Abstract
Rodents exhibit neophobia for novel tastes, demonstrated by an initial reluctance to drink novel-tasting, potentially-aversive solutions. Taste neophobia attenuates across days if the solution is not aversive, demonstrated by increased consumption as the solution becomes familiar. This attenuation of taste neophobia is context dependent, which has been demonstrated by maintained reluctance to drink the novel tasting solution if the subject has to drink it after being brought to a novel environment. This spatial context-dependent attenuation of taste neophobia has been described and likely depends on the integrity of the dorsal hippocampus because this brain area is crucial for representing space and spatial context associations, but is unnecessary for processing taste memories per se. Whether changing the non-spatial auditory context causes a similar effect on attenuation of taste neophobia and the potential role of the dorsal hippocampus in processing this decidedly non-spatial information has not been determined. Here we demonstrate that changing the non-spatial auditory context affects the attenuation of taste neophobia in mice, and investigate the consequence of hippocampal lesion. The results demonstrate that the non-spatial auditory context-dependent attenuation of taste neophobia in mice is lost following NMDA excitotoxic lesions of the CA1 region of the dorsal hippocampus. These findings demonstrate that the dorsal hippocampus is crucial for the modulation non-associative taste learning by auditory context, neither of which provide information about space.
Keywords: context, hippocampus, lesion, memory, neophobia, taste
INTRODUCTION
Taste recognition memory is a robust ethologically-grounded paradigm that has been exploited for studying neural mechanisms of learning and memory in rodents (Bermúdez-Rattoni, 2004). Taste neophobia is an unconditioned response that can be measured as an attenuation of fluid intake that is induced by a novel taste. Learning about the consequences of food and fluid ingestion leads to recognition of either aversive or safe tastes that manifests as changes in consumption. Specifically, safe taste recognition memory manifests as an attenuation of taste neophobia (ATN), measured as an increase in intake upon repeated exposures as a harmless taste becomes familiar.
Taste neophobia, along with taste aversion have been investigated for decades as neuroethologically-founded, non-associative types of learning that depend on non-declarative memory according to the declarative versus non-declarative memory dichotomy proposed by Squire (2004). However, recent evidence indicates that rats with excitotoxic lesions of the perirhinal cortex exhibit impairments of ATN that are comparable to the lesion-induced deficits that are observed in the novel object recognition memory task (Morillas et al., 2017), providing evidence that ATN also shares neural circuits that have traditionally been associated with declarative memory. Moreover, aging, which has been associated with selective alteration and decay of declarative memory (Dardou et al., 2008, 2010), leads to impaired ATN (Gómez-Chacón et al., 2015) in addition to other changes of taste learning (Moron et al., 2002; Manrique et al., 2009b, 2009a; Gámiz and Gallo, 2011).
The declarative versus non-declarative memory dichotomy is founded on the hypothesis that the hippocampal system is crucial for declarative memory and not required for nondeclarative memory. Furthermore, a somewhat alternative conception of the hippocampal function, the “cognitive mapping” theory, asserts that a central role of the hippocampus system is in computing and evaluating spatial information that is central to making spatially-informed and adaptive behavior. Attenuation of neophobia provides an opportunity to evaluate both cognitive mapping theory and the declarative-memory hypothesis for hippocampal function because evaluating taste memory is naturally accomplished without any overt physical changes to the testing environment, and without any conditioning or explicit reward. Thus, both the dominant declarative memory and cognitive mapping theories of hippocampal function predict no role of hippocampus in attenuation taste of neophobia.
In the present experiment we first investigated whether the attenuation of taste neophobia in mice is modulated by non-spatial changes to the auditory background. After observing that changing the auditory background reduces ATN, we investigated whether the non-spatial auditory modulation of this non-spatial taste recognition memory is sensitive to dorsal hippocampal lesion, thereby testing the two dominant theories of the hippocampal function.
1. MATERIALS AND METHODS
Subjects
Forty-eight adult male BALB/c mice (weighing 20-24g, Charles River, France) were used in this experiment. They were housed individually and maintained on a 12-hour dark-light cycle (lights from 8:00 am to 8:00pm). All the experimental procedures were performed during the light cycle at the same time each morning in the home cage. Mice were given ad libitum access to food and water until the experiment started, at which time access to water was restricted to a daily 10-minute morning drinking session. Four hours afterwards, all mice got additional access to water for one hour.
