of treatment for hydrocephalus for over 60 years.(1) CSF shunts allow children with congenital hydrocephalus to survive infancy and allow children with acquired hydrocephalus to avoid further brain injury. Despite their benefits, CSF shunts can cause new and chronic surgical and medical problems. Mechanical malfunction is frequent, and 60% of shunts require surgical revision within 4 years.(2–4) Infection develops in 5% to 15% of all CSF shunts.(5, 6)
The volume of pediatric surgeries related to CSF shunts is considerable in the United States, accounting for nearly 20,000 hospital admissions each year, of which approximately 4,500 are for initial CSF shunt placement, 10,000 for CSF shunt revision, and 3,200 for other CSF shunt surgeries.(7) Infections of CSF shunts account for over 2,000 hospital admissions each year and are associated with extensive resource utilization, including approximately 55,000 hospital days (mean of 14.2-15.1 days per admission) and up to $250 million in charges (mean of $46,000-$62,000 per admission).(7)
The true incidence of shunt infection is difficult to calculate, in part due to a lack of a standard definition for surveillance. The most common definition, put forth by the Centers for Disease Control and Prevention’s National Healthcare Safety Network (CDC/NHSN), concerns post-operative (surgical site) infection, and does not attempt to address shunt infection specifically.(8) Other definitions, such as that put forth by the Hydrocephalus Clinical Research Network (HCRN),(9) focus solely on CSF shunts and the various ways infections are diagnosed.
The HCRN consensus definition for CSF shunt infection is: (a) microbiological determination of bacteria present in a culture or Gram stain of CSF, wound swab, and/or pseudocyst fluid; or (b) shunt erosion (visible hardware); or (c) abdominal pseudocyst (without positive culture); or for children with ventriculoatrial shunts, (d) presence of bacteria in a blood culture. The HCRN definition has not been widely adopted as a surveillance definition, presumably because the data needed to apply the HCRN definition are not routinely collected by infection prevention programs, and this definition has been tested only in pediatric patients. Nonetheless, common features of definitions of shunt infection include the recovery of microorganisms from the CSF of children with shunts.
Clinical Features of CSF Shunt Infections
The clinical features of CSF shunt infection depend on the mechanism of infection, the causative pathogen, and the type of shunt. The most common clinical symptoms are fever, headache, nausea, and lethargy.(5, 10) Shunt infection is identified as the etiology of shunt malfunction in 3% to 8% of cases of malfunction.(11) Shunt malfunction, which leads to development of symptoms consistent with shunt failure, typically yields culture-negative CSF and is attributed to either apparatus obstruction or disconnection.
According to current, commonly held criteria, diagnosis of CSF shunt infection generally relies on the recovery of a microorganism from conventional culture of CSF.(9, 12) The pathogens identified in CSF shunt infections most often are bacteria (12–16), with fungi a distant second (17)), and it is believed that organisms are introduced onto the shunt apparatus at the time of surgery. Staphylococcal species, especially coagulase-negative Staphylococcus and Staphylococcus aureus, account for almost two-thirds of all shunt infections.(10, 18) The most common infecting organism recovered from conventional aerobic cultures of CSF is Staphylococcus epidermidis.(19–21) Cutibacterium acnes [formerly Propionibacterium acnes(22)] has been isolated more often in recent series of ventriculoperitoneal shunt infections; this bacterium generally causes low-grade, indolent infections with few overt signs or symptoms.(23) Although most bacteria causing shunt infections produce visible growth in broth or on agar within 48 to 72 hours, it is recommended that anaerobic cultures should be ordered and monitored for growth for up to 10 days because fastidious organisms such as C. acnes grow relatively slowly.(24) Despite prolonged incubation, CSF cultures may yield no bacteria despite clinical symptoms of infection, particularly if the patient was pretreated with antibiotics. In such instances, diagnosis typically is made using clinical judgment, close observation, and repeated CSF samples for Gram stain and culture.
Signs and symptoms of shunt infections are sometimes considered in relation to the location involved -i.e. proximal, meaning the portion of the shunt extending from the intracranial ventricle to valve, versus distal, meaning the portion of the shunt from the valve to the cavity into which CSF drains. Signs and symptoms of proximal shunt infection are less frequent than for distal infection, and usually include external signs of local soft tissue inflammation such as focal swelling, pain, erythema and purulent drainage from around the scalp incision site. Such surface shunt infections usually are a complication of surgery, due to direct inoculation of bacteria at the insertion site during shunt placement. Signs and symptoms of distal shunt infection depend on the location of the distal shunt tip and whether the internal lumen or the external surface is infected. Intraluminal infection of a ventriculoatrial shunt can result in bacteremia and systemic signs of toxicity, although septic shock is uncommon. Intraluminal infection of a ventriculoperitoneal shunt usually produces signs of peritonitis. Infection related to the external surface manifests with signs of local soft tissue inflammation along the shunt tubing tract.
