Abstract
Rationale & Objective:
Atrial fibrillation is common in patients with kidney failure treated by maintenance dialysis. Whether the incidence of AF differs between patients receiving hemodialysis and peritoneal dialysis is uncertain.
Study Design:
Retrospective cohort study.
Setting & Participants:
Using the United States Renal Data System we identified older patients (≥67 years) with Medicare Parts A+B who initiated dialysis (1996–2011) without a diagnosis of atrial fibrillation during the prior 2 years.
Exposure:
Dialysis modality at incident end-stage renal disease (ESRD) and maintained for at least 90 days.
Outcome:
Patients were followed for ≤36 months for a new diagnosis of atrial fibrillation.
Analytical Approach:
Time-to-event analysis using multivariable Cox proportional hazards regression to estimate cause-specific hazard ratios while censoring at modality switch, kidney transplantation, or death.
Results:
Overall, 271,722 older patients were eligible; 17,487 (6.9%) were treated with peritoneal dialysis and 254,235 (93.1%) with hemodialysis at the onset of ESRD. During 406,225 person-years of follow-up, 69,705 patients were newly diagnosed with atrial fibrillation. Since the proportionality assumption was violated, we introduced an interaction term between time period (first 90 days vs. thereafter) and modality. The atrial fibrillation incidence during the first 90 days was 187/1,000 person-years on peritoneal dialysis and 372/1,000 person-years on hemodialysis. Patients on peritoneal dialysis had an adjusted 39% (95%CI; 34–43%) lower incidence of atrial fibrillation than those on hemodialysis. From day 91 onwards, the atrial fibrillation incidence was ~140/1,000 person-years with no major difference between modalities.
Limitations:
Residual confounding from unobserved differences between exposure groups; ascertainment of AF from billing claims; study of first modality may not generalize to patients switching modalities; uncertain generalizability to younger patients.
Conclusions:
While patients initiating dialysis using peritoneal dialysis had lower atrial fibrillation incidence during the first 90 days of ESRD, there was no major difference in atrial fibrillation incidence thereafter. The value of interventions to reduce the early excess atrial fibrillation risk in patients receiving hemodialysis may warrant further study.
Keywords: Hemodialysis (HD), Peritoneal Dialysis (PD), Arrhythmia, Atrial fibrillation (AF), flutter, dialysis modality, Outcomes, Comparative Effectiveness, US Renal Data System (USRDS)
INTRODUCTION
Atrial fibrillation/flutter (AF) is the most common sustained arrhythmia in the general population and is particularly common in patients with end-stage renal disease (ESRD). More than 10% of prevalent U.S. patients on hemodialysis (HD) carry a confirmed diagnosis of AF, and the percentage increases steeply with age, reaching approximately one quarter of patients over 85 years of age.1 The cumulative incidence of newly diagnosed AF during the first year of dialysis among older patients initiating HD is almost 15%.2 Patients with AF experience poor health outcomes including higher mortality, excess rates of ischemic stroke, systemic thromboembolism, myocardial infarction, heart failure, kidney disease, and incur higher healthcare cost.3,4 Several risk factors for the development of AF have been identified, including socio-demographic characteristics (older age, female sex, white race, non-Hispanic ethnicity), and chronic conditions (e.g., heart failure, diabetes, hypertension) and it appears that most of these factors similarly increase AF risk in patients with kidney failure requiring maintenance dialysis.
One potential AF risk factor unique to patients with kidney failure that has not sufficiently been investigated is the dialysis modality used for kidney replacement therapy. Several considerations would support a hypothesized difference in AF risk between patients undergoing peritoneal dialysis (PD) vs. HD. Patients undergoing HD are exposed to considerable cyclical changes in fluid and electrolyte status, with accumulation of fluid and uremic toxins, including potentially pro-arrhythmogenic electrolytes (potassium, calcium, magnesium) during the intradialytic interval followed by rapid fluid removal and electrolyte shifts during the relatively short HD procedure.5 By contrast, PD confers a more continuous removal of excess fluids and maintenance of electrolyte balance, thus exercising less strain on the heart while reducing the burden of other potential AF triggers.
