Abstract
A key aspect of development in all metazoans is remodeling at the cellular level. During the development of gametes, remodeling occurs throughout the germ line. When C. elegans hermaphrodites become depleted of sperm after 4 days of adulthood, significant cellular remodeling occurs within the meiotically-arrested oocytes, including the formation of ribonucleoprotein (RNP) granules. Since major remodeling of the endoplasmic reticulum (ER) occurs in early embryos, we investigated the extent of ER remodeling in meiotically-arrested oocytes. We found, using a combination of fluorescence reporters and transmission electron microscopy (TEM), that the ER in arrested oocytes accumulates in patches and sheets that are enriched at the cortex. Our findings suggest this remodeling is not due to simple displacement by large amounts of yolk that accumulate in arrested oocytes, and instead may be genetically regulated. We further identified the Ddx6 RNA helicase, CGH-1, as a key regulator of ER in the germ line. In cgh-1(tn691) oocytes, we detected cortical ER patches as well as aberrant granules of the RNA-binding proteins, PAB-1, MEX-3, and CGH-1. Taken together, our results suggest the possibility that the spatial organization of RNA binding proteins may regulate the translation of mRNAs associated with the ER that in turn, controls the organization of the ER in the adult germ line.
Keywords: nematode, reproductive, early development
1. Introduction
Cellular remodeling is a critical part of development in all metazoans, including during the development of gametes. In unmated Caenorhabditis females such as C. remanei females, or in hermaphrodites that lack sperm such as C. elegans fog-2 hermaphrodites, oocytes arrest in meiosis and accumulate in the germ line. Several cellular differences have been described in these arrested oocytes, relative to non-arrested oocytes in mated C. remanei females, mated fog-2 hermaphrodites, or wild-type C. elegans hermaphrodites. In meiotically-arrested oocytes, microtubules are cortically enriched, large amounts of nuclear blebbing occur, and large, cortical RNP granules accumulate (Harris et al., 2006; M. Jud, Razelun, Bickel, Czerwinski, & Schisa, 2007; Patterson, Wood, & Schisa, 2011; Schisa, Pitt, & Priess, 2001). If meiosis resumes, microtubules are evenly dispersed throughout the cytoplasm, low levels of nuclear blebbing occur, and components of RNP granules are dissociated. While the function of cellular remodeling in meiotically-arrested oocytes is not completely understood, one hypothesis is that the remodeling may serve a protective function during the extended delay in fertilization (M. C. Jud et al., 2008). Remodeling of the endoplasmic reticulum (ER) has been characterized during the cell cycle of early embryos of C. elegans and Drosophila (Bobinnec, Marcaillou, Morin, & Debec, 2003; Poteryaev, Squirrell, Campbell, White, & Spang, 2005), but it has not been closely examined in the C. elegans germ line. The first goal of this project was to characterize the ER remodeling that occurs when C. elegans oocytes enter into an extended meiotic arrest. Our second goal was to identify genetic regulators of ER remodeling in the germ line.
2. Results and Discussion
To characterize ER remodeling when meiosis arrests an extended time, we used the GFP::SP12 strain. SP12 is a resident signal peptidase of ER commonly used as an ER marker (Poteryaev et al., 2005). In young hermaphrodites that contain sperm, here referred to as Adult Day 1 (see methods for details), the distribution of ER was generally diffuse throughout the oocytes, with subtle cortical enrichment and occasional small patches detected in some oocytes (Fig. 1a). In Adult Day 2 worms, we detected small patches in oocytes in 100% of worms (Fig. 1b). In Adult Day 5 worms, a time soon after sperm are depleted, larger cortical ER patches had accumulated in 80% of oocytes (Fig. 1c). These results demonstrate remodeling of ER occurs in the germ line prior to the ER cycling that occurs during meiotic maturation post-fertilization and during mitosis (Bonner, Han, & Skop, 2013; Poteryaev et al., 2005; Squirrell et al., 2006). To determine if the ER patches are simply a function of age, or if they could be a function of the oocyte meiotic arrest that occurs with sperm depletion, we crossed the GFP::SP12 strain into a fog-2(q71) background. No sperm are produced in fog-2 hermaphrodites, resulting in extremely low maturation rates and meiotically arrested oocytes, even at a young age (Schedl & Kimble, 1988). Throughout the first several days of adulthood, oocytes continue to accumulate in fog-2 germ lines and become more columnar in shape. In early Adult Day 1 worms, we observed diffuse distribution of GFP::SP12 (Fig. 1d), and, in 62.5% of oocytes, small ER patches were detected. In late Adult Day 1 worms, cortically-enriched ER patches were apparent in 96% of the worms (Fig. 1e), and in Adult Day 2 worms, ER patches similar to those observed in Adult Day 5 GFP::SP12 worms were detected in 100% of the worms (Fig. 1f). Cortical enrichment of the ER in the dorsal-ventral and anterior-posterior orientations was quantified in the second most-proximal oocyte using line intensity scans, and an enrichment ratio was calculated to compare meiotically arrested oocytes in Adult Day 1 fog-2; GFP::SP12 to non-arrested oocytes in Adult Day 1 GFP::SP12 (see methods). We calculated an enrichment ratio of 1.30 in the dorsal-ventral axis, and 1.49 in the anterior-posterior axis, confirming the reorganization of ER in meiotically arrested oocytes. Since other types of cellular remodeling, such as the assembly of RNP granules and increased nuclear blebs, are reversible upon mating into female worms lacking sperm (M. Jud et al., 2007; Patterson et al., 2011), we next mated males with fog-2 females to investigate if ER remodeling was reversible. We observed fewer ER patches in mated worms (Fig. 1g,h), suggesting that ER remodeling is a dynamic process strongly correlated with meiotic arrest.