Prevention of CSF Shunt Infections
Our Understanding of the Prevention of CSF Shunt Infection is Evolving
Studies related to the prevention of CSF shunt infections have been hampered by small sample sizes, and most have been performed retrospectively at single centers, limiting conclusions and generalizability. Results across studies often are equivocal. One example is the use of prophylactic antibiotics intravenously (IV) during shunt surgeries. Until the mid-1990s, equal numbers of studies demonstrated benefit (13, 25–30) and no benefit.(5, 31–37) Two meta-analyses subsequently demonstrated benefit,(26, 27) and in 1999 prophylactic IV antibiotics were recommended as standard care in the U.S.(38–40) Nonetheless, questions about the efficacy of intraoperative prophylactic IV antibiotics persist.(41) A 2012 National Institutes of Health-sponsored conference to assess research priorities in hydrocephalus highlighted a need for refinement of neurosurgical shunting procedures to improve survival and reduce infection rates.(42) Well-designed multicenter studies that can adjust analyses for variation between patient populations and centers and that provide adequate power will be needed to advance our understanding of effective infection prevention techniques.
Research on CSF Shunt Infection Prevention
Most infections become clinically apparent within 6 months of previous surgery.(6, 43) We and others have shown that relatively few patient, medical, or surgical risk factors are associated with CSF shunt infection. Factors associated with CSF shunt infections include a recent shunt insertion or revision, premature birth, young age, neuroendoscope use during shunt insertion, and prior shunt infection.(10, 44–48) Insertion of a shunt after a previous shunt infection is associated with a four-fold increase in the risk of shunt infection. To build on prior work using procedure-specific cohorts, we have assembled several cohorts of children undergoing initial CSF shunt placement to better understand the relative contribution of both patient and procedural factors to infection risk.(49) (Figure 1) Our multicenter observational studies using HCRN registry and administrative data have identified only three patient factors consistently associated with development of first CSF shunt infection: young age, intervening shunt revision surgeries, and the number of shunt revisions.(48–51) History of a single revision surgery is associated with a 3- to 4-fold higher risk of infection, whereas history of two or more revision surgeries is associated with a 6- to 13-fold higher risk of infection.(47, 48) The failure to identify additional patient, medical, or surgical risk factors for first infection is surprising and underscores the need for additional research.(44, 48–50)
Figure 1.
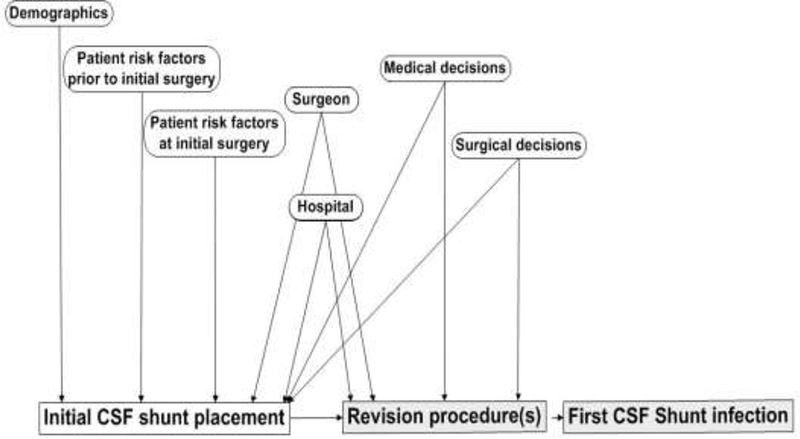
A framework for understanding patient and procedural risk factors for development of a first CSF shunt infection. Shaded boxes indicate possible surgeries; CSF infection can occur without an interval revision procedure.
Infection Prevention Quality Improvement Efforts
Substantial efforts have been taken to prevent CSF shunt infections in recent years that have led to reduction but not elimination of infections.(52, 53) Quality improvement methodology has been shown to prevent surgical site infection (SSI), including neurosurgical shunt infection. Much of the focus has been on standardizing intraoperative practice, which has shown success in HCRN and other cohorts.(9, 54)
Perhaps the largest effort to prevent pediatric shunt infection comes from Solutions for Patient Safety (SPS), a CMS-funded health engagement network of over 100 pediatric hospitals in North America.(55) Starting as an 8-hospital collaborative in Ohio, SPS has achieved measurable reduction of patient harm through partnerships to improve patient safety. SPS reported a 21% SSI reduction in a set of procedures within 10 months of implementation of a bundle to which adherence was high (>96%), with reduction of neurosurgical shunt infections from 3.2 to 2.3 per 100 procedures during the same time period.(56) Our subsequent work at a single institution showed that standardization of pre-operative activities such as bathing, S. aureus screening, and consistent communication with neurosurgical patients also reduced all post-operative infection, including shunt infections, significantly. (57)