All procedures were approved by the University of Granada Ethics Committee for Animal Research and Junta de Andalucía (CEEA17-02-15-195) and were in accordance with the European Communities Council Directive 86/609/EEC.
Surgery
Surgery was performed under general anesthesia with a mixture of ketamine and medetomidine (0.1% b.w.). The animals were randomly assigned to one of two groups: Lesion and Sham. They were placed in a stereotaxic apparatus (Stoeling Co. Instrument, Word Dale, IL, USA)with bregma and lambda at the same height. Small trephine openings were drilled in the exposed skull in order to perform bilateral injections of either NMD A (NMD A, Sigma–Aldrich, 0.077 M) or sterile 0.9% saline solution through 30-gauge injection needles that were connected to 10-μl Hamilton syringes, so that 0.4-μ1 of the NMDA (M3262 – 25mg, Sigma Aldrich, Spain) solution was infused in each hemisphere at a rate of 0.2μl/min using an injection pump (Harvard Apparatus, Holliston, MA, USA). The needles were left in place for an additional 90 seconds before being slowly withdrawn. The stereotaxic coordinates targeted dorsal CA1 according to Paxinos and Watson’s mouse brain atlas (2001). The coordinates relative to bregma were: AP: −1.70mm; ML: ±1.00mm; DV:−1.50mm. The skin was sutured and covered with povidone. After the surgery, all animals received an i.p. injection of 4% atipamezole (0.5% b.w.) in order to reverse the effects of anesthesia. They also received additional s.c. injections of 5% Baytril and Bupac (0.1ml) for four consecutive days in order to reduce post-surgical pain and prevent infection.
Behavioral Procedure
One week after surgery, all the animals were subjected to the same behavioral procedure consisting of baseline (4 days), Phase I (one day) and Phase II (3 days) protocol. Liquid was available from a drinking tube during daily 10-min drinking sessions and the amount ingested was recorded.
An experimentally-controlled auditory background was continuously present during the 10-min drinking sessions in all protocol phases. In a separate room adjacent to the colony room, two speakers were used to deliver the auditory background. They were positioned one meter from the mouse homecages. The speakers were separated by 50 cm, and slightly angled apart from each other, so that each speaker faced half of the rack that held the homecages. Two different tones created using MATLAB were used and counterbalanced amongst the subjects. One tone was a pure 600 Hz tone (PT) consisting of 3-secondpulses with an inter stimulus interval (ISI) of 3 seconds. The second tone was Gaussian white noise (WN) consisting of 2-second pulses with an ISI of 4 seconds. Each tone was delivered by the two speakers simultaneously.
Dorsal hippocampus and sham lesion were randomly assigned to experimental groups specified by the taste solution (Water or Vinegar) and whether the auditory background was the same or different in Phases I and II. Two sham groups received sham surgery to assess the impact of changing the auditory background on drinking behavior: Water-Same Tone (n=8) and Water-Different Tone (n=8). Four other groups were used to assess the impact of hippocampus lesion: Sham-Vinegar-Same Tone (n=8), Sham-Vinegar-Different Tone (n=8), Lesion-Vinegar-Same Tone (n=8) and Lesion-Vinegar-Different Tone (n=8). (see Table 1).
Table 1.
Table depicting the study groups as defined by the drinking solution and the auditory background.
| Groups | Surgery | Baseline (−4 to 0 days) | Day 1 (Phase 1) | Day 2 (Phase 2) | Day 3 (Phase 2) | Day 4 (Phase 2) |
|---|---|---|---|---|---|---|
| Same Tone | Lesion or Sham | Water Tone A | Vinegar Tone A | Vinegar Tone A | Vinegar Tone A | Vinegar Tone A |
| Different Tone | Lesion or Sham | Water Tone A | Vinegar Tone A | Vinegar Tone B | Vinegar Tone B | Vinegar Tone B |
| Same Tone | Sham | Water Tone A | Water Tone A | Water Tone A | Water Tone A | Water Tone A |
| Different Tone | Sham | Water Tone A | Water Tone A | Water Tone B | Water Tone B | Water Tone B |
Tones A and B were counterbalanced: half the animals experienced the PT (600 Hz) and the other half the WN.
If Tone A was PT, Tone B was WN, and vice versa.
During Phases I and II all mice assigned to the Vinegar groups had access to the3% cider vinegar solution (5° acidity) instead of water during the 10-min drinking sessions. The groups assigned to Water continued to be exposed to water. The mice assigned to the Same Tone groups were only exposed to one of the two auditory cues (either the PT or the WN). The mice assigned to the Different Tone groups experienced a change in the auditory background in Phase II. Due to counterbalancing half of the animals changed from PT to WN and the other half changed from WN to PT (see Table 1).