Figure 1.
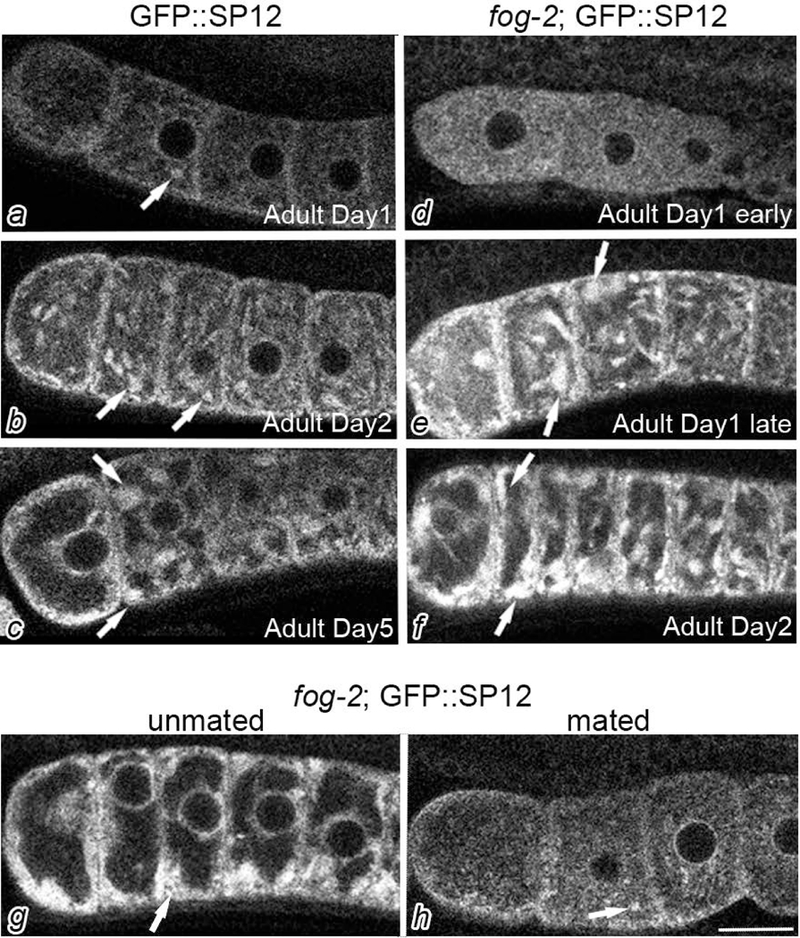
Cortical ER patches accumulate in meiotically-arrested oocytes. GFP::SP12 (a-c) and fog-2; GFP::SP12 (d-f) germ lines were imaged by confocal microscopy at different developmental stages as noted and described in the Methods. The most proximal oocyte of each germ line is oriented to the left in each image of this figure and in all figures. Arrows indicate ER patches. The accumulation of ER patches in arrested oocytes is reversible as shown after mating into an unmated fog-2;GFP::SP12 female (g,h). Scale bar is 10 μm.
To compare the ultrastructure of meiotically arrested and non-arrested oocytes, we performed transmission electron microscopy (TEM) studies. We first examined oocytes of C. remanei mated and unmated females. Since C. remanei is a male-female nematode species, it avoids any possible complications associated with age or the fog-2 mutation in C. elegans. We observed short stretches of membrane dispersed throughout the cytoplasm of mated females, including at the cortex (Fig. 2a, white arrows and inset). In contrast, we detected longer sheets of aligned membranes in arrested oocytes of unmated females (Fig. 2b). Similarly, in oocytes of wild-type C. elegans hermaphrodites, we detected short stretches of membrane dispersed throughout the cytoplasm (Fig. 2c, white arrows and inset); however, in the arrested oocytes of fog-2 females we detected sheets of aligned ER membranes (Fig. 2d). We quantitated the percent of oocytes in which we could detect at least two stacked ER membranes, and found substantial increases: from 30% in non-arrested oocytes to 94% in arrested C. elegans oocytes, and from 4% in non-arrested oocytes to 74% in arrested C. remanei oocytes. This analysis is limited by our examination of random thin sections, and by including oocytes in multiple positions in the proximal gonad arm, but nonetheless suggests a large change in ER morphology in arrested oocytes.
Figure 2.
