Abstract
Since 2011, the Centers for Medicare and Medicaid Services has provided reimbursement for renal dialysis services furnished to Medicare beneficiaries through a bundled payment system known as the Prospective Payment System. Medications that have no injectable equivalent, known as “oral-only medications,” are currently excluded from the bundle and are paid separately through Medicare Part D. Thus, prior to the development of etelcalcetide, the first injectable calcimimetic, calcimimetics were reimbursed outside the bundle. Etelcalcetide’s introduction and approval for use in Medicare triggered a transition payment for a minimum of two years that will eventually result in the incorporation of calcimimetics into the dialysis bundle. Consequently, providers may face incentives to reduce calcimimetic use once the transition period has expired. The complexity of bone-mineral management in conjunction with the paucity of evidence-based recommendations in this area makes it difficult to predict the impact of this transition. Because these medications are expensive, a poor transition could have financial ramifications for dialysis organizations and, potentially, patient health. To ensure that patients are not adversely affected, it is critical that Medicare incorporate these medications into the bundle carefully, with close monitoring of outcomes.
Keywords: etelcalcetide, calcimimetic, bundle, transition, payment, bone-mineral metabolism, end-stage renal disease (ESRD), Prospective Payment System (PPS), parathyroid hormone (PTH), health care incentive, medical parathyroidectomy, drug costs, hemodialysis, dialysis clinic, reimbursement rate
Introduction
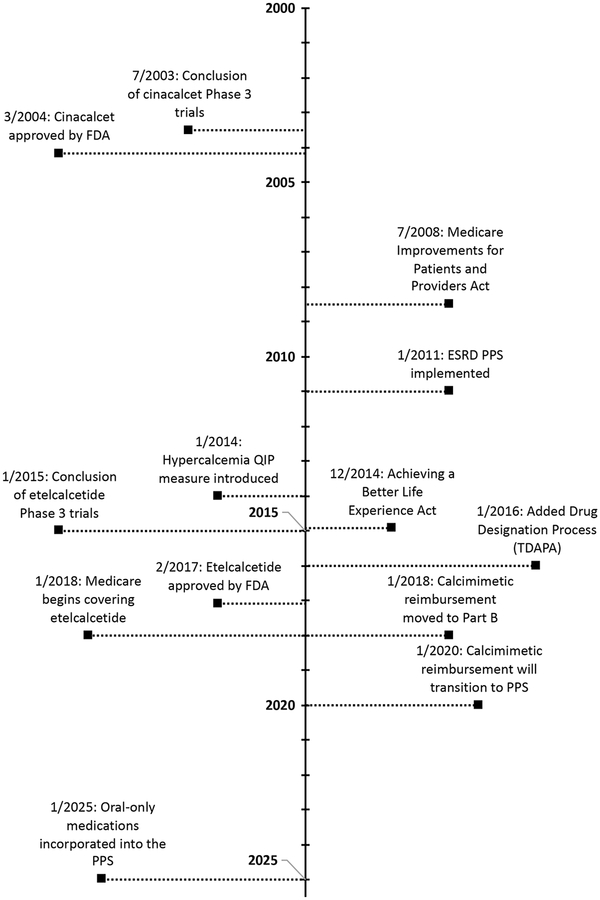
Etelcalcetide, the first injectable calcimimetic, was approved by the Food and Drug Administration (FDA) on February 7, 2017, for use in patients with end-stage renal disease (ESRD) receiving hemodialysis (Figure 1).1 Its introduction provided an intravenous alternative to cinacalcet for treating patients with bone-mineral metabolic derangements and elevated parathyroid hormone levels, particularly in patients with hypercalcemia.2–4 Because the Centers for Medicare and Medicaid Services (CMS), through the ESRD Prospective Payment System (PPS), “bundles” payment for injectable medications together with dialysis treatment,5 the emergence of etelcalcetide has important financial ramifications for dialysis organizations and the nephrology community at large.
Figure 1: Timeline of calcimimetic development and regulatory changes in Medicare reimbursement for dialysis services.