Histology
All the animals were euthanized after the last drinking session. They were deeply anesthetized with sodium pentobarbital (100mg/kg, i.p.). The animals were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. The brains were removed and placed in a 4% paraformaldehyde solution for 48 hours before being transferred to a 30% sucrose solution until they sank for cryoprotection. Brains were maintained at −80°C until 20pm coronal sections were cut on a cryostat (Leica CM1900). The brain sections were mounted on gelatin-coated slides, stained with cresyl violet, and cover slipped, using a standard protocol. The Neurolucida system (Micro Bright Field Inc., Williston, USA) was used to quantify the extent of the hippocampal lesions in each mouse using a light microscope (Olympus BX41) with a motorized stage interfaced to a computer (See Figure 1).
Figure 1.
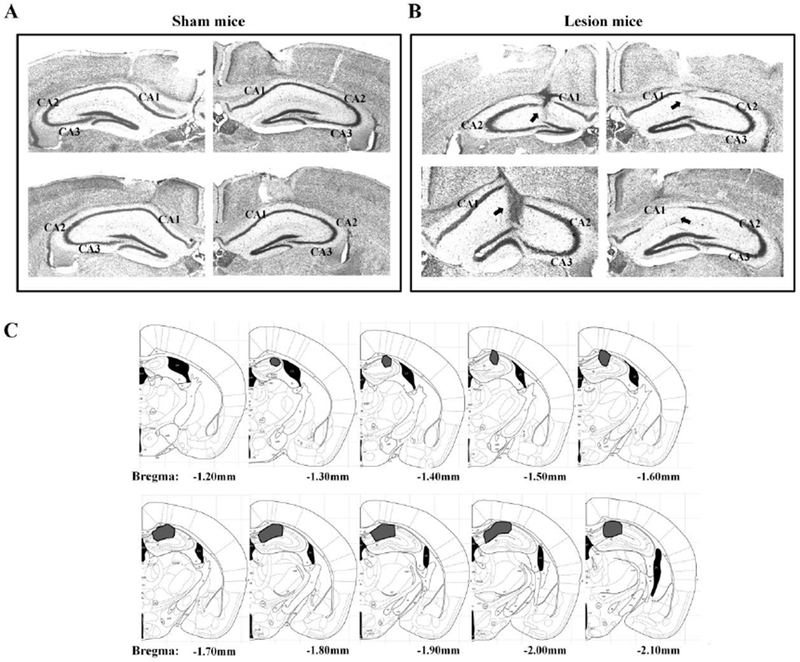
Example photomicrographs of the hippocampus in A) Sham and B) CA1 lesion mice. C) Mouse brain schematics with shading indicating the extent of the lesion.
2. RESULTS.
Water consumption: Phases I and II
We began by testing whether water consumption is affected by changing the background auditory noise (Fig. 2A). A global Mixed 4×2×2 (Day X Tone Change X Counterbalance Order) Repeated-Measures ANOVA comparing the water intake of the Water-Same Tone and Water-Different Tone groups on the four days after the baseline period did not reveal any significant effects or interaction (all p’s >.2). This indicates that changing the auditory background did not itself alter drinking (See Figure 2A,B) and allowed us to test the effect of changing the auditory background on taste neophobia.
Figure 2.
