Abstract
HIF-1α is a hypoxia-inducible protein that regulates many cellular processes, including neural stem cell maintenance. Previous work demonstrated constitutive stabilization of HIF-1α in neural stem cells (NSCs) of the adult mouse subventricular zone (SVZ) and hippocampal subgranular zone (SGZ). Genetic inactivation of NSC-encoded HIF-1α in the adult SVZ results in gradual loss of NSCs, but whether HIF-1α is required for the maintenance of SGZ hippocampal progenitors and adult hippocampal neurogenesis has not been determined. Here we tested the hypothesis that HIF-1α plays an essential role in the maintenance of adult hippocampal neurogenesis using Nestin-CreERT2/R26R-YFP/Hif1afl/fl triple transgenic mice, in which HIF-1α was genetically inactivated in nestin+ hippocampal progenitors and their downstream progeny following tamoxifen exposure. We found that disruption of HIF-1α gene expression resulted in a marked 50% reduction of adult-generated dentate granule cells (DGCs) that was highly correlated with impaired hippocampal function, as assessed using two behavioral assays of pattern discrimination. These behavioral tests included the A-B contextual fear-conditioning task and the trial-unique, delayed nonmatching-to-location (TUNL) touch-screen operant chamber task. Our findings identify HIF-1α as a novel regulator of adult hippocampal neurogenesis under non-pathological conditions, and underscore the importance of neurogenesis for pattern discrimination learning.
Keywords: adult neurogenesis, dentate granule cells, contextual fear-discrimination learning, touch-screen operant chamber
1. INTRODUCTION
The subgranular zone (SGZ) of the hippocampal dentate gyrus harbors neural progenitor cells that give rise to new dentate granule cells (DGCs) throughout life in most mammalian species studied to date (Boldrini et al., 2018; Spalding et al., 2013). In rodent models, newly generated DGCs are critical for many hippocampal-dependent behaviors, including certain forms of cognition and cognitive flexibility (Anacker & Hen, 2017; Clelland et al., 2009; Danielson et al., 2016; Denny, Burghardt, Schachter, Hen, & Drew, 2012; Nakashiba et al., 2012; Swan et al., 2014), anxiety, mood and stress resilience (Anacker et al., 2018; Hill, Sahay, & Hen, 2015; Santarelli et al., 2003; Snyder, Soumier, Brewer, Pickel, & Cameron, 2011; Surget et al., 2011). The rate of adult hippocampal neurogenesis is dynamically regulated by many factors, including environment, genetics, drugs and behavior (Christian, Song, & Ming, 2014). Adult hippocampal neurogenesis declines dramatically with age in both rodents and humans (Spalding et al., 2013), and has been implicated as a potential therapeutic target for mitigating cognitive decline and behavioral dysregulation in a growing number of brain pathologies (Braun & Jessberger, 2014). Although much is known regarding the cellular and molecular mechanisms that govern the production and circuit integration of adult-generated DGCs, many questions remain, particularly regarding mechanisms required for maintenance of progenitors and ongoing neurogenesis throughout life.
Among the many intrinsic and extrinsic molecular cues that regulate the production of newborn neurons from progenitor cells in the adult CNS, recent studies in experimental models have demonstrated a critical role for cellular metabolism (Knobloch & Jessberger, 2017). Hypoxic signaling plays an important role in stem cell maintenance and function in development and disease (Panchision, 2009). HIF-1α is a transcription factor best known for its role in the regulation of metabolic and angiogenic responses to hypoxia. Although HIF-1α normally undergoes proteosomal degradation under nonhypoxic conditions (Semenza, 2004), it is constitutively stabilized in multiple stem cell types in vivo, including bone marrow hematopoietic stem cells (Nombela-Arrieta et al., 2013), mesenchymal stem cells (Palomaki et al., 2013) and neural stem/progenitor cells (NSPCs) of both the adult subventricular zone (SVZ) and hippocampal SGZ (Roitbak, Surviladze, & Cunningham, 2011). Both embryonic and adult NSPCs are highly dependent upon glycolytic vs. oxidative metabolism for survival (Candelario, Shuttleworth, & Cunningham, 2013), and maintain close physical and functional association with specialized microvasculature within the neurogenic niches of the adult CNS (Kirby, Kuwahara, Messer, & Wyss-Coray, 2015; Licht & Keshet, 2015; Shen et al., 2008; Sun & Guo, 2005). Importantly, HIF-1α functions in many cell types to reprogram cellular metabolism to promote glycolysis and to repress oxidative metabolism (Majmundar, Wong, & Simon, 2010). Furthermore, HIF-1α activates vascular endothelial factor (VEGF) and other angiogenic growth factors that may function to maintain microvasculature within the neurogenic niche (Lange et al., 2016). Indeed, we previously demonstrated that NSPC-encoded HIF-1α is required for NSC maintenance and vascular stability in the adult mouse SVZ (Li et al., 2014), but whether NSPCencoded HIF-1α is required for adult hippocampal neurogenesis has not been determined.