Although SPS has focused on simple bundles for a variety of surgeries, the HCRN has focused on more extensive shunt infection prevention bundles that have included the restriction of operating room traffic, use of hair clipping, preparation of the surgical site, formal hand scrub, and double gloving.(9) Although the intrathecal instillation of broad-spectrum antibiotics into shunts upon placement had been reported in the literature,(34, 36, 58, 59) this practice was used rarely until recently. However, in 2007, the HCRN implemented a peri-operative infection prevention protocol that included one-time instillation of two intrathecal antibiotics (i.e. vancomycin and gentamicin) for all CSF shunt surgeries (totaling 1,571 surgeries).(9) The HCRN reported both a reduction in the overall Network infection rate from 8.8% prior to the protocol to 5.7% while using the protocol (p = 0.0028, absolute risk reduction 3.15%, relative risk reduction 36%), and reductions in per-procedure infection rates at three of four participating centers in 2011.(9) In the face of emerging evidence favoring the utility of antibiotic impregnated catheters in preventing CSF shunt infections, in January 2012 the HCRN discontinued the routine use of intrathecal antibiotics and initiated the use of antibiotic (clindamycin plus rifampin) impregnated shunt tubing in its shunt infection prevention protocol. Among 1,935 procedures performed at 8 centers between January 1, 2012, and September 30, 2013, the overall Network 6-month infection rate before and after implementation were 6.5% and 6.0%, respectively. Overall protocol compliance was 77%. The HCRN concluded that the change in the protocol from instillation of antibiotic through the shunt to antibiotic impregnated shunt tubing did not significantly reduce shunt infection, and that use of either procedure reduced shunt infection compared with use of neither.(54) The current HCRN protocol permits optional use of either technique
Management of CSF Shunt Infection
Management of CSF shunt infections is challenging. Because microorganisms adhere to the shunt itself, treatment of infection requires both surgical and medical management. Surgical management usually includes a minimum of two surgeries for removal and subsequent replacement of the infected shunt,(12, 15, 19–21, 60, 61) bridged by insertion of an external ventricular drain at the time of shunt removal. (20, 53, 62–66) The length of IV antimicrobial therapy often is based on the organism recovered and duration of positive cultures, with definitive drug choice based upon the susceptibilities of the recovered organism, its known bactericidal activity, and penetration of the blood-brain barrier. CSF shunt replacement generally does not occur until the CSF is culture-negative and treatment is complete, usually at 10 to 14 days. The Infectious Diseases Society of America (IDSA) recently published guidelines on their management in 2017.(24) Despite aggressive management, re-infection rates range from 20% to 25%.(19–21) Furthermore, CSF shunt infection negatively impacts neurocognitive outcomes (67) and quality of life.(68) In some cases, infection can result in death. (29, 31, 60, 67, 69, 70)
Investigation of Roles of Microbial Diversity and Biofilms
Increasing evidence suggests that CSF shunt infections often are polymicrobial, with most organisms not cultivatable, (71, 72) and that biofilms play an important role in in CSF shunt infection. (73–75) Until recently, the presence of bacteria during disease traditionally was determined by growth of bacteria in conventional culture. (76, 77) By use of culture-independent molecular approaches, the microbiota on and in human tissues, in both health and disease, in shown to more complex than is detectable by culture.(76, 79, 80) Sterility of CSF in the presence and absence of shunts in healthy individuals is difficult to confirm given current technological limitations in the study of low-abundance microbiota.
In a study of eight cases of CSF shunt infection and uninfected controls using quantitative PCR (qPCR) and high-throughput sequencing, we identified small amounts of bacterial and fungal DNA of both cultivatable and non-cultivatable species in CSF of all with CSF shunt infection and in no control CSF.(72) A representative example of the variety of bacterial DNA obtained from a child with Staphylococcus epidermidis culture-positive CSF shunt infection is shown in Figure 2. Surprisingly, the predominant organism detected by this analysis, a Paenibacillus sp., was markedly more abundant than was the isolated S. epidermidis.
Figure 2.
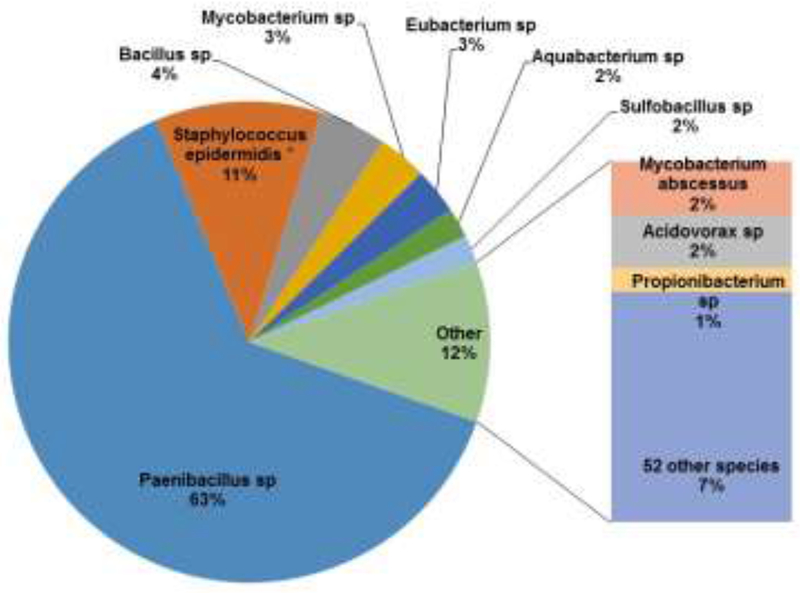
Microbiota in the CSF of a child with CSF shunt infection.