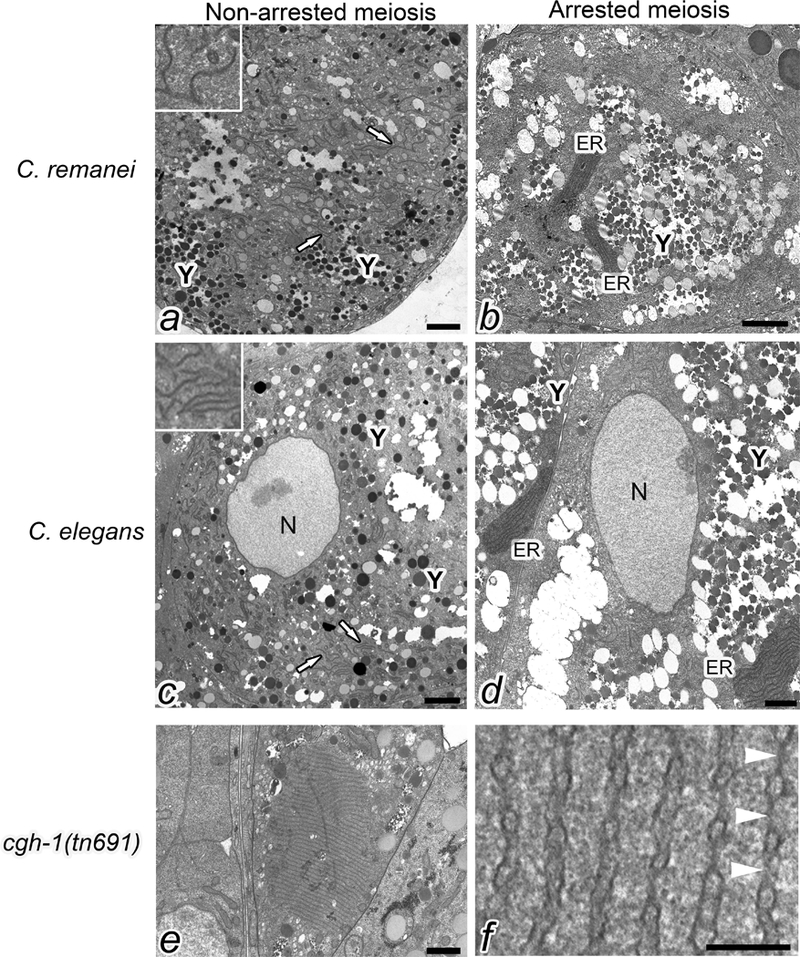
ER sheets accumulate in meiotically arrested oocytes. Transmission electron micrographs of oocytes in C. remanei mated and unmated females (cross section at the cortex, lacking nuclei) (a, b), C. elegans wild type and fog-2 worms (cross section through nuclei) (c, d), and cgh-1(tn691) (cross section at the cortex) (e). The blending option in Adobe Photoshop was used to highlight the stacks of ER in b, d. White arrows in a and c indicate dispersed ER. Y indicates yolk, which occupies large areas of the cytoplasm in arrested oocytes (b, d). Scale bars are 1500 nm. The insets in a and c show higher magnification view of dispersed ER. A higher magnification view of the annulate lamellae in panel e shows nuclear pore complexes indicated by white arrowheads (f). Scale bar in panel f is 125 nm.
In our TEM micrographs of arrested oocytes, we noted large areas of the cytoplasm filled with electron-dense yolk granules (Fig. 2b, d). One explanation for seeing ER accumulated at the cortex of arrested oocytes could be simple displacement due to an increased amount of yolk that accumulates in meiotically-arrested oocytes. To explore this possibility, we first used the GFP::VIT-2 strain to visualize the amount of yolk accumulation (Grant & Hirsh, 1999). VIT-2 is a vitellogenin protein abundant in yolk and commonly used as a marker of yolk. We found oocyte GFP levels increased 9-fold in sperm-depleted GFP::VIT-2 hermaphrodites (p<0.01), and 10-fold in the fog-2; GFP::VIT-2 females (p<0.01), compared to young GFP::VIT-2 hermaphrodites. To determine if cortical ER in arrested oocytes results from yolk accumulation, we blocked yolk transport from the intestine into the oocytes using rab-5(RNAi). rab-5 regulates the early endocytosis pathway and is required for yolk transport (Bucci et al., 1992; Grant & Hirsh, 1999; Hall et al., 1999). After RNAi of rab-5 in fog-2; GFP::VIT-2 oocytes, we observed high levels of yolk in the hypodermis outside of the germ line, and yolk was detected within oocytes in only 3% of worms, a significant decrease from the vector (RNAi) control of 93%, P<0.0001 (Fig. 3a, b), and confirming the efficacy of rab-5 RNAi. After rab-5(RNAi) in fog-2; GFP::SP12 females, ER patches were detected in the cortical regions of oocytes of 99% of worms, compared to 100% of control oocytes (Fig. 3c, d, arrows). Thus, accumulation of large amounts of yolk in the germ line is not required for the reorganization of ER in arrested oocytes, and a genetically regulated process rather than simple displacement may occur to promote remodeling.
Figure 3.