To ascertain whether NSPC-encoded HIF-1α is required for optimal adult hippocampal neurogenesis, we utilized Nestin-CreERT2/R26R-YFP/Hif1afl/fl triple transgenic mice, in which tamoxifen administration abolishes HIF-1α transcriptional activity within nestin+ NSCs and their downstream progeny via Cre-mediated excision of the floxed Hif1a exon 2, with concomitant expression of a yellow fluorescence protein (YFP) reporter, as previously described (Li et al., 2014). In addition, we utilized impaired pattern discrimination learning in either contextual and/or spatial domains as potential behavioral readouts for impaired neurogenesis. Specifically, we utilized the A-B contextual fear discrimination paradigm, previously demonstrated to be dependent upon adult hippocampal neurogenesis (Gustus et al., 2018; Kheirbek, Tannenholz, & Hen, 2012; McHugh et al., 2007; Niibori et al., 2012; Sahay, Scobie, et al., 2011; Tronel et al., 2012), and a more recently developed hippocampal-dependent novel trial-unique nonmatching- to-location (TUNL) paradigm using a touch-screen operant chamber (Josey & Brigman, 2015). Our findings demonstrate impaired adult hippocampal neurogenesis following genetic activation of NSPC-encoded HIF-1α, which is directly correlated with impaired discrimination learning.
2. MATERIALS and METHODS
2.1. Animals
Animal protocols were approved by the University of New Mexico HSC Institutional Animal Care and Use Committee, in accordance with the NIH Guide for the Care and use of Laboratory animals. Nestin-CreERT2/R26R-YFP/Hif1afl/fl (HIF-1α ciKO) triple transgenic mice and nestinCreERT2/R26R-YFP/Hif1awt/wt (HIF-1α WT) control mice were maintained at homozygosity for all three alleles as previously described (Li et al., 2014). Original strains comprised C57BL/6Tg(Nes-cre/ERT2)KEisc/J (Lagace et al., 2007) and B6.129-Hif1atm3Rsjo/J (Ryan et al., 2000) (The Jackson Laboratory, Bar Harbor, ME; stock #016261 and #007561, respectively). All mice were housed in a reverse 12-hour dark/12-hour light cycle (lights off at 8:00 hours). Only male mice were used for the current study. Food and water were available ad libitum, unless undergoing supervised food restriction as described (Josey & Brigman, 2015).
2.2. Tamoxifen Administration
Tamoxifen dissolved in 10%EtOH/90% sunflower seed oil (Sigma-Aldrich, St. Louis, MO) was administered to 7-to-8-week-old HIF-1α icKO and HIF-1α WT mice by single intraperitoneal injection (180 mg/kg) daily for 5 consecutive days. This tamoxifen regimen results in deletion of the floxed Hif1a exon 2 with abolishment of HIF-1α transcriptional activity in HIF-1α icKO mice, with concomitant induction of YFP reporter gene expression in adult nestin+ stem/progenitor cells and their progeny (Candelario et al., 2013; Lagace et al., 2007; Li et al., 2014). Tamoxifen administration to HIF-1α WT control mice results in YFP reporter gene expression only.
2.3. A-B Contextual Fear-Discrimination Task
Forty-five days following the final tamoxifen injection, HIF-1α WT (n=7) and HIF-1α icKO (n=6) mice were trained in the A-B contextual fear discrimination learning task, as modified from Sahay et al., (Sahay, Scobie, et al., 2011). Each mouse was individually placed into either Context A (foot shock) or Context B (no foot shock) for 90 s, followed immediately by foot shock (0.8 mA) for 2 s in Context A only. Following a second 90 s interval, mice in Context A received a second foot shock for 1 s. Mice in Context B received no shock or other aversive stimulus during the trial. Three hours following the first session, mice were exposed to a second identical training session, except that the mouse initially subjected to Context A (foot shock) was now subjected to Context B (no foot shock). This sequence was repeated once per day for 5 consecutive days, with the order of testing in Context A vs. Context B reversed every day for each individual mouse. Context A was a standard chamber with a stainless steel floor, clear Plexiglas front wall, and aluminum side and back walls. Context B was a similar chamber, except the floor was a wire mesh non-shock floor, with side and back walls covered in striped black and white contact paper. All mice were acclimated to the holding room (adjacent to the fear chamber testing room) for 2 hours 24 hours prior to Day 1 of training. Thereafter, mice were moved into the holding room and allowed to acclimate for 2 hours prior to training on each day. Chambers were thoroughly cleaned with 70% isopropyl alcohol between each session to remove scent contamination. All trials were recorded using a digital camera mounted above each chamber. Behavior was scored as time spent freezing during the full duration of the trial (observer blinded to treatment). A mean discrimination score was calculated as: (freezing score in Context A – freezing score in Context B)/(freezing score in Context A + freezing score in Context B) for each day. Higher discrimination scores indicated better contextual discrimination. All mice were sacrificed one week following the final training session for histological analysis of hippocampal neurogenesis as described below.