Shown are bacterial taxa identified by 16S bacterial tag-encoded FLX-Titanium amplicon pyrosequencing (bTEFAP) at the time of an initial CSF sample, presented as a proportion of all sequence detections in the sample representing bacteria for which detection comprised 1% or more of the total. The sole bacterium that was identified in conventional culture (S. epidermidis) is designated with an asterisk. Negative controls of donated CSF that underwent concurrent DNA extraction revealed no detection by bTEFAP analysis (data not shown).
Emerging evidence suggests that the microbes that cause CSF shunt infections live in complex, adherent assemblages of microbes encased in an extracellular matrix (81), known as biofilms, associated with the shunt catheter surface.(73–75) Biofilms are thought to be responsible for many persistent and chronic infections,(79, 82) and are increasingly understood to play a role in other medical device infections.(83, 84) Biofilm-dwelling bacteria grown in vitro are tolerant to antimicrobial activity,(85) a characteristic common among CSF infections. Currently, conventional culture techniques are designed to detect rapidly-growing, liquid-suspended clonal populations of individual microbial species,(76) and may not detect slow-growing organisms residing in surface-adherent biofilms.(81)
Using bacterial PCR-based quantitation, we reported that bacterial DNA was only detectable early in the course of infection, if at all, in most samples of CSF from shunt infections,(71) strengthening the concept that bacteria predominantly occupy shunt-adherent biofilms.
A variety of chronic (79, 82), device-associated (83, 84), biofilm associated infections are observed to be highly resistant to clearance by the immune system, with decreased phagocytosis.(86, 87) Biofilm structure, immunomodulation, and extracellular molecules produced by biofilm-forming bacteria likely contribute to increased fitness in the presence of immune cells. Leukocytes are observed to penetrate biofilms, but phagocytosis and bactericidal activity is decreased compared with activity against planktonic bacteria. Additionally, decreased cytokine activity has been measured in response to biofilm-grown compared with planktonic S. epidermidis. (88)
For S. epidermidis, the exopolysaccharide intercellular adhesin (PIA) also may contribute to immune evasion. PIA is secreted by biofilm-producing cells and is believed to mediate adherence to surfaces and other organisms participating in the biofilm.(89) S. epidermidis PIA knockout mutants (ica-) grown in biofilm were observed to be more susceptible to phagocytosis than wild-type biofilm S. epidermidis and were also more susceptible to killing by antimicrobial peptides.(86) In another study, ica- mutants were observed to be more susceptible to complement killing than wild-type S. epidermidis.(87)
Other antimicrobial resistance factors in biofilm infections have been identified: slow growth of organisms, glycocalyx production, high density, and adherence to surfaces.(90) Biofilm matrix production has been shown to inhibit the penetrance of oxacillin, cefotaxime, and vancomycin into the deeper layers of the biofilm, but not amikacin and ciprofloxacin.(91) Slow rate of growth and anaerobic growth of microbes in biofilm communities also likely add to antibiotic resistance. P. aeruginosa grown in anaerobic conditions is associated with decreased susceptibility to tobramycin and ciprofloxacin, likely due to slow growth rate, because most antibiotics act on actively dividing cells. However, when nitrate is added to the anaerobically grown cells, growth rate increases but resistance to tobramycin and ciprofloxacin increases(92), suggesting that, slow growth rate is not the only factor contributing to antibiotic resistance. It is possible that an abundance of nutrients upregulates the expression of antimicrobial resistance genes. More experimentation is needed to clarify these issues.
The presence of biofilms on CSF shunts(73–75), the detection of non-cultivatable organisms and biofilms in recalcitrant infections (76, 79, 80, 82), as well as other medical device-associated infections (83, 84) have been reported previously. Biofilm infections in the CSF pose a special challenge because of limited achievable antibiotic concentrations in the CSF. Although treatment of meningitis may be enhanced by a more permissive blood-brain barrier, shunt infection and ventriculitis may not generate significant meningeal inflammation. Few of the agents effective for bloodstream or tissue infection, such as β-lactam agents, carbapenems, and aminoglycosides, cross efficiently into the CSF. Fluoroquinolones demonstrate better CSF penetration, but typically are avoided in younger patients. Likewise, linezolid can achieve relatively high CSF levels, but its bacteriostatic properties make it less desirable as first-line therapy. Higher doses of drugs such as β-lactams and aminoglycosides can be given to drive CSF penetration, but often are limited by systemic toxicity.(93) Additionally, higher concentrations of antibiotics are not singularly effective against biofilm infections. Thus, it is recommended that antibiotic therapy be coupled with removal of the contaminated device. Meticulous care during shunt insertion to prevent procedure-associated infection, and more research into non-surgical treatment of biofilm and shunt infections, are needed.