Little is known, however, about whether the AF incidence differs between patients undergoing HD vs. PD. We conducted this study to specifically challenge the null hypothesis of no difference in AF incidence between incident ESRD patients using PD vs. HD in a large ESRD registry.
METHODS
Source population and study design
We identified from the United States Renal Data System (USRDS) older individuals with incident ESRD aged 67–99 years who initiated chronic dialysis between 1/1/1996 and 12/31/2011 in the 50 states and the District of Columbia. The USRDS is the national registry of persons with ESRD and includes almost all patients with ESRD undergoing dialysis treatment or kidney transplantation.6 We defined the first day of dialysis services as the index date.
We restricted the cohort to patients with uninterrupted Medicare Part A&B (MPAB) coverage between 24 months prior to and 3 months after the index date. Adjacent MPAB coverage periods with a coverage gap of ≤3 days were considered as uninterrupted coverage. We ascertained minimum active system use of MPAB by requiring at least one billing claim to Medicare in each of the 3 intervals: 24–13 and 12–0 months preceding the first service day, as well as during the 3 months following the index date. We identified eligible patients for incident AF by excluding all patients with any billing claims containing an International Classification of Diseases, 9th Revision (ICD-9) diagnosis code of 427.3x during the 24 months prior to the index date.2
Outcome of Interest
Patients were followed for the occurrence of a new diagnosis of AF, which could be from a single inpatient diagnosis code or from an outpatient diagnosis code that was a subsequently confirmed.2
Exposure of Interest
We required that eligible patients maintained dialysis using a single modality (HD or PD) until at least 90 days after the index date, unless they died or underwent kidney transplantation in this period, in which case they were censored. Individuals receiving HD could have had center HD, center-self HD, or home HD, and those using PD underwent either continuous ambulatory, continuous cyclic, or another type of PD. We excluded patients who died on the index date or received preemptive kidney transplantation, had a period with “uncertain” dialysis, discontinued dialysis, recovered kidney function, or were lost to follow-up during the 90 days following the index date.
Covariates
Demographic and health-relevant characteristics were abstracted from the ‘patients’ and the Medical Evidence Report files in the USRDS and categorized as follows: age (67–69, 70–74, 75–79, ≥80 years), sex, race/ethnicity (Hispanic White, non-Hispanic White, Black, Asian, Other), body mass index (BMI; <18.5, 18.5 to <25, 25 to <30, ≥30 kg/m2), current smoking, alcohol dependence, drug dependence, geographic region (nine census divisions: New England, Middle Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, Pacific), inability to ambulate or transfer, number of outpatient nephrologist visits in the 24 months preceding dialysis (0, 1–4, 5–9, ≥10), Medicaid dual eligibility, hemoglobin (<8, 8 to <9.5, 9.5 to < 12, ≥12 g/dl), serum albumin (<3.5, ≥3.5 mg/dl), estimated glomerular filtration rate at start of dialysis (eGFR; <15, ≥15 ml/min/1.73 m2). Comorbidities such as diabetes mellitus, hypertension, heart failure, cardiovascular disease, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease and cancer, were considered as potential confounders and ascertained from the Medical Evidence Report file or any corresponding recorded diagnoses from Medicare claims preceding the first service date by <24 months.
Statistical analysis
We described baseline characteristics among all patients as well as by dialysis modality, HD vs. PD, using means and standard deviations or medians and interquartile ranges for continuous variables and counts and percent for categorical variables. Patients were followed for incident AF from the index date until the earliest occurrence of: loss of uninterrupted MPAB coverage, any sustained switch in dialysis modality (>60 days duration; variable ‘rxhist60’ in the USRDS), kidney transplantation, death, or three years after their index date.
We calculated the incidence rate of AF as the number of incident AF events divided by total eligible person-time. With death as a competing event, we first applied the cause-specific hazard regression model to estimate the cause-specific hazard ratio (HR) of dialysis modality (PD vs. HD) on incident AF. This model considers the competing events as non-informative censoring and is “better suited for studying the etiology of diseases.”7 Then we applied the Fine-Gray model to estimate the sub-distribution hazard ratio. This model considered persons with competing events as being still in the risk set when defining the hazard of AF, and is preferable in “predicting an individual’s risk.”7 We applied these competing risk models without adjustment (Model 1), then adjusted for demographic characteristics (age, sex, race/ethnicity; Model 2), and finally adjusted for all baseline demographic and health-relevant characteristics and comorbidities shown in Table 1 (Model 3).