2.4. Trial Unique Nonmatching-to-Location (TUNL) Task
Forty-five days following the final tamoxifen injection, separate cohorts of HIF-1α WT (n=7) and HIF-1α icKO (n=7) mice were subjected to trial-unique, delayed nonmatching-to-location (TUNL) pattern discrimination training using a touch-screen paradigm using procedures identical to those recently described in detail (Josey & Brigman, 2015; Kenton, Castillo, Holmes, & Brigman, 2018). Mice were reduced and maintained at 85% free-feeding body weight through duration of testing. Prior to testing, mice were acclimated to both the testing room and the pellet food reward (14 mg pellet, BioServ, Felmington, NJ) as described by (Josey & Brigman, 2015). Mice were then habituated to the operant chamber and eating from the reward magazine by being placed in the chamber for 30 min with 10 pellets already available to them. Mice that retrieved all 10 pellets in under 30 min were moved to the pre-training regimen (3–5 days). For pretraining mice were required to lever press to receive a food reward. Mice that made 30 lever presses and retrieved 30 rewards in under 30 min were moved to touch training. All mice reached criteria within 2–3 days and there was no difference between genotypes in their time to criterion (training days to reach criterion for lever press: HIF-1αWT=2.0 + 0.2 and HIF-1α icKO=1.75 + 0.3, p>0.05). In touch training, the lever press resulted in the presentation of a white square stimulus in 1 of 10 response windows (2.5cm × 2.5cm; spatially pseudorandomized). Criterion for this stage was touching and retrieving 30 pellets within 30 min. All mice reached pre-training criterion within ~4–5 days of training, with no significant difference between genotypes (training days to reach criterion for touch pre-training: HIF-1αWT=4.3 + 0.6 and HIF-1α icKO=5.25 + 1.1, p>0.05)
After completing pre-training, mice were tested on the TUNL task, which was initially adapted from studies in the rat (Talpos, McTighe, Dias, Saksida, & Bussey, 2010). As in pretraining, mice lever pressed to initiate the onset of a trial. In the sample phase, 1 of the 10 squares (i.e., response location designated the sample stimulus) was illuminated. Mice were required to nose poke the illuminated sample square in order to complete the sample phase. To ensure motivation, thirty-three percent of sample phase responses were immediately rewarded with a food pellet. After a 1 second delay, mice were required to lever-press a second time, at which point the choice phase began. In choice phase, the previously touched sample stimulus was presented concomitant with illumination of a novel choice response location. A nose poke to the choice stimulus resulted in delivery of food pellet reward concomitant with illumination of the magazine light and a 1 sec tone. Five seconds following a correct trial, the mice were allowed to initiate a new trial by pressing the lever bar once more. Incorrect trials in which the sample stimulus was selected during the choice trial resulted in a 15 second house-light on time out. To aid learning and extinguish position bias, incorrect responses were followed by correction trials in which the same stimulus configurations were repeated until a correct response sequence was made. All subjects were tested for 48 presentation trials or for 1 hour, whichever was completed first, once per day. Mice were first tested for 5 consecutive days on a problem of the task in which there was a large separation between the sample and choice stimulus (3–4 spaces between stimuli, designated MAX). Next, the mice were tested for 5 consecutive days on a more difficult version of the task in which there was decreased separation between the sample and choice stimulus (2 spaces, designated FAR). Throughout testing, all 10 response locations were utilized and touches (nose-pokes) at non-illuminated windows during any phase had no response. One week following completion of the FAR version of the TUNL task, each mouse was sacrificed for histological assessment of hippocampal neurogenesis as described below.
The following dependent measures were taken during each phase of TUNL testing: Accuracy (correct responses/first presentation trials attempted), Correction Trials, Sample Phase Stimulus Response (time from trial initiation to touch-screen response), Choice Phase Stimulus Response (time from trial initiation of choice phase to touch-screen response), and Reward Response (time from correct choice phase touch to reward retrieval). Main effects of session, genotype, separation and interactions were compared for all measures using analysis of variance (ANOVA) followed by Fisher’s PLSD post hoc tests.
2.5. Histology
Mice were injected with an overdose of sodium pentobarbital (150 mg/kg, i.p.; Fort Dodge Animal Health, Fort Dodge, IA), and perfused transcardially with 4% paraformaldehyde (PFA) in 0.1M phosphate-buffered saline (PBS) as previously described (Kajimoto, Allan, & Cunningham, 2013). Brains were removed and post-fixed in 4% PFA overnight, followed by cryoprotection in 30% sucrose (w/v) in 0.1 M PBS at 4oC. The right hemisphere was then sectioned at 30 μm thickness in the coronal plane using a freezing sliding knife microtome. For immunofluorescence staining, floating sections were permeabilized with 0.1% (v/v) Triton X100, blocked with 10% normal donkey serum, and incubated with primary antibodies at 4oC overnight. Primary antibodies and dilutions were as follows: mouse anti-NeuN (1:1000; Millipore, Temecula, CA), chicken anti-GFP (1:1000; Life Technologies, Eugene, OR). NeuN immunofluorescence was visualized using Cy3-conjugated donkey anti-mouse secondary antibody (1:250; Jackson ImmunoResearch Laboratories, West Grove, PA). YFP immunofluorescence was visualized using biotinylated donkey anti-chicken secondary antibody (1:1500 Jackson ImmunoResearch Laboratories) and Tyramide-Plus amplification (PerkinElmer Life Sciences, boston, MA) as previously described (Lagace et al., 2007; Li et al., 2014; Li et al., 2010). Histological sections were mounted onto glass slides and coverslipped with Fluoromount G® mounting media (Electron Microscopy Sciences, Hatfield, PA)
2.6. Stereology
Cell counting:
The number of YFP+/NeuN+ co-labeled dentate granule neurons was estimated using Optical Fractionator Stereo Investigator™ software (MicroBrightField, Williston, VT) linked to an Olympus DSU spinning disk confocal microscope as described in (Kajimoto et al., 2013). The contour of the septal dentate gyrus was manually outlined in each individual section using a 20X objective with reference to consistent anatomical structures across each mouse. The optical dissector height was set at 18 μm, with a top and bottom guard zone height set at 2 μm. YFP+/NeuN+ cells were counted in 2–3 coronal sections within the septal hippocampus from each mouse using a 40X objective, with counting and grid frame sizes of 30 × 30 μm and 120 × 120 μm, respectively.
2.7. Statistical Analysis
Data are expressed as means ± SEM. Statistical comparisons were made using Student’s t-test, ANOVA and Pearson correlation, as indicated in text. p values < 0.05 were considered significant.
