Introduction
Asthma was first described by Hippocrates as a disease of breathlessness, potentially caused by emotional distress1. The 17th century writings of Sir John Floyer describe bronchial constriction and the concepts of attacks and triggers2. In the 20th century, mounting evidence led to the characterization of asthma as an inflammatory condition2. Asthma symptoms were recognized as narrowing of the airways with distinctive sputum and swelling of the bronchial mucous membranes, triggered by a variety of circumstances or exposures that stimulate immune cells2. Despite many scientific advances, asthma remains a major public health concern both in the United States and globally. Asthma-related healthcare and socioeconomic costs remain substantial, costing an estimated $56 billion and 14 million work days missed each year in the U.S.3. Inadequate asthma treatment impacts quality of life and contributes to preventable deaths, especially among children and adolescents. Today, the field is once again at the threshold of a deeper understanding of asthma. Evolving paradigms link the origins of asthma to environmental exposures that shape immune responses, mediated by exogenous and endogenous stimuli including microbes. These same stimuli may modulate how asthma behaves once established, contributing to asthma’s heterogeneity. The coupling of clinical insights with new investigative tools has generated new hypotheses and expanded the potential to understand better the different mechanisms underlying asthma and to inform new treatment strategies to revolutionize patient care.
There is no known singular cause of asthma. Existing data points to a combination of genetic risk and environmental factors that influence asthma susceptibility in children. For many, asthma onset in childhood and its persistence into adulthood is typically atopic in nature, driven by type 2 immune responses to environmental allergens and microbes4. However, the mechanisms underlying asthma in other scenarios are often less clear. For example, gender influences asthma prevalence, which is higher in boys than girls in childhood but begins to reverse in adolescence5. Moreover, adult onset of asthma is common and linked in some cases to new environmental exposures, including viral respiratory illnesses, irritant exposures, and newly developed allergies or obesity6. A large knowledge gap exists on mechanisms of adult-onset asthma, which can range from clinically mild to severe.
Ultimately, continued progress in asthma research depends on recognition of the disease as a collection of heterogeneous presentations, as highlighted in a recent commission published in the Lancet7. Integration of cross-disciplinary approaches and scientific tools has generated new insights into the complexities of asthma pathogenesis in children, involving interactions amongst environmental factors, genetics, and trajectories of human microbiota establishment8–11. Similar integration of methods is generating new insights and hypotheses into the role of human microbiota in asthma phenotypes. The purpose of this review is to highlight the latest evidence in these areas and provide some perspective on next directions of research and potential impact on future patient care.
Overview of the human microbiome
The human body is home to a diverse array of microorganisms, revealed by culture-independent methods to comprise bacteria, viruses, and fungi that inhabit our internal and external surfaces. Studies have shown that the microbiota is individualized, dynamic, different between body sites, and both reflective of and sensitive to the environment12. There is ample evidence for the role of human microbiota in homeostasis, immunity, and metabolism13. In the interest of clarity, Table 1 defines some commonly used terms in microbiome investigation14,15that will be referred to in this review.
Table 1.