Table 1.
Patient Characteristics, Stratified by Dialysis Modality
| Characteristics | All (N= 271,722) |
PD (n = 17,487) |
HD (n = 254,235) |
P-value |
|---|---|---|---|---|
| Baseline | ||||
| Age at first ESRD service | 75.0 [71.0, 80.0] | 74.0 [70.0, 78.0] | 76.0 [71.0, 81.0] | <.001 |
| Female sex | 51.0 | 46.1 | 51.4 | <.001 |
| Race | <.001 | |||
| White | 72.8 | 84.4 | 72.0 | |
| Black | 23.1 | 11.7 | 23.9 | |
| Asian | 2.8 | 2.9 | 2.8 | |
| other | 1.3 | 1.1 | 1.3 | |
| Hispanic ethnicity | 8.1 | 5.9 | 8.3 | <0.001 |
| Diagnosed/reported comorbid conditions |
||||
| Hypertension | 98.2 | 98.5 | 98.1 | <0.001 |
| Heart failure | 64.0 | 48.7 | 65.1 | <0.001 |
| Coronary arterial disease | 47.6 | 42.1 | 48.0 | <0.001 |
| Cerebrovascular disease | 29.8 | 23.0 | 30.3 | <0.001 |
| Peripheral vascular disease | 40.0 | 31.7 | 40.5 | <0.001 |
| Diabetes | 63.0 | 56.8 | 63.5 | <0.001 |
| Chronic obstructive lung disease | 10.4 | 7.1 | 10.7 | <0.001 |
| Cancer | 22.5 | 18.8 | 22.7 | <0.001 |
| Current smoking | 3.6 | 3.9 | 3.6 | <0.001 |
| Alcohol dependence | 0.6 | 0.3 | 0.6 | <0.001 |
| Drug dependence | 0.1 | 0.0 | 0.1 | <0.001 |
| Inability to ambulate or transfer | 6.5 | 2.2 | 6.8 | <0.001 |
| Cause of end-stage renal disease | <0.001 | |||
| Diabetes | 41.6 | 39.1 | 41.8 | |
| Hypertension | 34.4 | 33.5 | 34.4 | |
| Glomerulonephritis | 6.0 | 9.9 | 5.7 | |
| Other | 17.9 | 17.3 | 18.0 | |
| Missing | 0.1 | 0.1 | 0.1 | |
| Count of nephrology visits before index |
3.0 (0.0, 9.0) | 7.0 (2.0, 13.0) | 3.0 (0.0, 9.0) | <0.001 |
| Body mass index, kg/m2 | 25.4 (22.1, 29.7) | 25.7 (22.8, 29.4) | 25.4 (22.1, 29.7) | <0.001 |
| Serum albumin, g/dl | 3.2 (2.8, 3.6) | 3.6 (3.2, 4.0) | 3.2 (2.7, 3.6) | <0.001 |
| Hemoglobin, g/dl | 10.0 (8.9, 11.0) | 10.6 (9.6, 11.6) | 9.9 (8.9, 11.0) | <0.001 |
| eGFR, ml/min/1.73m2 | 7.7 (5.6, 10.5) | 8.1 (6.0, 10.9) | 7.7 (5.6, 10.5) | <0.001 |
| Follow-up during up to 3 years | <0.001 | |||
| Incident AF | 69,705 (25.7) | 3876 (22.2) | 65,829 (25.9) | <0.001 |
| Death before incident AF | 97,704 (36.0) | 5009 (28.6) | 92,695 (36.5) | <0.001 |
| Follow-up duration, y | 1.3 (0.4, 3.0) | 1.2 (0.6, 2.4) | 1.3 (0.4, 3.0) | <0.001 |
| During first 90 days | <0.001 | |||
| Incident AF | 21,709 (8.0) | 769 (4.4) | 20,940 (8.2) | <0.001 |
| Death before incident AF | 22,241 (8.2) | 622 (3.6) | 21,619 (8.5) | <0.001 |
| From 91 days to 3 years | ||||
| Incident AF | 47,996 (21.4) | 3107 (19.4) | 44,889 (21.6) | 0.7 |
| Death before incident AF | 75,463 (33.7) | 4387 (27.4) | 71,076 (34.1) | <0.001 |
Values for continuous variables given as median [interquartile range]; categorical variables as percentage or count (percentage).