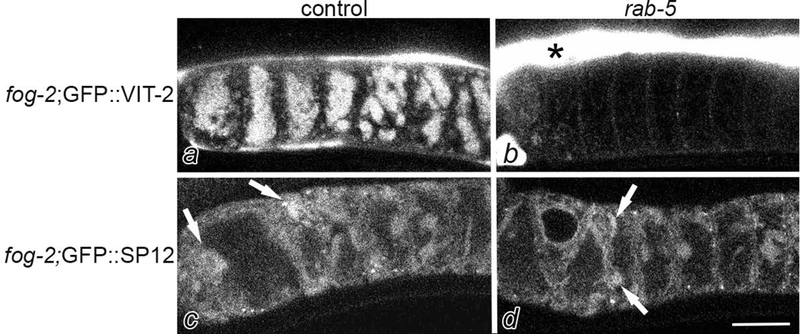
ER patches can form in meiotically-arrested oocytes in the absence of yolk accumulation. Confocal images of fog-2; GFP::VIT-2 germ lines after RNAi of vector control (a) and rab-5 (b). Yolk accumulates within oocytes after control RNAi; in contrast, yolk accumulates in the hypodermis outside of the germ line after rab-5(RNAi). fog-2;GFP::SP12 germ lines after RNAi of vector control (c) and rab-5 (d). Arrows indicate ER patches in both germ lines. Scale bar is 10 μm.
To identify regulators of ER remodeling, we screened a short list of candidates (Table 1), some of which had emerged from a prior RNAi screen suggesting a link between regulators of RNP granules and the ER (Wood, Hollis, Severance, Karrick, & Schisa, 2016) and some of which were identified as regulators of ER in embryos in the literature (A. Trombley, D. Leroux and J. Schisa, unpublished observations). Here, we discuss our results with cgh-1(RNAi). cgh-1 (conserved germ line helicase-1) encodes a DEAD-box RNA helicase orthologous to human Ddx6 (Navarro, Shim, Kohara, Singson, & Blackwell, 2001). We first asked if CGH-1 regulates ER remodeling in arrested oocytes. After RNAi of cgh-1 in fog-2: GFP::SP12, we did not observe a significant change compared to the vector RNAi control (data not shown). We next explored regulation by CGH-1 in non-arrested oocytes by knocking down expression of cgh-1 by RNAi in GFP::SP12 worms. As expected from the literature, we observed some disorganization of the germ line, and oocytes of various sizes (Navarro, Shim, Kohara, Singson, & Blackwell, 2001). In a subset of worms, we detected ER patches not seen in the vector(RNAi) control (Fig. 4a,b). To more thoroughly explore the cgh-1 phenotype, we crossed cgh-1(tn691) into the GFP::SP12 strain. After shifting worms to the non-permissive temperature, we observed cortically-enriched oocyte ER patches in 88% of the worms, compared to 12% in GFP::SP12 controls at the non-permissive temperature, p<0.0001 (Fig. 4c-f). Thus, CGH-1 appears to regulate the reorganization of ER in non-arrested oocytes. To examine the ultrastructure of cgh-1(tn691) oocytes, we used TEM. The most striking observation was that in 19% of cgh-1 oocytes, we detected large stacks of annulate lamellae at the cortex that were never observed at the cortex of wild-type, non-arrested oocytes (compare center oocyte in Fig. 2e to 2a). Annulate lamellae (AL) are specialized ER membranes with numerous aligned nuclear pore complexes (see arrowheads, Fig. 2f). The sheets of AL detected in cgh-1 oocytes, up to 40 aligned membranes, were much larger than the small stacks of AL sheets previously seen in a subset of arrested oocytes (Patterson et al., 2011; Pitt, Schisa, & Priess, 2000). Annulate lamellae have been detected in oocytes of many species, in some male germ cells, and also in several types of tumor cells (Kessel, 1989). The function of AL is not known; however, AL is often observed in cells where there is a substantial delay between transcription and translation. This association could suggest that the translational regulation of a subset of mRNAs is altered in cgh-1(tn691) oocytes.
Table 1.
Candidate genes screened as regulators of ER remodeling.
| Gene | Rationale for screening |
|---|---|
| nra-2 | Identified in RNAi screen as regulator of RNP granules, GO class ER |
| ufd-1 | Identified in RNAi screen as regulator of RNP granules, GO class ER |
| W03F9.1 | Identified in RNAi screen as regulator of RNP granules, GO class ER |
| ooc-3 | Identified in RNAi screen as regulator of RNP granules, GO class ER |
| arf-1 | Regulator of ER in embryos (Poteryaev et al 2005) |
| cdc-48.3 | Regulator of ER in embryos (Poteryaev et al 2005) |
| car-1 | Regulator of ER in embryos (Squirrel et al., 2006) |
| cgh-1 | Potential regulator of ER; associates with CAR-1 (Boag et al., 2005) |
Figure 4.