3. RESULTS
3.1. HIF-1α icKO mice display impaired learning in an A–B contextual fear-discrimination task
Contextual fear-discrimination learning is dependent upon the function of adult-generated DGCs (Danielson et al., 2016; Kheirbek et al., 2012; Sahay, Scobie, et al., 2011; Sahay, Wilson, & Hen, 2011; Tronel et al., 2012). Here, we tested whether deletion of HIF-1α from adult hippocampal progenitor cells leads to impaired learning in an A-B contextual fear-discrimination paradigm. HIF-1α WT and HIF-1α icKO mice were treated with tamoxifen for five consecutive days and tested on an A-B context discrimination task 45 days following the final tamoxifen injection (Fig. 1A). We chose 45 days following tamoxifen-induced recombination based on our previous work demonstrating gradual loss of neural stem cells within the SVZ of HIF-1α icKO mice, reaching ~50% depletion by 45 days following Hif1a gene inactivation (Li et al., 2014). All mice were subjected to two training sessions each day for five consecutive days, alternating between Context A (shock), and Context B (non-shock; Fig. 1B). HIF-1αWT and HIF-1α icKO mice displayed similar levels of freezing in each of the two contexts on day 1 of testing, indicating no effect of genotype on general fear response (Supplementary Figure 1). However, HIF-1α icKO mice displayed marked impairment in their ability to learn to discriminate between these contexts, as indicated by significantly lower discrimination scores by day 5 compared to HIF-1αWT mice (F(2.186, 24.04) =19.83, p<0.0001), genotype (F(1,11)=10.42, p=0.008; Holm-Sidak post-hoc comparison at Day 5, p=0.0004) (Fig. 1C).
Figure 1. HIF-1α icKO mice display impaired contextual fear discrimination learning.
A. Experimental timeline. Mice were treated for 5 consecutive days with tamoxifen to induce Cre-mediated recombination within nestin+ hippocampal progenitors. Forty-five days following the final tamoxifen injection, mice were tested on the A-B contextual fear conditioning task. Mice were sacrificed 7 days following the last day of behavioral testing for histological analysis. B. Exposures to context A (foot shock) and context B (no foot shock) were alternated daily for each mouse throughout the 5 days of behavioral testing. C. Context discrimination scores for HIF-1α WT (n=7) and HIF-1α icKO (n=6) mice on training days 1 through 5. Two-way ANOVA revealed significant effects of time (F(2.186, 24.04) =19.83, p<0.0001), genotype (F(1,11)=10.42, p=0.008). Interaction did not reach significance (F(4,44)=2.572, p=0.0508), **p=0.0004, Holm-Sidak post-hoc comparison.
3.2. Hippocampal neurogenesis is impaired HIF-1α icKO mice
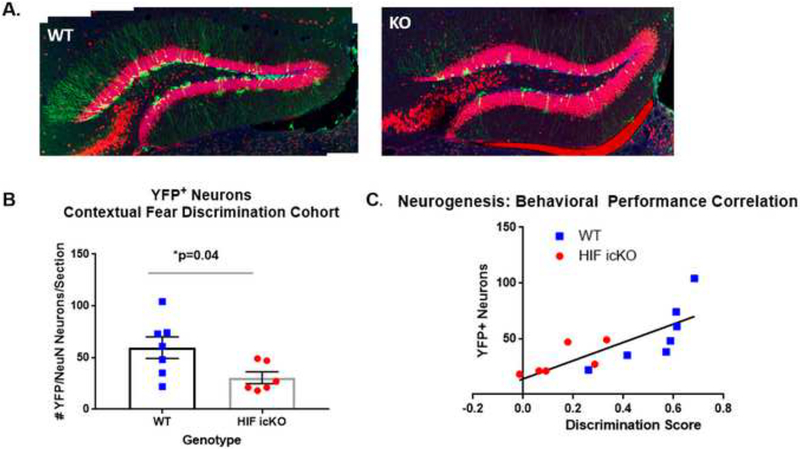
To determine whether impaired context discrimination learning in HIF-1α icKO mice was due to impaired production of adult-generated DGCs, we sacrificed mice 7 days following behavioral +/YFP+ co-labeled cells (~7 weeks post testing and performed stereological cell counts of NeuN recombination). As shown in Fig. 2A, YFP reporter was robustly expressed in tamoxifen-treated mice from both genotypes, but with many fewer YFP+ cells in HIF-1α icKO mice. Stereological assessment revealed a marked 50% reduction in the number of newly generated YFP+/NeuN+ DGCs in HIF-1α icKO mice compared to controls (59.57 + 10.37 vs. 30.5 + 5.67 YFP+/NeuN+ DGCs/section in HIF-1αWT vs. HIF-1α icKO mice, respectively; p=0.04). Importantly, the number of YFP+ neurons in each mouse was directly correlated with the level of performance on the A-B contextual fear-discrimination task at training day 5 (R2=0.615, p=0.002; Fig. 2C).
Figure 2. HIF-1α icKO mice display impaired adult hippocampal neurogenesis.
A. High resolution confocal images of hippocampal dentate gyrus from HIF-1α WT (WT) and HIF-1α icKO (KO) mice at 7 days following behavioral testing on the A-B contextual fear discrimination task. Sections were immunostained for YFP (green) and NeuN (red). B. Quantification of the number of YFP/NeuN adultgenerated neurons in HIF-1α WT (WT) and HIF-1α icKO. *p=0.04 by Student’s unpaired t-test, n= 6–7 mice/group). C. Correlation analysis of #YFP adult-generated neurons as a function of discrimination score for each mouse. R2=0.615, p<0.002.