Abbreviations: FDA = Food and Drug Administration, ESRD = end-stage renal disease, PPS = Prospective Payment System, QIP = Quality Incentive Program, TDAPA = Transitional Drug Add-on Payment. Data for the figure come from the following references:1–3,19,43,44
Medicare Payment Prior To Etelcalcetide
The management of bone-mineral metabolism in ESRD often requires a combination of dietary changes, phosphorus binders, activated Vitamin D analogs, and/or calcimimetics.6,7 For patients with Medicare, the reimbursement is complex because medications with an injectable equivalent are covered under the PPS bundle while those without any injectable equivalent (oral-only) remain separately covered under Medicare Part D.5 The Medicare Improvements for Patients and Providers Act of 2008 mandated the creation of a PPS bundle that combined reimbursement for dialysis treatments with medications intended to treat ESRD.8 When the PPS was implemented in 2011, CMS originally planned incorporation of oral-only medications into the bundle by 2014, a policy that was intended to ease the transition from fee-for-service to bundled payments.5 Subsequently, the American Taxpayer Relief Act of 2012,9 the Protecting Access to Medicare Act of 2014,10 and the Achieving a Better Life Experience Act of 201411 delayed the incorporation of oral-only medications into the PPS until January 1, 2025. Thus, prior to the advent of etelcalcetide, phosphorus binders and calcimimetics (oralonly medications) were reimbursed separately by Part D, while activated Vitamin D was incorporated into the PPS (Table 1).
Table 1:
Drugs used to treat bone-mineral metabolism in ESRD and Medicare payment
| Drug | Class | Route of Administration | Reimbursement |
|---|---|---|---|
| Calcium Carbonate | Phosphorus Binder | Oral | Over the counter |
| Calcium Acetate | Phosphorus Binder | Oral | Part D (oral-only) |
| Sevelamer | Phosphorus Binder | Oral | Part D (oral-only) |
| Lanthanum | Phosphorus Binder | Oral | Part D (oral-only) |
| Aluminum Hydroxide | Phosphorus Binder | Oral | Part D (oral-only) |
| Calcitriol | Activated Vitamin D | Oral / IV | PPS bundle |
| Paricalcitol | Activated Vitamin D | Oral / IV | PPS bundle |
| Doxercalciferol | Activated Vitamin D | Oral / IV | PPS bundle |
| Cinacalcet | Calcimimetic | Oral | TDAPA |
| Etelcalcetide | Calcimimetic | IV | TDAPA |
Abbreviations: IV = intravenous, PPS = prospective payment system, TDAPA = transitional drug add-on payment; ESRD, end-stage renal disease
Notes: This list of phosphorus binders is not exhaustive but is meant to represent those most commonly prescribed. All phosphorus binders have an “oral-only” designation and are thus reimbursed outside the ESRD PPS bundle through Part D.
The PPS incentivizes dialysis providers to reduce the use of medications covered under the bundle, specifically those with injectable equivalents such as erythropoietin-stimulating agents (ESAs) and activated Vitamin D.5,12 For instance, after 2011, evidence has emerged suggesting that the PPS, in combination with the Food and Drug Administration’s (FDA’s) black box warnings, led dialysis providers to dramatically reduce their use of ESAs with no corresponding long-term increase in blood transfusions.13–15 More recent evidence has shown similar trends in activated Vitamin D use after implementation of the PPS.16 On the other hand, the bundle likely exerted no financial pressure to reduce the use of oral-only medications including cinacalcet. This complex interplay of incentives may lead providers to preferentially use certain bone-mineral medications over others. (In contrast, ESAs and iron, the two mainstays of anemia treatment, are covered by the PPS and subject to the same incentive structure). To the extent that cinacalcet can act as a partial substitute for Vitamin D, it is possible that the PPS may have even increased the use of cinacalcet by providers trying to reduce their own expenses in administering activated Vitamin D. While 23% of Part D–enrolled patients with ESRD received cinacalcet ($295.1 million) in 2011, the proportion receiving cinacalcet increased to 30% of Part D–enrolled patients in 2015 ($495.7 million).17
The incorporation of hypercalcemia as a clinical measure in the Quality Incentive Program (QIP) beginning in calendar year 2014 also may have incentivized cinacalcet use. The QIP financially penalizes providers who have a disproportionate number of patients with an uncorrected serum calcium of greater than 10.2 mg/dL, a measure endorsed by the National Quality Forum (NQF).18 Because calcimimetics can achieve reductions in parathyroid hormone (PTH) levels without causing hypercalcemia,19–24 it is likely that the QIP has partially driven substitution of activated Vitamin D by cinacalcet. Despite the implementation of these payment incentives, we still do not know the impact of changes in bone-mineral medication use on patient outcomes or whether the incentives created by the ESRD PPS and QIP align with improved care. Many in the nephrology community have indicated the need for additional research to establish evidence-based guidelines on the effective management of bone-mineral metabolism.6,25 Although observational data suggest that patients with hypercalcemia have worse outcomes,26–29 it is unclear whether and to what extent reducing calcium levels leads to improved outcomes in ESRD. Calcimimetic-associated hypocalcemia occurs frequently, though often is self-limited,30 and further research is required to understand whether current payment policy has led to unintended negative consequences.