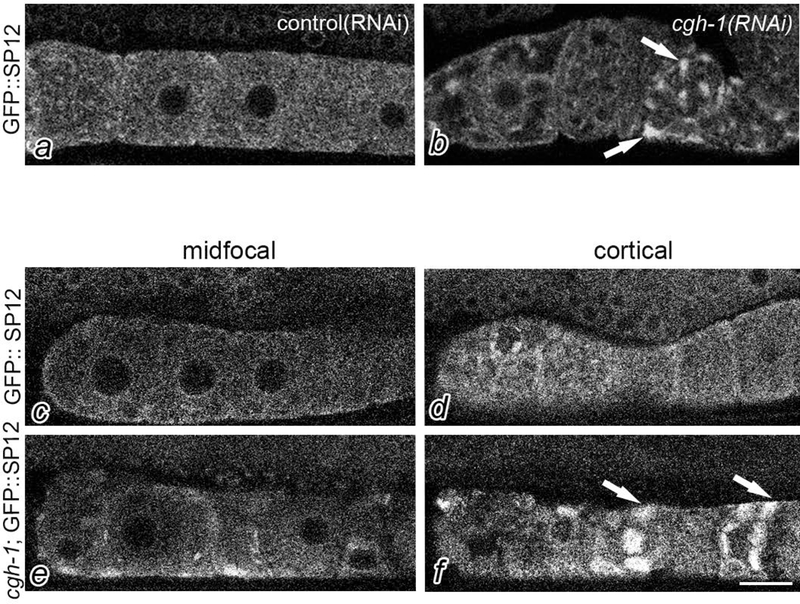
CGH-1 regulates ER remodeling in oocytes. Confocal imaging of GFP::SP12 germ lines after control RNAi results in diffuse ER (a); however, cgh-1(RNAi) results in patches of ER (arrows). Confocal images of GFP::SP12 (c,d) and cgh-1(tn691); GFP::SP12 (e,f). Cortical slices of cgh-1(tn691); GFP::SP12 reveal enrichment of cortical ER patches (arrows), as compared to controls or mid-focal slices (e). Scale bar is 10 μm.
Remodeling of the ER in arrested oocytes is correlated with a second type of cellular remodeling, the assembly of RNA binding proteins into cortical RNP granules (Schisa et al., 2001). One possibility is that the reorganization of the ER and RNA binding proteins is functionally linked. Therefore, after characterizing the reorganization of the ER in non-arrested cgh-1(tn691) oocytes, we asked if the localization of RNA binding proteins also changes. We first asked if PAB-1 (poly(A) binding protein), a common marker of stress granules, is regulated by CGH-1. In the vector (RNAi) control, we observed diffuse distribution of GFP::PAB-1 throughout the germ line (Fig. 5a). After cgh-1(RNAi), filamentous granules of GFP::PAB-1 were detected in approximately 50% of the oocytes (Fig. 5b). These granules appeared distinct from the square granules and thin sheets of CAR-1 previously described in cgh-1(tn691) oocytes (Fig. 5f) (Hubstenberger, Noble, Cameron, & Evans, 2013). CAR-1 is a homolog of RAP55, an evolutionarily conserved protein that localizes to P bodies (Marnef et al., 2010). CGH-1 appears to prevent the transformation of dynamic, liquid granules of CAR-1 into nondynamic solid, square structures (Hubstenberger, Noble, Cameron, & Evans, 2013). In several contexts, solid complexes of RNPs are associated with pathological states including several neurodegenerative disorders (Alberti & Carra, 2018). In the distal core dramatic reorganization of GFP::PAB-1 was detected, with discrete tubules in sheets and patches in nearly all of the worms (Fig. 5d). The sheet-like structures of GFP::PAB-1 appeared similar to the sheets of CAR-1 in the distal core (Fig. 5h). MEX-3 is a conserved KH domain RNA binding protein that strongly localizes to RNP granules in arrested oocytes (M. Jud et al., 2007; Schisa et al., 2001). Therefore, we next examined GFP::MEX-3 worms after cgh-1(RNAi) and observed a striking difference from the diffuse GFP::MEX-3 observed in control oocytes (Fig. 5i). After cgh-1(RNAi), a mixed population of GFP::MEX-3 granules were detected; some were spherical like the RNP granules in arrested oocytes (M. Jud et al., 2007), and in 70% of germ lines, square-shaped granules were also observed (Fig. 5j). This phenotype was recapitulated in a cgh-1(tn691); GFP::MEX-3 strain (Fig. 5l), and the shape and size of the square granules appeared similar to the square CAR-1 granules (Fig. 5f). Therefore, the CGH-1/Ddx6 RNA helicase may modulate the phase transition of several RNA binding proteins, as evidenced by the filamentous and square granules of PAB-1 and MEX-3. In the future, colocalization experiments could address whether CAR-1 and MEX-3 localize to the same square granules, and whether CAR-1 and PAB-1 localize to the same sheets in the distal core.
Figure 5.