| Term | Definition |
|---|---|
| Microbiota | The assemblage of all microorganisms present in a defined site or niche. Current usage often implies only bacteria. |
| Microbiome | The entire habitat, including the microbiota, their genomes (genes) and the surrounding site-specific conditions. Current usage often refers only to bacterial members of the ‘biome’. |
|
Mycobiota Mycobiome |
Variation on the above definitions referring specifically to fungi present in a habitat. |
|
Metagenome Metagenomics |
Collection of genomes and genes from the members of a microbiota, characterized by the process of ‘metagenomics’ (i.e. shotgun DNA sequencing) to obtain information on potential functions of the microbiota |
| Dysbiosis | Descriptive for imbalance in a microbiome, such as lack of homeostasis in microbial composition or functions |
| Diversity | Multiple types of measures exist. A calculated index or measure reflecting the types, numbers and distribution of microbiota present within a sample or site (oc-diversity) or between different samples or sites (β-diversity). |
The microbiome is critical to metabolic and immune system programming from birth. Some evidence suggests that this relationship may be initiated in the womb. While there is not yet a consensus, some human studies suggest that maternal-fetal microbiota transfer may occur in utero16,17. Peri-natal and early life disturbances, such as maternal antibiotic use and mode of delivery, have been shown to alter the microbiome. Babies born vaginally have a distinct microbiome from those born via cesarean section, and these differences can persist weeks after birth18,19. Breastfeeding has been shown to directly impact the infant gut microbiome, protecting the infant from infections and encouraging maturation of the immune system20. Overall it has been widely shown that the presence and composition of human microbiota modulate immune tone and shape inflammatory responses, impacting later health outcomes21,22. Therefore, it is reasonable to conceptualize microbiota as a dynamic entity that at the very least mediates, if not potentially drives, mechanisms to promote either health or disease.
The microbiome in asthma pathogenesis
A wealth of evidence now exists implicating gut and respiratory dysbiosis in the pathogenesis of asthma in childhood, highlights of which are discussed here.
Gut microbiota
The gastrointestinal tract is colonized by a variety of microorganisms which most densely populate the colon. Bacterial members of the gut microbiota have been extensively studied, and their role in shaping both local and systemic immune responses is well established. In studies of childhood allergy and asthma, findings from different birth cohorts have consistently demonstrated a relationship between altered patterns of gut microbiota composition in the first years of life and the development of allergy and asthma8–10. In one of the largest prospective studies to date involving nearly 700 children, the gut microbiota configuration at 1 year of age was associated with asthma development at age 59. These and other clinical findings are supported by work in mouse models that show age-dependent regulation of IgE production by the gut microbiota23 and a potential direct role for specific bacteria, such as Lachnospira, Veillonella, Faecalibacterium, and Rothia, in mitigating asthma development during the first 100 days of life8. Table 2 summarizes several additional prominent taxa and evidence for links to asthma.
Table 2.
Bacterial genera associated with asthma.
| Bacterial Genus | Compartment | Link to Asthma |
|---|---|---|
| Bifidobacterium9,14 | Gastrointestinal | Decrease abundance associated with risk for asthma in childhood |
| Dolosigranulum30,34, Corynebacterium (C)27,30,34 | Nasopharyngeal | Prevalence associated with lower risk of viral respiratory infections and asthma in children; (C) negatively associated with eosinophilic lung inflammation in adults |
| Faecalibacterium8,9 | Gastrointestinal | Decreased abundance in children at risk for asthma |
| Haemophilus43,34 | Nasopharyngeal | Increased abundance in early life associated with increased frequency of viral infections and likelihood of developing persistent wheeze |
| Moraxella30,34 | Nasopharyngeal, Respiratory | Increased abundance in early life associated with increased frequency of viral infections and likelihood of developing persistent wheeze |
| Neisseria27,30,34 | Respiratory | Increased abundance associated with asthma in adults |
| Rothia8 | Gastrointestinal | Decreased abundance in children at risk for asthma |
| Streptococcus34 | Nasopharyngeal, Respiratory | Increased abundance in early life associated with increased frequency of viral infections and likelihood of developing persistent wheeze |
| Veillonella8 | Respiratory (R), Gastrointestinal (G) | (G) Decreased abundance in children at risk for asthma |
What are the mechanisms by which gut microbiota mediate asthma pathogenesis? The effects of short-chain fatty acids (SCFAs), produced by fermentation of fiber by specific gut bacteria, has received particular attention with supporting data from both murine models and human studies24. Altered levels of SCFAs have been linked to gut dysbiosis in children at-risk for asthma8. Important work in mouse models has delineated putative mechanisms by which fiber supplementation, via the generation of specific SCFAs, can shape naïve immune cell responses, including dendritic cell phenotype in the lungs25. Other mediators linked to gut microbial metabolism, such as histamine and tryptophan metabolites, have been implicated in mechanisms of asthma24,26, but further studies are needed.