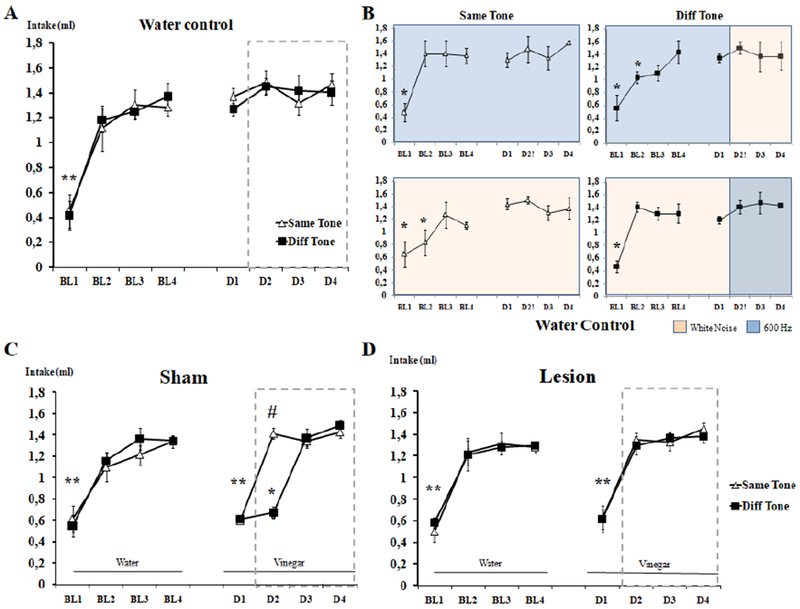
Water intake (±SEM) across the experimental days A) in the water only control mice demonstrating no effect of changing the auditory background, and B) that there is no effect of the specific auditory backgrounds; and demonstrating attenuation of taste neophobia in C) sham and D) hippocampus lesion mice. *Symbol represents statistically significant differences compared to Day 4.** indicates that both the Same Tone and Diff Tone groups were statistically significant compared to Day 4. # Symbol represents statistically significant differences between groups. Dashed-line boxes represent the days in which the tone was changed for the Different groups only (in a counterbalanced way).
Vinegar consumption: Phases I and II
We tested the effects of changing the auditory background and dorsal hippocampus lesion on taste neophobia, using vinegar as a novel taste (Figures 2C,D). By inspection, taste neophobia is clearly observed in response to introducing vinegar, and reduced drinking appears to persist longer if the auditory background is changed in control animals (see Figure2C), but not in mice with dorsal hippocampus lesions (Figure 2D). We confirmed these impressions starting with a global Mixed 4 × 2 × 2 × 2 (Day × Lesion × Tone Change × Counterbalance Order) Repeated Measures ANOVA that compared the intake of vinegar amongst the groups on the four days after the baseline period. There was a significant effect of the main factors Days [F(3,60)=104.51; p;<.0001]. Tone Change [F(1,20)=11.5; p=.003], the interactions Day × Tone Change [F(3,60)=10.12; p<.001], Tone Change × Lesion [F(1,20)=7.32;p=.014] and DayX Tone Change × Lesion [F(3,60)=8.60; p=.004].
To analyze the interactions, additional 4×2 (Day × Tone Change) Repeated Measures ANOVAs were performed for the Sham and Lesion groups separately. The analysis performed for the Sham groups confirmed a significant effect of the main factors Day [F(3,36)=72.07; p<.001] and Tone Change [F(1,12)=26.7; p<001] as well as the Day × Tone Change interaction [F(3,36)=24,64; p<.001]. Analysis of the interaction by Repeated Measures ANOVAs of the vinegar consumption was performed on the factor Day for each of the Tone Change groups separately. The analyses confirmed a significant effect of Day in the Sham-Vinegar-Same Tone group [F(3,18)=48.92; p<001] as well as the Sham-Vinegar-Different Tone [F(3,18)=47.81; p<.001], indicating attenuation of neophobia. Further comparisons using Bonferroni-corrected tests identified significantly less vinegar was consumed on Day 1 compared to Days 2, 3 and 4 (all p’s<.001) in the Sham-Vinegar-Same Tone group, and this confirms that the neophobic response to the vinegar taste was completely attenuated on Day 2 and its consumption remained stable across the rest of days. In contrast, the same analysis performed in the Sham-Vinegar-Different Tone group identified that the amount of vinegar consumed on Days 1 and 2 were indistinguishable (p=1) and less than on Days 3 and 4 (p‘.s≤.009). Thus unlike the mice that did not experience a change of auditory background, the animals that experienced the change maintained the neophobic response for one more day; the attenuation of taste neophobia occurred on Day 3, when the novel auditory background became familiar.
Because hippocampus is specialized for spatial computations, and declarative-type learning and memory, and neither taste novelty nor changes in auditory background carry information about space, we investigated whether dorsal hippocampus lesion affects the attenuation of taste neophobia and its delay by changing the auditory background, a test of nondeclarative memory. We repeated the above analysis for the Lesion groups. There was a significant effect of Day [F(3,24)=39.195; p<.001] and no other effect or interaction (all p‘s>.6). Post-hoc analysis of the effect of Day using Bonferroni-corrected t tests confirmed less vinegar intake on Day 1 compared to Days 2, 3 and 4 (all p‘s<.001) but no other comparisons were significant. This indicates that unlike the sham mice, the lesion animals attenuated the neophobic response to the vinegar taste on Day 2, regardless of whether the background tone was or was not changed (see figure 3). These results demonstrate that lesions of dorsal CA1 impairs the auditory background-dependent attenuation of taste neophobia (see Figure 2D).