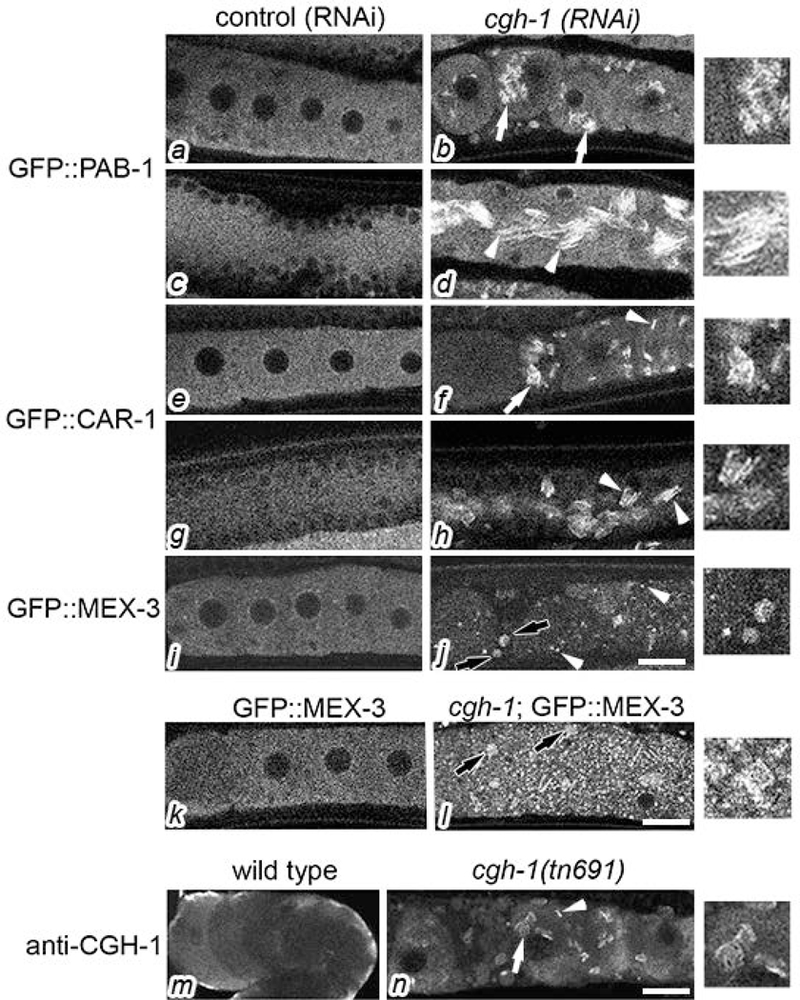
Characterization of cgh-1 oocytes. In vector (RNAi) controls, confocal images show diffuse distribution of GFP::PAB-1, GFP::CAR-1, and GFP::MEX-3 in the proximal oocytes (a, e, i) and distal gonad core (c, g). After RNAi of cgh-1, filamentous granules of GFP::PAB-1 accumulate in oocytes (b, white arrows); in the distal core, filamentous sheets accumulate (d, arrowheads). RNP structures induced by cgh-1(RNAi) are shown at two-fold magnification (digitally increased magnification) in the far right column. After RNAi of cgh-1, GFP::CAR-1 assembles into oocyte granules that appear square-shaped from top/bottom views (f, arrows) and appear as thin sheets from lateral views (f, arrowheads). GFP::CAR-1 localizes to filamentous sheets in the distal gonad core (h, arrowheads). After cgh-1(RNAi) GFP::MEX-3 accumulates in a mixed population of square granules (j, black arrows) and small spherical granules (j, arrowheads). GFP::MEX-3 is normally diffuse in oocytes (k), but a mixed population of square (black arrows) and spherical granules are detected in cgh-1(tn691);GFP::MEX-3 oocytes (l). Confocal images after anti-CGH-1 antibody staining in wild type (m) and cgh-1(tn691) oocytes (n). CGH-1 is enriched at the cortex of wild-type oocytes, and in cgh-1(tn691) CGH-1 assembles into granules that appear square-shaped from top/bottom views (n, arrows) and as thin sheets from lateral views (n, arrowheads). Scale bars are 10 μm.
To better understand the nature of the cgh-1(tn691) allele, we next examined levels of CGH-1 in cgh-1 worms at the non-permissive temperature. We observed low levels of cortically-enriched staining in wild-type oocytes, as has been described (Fig. 5m) (Navarro et al., 2001). Interestingly, in cgh-1 oocytes we observed CGH-1 particles similar in size and shape as the square granules and thin sheets of GFP::CAR-1, suggesting CGH-1 and CAR-1 may colocalize in cgh-1 oocytes, as the proteins do in wild-type germ lines (Boag, Nakamura, & Blackwell, 2005) (Fig. 5n). This result clarifies that the cgh-1(tn691) allele is not a translational null, and that some form of the CGH-1 protein is stable, yet not fully functional. The P68L mutation of tn691 is in the Q motif which is the proposed MAPK phosphorylation site of CGH-1; in other DEAD-box helicases, the Q motif regulates ATP-binding and hydrolysis (Arur et al., 2009). This suggests that phosphorylation of CGH-1 by MAPK may be critical to prevent the assembly of RNP complexes in the absence of meiotic arrest. The aberrant CGH-1 protein in cgh-1(tn691) may be a nucleating factor that attracts other RNA-binding proteins to the square, solid granules.
In summary, our studies reveal dynamic remodeling of the ER in the C. elegans germ line. The novel phenotype of cortical ER patches accumulating in meiotically-arrested oocytes can be added to the previously documented changes in the microtubule cytoskeleton, increased nuclear blebbing, and formation of large RNP granules. While the function of cellular remodeling in arrested oocytes is not known, the reversible nature of the changes upon resumption of meiosis is consistent with a protective role in maintaining oocytes when there is an extended delay in fertilization. We have also identified CGH-1 as a regulator of cellular remodeling in non-arrested oocytes. The ER patches identified using the GFP::SP12 reporter may correspond to the sheets and/or AL observed in transmission electron micrographs. In addition to effects of cgh-1 on the ER, we also identified effects on RNP complexes; square-shaped granules of GFP::CGH-1 and GFP::MEX-3 accumulate at the oocyte cortex, similar to GFP::CAR-1 granules (Hubstenberger et al., 2013). Although we do not yet know if the ER and RNP granule phenotypes are functionally linked, in early embryos of worms and flies, the Rap55 homologs CAR-1 and Trailerhitch, regulate ER organization (Squirrell et al., 2006; Wilhelm, Buszczak, & Sayles, 2005). Our results suggest the possibility that the RNA binding proteins CGH-1, CAR-1, MEX-3, and PAB-1 may regulate the translation of mRNAs associated with the ER that in turn, controls the organization of the ER in the adult germ line.