Respiratory microbiota
In pediatric studies the role of respiratory microbiota in asthma pathogenesis has largely focused on the nasopharyngeal (NP) niche. Not only is the nasopharynx easier to sample but it is also the most proximal point of contact between inhaled material and respiratory epithelia. While the composition of NP microbiota is overall very different from the lower respiratory tract (defined as below the glottis), there is some overlap, particularly in the setting of lung disease including asthma27,28. Thus study of the NP microbiome has the potential to inform biological pathways associated with asthma risk, as further detailed below29,30.
Given the known strong association between childhood asthma and viral respiratory infections, in particular human rhinovirus and respiratory syncytial virus31,32, recent data highlight the importance of interactions between bacteria and viruses in the nasopharynx. Analyses of NP samples from several pediatric cohorts have demonstrated important associations amongst bacterial microbiota composition, asthma risk, and/or frequency or severity of viral respiratory infections including bronchiolitis29–31,33,34. Notably, a consistent observation has been dynamic changes in NP microbiota composition early in life, followed by a settling into one of several microbiota patterns defined by relative dominance of one or more members. For example, as reported in one study34, six bacterial genera generally define NP microbiota patterns in early childhood (Moraxella, Streptococcus, Corynebacterium, Alloiococcus, Haemophilus, and Staphylococcus). However, frequency of viral respiratory infections and/or likelihood of developing persistent wheeze both have been strongly associated with the prevalence of Haemophilus, Moraxella, and/or Streptococcus in the nasopharynx30,34.
Strikingly, the associations between a dysbiotic NP microbiota pattern and frequency of viral illness or asthma risk appear to be linked very early, within the first one or two months of life38–40. Moreover, in at least one study34, a shift in NP bacterial microbiota composition towards Moraxella dominance was detected in the weeks preceding the clinical onset of viral illness. Finally, only in children who developed early sensitization to aeroallergens did a dysbiotic NP microbiota pattern associate with persistent wheeze in later childhood. Together, collective findings from studies of the NP microbiome suggest that, as seen in the gut, a ‘critical window’ of upper airway microbiota–immune interactions exists that may also influence asthma pathogenesis in children, although the mechanisms are not understood.
The microbiome in asthma phenotype
Respiratory microbiota
The relationships between microbial dysbiosis and asthma phenotype have predominantly been explored in studies of adults amongst whom the heterogeneity of asthma is well recognized. In contrast to the focus on upper airway sampling in pediatric studies, analyses of lower respiratory tract samples in adults have established asthma-associated differences in bronchial microbiota composition35–38. It is important to state upfront that many studies now have refuted the long-taught supposition that the lungs, even in health, are sterile or free from microorganisms35,36,39. Using a culture-independent approach to survey bacterial composition in bronchoscopic samples, Hilty et al. first showed that the bronchi of healthy individuals indeed harbor bacteria, but that the bacterial composition detected from the airways of both adults and children with asthma differed from that in healthy subjects35. Subsequent investigations confirmed that, although lower in microbial burden compared to the oral cavity40, the lower respiratory tract harbors bacteria whose compositional configuration differs in asthmatic subjects36–38,41–44. Notably, this bronchial dysbiosis associated with asthma has been reported consistently across analyses of different specimen types (e.g. bronchoalveolar lavage, bronchial epithelial brushings, sputum), despite incomplete overlap in the identities of microbes detected27.