Figure 3.
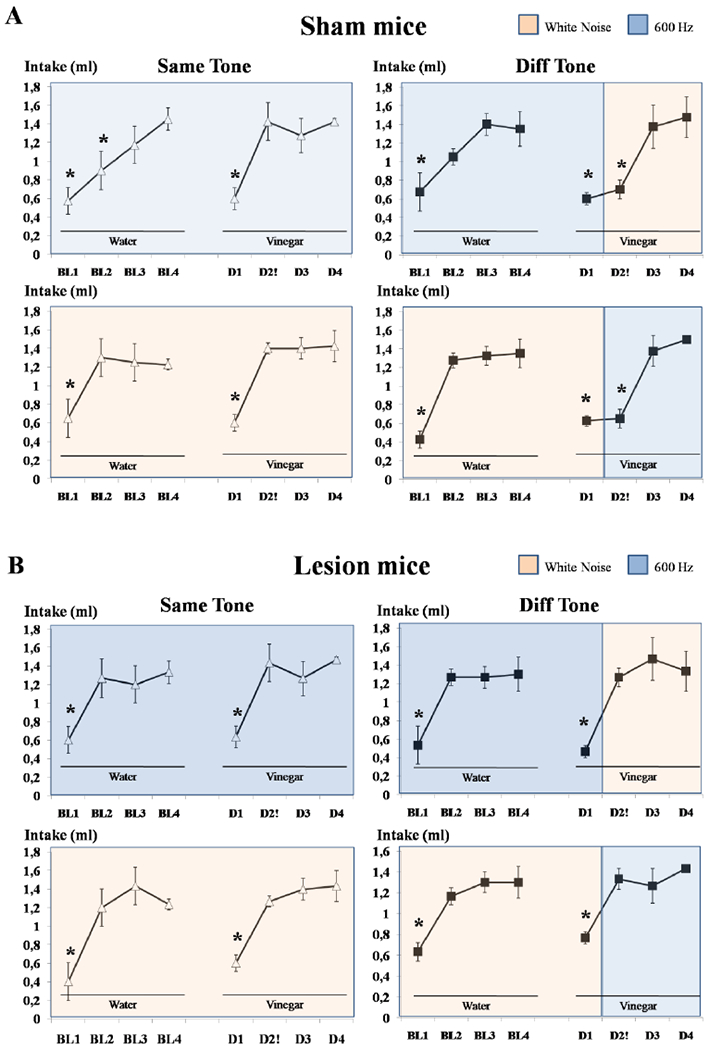
Water and vinegar intake (±SEM) across the experimental days A) in Sham animals demonstrating that both Different groups continued to show taste neophobia to vinegar on Day 2 regardless the presentation order of the auditory background and B) that there is no effect of the specific auditory backgrounds in the Lesion animals, regardless the presentation order of the auditory background, demonstrating attenuation of taste neophobia on Day 2. *Symbol, represents statistically significant differences compared to Day 4.
Baseline: water consumption.
Finally, we examined whether the differences between the Sham and Lesion groups or any other groups for that matter, could be due to group differences in baseline water consumption. A global Mixed 4 × 2 × 2 × 2 × 2 (Day X Lesion X Tone Change X Counterbalance Order) Repeated Measures ANOVA comparing the amount of water intake between all the groups during the four days of baseline (BL) revealed only a significant effect of Day [F(3,93)=85.86; p<.001]. No other effect or interaction was significant (all p‘s>.2). Further analyses of the main effect Day using Bonferroni-corrected t tests revealed that all groups consumed less amounts of water on BL Day 1 compared to BL Days 2, 3 and 4 (all p‘s<.001). This indicates adaptation to the water deprivation procedure was indistinguishable across the groups, and so cannot easily account for the observed differences.
3. DISCUSSION.
The present findings demonstrate for the first time that the auditory background influences attenuation of neophobia, a non-associative form of recognition memory and that dorsal hippocampus integrity is required for this influence of the auditory background. Because the auditory background can provide contextual information, we interpret these findings as evidence that the auditory context can influence the attenuation of taste neophobia and that the hippocampus is crucial for this effect, despite the absence of spatial information in the taste or auditory background.