AF – atrial fibrillation; eGFR – estimated glomerular filtration rate (calculated using the _____ equation); ESRD – end-stage renal disease; HD – hemodialysis; PD – peritoneal dialysis.
Missing data included 30 (0.01%) for sex, 38 (0.01%) for race, 10,892 (4.0%) for body mass index, 68,247 (25.1%) for serum albumin, 21,781 (8.0%) for hemoglobin, and 3,878 (1.4%) for estimated glomerular filtration rate.
For covariates with missing values, we assumed data were missing at random and used fully conditional specification method to generate five multiple imputed data sets.8 All variables in the analysis were included in the imputation model.
The validity of the proportional hazards assumption was examined by including an interaction term between exposure and follow-up time in the regression model, as well as analyzing Schoenfeld residuals for the exposure and all covariates. Any violation of the proportional hazards assumption would induce stratification or inclusion of an interaction term with follow-up time.
The study was approved by an institutional review board at Baylor College of Medicine (protocol #H-36408), which waived the requirement for informed consent, and an active Data Use Agreement with the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) was in place. Analyses were conducted using SAS for Windows (version 9.4) or R software (version 3.3; R-project). Significance was at 2-sided α=0.05.
RESULTS
Study Cohort
We identified 271,722 patients eligible for inclusion in our study of incident AF (Figure 1), of whom 17,487 (6.4%) patients initiated dialysis using PD and 254,235 (93.6%) using HD. Differences between included and excluded patients are shown in Table S1. The median age was 75 years, 51% were women, 73% were white, 23% black, and 8% were Hispanic. PD patients were younger, more likely to be male, white, had fewer comorbidities, and were less likely to be impaired in their ambulation or ability to transfer (Table 1). While most characteristics differed significantly between modalities owing to the large sample size, it is notable that HD patients had fewer visits with nephrologists prior to dialysis initiation and lower serum albumin concentrations.
Figure 1:
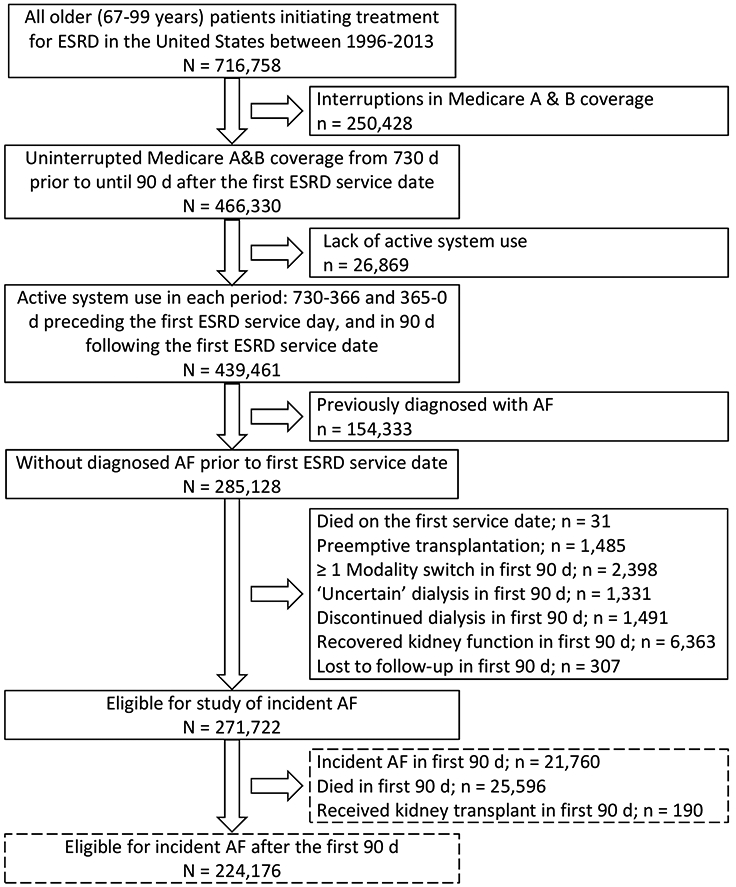
Flow chart illustrating cohort assembly.