Areas for Further Study
Using recent advances in molecular and microscopic microbiology, careful characterization of the CSF microbiota over time may lead to a better understanding of the qualitative and quantitative changes in the microbial community that contribute to patient morbidity. The practical implications of a diverse CSF microbiota in hydrocephalus currently are unclear. One possibility is that if a CSF microbiota is present before infection, CSF shunt revision surgeries may disrupt the local environment either by introducing new organisms or permitting further growth of extant organisms, thus increasing infection risk. One theoretical infection prevention approach worthy of study might be to reduce bacterial load at the time of CSF shunt revision by a short peri-operative courses of broad spectrum antibiotics.
A variety of related clinical questions arise if the plastic surface of a shunt acts as a nucleation site for microbial biofilm formation. Preventing infection may require surgical approaches that disrupt biofilms. Potentially the use of a different shunt tract at the time of CSF shunt revision could reduce infection risk because biofilm may be left behind in the tract following shunt removal.
The implications of CSF microbiota and/or biofilm formation may also impact how we approach treatment of CSF shunt infections. Might there be a role for identification of non-cultivatable species to better define treatment? How does the establishment of biofilm lead to overgrowth (i.e. infection) of a predominant organism? Are biofilm polymicrobial integrated communities or do they consist of single species aggregates dispersed across the shunt surface? Might shunts be epithelialized (as are vascular conduits)? If so, does epithelialization increase or decrease the risk of infection? Does culture-negative shunt malfunction reflect infection of the shunt tubing by a biofilm, with resulting occlusion? Does the passive nature of CSF flow through the catheter influence biofilm formation? Do the tissues surrounding the shunt become colonized or infected with these microbiota, either during the infection itself or during shunt surgeries, serving as a nidus for re-infection?
Can certain antimicrobial agents better target biofilms? Does different hardware and/or surgical approaches affect microbial load and hence microbiota/biofilm formation? What impact does antibiotic-impregnated catheter tubing have on biofilm microbiota and/or biofilm antimicrobial resistance? What other factors contribute to biofilm antimicrobial resistance? And finally, is there a better approach to surveillance of shunts given this microbial hypothesis?
Knowledge of the involvement of a microbiota and polymicrobial biofilm in the pathogenesis of CSF shunt infections represents a paradigm shift in this field. Given the high burden of disease and the inadequacies of current pathophysiologic models, diagnostic modalities, and treatments in the prevention and cure of these infections, this conceptual shift provides a promising way forward to improving the care of children with hydrocephalus.
Acknowledgments
T.S., M.P., and L.H. were supported by the National Institute of Neurological Disorders And Stroke (R01NS095979). The study funders played no role in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the funding sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Kestle JR. Pediatric hydrocephalus: current management. Neurol Clin. 2003;21(4):883–95, vii. [DOI] [PubMed] [Google Scholar]
- 2.Browd SR, Ragel BT, Gottfried ON, Kestle JR. Failure of cerebrospinal fluid shunts: part I: Obstruction and mechanical failure. Pediatr Neurol. 2006;34(2):83–92. [DOI] [PubMed] [Google Scholar]
- 3.Browd SR, Gottfried ON, Ragel BT, Kestle JR. Failure of cerebrospinal fluid shunts: part II: overdrainage, loculation, and abdominal complications. Pediatr Neurol. 2006;34(3):171–6. [DOI] [PubMed] [Google Scholar]
- 4.Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33(5):230–6. [DOI] [PubMed] [Google Scholar]
- 5.Kontny U, Hofling B, Gutjahr P, Voth D, Schwarz M, Schmitt HJ. CSF shunt infections in children. Infection. 1993;21(2):89–92. [DOI] [PubMed] [Google Scholar]
- 6.Mancao M, Miller C, Cochrane B, Hoff C, Sauter K, Weber E. Cerebrospinal fluid shunt infections in infants and children in Mobile, Alabama. Acta Paediatr. 1998;87(6):667–70. [DOI] [PubMed] [Google Scholar]
- 7.Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatrics. 2008;1(2):131–7. [DOI] [PubMed] [Google Scholar]
- 8.Prevention CfDCa. Surgical Site Infection (SSI) event. National Healthcare Safety Network Patient Safety Component Manual 2016. p. 9-1 to 9-14. [Google Scholar]
- 9.Kestle JR, Riva-Cambrin J, Wellons JC, 3rd, Kulkarni AV, Whitehead WE, Walker ML, et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr. 2011;8(1):22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris A, Low DE. Nosocomial bacterial meningitis, including central nervous system shunt infections. Infect Dis Clin North Am. 1999;13(3):735–50. [DOI] [PubMed] [Google Scholar]