The modulation of ATN by auditory context was assessed using two different auditory backgrounds. Changing the auditory background reduced ATN in the Sham control groups while the group of mice that experienced a constant auditory background exhibited rapid ATN on day 2. These findings are consistent with a prior demonstration of the spatial context dependency of ATN (De la Casa and Díaz, 2013) and they extend the phenomenon to non-spatial auditory background as a contextual cue. To our knowledge, there is only one previous report using an auditory background as part of the context in taste learning (Bonardi et al., 1990). Most previous research used visual cues (Quintero et al., 2011; De la Casa and Díaz, 2013), as well as temporal information to define context (Moron et al., 2002; De la Casa et al., 2003; Manrique et al., 2004).
The auditory backgrounds were distinct, differing in frequency (600 Hz versus Gaussian white noise), duration (three versus two seconds) and ISI (three versus four seconds). As we were interested in the effect of changing the auditory background on taste neophobia attenuation, the context change occurred after the mice had consumed the novel taste for the first time with the same auditory background as the baseline period. The fact that the ATN was delayed by changing the auditory background on the second exposure day confirms that the animals were able to distinguish the auditory backgrounds. Also, all groups exhibited similar consumption of water and eventually vinegar, regardless of the auditory background. This indicates that the modulation of the taste memory by changing the auditory background was not specific for a single auditory frequency; the presentation order was counterbalanced and this had no effect (see Figure 3) allowing one to conclude that the influence of auditory background is not unique to a particular tone and that this influence is specific to attenuation of neophobia, rather than a general disruption of drinking behavior (see Figure 2A,B).
Prior work has indicated a role for hippocampal function in complex taste learning phenomena, such as blocking (Gallo and Cándido, 1995; Moron et al., 2002) and in taste learning tasks that critically depend on contextual information (Gallo et al., 1999). Electrolytic lesions of the dorsal hippocampus impaired both learned taste aversions to the physical context and the blocking of the context in taste aversion learning (Aguado et al., 1998). The context-dependence of taste aversion’s extinction was also disrupted by electrolytic lesions of the dorsal hippocampus (Fujiwara et al., 2012) as well as the context-dependent extinction itself (Garcia-Delatorre et al., 2010). Finally, excitotoxic dorsal hippocampal lesions disrupted the context dependency of both taste aversions and latent inhibition of taste aversion (Molero et al., 2005; Manrique et al., 2009b, 2009a).
What defines a context? Context is commonly defined as the set of background stimuli that comprises the environment during a behavior. These same stimuli can of course also become foreground conditioned stimuli, depending on the task (Nadel and Willner, 1980; De la Casa et al., 2018). The study of context in different taste recognition memory tasks has primarily investigated spatial contexts, often defined only by visual cues (Quintero et al., 2011; De la Casa and Díaz, 2013), as well as temporal contexts, defined either as time elapsed (De la Casa et al., 2003) or the time of day (Moron et al., 2002; Manrique et al., 2004). In this context, it is important that memory, spatial and temporal task information are signaled in the discharge of hippocampus CA1 cells, as well as other hippocampus subfields (Lenck-Santini et al., 2008; Pastalkova et al., 2008; Jezek et al., 2011; Eichenbaum, 2017; van Dijk and Fenton, 2018). This, as well as other robust behavioral evidence that hippocampus is crucial for context-based memory (Kim and Fanselow, 1992), is consistent with the present finding that dorsal hippocampus lesions interfere with the auditory context modulation of ATN.
What other evidence is there for a role of hippocampus in the auditory context modulation of ATN? A role for dopamine has been reported in the consolidation of contextual memories in hippocampus (Kempadoo et al., 2016; Takeuchi et al., 2016; Yamasaki and Takeuchi, 2017). Fike we observed for the auditory background, the attenuation of the neophobic response to a novel saccharin solution was weaker when the novel taste was encountered in a novel cage compared to the familiar homecage. In that work the contexts differed in spatial (size of the cages), visual (red vs white light) and somatosensory (different bedding) dimensions but a crucial role for hippocampus was not established. At present there is no evidence that specific hippocampal subfields have a particular role in contextual taste learning, and frankly this would not be expected given that hippocampal subfields have distinctive computational roles such as pattern separation, pattern completion, and model-data comparisons that transcend specific classes of information and learning (Aronov et al., 2017; Colgin et al., 2009; Dvorak et al., 2018; Guzowski et al., 2004; Lenck-Santini et al., 2008). The effect of changing contexts on ATN is disrupted by systemic administration of the D1/D5 dopamine receptor antagonist SCH-23390 (De la Casa and Díaz, 2013), but rather little is known about the contextual modulation of ATN and the brain areas involved.