Eosinophilic asthma is the best understood clinical phenotype, rooted in activation of type 2 immune responses that lead to airway infiltration by eosinophils that generally is responsive to corticosteroid treatments. Exposure to allergens stimulates type 2 inflammatory responses, as do other potentially concomitant factors45,46. For example, the diversity of bacterial content in allergenic environmental dust may modulate the degree of subsequent allergic sensitization46. In contrast there is a surprising lack of association found to date between asthma-associated lung bacterial dysbiosis and markers of type 2 airway inflammation or eosinophilic asthma41,43,44. This raises the question whether fungi, rather than bacteria, may play a greater role in perpetuating type 2 inflammation in the eosinophilic asthma phenotype46,47. Fungal proteases, such as that from Aspergillus fumigatus, can induce a strong eosinophilic allergic airway response in mice53 This correlates with clinical phenotypes characterized in certain geographic areas and described as severe asthma with fungal sensitization (SAFS)46–49. Given the estimates that more than 50,000 fungal spores/m3 of air may be inhaled each day50, this raises the question whether the links between fungal sensitization and allergic asthma may implicate dysbiosis related to airway mycobiota, which have yet to be well characterized.
The high prevalence in asthma of sensitization to aeroallergens leads one to query if atopic status, once established, might in itself be linked to alterations in airway microbiota composition. This was examined in a recent study comparing the bronchial bacterial microbiota of subjects with mild atopic asthma, atopy without asthma, and non-atopic healthy controls27. Significant differences were observed among these groups, including a pattern of bacterial dysbiosis strongly associated with atopy alone that was distinct from that observed in association with asthma. The findings suggest that the airway mucosal environment in allergen-sensitized subjects allows for establishment of a different microbiota configuration from that in nonsensitized subjects. However, what factors potentially drive microbiome differences between atopic subjects with or without asthma are unknown.
Neutrophilic asthma has generally been associated with more severe asthma and poor response to corticosteroid treatment44,51. Based on analyses of either sputum or samples obtained by bronchoscopy, studies from different cohorts have demonstrated that airway neutrophilia is associated with an altered bacterial microbiota composition41,44,52. Specifically, organisms belonging to the phylum Proteobacteria, such as Haemophilus, Moraxella, Pseudomonas, and Klebsiella, are proportionally enriched in neutrophilic asthma. In one study of severe asthma subjects with different sputum inflammatory phenotypes, those with neutrophilic asthma displayed significant differences in sputum bacterial diversity and composition44. Notably, no differences in airway bacterial composition were observed amongst the remaining phenotypes, including eosinophilic and pauci-granulocytic which together characterized the majority of subjects in this study44. In another study of severe asthma, significant associations between the proportional abundance of Proteobacteria members and epithelial expression of IL-17 pathway-related genes were observed41. Enrichment in Proteobacteria also correlated with less stable asthma control in this cohort. Findings from these and other studies together suggest that airway enrichment in potentially pathogenic members of the Proteobacteria, once established, may promote neutrophilic inflammation, possibly via activation of IL-17-driven pathways. Since airway neutrophilia tends to be observed in more severe asthma, it raises further questions regarding the role of corticosteroid therapy in potentially fostering an airway microenvironment conducive to the outgrowth of particular members.
Gut Microbiota
In contrast to investigations of other chronic medical conditions or of pediatric allergy and asthma, far less evidence exists currently on possible relationships between gut dysbiosis and asthma phenotype in adults. However, such relationships are plausible given known associations between altered gut microbiota patterns and allergic sensitization as well as obese status. The potential for intestinal microbiota to modulate allergic airway inflammation has been demonstrated in murine models, mediated by SCFAs for example25 and potentially modifiable by helminth co-infection53. In clinical studies, asthmatic adults have been found to harbor a higher burden of histamine-secreting bacteria in their gut than healthy subjects, suggesting a microbial source of histamine that affect manifestations of allergic asthma26. Intestinal microbiota differences also have been associated with sensitization to a greater number of aeroallergens and with differences in lung function amongst asthmatic adults26,54. These early findings invite a closer look into possible mechanisms by which the intestinal microbiota may influence features of asthma in adult populations.