Overall, patients were followed for 1.5 years, on average, and contributed 406,225 person-years during which 69,705 patients were newly diagnosed with AF. The PD group had lower unadjusted rates of AF in the first months after the index date, but this difference narrowed over the remaining follow up to 36 months (Figure 2). The unadjusted incidence rate of AF was lower among PD patients compared with HD patients (152.0 vs. 173.2 per 1000 person-years), which was confirmed by formal Cox regression models (multivariable-adjusted cause-specific HR, 0.96 [95%CI, 0.93–0.99]; sub-distribution HR, 0.92 [95%CI, 0.89–0.95]; Table S2). However, the interaction term between dialysis modality and follow-up time indicated violation of the proportional hazards assumption (p<0.001), which was confirmed by inspection of a smoothed plot of Schoenfeld residuals versus time. Hence, we conducted the analyses by separating follow-up time into two periods at 3 months (≤90 vs. >90 days) and including an interaction term between modality and time period into the model. No residual violation of the proportionality assumption was observed within either of the two time periods. During the first 3 months of dialysis, 21,709 patients developed incident AF and 22,241 patients died without having developed AF. The unadjusted AF incidence rate was 186.6 per 1000 person-years in the PD and 372.0 per 1000 person-years in the HD group. Estimates of association from both cause-specific models indicated that patients on PD had an adjusted 43% (95%CI, 38%−47%) lower AF incidence than those on HD (Table 2). These were consistent with the sub-distribution HR from Fine and Gray models that considered death as a competing risk (Table S3).
Figure 2:
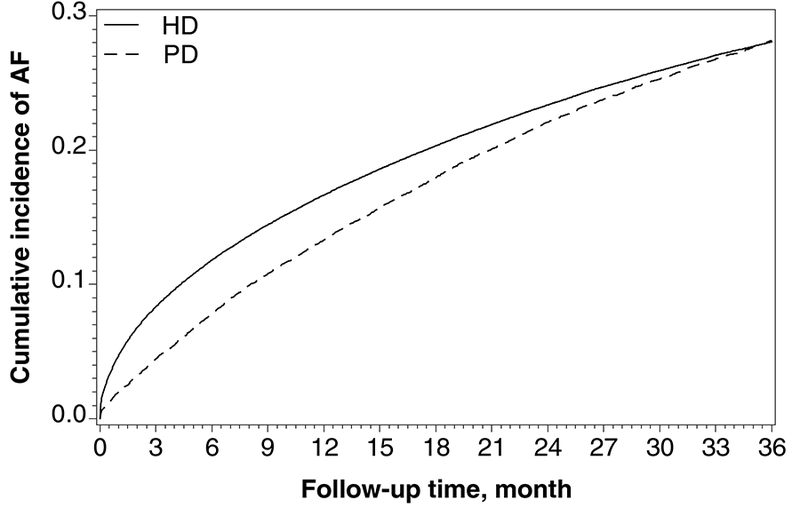
Actuarial incidence plot of newly diagnosed atrial fibrillation, by dialysis modality.
Table 2.
Association of the first modality and incident AF
| Dialysis Modality |
Person-y | No. of incident AF |
Incidence rate of AF, per 1000 person-y |
Cause specific hazard model: HR (95 % CI) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| Incident AF during the first 90 days of dialysis | ||||||
| HD | 56,281 | 20,940 | 372.0 | 1.00 (reference) |
1.00 (reference) | 1.00 (reference) |
| PD | 4122 | 769 | 186.6 | 0.51 (0.48–0.55) | 0.50 (0.47–0.54) | 0.57 (0.53–0.62) |
| Incident AF from 91 days until 3 years from first dialysis | ||||||
| HD | 323,883 | 44,889 | 138.6 | 1.00 (reference) |
1.00 (reference) | 1.00 (reference) |
| PD | 21,393 | 3107 | 145.2 | 1.04 (1.00–1.08) | 1.01 (0.97–1.04) | 1.14 (1.10–1.19) |
Model 1:unadjusted
Model 2:adjusting for age, sex, race/ethnicity
Model 3:adjusting for age, sex, race/ethnicity, cause of end-stage renal disease, current smoking, alcohol dependence, drug dependence, geographic region, inability to ambulate or transfer, count of pre-ESRD visits to nephrologist, Medicaid dual eligibility, body mass index, hemoglobin, albumin, estimated glomerular filtration rate at dialysis initiation, comorbidities (diabetes, hypertension, heart failure, cardiovascular disease, cerebral vascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer).