3.3. HIF-1α icKO mice display impaired performance on a touch-screen pattern separation learning task.
To determine whether impaired pattern discrimination in HIF-1α icKO extends to other behavioral tasks, we tested pattern separation using a TUNL touch-screen paradigm (Josey & Brigman, 2015). The TUNL task tests working memory, and has been previously shown to be hippocampal-dependent (Josey 2015). Previous studies using a similar, albeit less challenging touch-screen pattern discrimination paradigm, have demonstrated reliance on adult hippocampal neurogenesis (Clelland et al., 2009).
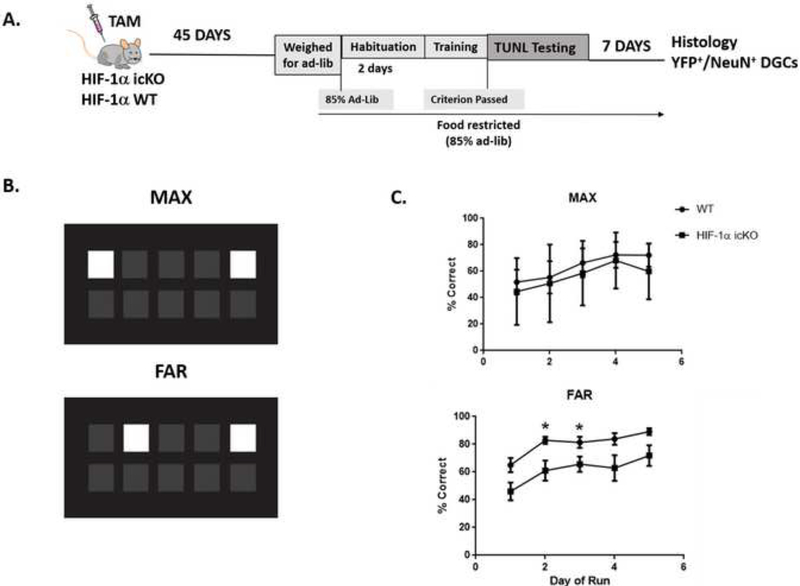
For these experiments, we treated new cohorts of HIF-1α WT and HIF-1α icKO mice with tamoxifen for five consecutive days, followed by touch-screen training at 45 days after the final tamoxifen injection (Fig. 3A). Mice were placed on food-restricted diets to ensure motivation, and were subsequently trained to perform the TUNL task using predetermined stage-dependent performance criterion to determine progression as previously described (Josey & Brigman, 2015). Mice were first tested on the simplest task, with maximum separation between sample and choice stimuli (Fig. 3B). Over the course of five days, the performance of HIF-1α WT and HIF-1α icKO mice were not statistically different on this maximal (MAX) separation (Fig. 3C). Next, the mice were tested for increasing difficulty by decreasing the separation between sample and choice stimuli by one space (FAR separation; Fig. 3B). Two-way ANOVA revealed significant effects of time (F(4,52)=7.587, p<0.0001) and genotype (F(1,13)=13.52, p=0.0028), but no significant interaction for performance on the FAR separation test.
Figure 3. HIF-1α icKO mice display impaired performance on TUNL touch screen pattern separation task.
A. Experimental timeline. Mice were treated for 5 consecutive days with tamoxifen to induce Cre-mediated recombination within nestin+ hippocampal progenitors. Forty-five days following the final tamoxifen injection, mice began habituation and training on the TUNL pattern separation task using a touchscreen paradigm. All mice were sacrificed seven days following the final day of behavioral testing. B. Cartoon depiction of computer touch screen depicting an example of lighted squares during testing for discrimination of maximally separated squares (MAX) vs discrimination of pattern in which lighted squares are closer together (FAR). C. Percent correct choices for mice tested for pattern separation with MAX vs. FAR touchscreen patterns over 5 consecutive days. Two-way ANOVA revealed significant effects of time (F(4,52)=7.587, p<0.0001), genotype (F(1,13)=13.52, p=0.0028) with no significant interaction for performance on the FAR separation test. There was no significant effects of genotype in performance of MAX separation TUNL test.
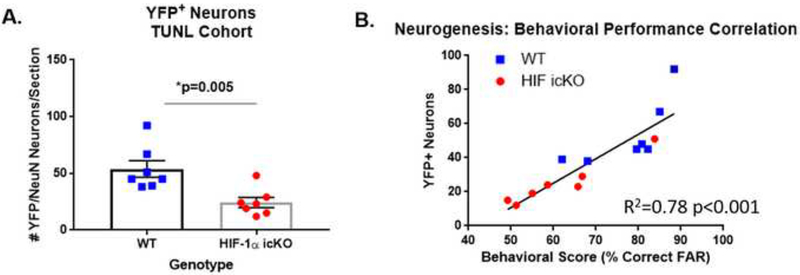
To confirm impaired neurogenesis in HIF-1α icKO mice in this second cohort of mice, we sacrificed all mice at 7 days after their final day of behavioral testing and assessed the number of YFP+ DGCs as described above. As shown in Fig. 4A, HIF-1α icKO mice displayed an ~50% reduction in the number of newly generated YFP+/NeuN+ DGCs, as in the previous cohort (53.86 + 7.34 vs. 24.29 + 4.50 YFP+/NeuN+ DGCs/section in HIF-1αWT vs. HIF-1α icKO mice, respectively; p=0.005). As with the context discrimination, the number of YFP+ neurons in each mouse was highly correlated with the level of performance on the TUNL pattern separation task as assessed at training day 3 (R2=0.78, p<0.001; Fig. 4B).