Etelcalcetide And Changes To Calcimimetic Payment
The development of etelcalcetide introduced an injectable equivalent to cinacalcet, making calcimimetics no longer oral-only medications. Consequently, on January 1, 2018, calcimimetics furnished to patients with ESRD officially transitioned from Medicare Part D to Part B reimbursement.31 When a product is no longer an oralonly drug, CMS provides payment using a transitional drug add-on payment adjustment (TDAPA) for additional injectable or intravenous drugs and biological products.32,33 To be considered for this designation, the product must meet 4 criteria: the product must be 1) approved by the FDA, 2) commercially available, 3) assigned a Healthcare Common Procedure Coding System (HCPCS) code, and 4) designated by CMS as a “renal dialysis service”.
For drugs approved by the FDA before January 1, 2020, CMS will not provide a TDAPA payment over and above the bundle if the drug covers a functional category (such as anemia) already in the PPS. Although calcimimetics are included in the bone and mineral metabolism functional category, the 2016 ESRD PPS Final Rule (80 FR 69025, 29027) explicitly created an exception to the drug designation process for calcimimetics because inclusion of oral-only medications into the PPS has been delayed until 2025.33 Currently, calcimimetics are the only drugs that qualify for payment under TDAPA.
The TDAPA payment is applicable for a minimum of two years, during which time CMS will gather data to determine the appropriate increase into the PPS bundled payment rate. Many in the nephrology community have concerns that CMS will not have adequate data to accurately calculate the increase to the bundled payment, given that CMS may have access to less than 9 months of data to base their calculations during this two year period. Additionally, data gathered during the first few months may not be reflective of standardized operational mechanisms to supply calcimimetics to patients. One potential solution is requesting that CMS consider an extension to the TDAPA period, to gather adequate data and better calculate an appropriate bundled rate for the long-term.
With the implementation of the TDAPA payment in January 2018, dialysis organizations around the country have had to address operational challenges as they have become responsible for patients’ calcimimetics. Strategies have differed depending on a facility’s access to pharmacy services. Some dialysis organizations have converted wholly to intravenous administration, some have provided daily oral calcimimetics for administration at home, while others have provided oral calcimimetics thrice daily incenter. Some who provide oral calcimimetics offer the intravenous form as a second line agent.
Dialysis facilities have already faced fundamental challenges that may be beyond their control. Many physicians will opt to prescribe oral forms of calcimimetics (i.e., cinacalcet), which will require an upfront prescription of at least 30 days. Because CMS pays a prorated amount for calcimimetics supplied during a calendar month, facilities may face financial losses if their patients die, are hospitalized, switch dialysis organizations, or change medication dosages before the end of the calendar month.