We observed, to our knowledge for the first time, that dorsal CA1 subfield lesions disrupt the non-spatial contextual dependence of ATN, which on the surface appears to contradict cognitive mapping theory (O’Keefe and Nadel, 1978), but is consistent with the view that hippocampus is critical for processing complex associative representations of stimuli involving context (Eichenbaum et al., 1999; Eichenbaum, 2017; Jezek et al., 2011; Lenck-Santini et al., 2008; Pastalkova et al., 2008; van Dijk and Fenton, 2018). There are of course, also non-associative explanations for the differential role of auditory context, which when changed, could increase levels of arousal, and lead to the recovery of taste neophobia. This is supported by the finding thatif the context is familiar, neophobia can persist despite the change of context (Honey et al., 1992).
We find that the relationship between taste and auditory cues whatever its nature, requires dorsal hippocampus. Indeed, the present findings suggest that changes in the auditory background has similar effects on taste learning as what was previously observed by manipulating the physical properties of the environment. To our knowledge, this is the first evidence that mice use the auditory information that is present in the environment to define context sufficient to modulate attenuation of taste neophobia. Although more research is needed to identify the particular procedural features that might be critical for auditory modulation of taste memory, the present results introduce a new paradigm for exploring the hippocampus-dependent mechanisms that underlie how non-spatial memories are stored and modulated by non-spatial environmental cues.
Highlights.
To our knowledge, this is the first evidence that mice use the auditory information present in the environment to define context sufficient to modulate attenuation of taste neophobia (AN).
- Dorsal CA1 subfield lesions disrupt the non-spatial contextual dependence of AN:
-
○It appears to contradict cognitive mapping theory (O’Keefe and Nadel, 1978),
-
○However, it is consistent with the view that hippocampus is critical for processing complex representations of stimuli involving context (Eichenbaum et al., 1999; Eichenbaum, 2017).
-
○
The present results introduce a new paradigm for exploring the hippocampus-dependent mechanisms that underlie how non-spatial memories are stored and modulated by non-spatial environmental cues.
4. ACKNOWLEDGEMENTS.
This work was supported by the research projects PSI2014-57643-P and PSI2017-86381-P (MINECO.Spain). A.B. Grau-Perales was recipient of a predoctoral fellowship (FPU14/1531, MECD, Spain) and a predoctoral travel grant (ETS16/00256, MECD, Spain). This series of experiments are part of the Ph.D. research performed by A.B. Grau-Perales. E. Levy and A.A. Fenton are supported by NIH grant R01NS105472. The authors of this manuscript state that there are no actual or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES.
- Aguado L, Hall G, Harrington N, Symonds M (1998) Illness-induced context aversion learning in rats with lesions of the dorsal hippocampus. Behav Neurosci 112:1142–1151. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F (2004) Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci 5:209–217. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Honey RC, Hall G (1990) Context specificity of conditioning in flavor-aversion learning: Extinction and blocking tests. Anim Learn Behav 18:229–237. [Google Scholar]
- Dardou D, Datiche F, Cattarelli M (2008) Memory is differently impaired during aging according to the learning tasks in the rat. Behav Brain Res 194:193–200. [DOI] [PubMed] [Google Scholar]
- Dardou D, Datiche F, Cattarelli M (2010) Does the olfactory cue activate the same brain network during aging in the rat aftertaste potentiated odor aversion retrieval? Neurobiol Learn Mem 93:137–150. [DOI] [PubMed] [Google Scholar]
- De la Casa LG, Cárcel L, Ruiz-Salas JC, Vicente L, Mena A (2018) Conditioned increase of locomotor activity induced by haloperidol. PloS One 13:e0200178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Casa LG, Díaz E (2013) Contextual control of flavor neophobia. Physiol Behav 118:45–51. [DOI] [PubMed] [Google Scholar]