Figure 4. Impaired performance on TUNL pattern separation is correlated with impaired neurogenesis in HIF-1a icKO mice.
A. Quantification of the number of YFP/NeuN adult-generated neurons in HIF-1α WT (WT) and HIF-1α icKO as assessed 7 days following the final TUNL testing day. *p=0.005 by Student’s unpaired t-test, n=7/group. B. Correlation analysis of TUNL performance with #YFP adult-generated DGCs. R2=0.78, p<0.001.
4. DISCUSSION
In the present study, we demonstrate impaired context and spatial discrimination learning associated with impaired adult hippocampal neurogenesis following selective and inducible HIF1α gene deletion in adult hippocampal progenitor cells and their downstream progeny. Genetic inactivation of NSC-encoded HIF-1α resulted in a marked 50% decrease in the number of adult-generated DGCs by 45 days post-recombination, which was highly correlated with impaired performance on pattern discrimination tasks in both contextual and spatial domains. These results demonstrate that NSC-encoded HIF-1α is required for the maintenance of adult hippocampal neurogenesis, and further support a critical role for adult-generated DGCs in pattern discrimination, as previously documented by others (Clelland et al., 2009; Danielson et al., 2016; Kheirbek et al., 2012; Sahay, Scobie, et al., 2011; Sahay, Wilson, et al., 2011; Tronel et al., 2012). Thus, HIF-1α expression is critical in maintaining neurogenic processes necessary for pattern discrimination learning in adult mouse brain.
Genetic inactivation of HIF-1α in adult hippocampal progenitors resulted in pronounced impairment in discrimination learning as assessed by the A-B contextual fear-discrimination learning task, where average discrimination scores in HIF-1α icKO mice were decreased by >50% compared to controls. Importantly, impaired performance on the A-B contextual fear-discrimination task was highly correlated with impaired hippocampal neurogenesis in HIF-1α icKO mice, as assessed by quantification of newborn YFP+ DGCs. This correlation suggests that it is the reduction in the number of adult-generated DGCs rather than loss of HIF-1α per se, that underlies impaired learning in this task. Contextual fear conditioning is a widely used hippocampal-dependent task strongly influenced by adult hippocampal neurogenesis. Loss of neurogenesis functions following either x-irradiation or genetic ablation of adult hippocampal progenitors (Saxe et al., 2006), or following silencing of newborn DGCs via genetic or optogenetic methods (Gu et al., 2012; Gustus et al., 2018; Huckleberry et al., 2018), results in impaired contextual fear-discrimination learning. Conversely, increasing adult hippocampal neurogenesis by genetic inhibition of apoptosis improves performance on this task (Sahay, Scobie, et al., 2011). Adult-generated DGCs are thought to contribute to hippocampal function both by encoding new information and by modifying dentate activity (Anacker & Hen, 2017). New DGCs undergo a period of enhanced excitability and plasticity at approximately 4–6 weeks of cellular age, during which they uniquely contribute to hippocampal function and potentiate the ability of the dentate gyrus to decorrelate neural inputs for optimal pattern separation (Denny et al., 2012; Gu et al., 2012).
As with the contextual fear-discrimination, we found that impaired discrimination learning in the TUNL task was also highly correlated with impaired neurogenesis in HIF-1α icKO mice. The touch-screen TUNL task involves rigorous testing of visual-spatial discrimination by varying the distance between two stimuli in the touch-screen operant chamber in a trial-unique manner (Josey & Brigman, 2015). Seminal work by Clelland et al., (2009) initially demonstrated a functional role for adult hippocampal neurogenesis in spatial pattern separation using a touchscreen paradigm. In that study, spatial discrimination was impaired in neurogenesis-impaired mice only when target and distracter locations were proximal. Using a slightly different version of the same touch-screen operant task, Swan et al. (2014) further demonstrated that reversal learning is particularly sensitive to arrested hippocampal neurogenesis. Although others have demonstrated an important role for adult hippocampal neurogenesis in learning a navigable delayed non-matching-to-place radial arm maze task (Clelland et al., 2009; Lemaire et al., 2012), our study demonstrates that adult hippocampal neurogenesis is also required for TUNL touchscreen operant learning, which also requires working spatial memory. Although HIF-1α icKO mice performed normally on the TUNL task under the least demanding conditions (MAX separation of stimuli at 3–4 spaces apart), performance was significantly impaired when the separation was reduced (FAR separation of 2 spaces apart). Previous work has demonstrated that performance on this task is also impaired in mice with complete lesion of the dorsal hippocampus (Josey & Brigman, 2015) and following selective inactivation of the GluN2B Nmethyl-D-aspartate (NMDA) receptor subunit in cortical and hippocampal CA1 principle neurons in mice (Kenton et al., 2018). Our studies demonstrate that performance on this task is also correlated with the number of adult-generated DGCs.