AF – atrial fibrillation; HD – hemodialysis; PD – peritoneal dialysis
Subsequently (i.e., from month 4 onwards), we followed 224,176 patients for incident AF. During up to 33-months of follow-up, 47,996 incident AF events occurred over 345,276 person-years. The unadjusted AF incidence rate was 145.2 per 1000 person-years in patients on PD and 138.6 per 1000 person-years among those on HD. After controlling for all demographic and recorded health-related characteristics and comorbidities, the multivariable-adjusted cause-specific HR for PD vs. HD was 1.14 (95%CI, 1.10–1.19). When accounting for the competing risk of death the difference in the risk of incident AF between patients receiving PD vs. HD essentially disappeared (sub-distribution HR, 1.05; 95%CI, 1.01–1.09).
We found that the unadjusted associations were relatively robust when adjusting for demographic characteristics (Model 2), but that adjustment for additional clinical variables attenuated the favorable association between PD and incident AF in the first 90 days and led to a small, but significant association between PD and higher AF risk thereafter. We identified presence of diagnosed heart failure prior to and baseline serum albumin at dialysis initiation to be the main confounders driving the differences between models 2 and 3 for both time periods (Table S4).
DISCUSSION
Using a large, national registry of persons with ESRD in the United States, we examined potential differences in the incidence of AF by dialysis modality in older patients initiating dialysis. We found evidence of a sizable, approximately 40% lower risk for incident AF among patients using PD during the first 3 months of dialysis treatment compared with HD. Beyond the first 3 months, however, the rates of incident AF were roughly similar between patients undergoing PD versus HD. These findings arose from a large cohort representative of older patients with kidney failure initiating maintenance dialysis and from analyses carefully adjusted for a large number of patient and health care characteristics that were captured in 2 years of pre-dialysis Medicare claims.
Atrial fibrillation is very common in patients with kidney failure on dialysis. While previous registry-based studies have indicated that approximately 10% among point prevalent HD patients had AF identified using billing claims,1 a study using electrocardiograms to identify AF showed a prevalence of 26%.9 Using new implantable technology, loop recorders, the Monitoring in Dialysis Investigators found that 41% of prevalent HD patients had at least one episode of >6 minutes of AF over 6 months of monitoring.10 Patients with AF on HD have worse morbidity and mortality outcomes compared with patients without AF, especially soon after a new diagnosis of AF.11,12 In addition, established interventions to reduce morbidity and mortality in patients with AF in the general population, such as oral anticoagulation, are not proven to be effective or net-beneficial in patients on dialysis.13–16 Hence, any intervention towards reducing the risk of AF could be quite impactful in this high-risk population. Since many patients have a choice among dialysis modalities, our results may be informative for medical decision making. The results from our study may also have major implications for the direction of future investigation, including interventional trials, towards reducing this excessively high incidence of AF especially in patients initiating maintenance dialysis using HD.
Our study contributes novel information regarding the risk of incident AF between the dialysis modalities. A retrospective cohort analysis of maintenance dialysis patients using Medicaid claims to identify chronic AF found 29% lower odds of prevalent AF in patients with self-care dialysis (either PD or HD) suggesting the possibility that there may also be modality differences in incident AF.17 A smaller and underpowered study of 225 patients without AF at dialysis initiation identified no significant difference for incident AF between dialysis modalities.18 A recent study from Taiwan compared the incidence of AF of patients on HD with age- and sex-matched persons from the general population, and – separately – did the same for patients on PD.19 The adjusted HR for AF risk was 1.46 (95%CI, 1.32–1.61) among HD patients and 1.32 (95%CI, 1.00–1.83) for PD, versus the general population, but no formal direct comparison between the dialysis modalities was conducted, and the non-dialysis reference groups differed. There was also no consideration of the time dependency of AF risk over time.