For example, if a patient is given a 30-day supply of a calcimimetic on June 16, and dies on June 27, the dialysis facility will only be reimbursed for the rest of the supply prescribed for the calendar month of death, in this case June 16 to June 30. Any additional prescribed medication into subsequent months (i.e., July 1 to July 15) becomes a loss for the facility. This becomes a financial challenge when the medication is costly, like the calcimimetics. Anecdotal reports suggest that some dialysis organizations are trying to administer prescriptions for all calcimimetics at the beginning of the calendar month to avoid taking a loss, a practice which may not be ideal for patients who require a new prescription during the middle of the month.
CMS and the nephrology community should be prepared for changes in calcimimetic use during each phase of this transition. During the TDAPA period, dialysis facilities are being reimbursed for calcimimetics, and some can achieve profit by obtaining small margins. This introduces an incentive for providers to increase calcimimetic use, to the extent that calcimimetics are profitable. Because calcimimetic use during this time period will be used to project future use, dialysis providers might have an additional incentive to further increase calcimimetic prescriptions in an effort to increase the future overall base rate.
On the other hand, once the TDAPA period has expired, incentives will change, much as they did when ESA reimbursement converted from a separately billable item to a bundled expense on January 1, 2011. At that time, the community observed a marked decrease in ESA use.13 We might expect the same with calcimimetics. It is imperative that CMS carefully incorporate calcimimetics into the bundle with appropriate risk-adjustment, to ensure that facilities are not incentivized to risk-select (or “cherry-pick”) patients less likely to require calcimimetics.
Without risk adjustment, incorporating a medication into the bundle effectively takes its total cost to Medicare and divides the amount evenly among patients, a redistribution of payment. Risk adjustment ensures that the costs are not redistributed from those most likely to use the medication to those least likely. In the case of ESAs, this redistribution was not as pronounced because the vast majority of patients receive ESAs. Still, high-risk patients, such as those with hereditary hemolytic anemia, sickle cell anemia, and myelodysplastic syndromes, are accounted for in the PPS payment formula. In contrast, only a minority of patients (25%−30%) receive calcimimetics. Therefore, a poor risk-adjustment model could lead providers to avoid high-cost patients or, even worse, to withhold calcimimetics when medically indicated. This could negatively synergize with the incentive to avoid prescribing calcimimetics to patients without secondary insurance who must pay Medicare’s 20% co-insurance out-of-pocket. Additionally, it could set a negative precedent that deters entrepreneurs and researchers from developing future therapeutics relevant to ESRD.
Because calcimimetics may obviate the need for parathyroidectomies (indeed, some have referred to these medications as a form of “medical parathyroidectomy”),34–38 CMS should also monitor for increases in parathyroidectomies and their respective outcomes, particularly since these procedures are not part of the bundle. It may be the case that an increase in parathyroidectomy rate is clinically appropriate, as some recent studies suggest.39,40 Thus, any monitoring of cinacalcet utilization and parathyroidectomy rate should be done in conjunction with the reporting of clinical adverse events such as hypocalcemia, seizures, and cardiovascular complications. To this end, CMS or the nephrology research community could also commission an outcomes study comparing long-term clinical and quality of life outcomes in patients receiving oral or IV calcimimetic to those receiving no therapy or a parathyroidectomy.
Finally, with a generic calcimimetic nearing production, we may see increased use of oral calcimimetics over intravenous ones due to increased profit margins, particularly if the community does not have robust data to show that one form of calcimimetic is clearly superior to another. Improved data on whether etelcalcetide demonstrates superior outcomes over oral calcimimetics (e.g., through improved patient adherence) could help inform CMS on how to most effectively incorporate calcimimetics into the bundle to achieve the best patient outcomes.
Conclusions
Moving calcimimetic reimbursement from Part D to Part B will change the way providers treat bone-mineral metabolism derangements in patients receiving dialysis. The TDAPA period gives dialysis providers a short time window to adjust. Once the TDAPA expires, calcimimetics will be bundled into the PPS base rate, likely resulting in reduced calcimimetic use. Because the optimal treatment of bone-mineral metabolism is largely an evidence-free zone, CMS should closely monitor whether constraining calcimimetic use leads to an increase in adverse outcomes, such as hypercalcemia, cardiovascular events, and parathyroidectomies. Such monitoring is important, since it is unclear whether calcimimetics are currently overused and due for a correction. It may be the case that calcimimetics are being used appropriately in the status quo and that TDAPA leads providers to withhold calcimimetics from patients who need them.