The mechanisms by which inactivation of NSC-encoded HIF-1α impairs adult hippocampal neurogenesis have yet to be elucidated, but may involve diminishment of the stem cell pool, and/or impaired survival of newly-generated DGCs through cell-autonomous and/or paracrine mechanisms. In the present study, we found that inactivation of NSC-encoded HIF-1α resulted in a 50% reduction in the number of adult-generated DGCs within 45 days following tamoxifen-induced recombination. Utilizing the same strain of HIF-1α icKO mice, we previously demonstrated a significant depletion of YFP+ NSCs within the SVZ by 45 days post-recombination, concomitant with decreased VEGF expression and preceded by significant regression of the SVZ vasculature (Li et al., 2014). The importance of healthy vasculature for maintenance of NSCs within the SVZ has been well-documented (Apple & Kokovay, 2017; Kokovay et al., 2010; Shen et al., 2004; Shen et al., 2008; Tavazoie et al., 2008), but much less is known about the role of the vasculature in the maintenance of NSCs neurogenesis in the adult hippocampus. HIF-1α is an important transcriptional driver of VEGF and other angiogenic factors (Simon & Keith, 2008). Recent studies have demonstrated that NSC-encoded VEGF is necessary for NSC maintenance within the SGZ of the adult hippocampus (Kirby et al., 2015), and may promote vascular-NSC paracrine communication important for neurogenesis within the adult hippocampal dentate gyrus (Licht & Keshet, 2015; Licht et al., 2016; Sun & Guo, 2005). Previous studies have also implicated HIF-1α in the regulation of neural stem cell function via modulation of Wnt/β-catenin activity, which can alter NSC proliferation, differentiation and neuronal maturation (Mazumdar et al., 2010). It is also important to note that although we utilized hippocampal-dependent discrimination learning tasks as a behavioral readout of impaired hippocampal neurogenesis in this study, we cannot rule out the possibility that HIF-1α gene deletion within the other nestin+ progenitor cells within the SVZ or hypothalamic neurogenic zones of adult mouse brain may have contributed to the behavioral impairments.
In sum, our findings support a novel role for NSC-encoded HIF-1α in the maintenance of adult hippocampal neurogenesis in mice, and further support a role for neurogenesis in pattern discrimination learning in both contextual and spatial domains. It will be important in future studies to determine whether HIF-1α dysregulation leading to impaired neurogenesis may contribute to age-related or pathology-related cognitive decline, and the molecular mechanisms by which NSC-encoded HIF-1α promotes neurogenesis under non-pathological conditions. Ultimately, small molecule regulators that target HIF-1α may be useful in therapeutic targeting of adult hippocampal neurogenesis for improved hippocampal function.
Supplementary Material
Highlights:
Genetic inactivation of NSPC-encoded HIF-1α impairs hippocampal neurogenesis
Genetic inactivation of NSPC-encoded HIF-1α impairs pattern discrimination.
Direct correlation between learning performance and numbers of newborn hippocampal DGCs
HIF-1α is a novel regulator of adult hippocampal neurogenesis.
Findings underscore the importance of neurogenesis for pattern discrimination learning.
7. ACKNOWLEDGMENTS
Confocal images in this article were generated in the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on: http://hsc.unm.edu/crtc/microscopy/acknowledgement.shtml. We thank Ms. Lilly Lu for mouse illustration.
6. FUNDING SOURCES
This research was supported by NIH-NIGMS (R25-GM60201), NIH-NIGMS (1P20 GM109089), NIH-NIAAA (1F31AA027127) and (P50-AA022534), American Heart Association (09GRNT2290178).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. CONFLICT OF INTEREST STATEMENT
The authors have no affiliations with or involvement in any entity with financial interest or nonfinancial interest in the subject matter or materials in this article.
5. REFERENCES
- Anacker C, & Hen R (2017). Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci, 18(6), 335–346. doi: 10.1038/nrn.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, … Hen R (2018). Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature, 559(7712), 98–102. doi: 10.1038/s41586-018-0262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple DM, & Kokovay E (2017). Vascular niche contribution to age-associated neural stem cell dysfunction. Am J Physiol Heart Circ Physiol, 313(5), H896–H902. doi: 10.1152/ajpheart.00154.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, … Mann JJ (2018). Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell, 22(4), 589–599 e585. doi: 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun SM, & Jessberger S (2014). Adult neurogenesis and its role in neuropsychiatric disease, brain repair and normal brain function. Neuropathology and applied neurobiology, 40(1), 3–12. doi: 10.1111/nan.12107 [DOI] [PubMed] [Google Scholar]
- Candelario KM, Shuttleworth CW, & Cunningham LA (2013). Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1alpha expression. Journal of neurochemistry, 125(3), 420–429. doi: 10.1111/jnc.12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Song H, & Ming GL (2014). Functions and dysfunctions of adult hippocampal neurogenesis. Annual review of neuroscience, 37, 243–262. doi: 10.1146/annurev-neuro-071013014134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P,… Bussey TJ (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 325(5937), 210–213. doi: 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, … Kheirbek MA (2016). Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron, 90(1), 101–112. doi: 10.1016/j.neuron.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, & Drew MR (2012). 4- to 6-week-old adultborn hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus, 22(5), 1188–1201. doi: 10.1002/hipo.20964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, & Ge S (2012). Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nature neuroscience, 15(12), 1700–1706. doi: 10.1038/nn.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustus KC, Li L, Chander P, Weick JP, Wilson MC, & Cunningham LA (2018). Genetic inactivation of synaptosomal-associated protein 25 (SNAP-25) in adult hippocampal neural progenitors impairs pattern discrimination learning but not survival or structural maturation of newborn dentate granule cells. Hippocampus. doi: 10.1002/hipo.23008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, & Hen R (2015). Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology, 40(10), 2368–2378. doi: 10.1038/npp.2015.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckleberry KA, Shue F, Copeland T, Chitwood RA, Yin W, & Drew MR (2018). Dorsal and ventral hippocampal adult-born neurons contribute to context fear memory. Neuropsychopharmacology. doi: 10.1038/s41386-018-0109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josey M, & Brigman JL (2015). Loss of hippocampal function impairs pattern separation on a mouse touch-screen operant paradigm. Neurobiology of learning and memory, 125, 85–92. doi: 10.1016/j.nlm.