The microbiome and asthma treatments
Current immunomodulatory treatments for asthma largely target mechanisms of type 2-related eosinophilic inflammation, such as corticosteroids and antibodies directed at specific cytokine mediators (e.g. IL-5). However, these strategies are not effective for patients without evidence of significant type 2 pathway activation and become limited for those who demonstrate persistent eosinophilic inflammation despite such treatments. Improved understanding of the biological drivers underlying different asthma phenotypes will advance more precise approaches to defining asthma in a given patient and determining best treatment options. Until then, the efficacy of current treatments in each patient should be considered alongside potential harms. For example, in addition to known clinical side effects of corticosteroids, emerging evidence suggests that use of inhaled corticosteroids even after just six weeks alters the bronchial microbiome43. While the long-term consequences of this remain unclear, prior work has shown that at least one member of the airway microbiota found to be enriched in steroid-resistant asthma may alter macrophage responses to corticosteroids42.
Another therapeutic strategy of long-standing interest is macrolide antibiotics, which carry both antimicrobial and anti-inflammatory properties. While meta-analyses have failed to find overall evidence in support of their efficacy in asthma55, individual large clinical trials have, such as recent demonstration that azithromycin reduces exacerbations in subjects with severe asthma56. However, concerns regarding the development of microbial resistance to macrolides persist, as increased carriage of macrolide resistance genes in the oropharyngeal microbiome has been shown57. Other microbiome-modifying effects of macrolides may exist. Better means of determining which asthmatic patients may benefit from macrolide treatment is needed, which may relate to harboring a higher burden or diversity of airway microbiota at baseline amongst those using corticosteroid36.
Probiotic therapies offer a promising approach to therapeutic microbiome manipulation, and interest in probiotic therapies for allergic diseases continues to increase. There is ample evidence from mouse studies that link intestinal microbiota to immune tolerance58,59. The World Allergy Organization recognizes net benefit of probiotics for possible eczema prevention and recommends probiotic use in pregnant and lactating women, as well as in infants with high risk of allergy60. Studies regarding the use of probiotics for the treatment of asthma have been inconclusive. Pre-clinical studies have reported positive effects of probiotics on manifestations of asthma. For example, Lactobaccilus rhamnosus GG has been shown to decrease airway inflammation in a mouse asthma model61. However, several clinical trials have found no evidence for a positive effect62. Similarly, clinical studies of probiotics for primary prevention of asthma have yet to show positive benefits63. Overall, there is not yet sufficient clinical evidence to support the use of probiotics to treat or prevent asthma. For further in-depth discussion on this topic, we recommend a recent review63.
Outstanding questions and challenges
Reflection on the recent recommendations published in the Lancet7 highlights, in our view, the significant impact that incorporation of microbiome-centered lines of investigation can have on asthma research. Importantly, this should not be pursued as an isolated discipline but rather integrated with other investigative methods to attain a more holistic understanding of asthma behaviors and the biological drivers of disease instability or progression. To this end we endorse the value in “complementary use of [both] reductionist and system-based approaches”7, the latter already being pursued to better understand the complexities of microbiome-host interactions.
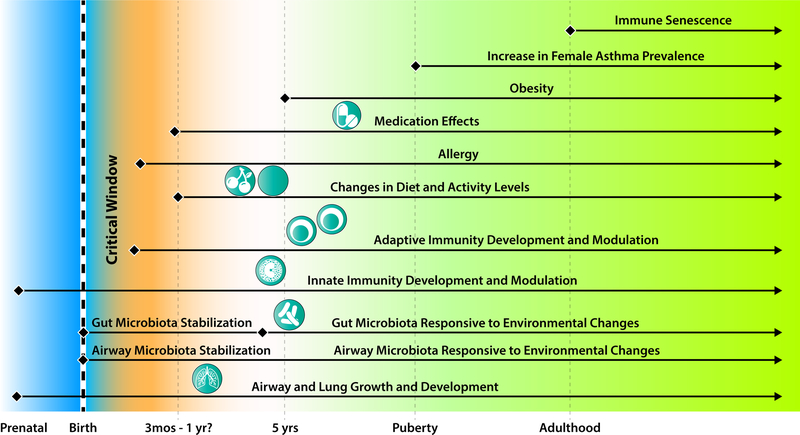
Existing evidence clearly implicates the gut and respiratory microbiota in shaping asthma pathogenesis in childhood, but their role in the march towards phenotypic differentiation in later life is less clear. Findings from adult studies also implicate these microbiome compartments in asthma phenotype, but mechanistic insights have been more difficult. One challenge is the absence of clinical studies with built-in capacity to investigate the interactions among dynamic lifestyle changes and exposures, immune responses to various stimuli, and of course functions conferred by microbiota in a given individual that may tune responses in these interactions (Figure 1). Secondly, model systems that better reflect human asthma behaviors could provide complementary mechanistic insights. Ultimately, in our view, these two challenges must be addressed if clinical strategies to manipulate microbiota or apply microbial products are to be successful in modifying trajectories of asthma in which the microbiome is demonstrated to play a strong role.