There are at least two major explanations for the increased risk during the early dialysis period. First, patients with PD tend to be healthier that patients on HD on several domains that are measurable and others that may be less well captured. However, several recent studies comparing all-cause mortality between patients using PD vs. HD have found little difference in outcomes. Over the same duration of follow-up (3 years), Weinhandl et al. described cumulative survival probabilities of 58% vs. 57% for well-matched PD vs. HD patients in the US registry (HR, 1.00; 95%CI, 0.88–1.13), although earlier mortality appeared to favor PD.20 Results in older patients were generally more in favor of HD, with less benefit for PD early on and increased mortality among PD patients beyond 1 year. Using the Canadian dialysis registry, Perl et al. further differentiated between vascular access types among patients on HD and found that over 3 years of follow up, patients with arteriovenous fistula generally had lower mortality compared with patients on PD (HR, 0.8; 95%CI, 0.7–0.9), whereas patients with a central venous hemodialysis catheter had higher mortality (HR, 1.2; 95%CI, 1.2–1.3).21 Neither of these studies specifically looked at early (first 90 days) vs. later mortality, although the patients with a central venous catheter appeared to have even higher excess mortality in earlier than in later years.21 These findings appear to be corroborated by Chan et al. in data from one large U.S. dialysis provider, where patients with an arteriovenous fistula have similar mortality compared with PD patients, with little apparent time-dependency over the first year.22 However, none of these studies provided any more granular information about patient outcomes other than death, including AF.
Second, it is possible that among older patients initiating maintenance HD, a certain subgroup may be particularly susceptible to AF, especially in the first 3 months after dialysis initiation. Thus, these susceptible patients developed AF in the first 3 months, leaving a remaining risk set that is more tolerant of the stresses of the rapid fluid and electrolyte swings associated with thrice weekly HD, and therefore, more AF-resistant (‘depletion of susceptibles’).23 Indeed, the electrocardiographic changes with intermittent dialysis and its weight and electrolyte changes have been described as longas 60 years ago,5 and its association with adverse outcomes in hemodialysis patients, such as increased sudden (cardiac) death and hospitalization remain of high interest to clinical investigators in HD today.
Our findings of an increased incidence of AF during the first 3 months after initiation of HD may inform a movement adopting an older idea that appears to have gained traction recently.24 It has been proposed to initiate HD in a manner that is gentler than the one-fits-all thrice weekly HD approach, for example by starting patients with lesser frequency.25,26 Such ‘incremental dialysis’ may facilitate the transition from untreated to treated kidney failure and takes into account the residual kidney function that most of the patients initiating dialysis possess. The usual approach towards prescribing PD incorporates the contribution in clearance from the residual function of the kidney whereas prescription of HD usually does not, partly in response to clearance criteria mandated and monitored as part of the Medicare Quality Improvement Program. It has been shown in the Monitoring in Dialysis study that AF episodes are most frequent during the 12 hour interval that begins with the start of the HD session, with the HD procedure being a particular strong trigger of AF episodes.10 Thus, it would be interesting to examine whether the incidence of AF can be reduced by a more personalized approach to initiating HD, by reducing the frequency or, perhaps, the dose.
The lack of any major difference in AF incidence between the dialysis modalities beyond 3 months was perhaps unexpected. We had hypothesized that the cyclical swings in fluid status and electrolyte concentrations in patients on HD would contribute to increased AF risk, especially after the findings from smaller studies using implanted rhythm monitors that showed such a strong dependence of AF episodes relative to the specific time during the interval between 2 HD sessions.27 By contrast, PD is a much more continuous procedure with less swings in fluid status and electrolyte concentrations which, apparently, does not translate into reduced AF risk. Why the benefit of PD for AF risk is not sustained is unclear. Perhaps it is related to the complex pathophysiology of AF. As kidney failure patients chronically have volume overload and neurohormonal alterations leading to cardiac structural abnormalities, modality differences for dialysis may not be enough to reduce the risk of AF. It has been shown that chronic inflammation and oxidative stress may be implicated in the pathophysiology of AF, both of which are present in kidney failure patients on any dialysis modality.28 It is possible that these processes overcome the initial modality benefit for AF risk. Another possibility for the initial benefit of PD in decreasing AF risk is better preservation of residual kidney function in PD.29 The decreased initial risk of incident AF in PD may be related to residual kidney function and further warrants study of incremental HD as previously discussed.