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto K, Allan A, & Cunningham LA (2013). Fate analysis of adult hippocampal progenitors in a murine model of fetal alcohol spectrum disorder (FASD). PLoS ONE, 8(9), e73788. doi: 10.1371/journal.pone.0073788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton JA, Castillo R, Holmes A, & Brigman JL (2018). Cortico-hippocampal GluN2B is essential for efficient visual-spatial discrimination learning in a touchscreen paradigm. Neurobiol Learn Mem, 156, 60–67. doi: 10.1016/j.nlm.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, & Hen R (2012). NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(25), 8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ED, Kuwahara AA, Messer RL, & Wyss-Coray T (2015). Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci U S A, 112(13), 4128–4133. doi: 10.1073/pnas.1422448112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, & Jessberger S (2017). Metabolism and neurogenesis. Curr Opin Neurobiol, 42, 45–52. doi: 10.1016/j.conb.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, … Temple S (2010). Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell, 7(2), 163–173. doi:S1934–5909(10)00279–1 [pii] 10.1016/j.stem.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, … Eisch AJ (2007). Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci, 27(46), 12623–12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, Quaegebeur A, … Carmeliet P (2016). Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. The EMBO journal, 35(9), 924–941. doi: 10.15252/embj.201592372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Tronel S, Montaron MF, Fabre A, Dugast E, & Abrous DN (2012). Long-lasting plasticity of hippocampal adult-born neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(9), 3101–3108. doi: 10.1523/JNEUROSCI.4731-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Candelario KM, Thomas K, Wang R, Wright K, Messier A, & Cunningham LA (2014). Hypoxia inducible factor-1alpha (HIF-1alpha) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ. The Journal of neuroscience : the official journal of the Society for Neuroscience, 34(50), 16713–16719. doi: 10.1523/JNEUROSCI.4590-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, & Cunningham LA (2010). Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia, 58(13), 1610–1619. doi: 10.1002/glia.21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, & Keshet E (2015). The vascular niche in adult neurogenesis. Mech Dev, 138 Pt 1, 56–62.doi: 10.1016/j.mod.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Licht T, Rothe G, Kreisel T, Wolf B, Benny O, Rooney AG, … Keshet E (2016). VEGF preconditioning leads to stem cell remodeling and attenuates age-related decay of adult hippocampal neurogenesis. Proc Natl Acad Sci U S A, 113(48), E7828–E7836. doi: 10.1073/pnas.1609592113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, & Simon MC (2010). Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell, 40(2), 294–309. doi:S1097–2765(10)00750–1 [pii] 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, & Simon MC (2010). O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol, 12(10), 10071013. doi:ncb2102 [pii] 10.1038/ncb2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, … Tonegawa S (2007). Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science, 317(5834), 94–99. doi: 10.1126/science.1140263 [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, … Tonegawa S (2012). Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell, 149(1), 188–201. doi: 10.1016/j.cell.2012.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niibori Y, Yu TS, Epp JR, Akers KG, Josselyn SA, & Frankland PW (2012). Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun, 3, 1253. doi: 10.1038/ncomms2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, … Silberstein LE (2013). Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature cell biology, 15(5), 533–543. doi: 10.1038/ncb2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomaki S, Pietila M, Laitinen S, Pesala J, Sormunen R, Lehenkari P, & Koivunen P (2013). HIF1alpha is Upregulated in Human Mesenchymal Stem Cells. Stem Cells. doi: 10.1002/stem.1435 [DOI] [PubMed] [Google Scholar]
- Panchision DM (2009). The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol, 220(3), 562–568. doi: 10.1002/jcp.21812 [DOI] [PubMed] [Google Scholar]
- Roitbak T, Surviladze Z, & Cunningham LA (2011). Continuous Expression of HIF-1alpha in Neural Stem/Progenitor Cells. Cell Mol Neurobiol, 31(1), 119–133. doi: 10.1007/s10571-010-9561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, & Johnson RS (2000). Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res, 60(15), 4010–4015. [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, … Hen R (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature, 472(7344), 466–470. doi: 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, & Hen R (2011). Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron, 70(4), 582–588. doi: 10.1016/j.neuron.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, … Arancio O (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science, 301(5634), 805–809. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, … Drew MR (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America, 103(46), 17501–17506. doi: 10.1073/pnas.0607207103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2004). Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda), 19, 176–182. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, … Temple S (2004). Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science, 304(5675), 13381340. doi: 10.1126/science.1095505 [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK,… Temple S (2008). Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell, 3(3), 289–300. doi: 10.1016/j.stem.2008.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MC, & Keith B (2008). The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol, 9(4), 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, & Cameron HA (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature, 476(7361), 458–461. doi: 10.1038/nature10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, … Frisen J (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell, 153(6), 1219–1227. doi: 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FY, & Guo X (2005). Molecular and cellular mechanisms of neuroprotection by vascular endothelial growth factor. J Neurosci Res, 79(1–2), 180–184. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, … Belzung C (2011). Antidepressants recruit new neurons to improve stress response regulation. Molecular Psychiatry, 16(12), 1177–1188. doi: 10.1038/mp.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, & Drew MR (2014). Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus, 24(12), 1581–1591. doi: 10.1002/hipo.22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, & Bussey TJ (2010). Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiology of learning and memory, 94(3), 341–352. doi: 10.1016/j.nlm.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, … Doetsch F (2008). A specialized vascular niche for adult neural stem cells. Cell Stem Cell, 3(3), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, & Abrous DN (2012). Adultborn neurons are necessary for extended contextual discrimination. Hippocampus, 22(2), 292298. doi: 10.1002/hipo.20895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.