3. Methods
3.1. C. elegans growth and strains used
C. elegans strains were cultured using standard methods (Brenner, 1974), grown on nematode growth media (NGM) plates with E.coli OP50, or HT115 in the case of RNAi experiments. Strains were maintained at 20°C unless otherwise indicated. For simplicity, the developmental stages of 1, 2, and 5 days post-L4 are indicated as Adult Day 1, 2, or 5. The Adult Day1 early worms were imaged at 2 hours post-L4, and the Adult Day1 late worms were imaged at 8 hours post-L4. The temperature sensitive cgh-1(tn691) strain was shifted from 15°C to the non-permissive temperature of 24°C at the L4 stage. The following strains were used in this work:
DG1701 cgh-1(tn691) III
WH327 unc-119(ed3) III; ojIs23 [pie-1p::GFP::C34B2.10]
CB4108 fog-2(q71) V
SB146 C. remanei
Bristol strain N2, C. elegans
RT130 pwIs23[vit-2::GFP]
JH2338 unc-119(ed3); axIs1489[pCG61] pie-1prom:LAP:PAB-1
WH346 unc-119(ed3) III; ojIs23 [pie-1p::GFP::CAR-1 (Y18D10A.17)]
GFP::MEX-3 (M. C. Jud et al., 2008)
Crosses were conducted using standard techniques to generate the following strains:
fog-2; GFP::SP12
fog-2; GFP::VIT-2
cgh-1(tn691); GFP::SP12
3.2. Imaging and analysis
Transgenic worms carrying GFP reporters were imaged using either an Olympus BX51 fluorescence microscope or a Nikon A1R confocal microscope. GFP levels were determined using NIS Elements software, and an ANOVA was used to assess significance. The sizes of ER patches were determined using Image J, and the Fisher exact test was used with Bonferroni correction. Adobe Photoshop was used to assemble plates for figures. The ER cortical enrichment ratios were determined using Image J. The second-most proximal oocyte was measured in five fog-2; GFP::SP12 worms and five GFP::SP12 worms. Six line intensity scans were drawn in the dorsal-ventral and the anterior-posterior orientations, and averaged. For each orientation, the cortical enrichment ratio was calculated by dividing the average for the arrested oocytes by the average for the non-arrested oocytes. A ratio greater than 1.0 indicates cortical enrichment in arrested oocytes.
3.3. Transmission electron microscopy
Worms were prepared according to Pitt et al., 2000. Worms were oriented for longitudinal sections during embedding in Spurr’s resin (Spurr, 1969). The samples were sectioned with a Sorvall MT-2B or PowerTome X ultramicrotome. Sections were collected on copper grids (100, 200, or 600 mesh hex), stained with uranyl acetate (saturated) for 25 min., rinsed with ddH2O, stained with Reynold’s lead citrate for 2 min., and rinsed with ddH2O (Reynolds, 1963). After grids dried, they were imaged using either a Phillips CM10 or a Hitachi HT7700 transmission electron microscope. Images of 20 oocytes of each genotype were taken on Kodak 4489 Electron Microscope Film, or digitally. For the former, negatives were developed with Kodak D19 Developer, fixed with Kodak Professional Rapid Fixer, and rinsed with Kodak Photo Flo, then digitally scanned. The images were compiled and formatted using Adobe Photoshop® CS2.
3.4. RNAi
Synchronized L1-stage worms were grown on NGM until the L4-stage when females were transferred to RNAi plates for two days at 24°C, as described for feeding RNAi (Kamath & Ahringer, 2003). The rab-5 and cgh-1 RNAi clones were obtained from the Ahringer RNAi library (Kamath & Ahringer, 2003). Controls for RNAi experiments included gfp(RNAi) and the empty “feeding” vector L4440. All RNAi clones were sequenced to confirm the gene identities.
3.5. Immunofluorescence
The anti-CGH-1 antibody was a gift from Dr. David Greenstein, and used at 1:5000 with fixation conditions as described (M. C. Jud et al., 2008). The secondary antibodies were from Molecular Probes and used at 1:200.
4. Acknowledgements
The authors thank Dr. Jamie Alan for reagents, Phil Oshel, Andrea Montalbano, and members of the Schisa lab for advice and support. Some strains were provided by the Caenorhabditis Genetics Center which is funded by NIH Office of research infrastructure programs, P40 OD010440. Funding for this work was provided in part by NIH 2R15GM109337 and NSF MRI1531277.