- De la Casa LG, Díaz E, Lubow RE (2003) Effects of post-treatment retention interval and context on neophobia and conditioned taste aversion. Behav Processes 63:159–170. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2017) On the Integration of Space, Time, and Memory. Neuron 95:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H (1999) The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23:209–226. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Sawa K, Takahashi M, Lauwereyns J, Tsukada M, Aihara T (2012) Context and the renewal of conditioned taste aversion: the role of rat dorsal hippocampus examined by electrolytic lesion. CognNeurodyn 6:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M, Cándido A (1995) Reversible inactivation of dorsal hippocampus by tetrodotoxin impairs blocking of taste aversion selectively during the acquisition but not the retrieval in rats. Neurosci Lett 186:1–4. [DOI] [PubMed] [Google Scholar]
- Gallo M, Marquez SL, Ballesteros MA, Maldonado A (1999) Functional blockade of the parabrachial area by tetrodotoxin disrupts the acquisition of conditioned taste aversion induced by motion-sickness in rats. Neurosci Lett 265:57–60. [DOI] [PubMed] [Google Scholar]
- Gámiz F, Gallo M (2011) Taste learning and memory: a window on the study of brain aging. Front Syst Neurosci 5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Delatorre P, Rodriguez-Ortiz CJ, Balderas I, Bemnidez-Rattoni F (2010) Differential participation of temporal structures in the consolidation and reconsolidation of taste aversion extinction. Eur J Neurosci 32:1018–1023. [DOI] [PubMed] [Google Scholar]
- Gómez-Chacón B, Morillas E, Gallo M (2015) Altered perirhinal cortex activity patterns during taste neophobia and their habituation in aged rats. Behav Brain Res 281:245–249. [DOI] [PubMed] [Google Scholar]
- Honey RC, Pye C, Lightbown Y, Rey V, Hall G (1992) Contextual factors in neophobia and its habituation: the role of absolute and relative novelty. Q J Exp Psychol B 45:327–347. [PubMed] [Google Scholar]
- Jezek K, Henriksen EJ, Treves A, Moser El, Moser M-B (2011) Theta-paced flickering between place-cell maps in the hippocampus. Nature 478:246–249. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113:14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS (1992) Modality-specific retrograde amnesia of fear. Science 256:675–677. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini P- P, Fenton AA, Muller RU (2008) Discharge properties of hippocampal neurons during performance of a jump avoidance task. J Neurosci Off J Soc Neurosci 28:6773–6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique T, Gamiz F, Morón I, Ballesteros MA, Gallo M (2009. a) Peculiar modulation of taste aversion learning by the time of day in developing rats. Dev Psychobiol 51:147–157. [DOI] [PubMed] [Google Scholar]
- Manrique T, Molero A, Ballesteros MA, Morón I, Gallo M, Fenton AA (2004) Time of day-dependent latent inhibition of conditioned taste aversions in rats. Neurobiol Learn Mem 82:77–80. [DOI] [PubMed] [Google Scholar]
- Manrique T, Morón I, Ballesteros MA, Guerrero RM, Fenton AA, Gallo M (2009. b) Hippocampus, aging, and segregating memories. Hippocampus 19:57–65. [DOI] [PubMed] [Google Scholar]
- Molero A, Moron I, Angeles Ballesteros M, Manrique T, Fenton A, Gallo M (2005) Hippocampus, temporal context and taste memories. Chem Senses 30 Suppl 14160–161. [DOI] [PubMed] [Google Scholar]
- Morillas E, Gómez-Chacón B, Gallo M (2017) Flavor and object recognition memory impairment induced by excitotoxic lesions of the perirhinal cortex. Neurobiol Learn Mem 144:230–234. [DOI] [PubMed] [Google Scholar]
- Moron I, Ballesteros MA, Candido A, Gallo M (2002) Taste aversion learning and aging: a comparison with the effect of dorsal hippocampal lesions in rats. Physiol Res 51 Suppl l:S21–27. [PubMed] [Google Scholar]
- Nadel L, Willner J (1980) Context and conditioning: A place for space. Physiol Psychol 8:218–228. [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G (2008) Internally generated cell assembly sequences in the rat hippocampus. Science 321:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero E, Díaz E, Vargas JP, Schmajuk N, López JC, De la Casa LG (2011) Effects of context novelty vs. familiarity on latent inhibition with a conditioned taste aversion procedure. Behav Processes 86:242–249. [DOI] [PubMed] [Google Scholar]
- Squire LR (2004) Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem 82:171–177. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonnebom A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernandez G, Deisseroth K, Greene RW, Morris RGM (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MT, Fenton AA (2018) On How the Dentate Gyrus Contributes to Memory Discrimination. Neuron 98:832–845.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Takeuchi T (2017) Locus Coeruleus and Dopamine-Dependent Memory Consolidation. Neural Plast 2017:8602690. [DOI] [PMC free article] [PubMed] [Google Scholar]