Additionally, correctly implementing this transition payment could set an important precedent for future dialysis-related treatments and how they are incorporated into the PPS bundle. CMS and the American Society of Nephrology recently announced a Kidney Innovation Accelerator partnership (“Kidney X”), aimed at developing novel treatments and potential cures for kidney disease.41 Its success will depend, in part, on ensuring that device and drug companies will receive fair reimbursement in exchange for investing in improving the health of patients with kidney disease. A poorly implemented drug transition could inadvertently deter innovation and delay other nephrology-related research ventures. Notably, CMS recently attempted to address some of these concerns through the 2019 PPS final rule, which will allow all (kidney dialysis) drugs approved by the FDA on or after January 1, 2020 to qualify for two years of TDAPA payments even if the drug belongs in a functional category.42 Afterwards, drugs that do not belong in a functional category will be rolled into the PPS base rate, similar to the calcimimetics. Drugs that belong in a functional category will be incorporated into the PPS without updating the base rate.
To ensure that TDAPA does not negatively impact patients’ present and future health, it is vital that CMS and the nephrology community work closely during this important transition.
Acknowledgements:
The authors would like to acknowledge the review of this manuscript by Srinikath Nagavarapu and Kyle Buika for content accuracy.
Support: EL is supported, in part, by the National Institutes of Health (NIH) through a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant: K08DK118213. The NIH had no role in writing or the decision to submit for publication. Financial Disclosure: EL provides limited consulting services to Acumen, LLC. SW is affiliated with Northwest Kidney Centers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. The content is solely the responsibility of the authors and does not necessarily represent the official views of Acumen, CMS, Northwest Kidney Centers, or the NIH.
REFERENCES
- 1.Food US & Administration Drug. Parsabiv (etelcalcetide) Injection [Internet]. 2017. [cited 2018 Jul 18]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208325Orig1s000TOC.cfm
- 2.Block GA, Bushinsky DA, Cunningham J, et al. Effect of Etelcalcetide vs Placebo on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: Two Randomized Clinical Trials. JAMA 2017;317(2):146–55. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Bushinsky DA, Cheng S, et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA 2017;317(2):156–64. [DOI] [PubMed] [Google Scholar]
- 4.Middleton JP, Wolf M. Second Chances to Improve ESRD Outcomes With a SecondGeneration Calcimimetic. JAMA 2017;317(2):139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; endstage renal disease prospective payment system. Final rule. CMS-1418-F. Fed Regist 2010;75(155):49029–214. [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving, Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2017;7:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 2017;92(1):26–36. [DOI] [PubMed] [Google Scholar]
- 8.110th Congress. Medicare Improvements for Patients and Providers Act of 2008. Public Law Number 110–275. 122 STAT. 2494–2597 [Internet]. 2008. [cited 2018 Feb 6]. Available from: https://www.gpo.gov/fdsys/pkg/PLAW-110publ275/pdf/PLAW110publ275.pdf
- 9.112th Congress. American Taxpayer Relief Act of 2012. Public Law 112–240. 126 Stat. 2313–2371 [Internet]. 2013. [cited 2018 Jul 18]. Available from: https://www.congress.gov/112/plaws/publ240/PLAW-112publ240.pdf
- 10.113th Congress. Protecting Access to Medicare Act of 2014. Public Law 113–93. 128 STAT. 1040–1084 [Internet]. [cited 2018 Jul 18]. Available from: https://www.congress.gov/113/plaws/publ93/PLAW-113publ93.pdf