- 11.Kim TY, Stewart G, Voth M, Moynihan JA, Brown L. Signs and symptoms of cerebrospinal fluid shunt malfunction in the pediatric emergency department. Pediatr Emerg Care. 2006;22(1):28–34. [DOI] [PubMed] [Google Scholar]
- 12.Fan-Havard P, Nahata MC. Treatment and prevention of infections of cerebrospinal fluid shunts. Clin Pharm. 1987;6(11):866–80. [PubMed] [Google Scholar]
- 13.Odio C, McCracken GH, Jr., Nelson JD. CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child. 1984;138(12):1103–8. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JD. Cerebrospinal fluid shunt infections. Pediatr Infect Dis. 1984;3(3 Suppl):S30–2. [DOI] [PubMed] [Google Scholar]
- 15.Sells CJ, Shurtleff DB, Loeser JD. Gram-negative cerebrospinal fluid shunt-associated infections. Pediatrics. 1977;59(4):614–8. [PubMed] [Google Scholar]
- 16.Jamjoom A, al-Abedeen Jamjoom Z, al-Hedaithy S, Jamali A, Naim Ur R, Malabarey T. Ventriculitis and hydrocephalus caused by Candida albicans successfully treated by antimycotic therapy and cerebrospinal fluid shunting. Br J Neurosurg. 1992;6(5):501–4. [DOI] [PubMed] [Google Scholar]
- 17.Chiou CC, Wong TT, Lin HH, Hwang B, Tang RB, Wu KG, et al. Fungal infection of ventriculoperitoneal shunts in children. Clin Infect Dis. 1994;19(6):1049–53. [DOI] [PubMed] [Google Scholar]
- 18.Yogev R Cerebrospinal fluid shunt infections: a personal view. Pediatr Infect Dis. 1985;4(2):113–8. [DOI] [PubMed] [Google Scholar]
- 19.Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, et al. Management of shunt infections: a multicenter pilot study. J Neurosurg. 2006;105(3 Suppl):177–81. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni AV, Rabin D, Lamberti-Pasculli M, Drake JM. Repeat cerebrospinal fluid shunt infection in children. Pediatr Neurosurg. 2001;35(2):66–71. [DOI] [PubMed] [Google Scholar]
- 21.Tuan TJ, Thorell EA, Hamblett NM, Kestle JR, Rosenfeld M, Simon TD. Treatment and microbiology of repeated cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 2011;30(9):731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz CF, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016;66(11):4422–32. [DOI] [PubMed] [Google Scholar]
- 23.Thompson TP, Albright AL. Propionibacterium [correction of Proprionibacterium] acnes infections of cerebrospinal fluid shunts. Childs Nerv Syst. 1998;14(8):378–80. [DOI] [PubMed] [Google Scholar]
- 24.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Michael Scheld W, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kestle JR, Hoffman HJ, Soloniuk D, Humphreys RP, Drake JM, Hendrick EB. A concerted effort to prevent shunt infection. Childs Nerv Syst. 1993;9(3):163–5. [DOI] [PubMed] [Google Scholar]
- 26.Langley JM, LeBlanc JC, Drake J, Milner R. Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: meta-analysis. Clin Infect Dis. 1993;17(1):98–103. [DOI] [PubMed] [Google Scholar]
- 27.Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery. 1994;34(1):87–92. [PubMed] [Google Scholar]
- 28.Savitz MH, Katz SS. Prevention of primary wound infection in neurosurgical patients: a 10-year study. Neurosurgery. 1986;18(6):685–8. [DOI] [PubMed] [Google Scholar]
- 29.Schoenbaum SC, Gardner P, Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975;131(5):543–52. [DOI] [PubMed] [Google Scholar]
- 30.Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. J Neurosurg. 1985;62(5):694–7. [DOI] [PubMed] [Google Scholar]
- 31.George R, Leibrock L, Epstein M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 1979;51(6):804–11. [DOI] [PubMed] [Google Scholar]
- 32.Di Rocco C, Marchese E, Velardi F. A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus. Cooperative survey of the 1991-1992 Education Committee of the ISPN. Childs Nerv Syst. 1994;10(5):321–7. [DOI] [PubMed] [Google Scholar]
- 33.Griebel R, Khan M, Tan L. CSF shunt complications: an analysis of contributory factors. Childs Nerv Syst. 1985;1(2):77–80. [DOI] [PubMed] [Google Scholar]
- 34.Shurtleff DB, Stuntz JT, Hayden PW. Experience with 1201 cerebrospinal fluid shunt procedures. Pediatr Neurosci. 1985;12(1):49–57. [DOI] [PubMed] [Google Scholar]
- 35.Rieder MJ, Frewen TC, Del Maestro RF, Coyle A, Lovell S. The effect of cephalothin prophylaxis on postoperative ventriculoperitoneal shunt infections. Cmaj. 1987;136(9):935–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Quigley MR, Reigel DH, Kortyna R. Cerebrospinal fluid shunt infections. Report of 41 cases and a critical review of the literature. Pediatr Neurosci. 1989;15(3):111–20. [PubMed] [Google Scholar]
- 37.Wang EE, Prober CG, Hendrick BE, Hoffman HJ, Humphreys RP. Prophylactic sulfamethoxazole and trimethoprim in ventriculoperitoneal shunt surgery. A double-blind, randomized, placebo-controlled trial. Jama. 1984;251(9):1174–7. [PubMed] [Google Scholar]