Figure 1.
Factors that can be critical influences on asthma pathogenesis and phenotype through effects on the gut and/or respiratory microbiomes. Mechanisms are understood for some but not all interactions.
Finally, many clinically relevant questions remain for which we speculate, based on at least some evidence, that further understanding of the human microbiome’s role will be informative.
What timeframe is the critical window in which modulation of the microbiome may have sufficient influence to avert the development of asthma? Recent evidence continues to push this critical window to very early life, at least soon after birth and even peri- or pre-natally. This speaks to much larger questions regarding societal and lifestyle habits.
Are microbiota changes related to differing clinical features of asthma a driver or consequence of underlying mechanisms? We submit that both are possible and likely differ by context. The strong associations observed to date between a “type 2-low” phenotype and differences in bacterial members of the lower airway microbiota suggest the possibility that potentially pathogenic members of the latter stimulate non-type 2 immune responses. This may reflect forms of severe asthma characterized by significant airway neutrophilia. On the other hand, sensitization to and the detection of fungi in the airways are associated with strong type 2 inflammatory responses, suggestive that fungi can drive such responses. Could this explain eosinophilic severe asthma that may persist despite directed treatments?
Could the microbiome affect responses to asthma treatment, or vice versa such that treatment-induced changes in the microbiome have important consequences? Again, we speculate both are possible based on some existing evidence. Responsiveness to corticosteroid or macrolide treatment has been linked to characteristics of the airway microbiota present at baseline36,42,43. Changes in the airway immune environment after institution of such treatments also could provide a selection pressure on microbiota composition, as reflected in the very definition of “microbiome” as a habitat. Future work will also need to consider the role of other members of the microbiome beyond bacterial, although further methodologic advances are needed to make pursuing such in clinical studies more feasible, accessible and cost-efficient.
Most of the challenges outlined above will require better integration of clinical research and laboratory tools, the latter continually evolving in the – ‘omic and bioinformatic space. However, combining advanced approaches (e.g. function-oriented ‘omic’ tools) with improved study designs or models, nested within a systems-level framework, will likely reveal connections that enable more rapid advancement towards microbiome-informed therapeutic or curative strategies for asthma.
Key Messages:
Collective evidence supports the ‘critical-window’ hypothesis that interactions between the immune system and the intestinal or airway microbiota in very early life influence asthma risk and pathogenesis.
Growing evidence suggests that particular asthma phenotypes may be driven by or are reflective of alterations in the lower airway microbiome, which can be further shaped by asthma treatments.
A large knowledge gap exists on mechanisms underlying adult-onset asthma, but changes in environmental exposures, lifestyle factors and immune response tendencies should be considered across the lifespan.
To maintain progress towards more precise therapeutic strategies for asthma, better integration of phenotyping tools, including molecular and microbial methods, with appropriate study designs is needed along with longitudinal assessments.
Acknowledgments
Funding:
YJH is supported by NIH R01AI129958 and R03HL138310.