- 11.113th Congress. Achieving a Better Life Experiencing Act of 2014. Public Law 113295. 128 STAT. 4056–4074 [Internet]. 2014. [cited 2018 Jul 18]. Available from: https://www.congress.gov/113/plaws/publ295/PLAW-113publ295.pdf
- 12.Chambers JD, Weiner DE, Bliss SK, Neumann PJ. What can we learn from the U.S. expanded end-stage renal disease bundle? Health Policy Amst Neth 2013;110(2–3):164–71. [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Liu J, Monda KL, et al. Epoetin Alfa and Outcomes in Dialysis amid Regulatory and Payment Reform. J Am Soc Nephrol JASN 2016;27(10):3129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisoni RL, Fuller DS, Bieber BA, Gillespie BW, Robinson BM. The DOPPS Practice Monitor for US Dialysis Care: Trends Through August 2011. Am J Kidney Dis 2012;60(1):160–5. [DOI] [PubMed] [Google Scholar]
- 15.Weiner DE, Winkelmayer WC. Commentary on ‘The DOPPS Practice Monitor for US Dialysis Care: Trends Through August 2011”: An ESA Confluence.’ Am J Kidney Dis 2012;60(1):165–7. [DOI] [PubMed] [Google Scholar]
- 16.Spoendlin J, Schneeweiss S, Tsacogianis T, et al. Association of Medicare’s Bundled Payment Reform With Changes in Use of Vitamin D Among Patients Receiving Maintenance Hemodialysis: An Interrupted Time-Series Analysis. Am J Kidney Dis 2018;72(2):178–87. [DOI] [PubMed] [Google Scholar]
- 17.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):S1–S672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services. ESRD QIP PY 2021 Proposed Rule Technical Specifications [Internet]. 2017. [cited 2018 Jul 18];Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-AssessmentInstruments/ESRDQIP/Downloads/PY-2021-Final-Rule-Tech-Specs.pdf
- 19.Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol JASN 2005;16(3):800–7. [DOI] [PubMed] [Google Scholar]
- 20.Sterrett JR, Strom J, Stummvoll HK, et al. Cinacalcet HCI (Sensipar/Mimpara) is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol 2007;68(1):10–7. [DOI] [PubMed] [Google Scholar]
- 21.Messa P, Macário F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol CJASN 2008;3(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprague SM, Evenepoel P, Curzi MP, et al. Simultaneous control of PTH and CaxP Is sustained over three years of treatment with cinacalcet HCl. Clin J Am Soc Nephrol CJASN 2009;4(9):1465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer SC, Nistor I, Craig JC, et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med 2013;10(4):e1001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetmore JB, Gurevich K, Sprague S, et al. A Randomized Trial of Cinacalcet versus Vitamin D Analogs as Monotherapy in Secondary Hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol CJASN 2015;10(6):1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int 2015;87(3):502–28. [DOI] [PubMed] [Google Scholar]
- 26.Fukagawa M, Kido R, Komaba H, et al. Abnormal Mineral Metabolism and Mortality in Hemodialysis Patients With Secondary Hyperparathyroidism: Evidence From Marginal Structural Models Used to Adjust for Time-Dependent Confounding. Am J Kidney Dis 2014;63(6):979–87. [DOI] [PubMed] [Google Scholar]
- 27.Lacson E, Wang W, Hakim RM, Teng M, Lazarus JM. Associates of Mortality and Hospitalization in Hemodialysis: Potentially Actionable Laboratory Variables and Vascular Access. Am J Kidney Dis 2009;53(1):79–90. [DOI] [PubMed] [Google Scholar]
- 28.Coen G, Pierantozzi A, Spizzichino D, et al. Risk factors of one year increment of coronary calcifications and survival in hemodialysis patients. BMC Nephrol 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallieni M, Caputo F, Filippini A, et al. Prevalence and progression of cardiovascular calcifications in peritoneal dialysis patients: A prospective study. Bone 2012;51(3):332–7. [DOI] [PubMed] [Google Scholar]