- 38.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97–132; quiz 3-4; discussion 96. [PubMed] [Google Scholar]
- 39.Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts. Cochrane Database Syst Rev. 2006;3:CD005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatr. 2008;1(1):48–56. [DOI] [PubMed] [Google Scholar]
- 41.Klimo P, Jr., Van Poppel M, Thompson CJ, Baird LC, Duhaime AC, Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 6: Preoperative antibiotics for shunt surgery in children with hydrocephalus: a systematic review and meta-analysis. JNS: Pediatrics Special Supplements. 2014;14(Suppl):44–52. [DOI] [PubMed] [Google Scholar]
- 42.McAllister JP, 2nd, Williams MA, Walker ML, Kestle JR, Relkin NR, Anderson AM, et al. An update on research priorities in hydrocephalus: overview of the third National Institutes of Health-sponsored symposium “Opportunities for Hydrocephalus Research: Pathways to Better Outcomes”. J Neurosurg. 2015:1–12. [DOI] [PubMed] [Google Scholar]
- 43.Ronan A, Hogg GG, Klug GL. Cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 1995;14(9):782–6. [DOI] [PubMed] [Google Scholar]
- 44.McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36(7):858–62. [DOI] [PubMed] [Google Scholar]
- 45.Naradzay JF, Browne BJ, Rolnick MA, Doherty RJ. Cerebral ventricular shunts. J Emerg Med. 1999;17(2):311–22. [DOI] [PubMed] [Google Scholar]
- 46.Dallacasa P, Dappozzo A, Galassi E, Sandri F, Cocchi G, Masi M. Cerebrospinal fluid shunt infections in infants. Childs Nerv Syst. 1995;11(11):643–8; discussion 9. [DOI] [PubMed] [Google Scholar]
- 47.Simon TD, Butler J, Whitlock KB, Browd SR, Holubkov R, Kestle JR, et al. Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study. The Journal of pediatrics. 2014;164(6):1462–8 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon TD, Whitlock KB, Riva-Cambrin J, Kestle JR, Rosenfeld M, Dean JM, et al. Revision surgeries are associated with significant increased risk of subsequent cerebrospinal fluid shunt infection. Pediatr Infect Dis J. 2012;31(6):551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon TD, Butler J, Whitlock KB, Browd SR, Holubkov R, Kestle JR, et al. Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study. J Pediatr. 2014;164(6):1462–8 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4(2):156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon T, Whitlock K, Riva-Cambrin J, Kestle J, Rosenfeld M, Dean J, et al. Association of intraventricular hemorrhage secondary to prematurity with cerebrospinal fluid shunt surgery in the first year following initial shunt placement. Journal of Neurosurgery: Pediatrics. 2012;9(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flannery AM, Mazzola CA, Klimo P, Jr., Duhaime AC, Baird LC, Tamber MS, et al. Foreword: Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. J Neurosurg Pediatr. 2014;14 Suppl 1:1–2. [DOI] [PubMed] [Google Scholar]
- 53.Williams MA, McAllister JP, Walker ML, Kranz DA, Bergsneider M, Del Bigio MR, et al. Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. Journal of Neurosurgery. 2007;(5 Suppl Pediatrics)(107):345–57. [DOI] [PubMed] [Google Scholar]
- 54.Kestle JR, Holubkov R, Cochrane DD, Kulkarni AV, Limbrick D, Luerssen TG, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. Journal of Neurosurgery: Pediatrics. 2016;17(4):391–6. [DOI] [PubMed] [Google Scholar]
- 55.Website SfPS. [http://www.solutionsforpatientsafety.org/]. [Google Scholar]
- 56.Schaffzin JK, Harte L, Marquette S, Zieker K, Wooton S, Walsh K, et al. Surgical Site Infection Reduction by the Solutions for Patient Safety Hospital Engagement Network. Pediatrics. 2015;136(5):e1353–60. [DOI] [PubMed] [Google Scholar]
- 57.Schaffzin JK, Simon K, Connelly BL, Mangano FT, Team obotP-OSSIP. Standardizing preoperative preparation to reduce surgical site infections among pediatric neurosurgical patients. J Neurosurg Pediatr. 2017;19(4):399–406. [DOI] [PubMed] [Google Scholar]
- 58.Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg. 2006;105(2):242–7. [DOI] [PubMed] [Google Scholar]
- 59.Lambert M, MacKinnon AE, Vaishnav A. Comparison of two methods of prophylaxis against CSF shunt infection. Z Kinderchir. 1984;39 Suppl 2:109–10. [DOI] [PubMed] [Google Scholar]
- 60.Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60(5):1014–21. [DOI] [PubMed] [Google Scholar]
- 61.Tamber MS, Klimo P, Jr., Mazzola CA, Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 8: Management of cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2014;14 Suppl 1:60–71. [DOI] [PubMed] [Google Scholar]
- 62.Kanev PM, Sheehan JM. Reflections on shunt infection. Pediatr Neurosurg. 2003;39(6):285–90. [DOI] [PubMed] [Google Scholar]
- 63.Gardner P, Leipzig T, Phillips P. Infections of central nervous system shunts. Med Clin North Am. 1985;69(2):297–314. [PubMed] [Google Scholar]