Grant numbers: NIH 2R15GM109337 and NSF MRI1531277
5. References
- Alberti S, & Carra S (2018). Quality Control of Membraneless Organelles. J Mol Biol, 430(23), 4711–4729. doi: 10.1016/j.jmb.2018.05.013 [DOI] [PubMed] [Google Scholar]
- Arur S, Ohmachi M, Nayak S, Hayes M, Miranda A, Hay A, … Schedl T (2009). Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc Natl Acad Sci U S A, 106(12), 4776–4781. doi: 10.1073/pnas.0812285106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag PR, Nakamura A, & Blackwell TK (2005). A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development, 132(22), 4975–4986. doi:dev.02060 [pii] 10.1242/dev.02060 [DOI] [PubMed] [Google Scholar]
- Bobinnec Y, Marcaillou C, Morin X, & Debec A (2003). Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil Cytoskeleton, 54(3), 217–225. doi: 10.1002/cm.10094 [DOI] [PubMed] [Google Scholar]
- Bonner MK, Han BH, & Skop A (2013). Profiling of the mammalian mitotic spindle proteome reveals an ER protein, OSTD-1, as being necessary for cell division and ER morphology. PLoS One, 8(10), e77051. doi: 10.1371/journal.pone.0077051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics, 77(1), 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, & Zerial M (1992). The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell, 70(5), 715–728. [DOI] [PubMed] [Google Scholar]
- Grant B, & Hirsh D (1999). Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell, 10(12), 4311–4326. doi: 10.1091/mbc.10.12.4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, … Greenstein D (1999). Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol, 212(1), 101–123. doi: 10.1006/dbio.1999.9356 [DOI] [PubMed] [Google Scholar]
- Harris JE, Govindan JA, Yamamoto I, Schwartz J, Kaverina I, & Greenstein D (2006). Major sperm protein signaling promotes oocyte microtubule reorganization prior to fertilization in Caenorhabditis elegans. Dev Biol, 299(1), 105–121. doi:S0012-1606(06)00995-X [pii] 10.1016/j.ydbio.2006.07.013 [DOI] [PubMed] [Google Scholar]
- Hubstenberger A, Noble SL, Cameron C, & Evans TC (2013). Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev Cell, 27(2), 161–173. doi: 10.1016/j.devcel.2013.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud M, Razelun J, Bickel J, Czerwinski M, & Schisa JA (2007). Conservation of large foci formation in arrested oocytes of Caenorhabditis nematodes. Development genes and evolution, 217(3), 221–226. doi: 10.1007/s00427-006-0130-3 [DOI] [PubMed] [Google Scholar]
- Jud MC, Czerwinski MJ, Wood MP, Young RA, Gallo CM, Bickel JS, … Schisa JA (2008). Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev Biol, 318(1), 38–51. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, & Ahringer J (2003). Genome-wide RNAi screening in Caenorhabditis elegans. Methods, 30(4), 313–321. [DOI] [PubMed] [Google Scholar]
- Kessel RG (1989). The annulate lamellae--from obscurity to spotlight. Electron Microsc Rev, 2(2), 257–348. [DOI] [PubMed] [Google Scholar]
- Marnef A, Maldonado M, Bugaut A, Balasubramanian S, Kress M, Weil D, & Standart N (2010). Distinct functions of maternal and somatic Pat1 protein paralogs. RNA, 16(11), 2094–2107. doi: 10.1261/rna.2295410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro RE, Shim EY, Kohara Y, Singson A, & Blackwell TK (2001). cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development, 128(17), 3221–3232. [DOI] [PubMed] [Google Scholar]
- Patterson JR, Wood MP, & Schisa JA (2011). Assembly of RNP granules in stressed and aging oocytes requires nucleoporins and is coordinated with nuclear membrane blebbing. Developmental biology, 353(2), 173–185. doi: 10.1016/j.ydbio.2011.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JN, Schisa JA, & Priess JR (2000). P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Developmental biology, 219(2), 315–333. doi: 10.1006/dbio.2000.9607 [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Squirrell JM, Campbell JM, White JG, & Spang A (2005). Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans. Mol Biol Cell, 16(5), 2139–2153. doi: 10.1091/mbc.e04-08-0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol, 17, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T, & Kimble J (1988). fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics, 119(1), 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa JA, Pitt JN, & Priess JR (2001). Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development, 128(8), 1287–1298. [DOI] [PubMed] [Google Scholar]
- Spurr AR (1969). A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res, 26(1), 31–43. [DOI] [PubMed] [Google Scholar]
- Squirrell JM, Eggers ZT, Luedke N, Saari B, Grimson A, Lyons GE, … White JG (2006). CAR-1, a protein that localizes with the mRNA decapping component DCAP-1, is required for cytokinesis and ER organization in Caenorhabditis elegans embryos. Mol Biol Cell, 17(1), 336–344. doi: 10.1091/mbc.e05-09-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Buszczak M, & Sayles S (2005). Efficient protein trafficking requires trailer hitch, a component of a ribonucleoprotein complex localized to the ER in Drosophila. Dev Cell, 9(5), 675–685. doi: [DOI] [PubMed] [Google Scholar]
- Wood MP, Hollis A, Severance AL, Karrick ML, & Schisa JA (2016). RNAi Screen Identifies Novel Regulators of RNP Granules in the Caenorhabditis elegans Germ Line. G3 (Bethesda), 6(8), 2643–2654. doi: 10.1534/g3.116.031559 [DOI] [PMC free article] [PubMed] [Google Scholar]