AJK is supported by NIH T32HL007749.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
References
- 1.Douwes J, Brooks C, Pearce N. Stress and asthma: Hippocrates revisited. Journal of Epidemiology and Community Health (1979-). 2010;64(7):561–562. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. A Brief History of Asthma and Its Mechanisms to Modern Concepts of Disease Pathogenesis. Allergy Asthma Immunol Res. 2010;2(3):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012;(35):1–58. [PubMed] [Google Scholar]
- 4.Bantz SK, Zhu Z, Zheng T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J Clin Cell Immunol. 2014;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax. 2012;67(7):625–631. [DOI] [PubMed] [Google Scholar]
- 6.Coumou H, Westerhof GA, de Nijs SB, Amelink M, Bel EH. New-Onset Asthma in Adults: What Does the Trigger History Tell Us? J Allergy Clin Immunol Pract. September 2018. [DOI] [PubMed] [Google Scholar]
- 7.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. The Lancet. 2018;391(10118):350–400. [DOI] [PubMed] [Google Scholar]
- 8.Arrieta M-C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. [DOI] [PubMed] [Google Scholar]
- 9.Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nature Communications. 2018;9(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein MM, Hrusch CL, Gozdz J, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. New England Journal of Medicine. 2016;375(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consortium THMP, Huttenhower C, Gevers D, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YJ, Marsland BJ, Bunyavanich S, et al. The microbiome in allergic disease: Current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. Journal of Allergy and Clinical Immunology. 2017;139(4):1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rautava S, Walker WA. Academy of Breastfeeding Medicine Founder’s Lecture 2008: Breastfeeding—An Extrauterine Link Between Mother and Child. Breastfeed Med. 2009;4(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. 2013;131(1):23–30. [DOI] [PubMed] [Google Scholar]
- 23.Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Intestinal Microbial Diversity during Early-Life Colonization Shapes Long-Term IgE Levels. Cell Host Microbe. 2013;14(5):559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CH. Immune regulation by microbiome metabolites. Immunology. 2018;154(2):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Medicine. 2014;20(2):159–166. [DOI] [PubMed] [Google Scholar]
- 26.Barcik W, Pugin B, Westermann P, et al. Histamine-secreting microbes are increased in the gut of adult asthma patients. Journal of Allergy and Clinical Immunology. 2016;138(5):1491–1494.e7. [DOI] [PubMed] [Google Scholar]
- 27.Durack J, Huang YJ, Nariya S, et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh RL, Kaestli M, Chang AB, et al. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome. 2016;4(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart CJ, Mansbach JM, Wong MC, et al. Associations of Nasopharyngeal Metabolome and Microbiome with Severity among Infants with Bronchiolitis. A Multiomic Analysis. Am J Respir Crit Care Med. 2017;196(7):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch AATM, Piters WAA de S van Houten MA, et al. Maturation of the Infant Respiratory Microbiota, Environmental Drivers, and Health Consequences. A Prospective Cohort Study. Am J Respir Crit Care Med. 2017;196(12):1582–1590. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Garcia ML, Calvo C, Ruiz S, et al. Role of viral coinfections in asthma development. PLOS ONE. 2017;12(12):e0189083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CK, Callaway Z, Gern JE. Viral Infections and Associated Factors That Promote Acute Exacerbations of Asthma. Allergy Asthma Immunol Res. 2018;10(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Çalışkan M, Bochkov YA, Kreiner-Møller E, et al. Rhinovirus Wheezing Illness and Genetic Risk of Childhood-Onset Asthma. New England Journal of Medicine. 2013;368(15):1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teo SM, Tang HHF, Mok D, et al. Airway Microbiota Dynamics Uncover a Critical Window for Interplay of Pathogenic Bacteria and Allergy in Childhood Respiratory Disease. Cell Host & Microbe. 2018;24(3):341–352.