- 64.Gardner P, Leipzig TJ, Sadigh M. Infections of mechanical cerebrospinal fluid shunts. Curr Clin Top Infect Dis. 1988;9:185–214. [PubMed] [Google Scholar]
- 65.Morissette I, Gourdeau M, Francoeur J. CSF shunt infections: a fifteen-year experience with emphasis on management and outcome. Can J Neurol Sci. 1993;20(2):118–22. [DOI] [PubMed] [Google Scholar]
- 66.Venes JL. Infections of CSF shunt and intracranial pressure monitoring devices. Infect Dis Clin North Am. 1989;3(2):289–99. [PubMed] [Google Scholar]
- 67.Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22(7):692–7. [DOI] [PubMed] [Google Scholar]
- 68.Kulkarni AV, Cochrane DD, McNeely PD, Shams I. Medical, Social, and Economic Factors Associated with Health-Related Quality of Life in Canadian Children with Hydrocephalus. J Pediatr. 2008. [DOI] [PubMed] [Google Scholar]
- 69.Tuli S, Tuli J, Drake J, Spears J. Predictors of death in pediatric patients requiring cerebrospinal fluid shunts. J Neurosurg. 2004;100(5 Suppl Pediatrics):442–6. [DOI] [PubMed] [Google Scholar]
- 70.Renier D, Sainte-Rose C, Pierre-Kahn A, Hirsch JF. Prenatal hydrocephalus: outcomeand prognosis. Childs Nerv Syst. 1988;4(4):213–22. [DOI] [PubMed] [Google Scholar]
- 71.Simon TD, Van Yserloo B, Nelson K, Gillespie D, Jensen R, McAllister JP, 2nd, et al. Use of quantitative 16S rRNA PCR to determine bacterial load does not augment conventional cerebrospinal fluid (CSF) cultures among children undergoing treatment for CSF shunt infection. Diagn Microbiol Infect Dis. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon TD, Pope CE, Browd SR, Ojemann JG, Riva-Cambrin J, Mayer-Hamblett N, et al. Evaluation of microbial bacterial and fungal diversity in cerebrospinal fluid shunt infection. PLoS One. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fux CA, Quigley M, Worel AM, Post C, Zimmerli S, Ehrlich G, et al. Biofilm-related infections of cerebrospinal fluid shunts. Clin Microbiol Infect. 2006;12(4):331–7. [DOI] [PubMed] [Google Scholar]
- 74.Guevara JA, Zuccaro G, Trevisan A, Denoya CD. Bacterial adhesion to cerebrospinal fluid shunts. J Neurosurg. 1987;67(3):438–45. [DOI] [PubMed] [Google Scholar]
- 75.Stoodley P, Braxton EE, Jr., Nistico L, Hall-Stoodley L, Johnson S, Quigley M, et al. Direct demonstration of Staphylococcus biofilm in an external ventricular drain in a patient with a history of recurrent ventriculoperitoneal shunt failure. Pediatr Neurosurg. 2010;46(2):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhoads DD, Wolcott RD, Sun Y, Dowd SE. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci. 2012;13(3):2535–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Falkow S Molecular Koch’s postulates applied to microbial pathogenicity. Rev Infect Dis. 1988;10 Suppl 2:S274–6. [DOI] [PubMed] [Google Scholar]
- 78.Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19(1):165–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han A, Zenilman JM, Melendez JH, Shirtliff ME, Agostinho A, James G, et al. The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound Repair Regen. 2011;19(5):532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seng P, Rolain JM, Fournier PE, La Scola B, Drancourt M, Raoult D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010;5(11):1733–54. [DOI] [PubMed] [Google Scholar]
- 81.Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11(2):94–100. [DOI] [PubMed] [Google Scholar]
- 82.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22. [DOI] [PubMed] [Google Scholar]
- 83.Vergidis P, Patel R. Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Dis Clin North Am. 2012;26(1):173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stenehjem E, Armstrong WS. Central nervous system device infections. Infect Dis Clin North Am. 2012;26(1):89–110. [DOI] [PubMed] [Google Scholar]
- 85.Bayston R, Ullas G, Ashraf W. Action of linezolid or vancomycin on biofilms in ventriculoperitoneal shunts in vitro. Antimicrob Agents Chemother. 2012;56(6):2842–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004;279(52):54881–6. [DOI] [PubMed] [Google Scholar]
- 87.Kristian SA, Birkenstock TA, Sauder U, Mack D, Gotz F, Landmann R. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J Infect Dis. 2008;197(7):1028–35. [DOI] [PubMed] [Google Scholar]
- 88.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70(11):6339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rupp ME, Fey PD, Heilmann C, Gotz F. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J Infect Dis. 2001;183(7):1038–42. [DOI] [PubMed] [Google Scholar]
- 90.Konig C, Schwank S, Blaser J. Factors compromising antibiotic activity against biofilms of Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 2001;20(1):20–6. [DOI] [PubMed] [Google Scholar]
- 91.Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2010;65(9):1955–8. [DOI] [PubMed] [Google Scholar]
- 92.Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48(7):2659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