- 30.Floege J, Tsirtsonis K, Iles J, Drueke TB, Chertow GM, Parfrey P. Incidence, predictors and therapeutic consequences of hypocalcemia in patients treated with cinacalcet in the EVOLVE trial. Kidney Int 2018;93(6):1475–82. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Medicare & Medicaid Services. Implementation of the Transitional Drug Add-On Payment Adjustment. Pub 100–20 One-Time Notification. Transmittal 1999 [Internet]. 2018. [cited 2018 Jul 18]. Available from: https://www.cms.gov/Regulationsand-Guidance/Guidance/Transmittals/2018Downloads/R1999OTN.pdf
- 32.Centers for Medicare & Medicaid Services. Drug Designation Process. CFR 413.234 (c)(1) [Internet]. 2015. [cited 2018 Jul 27]. Available from: https://www.gpo.gov/fdsys/pkg/CFR-2016-title42-vol2/pdf/CFR-2016-title42-vol2sec413-234-.pdf
- 33.Centers for Medicare & Medicaid Services. Medicare Program; End-Stage Renal Disease Prospective Payment System, and Quality Incentive Program; Final Rule. 42 CFR Part 413. Fed Regist 2015;80(215):68968–9077. [PubMed] [Google Scholar]
- 34.Elder GJ. Parathyroidectomy in the calcimimetic era. Nephrol Carlton Vic 2005;10(5):511–5. [DOI] [PubMed] [Google Scholar]
- 35.Ballinger AE, Palmer SC, Nistor I, Craig JC, Strippoli GFM. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev 2014;(12):CD006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan R, Perkins RM, Berbano EP, et al. Parathyroidectomy versus cinacalcet hydrochloride-based medical therapy in the management of hyperparathyroidism in ESRD: a cost utility analysis. Am J Kidney Dis Off J Natl Kidney Found 2007;49(6):801–13. [DOI] [PubMed] [Google Scholar]
- 37.Wüthrich RP, Martin D, Bilezikian JP. The role of calcimimetics in the treatment of hyperparathyroidism. Eur J Clin Invest 2007;37(12):915–22. [DOI] [PubMed] [Google Scholar]
- 38.Ghani A, Baxter P. Surgical parathyroidectomy versus cinacalcet therapy: in the management of secondary hyperparathyroidism. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg 2012;146(2):220–5. [DOI] [PubMed] [Google Scholar]
- 39.van der Plas WY, Dulfer RR, Engelsman AF, et al. Effect of parathyroidectomy and cinacalcet on quality of life in patients with end-stage renal disease-related hyperparathyroidism: a systematic review. Nephrol Dial Transplant 2017;32(11):1902–8. [DOI] [PubMed] [Google Scholar]
- 40.Komaba H, Nakamura M, Fukagawa M. Resurgence of parathyroidectomy: evidence and outcomes [Internet]. 2017. [cited 2018 Oct 23];Available from: https://www.ingentaconnect.com/content/wk/mnh/2017/00000026/00000004/art00003 [DOI] [PubMed]
- 41.Department of Health Press Office. The U.S. Department of Health and Human Services and the American Society of Nephrology to Launch Kidney Innovation Accelerator [Internet]. 2018. [cited 2018 Aug 5];Available from: https://www.hhs.gov/about/news/2018/04/26/us-department-health-and-human-servicesand-american-society-nephrology-launch-kidney-innovation.html
- 42.Centers for Medicare & Medicaid Services. Medicare Program; End-Stage Renal Disease Prospective Payment System, Payment for Renal Dialysis Services Furnished to Individuals with Acute Kidney Injury, End-Stage Renal Disease Quality Incentive Program, Durable Medical Equipment, Prosthetics, Orthotics and Supplies (DMEPOS) Competitive Bidding Program (CBP) and Fee Schedule Amounts, and Technical Amendments to Correct Existing Regulations Related to the CBP for Certain DMEPOS. 42 CFR Parts 413–414. CMS-1691-F. [Internet]. Fed. Regist 2018. [cited 2018 Nov 5];Available from: https://s3.amazonaws.com/public-inspection.federalregister.gov/2018-24238.pdf [PubMed]
- 43.Food US & Administration Drug. Drug Approval Package: Sensipar (Cinacalcet HCl) Tablets [Internet]. 2004. [cited 2018 Jul 27];Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-688_Sensipar.cfm
- 44.Martin KJ, Jüppner H, Sherrard DJ, et al. First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int 2005;68(3):1236–43. [DOI] [PubMed] [Google Scholar]