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilty M, Burke C, Pedro H, et al. Disordered Microbial Communities in Asthmatic Airways. PLOS ONE. 2010;5(1):e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–381.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2):346–352.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denner DR, Sangwan N, Becker JB, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2016;137(5):1398–1405.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The Microbiome and the Respiratory Tract. Annu Rev Physiol. 2016;78(1):481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlson ES, Bittinger K, Haas AR, et al. Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Am J Respir Crit Care Med. 2011;184(8):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goleva E, Jackson LP, Harris JK, et al. The Effects of Airway Microbiome on Corticosteroid Responsiveness in Asthma. Am J Respir Crit Care Med. 2013;188(10):1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durack J, Lynch SV, Nariya S, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2017;140(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor SL, Leong LEX, Choo JM, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. Journal of Allergy and Clinical Immunology. 2018;141(1):94–103.e15. [DOI] [PubMed] [Google Scholar]
- 45.Lynch SV, Wood RA, Boushey H, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134(3):593–601.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kheradmand F, Kiss A, Xu J, Lee S-H, Kolattukudy PE, Corry DB. A Protease-Activated Pathway Underlying Th Cell Type 2 Activation and Allergic Lung Disease. The Journal of Immunology. 2002;169(10):5904–5911. [DOI] [PubMed] [Google Scholar]
- 47.Porter P, Lim DJ, Maskatia ZK, et al. Airway Surface Mycosis in Chronic Th2-Associated Airway Disease. J Allergy Clin Immunol. 2014;134(2):325–331.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.PORTER P, POLIKEPAHAD S, QIAN Y, et al. Respiratory tract allergic disease and atopy: experimental evidence for a fungal infectious etiology. Med Mycol. 2011;49(01): S158–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. European Respiratory Journal. 2006;27(3):615–626. [DOI] [PubMed] [Google Scholar]
- 50.Pashley CH, Fairs A, Free RC, Wardlaw AJ. DNA analysis of outdoor air reveals a high degree of fungal diversity, temporal variability, and genera not seen by spore morphology. Fungal Biol. 2012;116(2):214–224. [DOI] [PubMed] [Google Scholar]
- 51.Ray A, Kolls JK. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends in Immunology. 2017;38(12):942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson JL, Daly J, Baines KJ, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47(3):792–800. [DOI] [PubMed] [Google Scholar]
- 53.Zaiss MM, Rapin A, Lebon L, et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity. 2015;43(5):998–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begley L, Madapoosi S, Opron K, et al. Gut microbiota relationships to lung function and adult asthma phenotype: a pilot study. BMJ Open Respir Res. 2018;5(1):e000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015;(9):CD002997. [DOI] [PubMed] [Google Scholar]
- 56.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. [DOI] [PubMed] [Google Scholar]
- 57.Choo JM, Abell GCJ, Thomson R, et al. Impact of long-term erythromycin therapy on the oropharyngeal microbiome and resistance gene reservoir in non-cystic fibrosis bronchiectasis. mSphere. 2018;3(2):e00103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noval Rivas M, Burton OT, Wise P, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131(1):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. The Journal of Immunology. 1997;159(4):1739–1745. [PubMed] [Google Scholar]
- 60.Fiocchi A, Pawankar R, Cuello-Garcia C, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu C-T, Chen P-J, Lee Y-T, Ko J-L, Lue K-H. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. Journal of Microbiology, Immunology and Infection. 2016;49(5):625–635. [DOI] [PubMed] [Google Scholar]
- 62.Vliagoftis H, Kouranos VD, Betsi GI, Falagas ME. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Annals of Allergy, Asthma & Immunology. 2008;101(6):570–579. [DOI] [PubMed] [Google Scholar]
- 63.West CE, Jenmalm MC, Kozyrskyj AL, Prescott SL. Probiotics for treatment and primary prevention of allergic diseases and asthma: looking back and moving forward. Expert Rev Clin Immunol. 2016;12(6):625–639. [DOI] [PubMed] [Google Scholar]