The strengths of this study include a large population representative of the older US dialysis population, adjustment for a large number of patient characteristics measured over 2 years prior to initiation of dialysis, sophisticated statistical analysis including multiple imputation for missing data and consideration of competing risks, as well as careful dissection of the time dependency of the association found. However, there are also certain limitation that require consideration. This study is observational and, hence, susceptible to residual confounding by unmeasured, or imprecisely measured, characteristics. Too few patients used home-hemodialysis for us to specifically evaluate this sub-modality. We relied on information submitted in billing claims to Medicare, which assumes accurate capture of AF. Several validation studies have demonstrated that this may be a valid approach.30 Still, studies confirming and further refining our observation using direct measurement of heart rhythm may be warranted. It is further unclear from claims whether atrial fibrillation was persistent, permanent, or paroxysmal, or whether it should be considered valvular or not. Medication information was unavailable for the vast majority of patients. Finally, it is unclear how these findings generalize to younger populations and populations and health care settings other than the United States.
In conclusion, we found that AF incidence differed between older patients initiating dialysis using PD versus HD in the United States, specifically that patients had increased AF risk over the first 3 months. Thereafter, the adjusted incidence of AF was similar between patients receiving HD versus PD. This study highlights the opportunity to further investigate the high rate of mortality, in particular as it relates to arrhythmia, in patients with ESRD new to dialysis.
Supplementary Material
Difference in select baseline characteristics between eligible vs excluded patients.
Association of dialysis modality and incident AF over 3 years.
Association of the first modality and incident AF from subdistribution hazard model.
Influence analysis of single confounders driving the differences in cause-specific hazard ratios between models 2 and 3.
Acknowledgments
Support: This work was supported by grants R01DK095024 (to Dr. Winkelmayer) and K23DK095914 (to Dr. Chang) from the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK, Bethesda, MA). Dr. Winkelmayer received salary and research support from the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine. The funders of this study did not have any role in study design; collection, analysis, and interpretation of the data; writing the report; or the decision to submit the report for publication.
Financial Disclosure: Dr. Winkelmayer reports having served as a scientific advisor to Akebia, AMAG Pharmaceuticals, Amgen, AstraZeneca, Bayer, Relypsa, and Vifor Fresenius Medical Care Renal Pharma, and on clinical trial committees (executive steering committees, event adjudication committees, data safety monitoring boards) for Akebia, the Duke Clinical Research Institute, and Medtronic. Dr. Navaneethan reports serving as a consultant to AbbVie, Akebia, Bayer, and Boehringer Ingelheim. Dr. Turakhia reports serving as a consultant to Precision Health Economics, Medtronic, and St. Jude Medical. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The manuscript was reviewed and approved for publication by an officer of the NIDDK. Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Prior Presentation: A preliminary version of this work titled, “Dialysis Modality and Incident Atrial Fibrillation in Older Patients with ESRD Initiating Dialysis” was presented on November 3 2017 as an abstract at the 2017 Kidney Week of the American Society of Nephrology in New Orleans, LA.
Peer Review: Received _______. Evaluated by 2 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Editorial Board Member Francis L. Weng, MD, MSCE). Accepted in revised form September 7, 2018. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
REFERENCES
- 1.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22(2):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein BA, Arce CM, Hlatky MA, Turakhia M, Setoguchi S, Winkelmayer WC. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation. 2012;126(19):2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res. 2017;120(9):1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320. [DOI] [PubMed] [Google Scholar]
- 5.Rubin AL, Lubash GD, Cohen BD, Brailovsky D, Braveman WS, Luckey EH. Electrocardiographic changes during hemodialysis with the artificial kidney. Circulation. 1958;18(2):227–234. [DOI] [PubMed] [Google Scholar]
- 6.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69(3 Suppl 1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montez-Rath ME, Winkelmayer WC, Desai M. Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol. 2014;9(7):1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konigsbrugge O, Posch F, Antlanger M, et al. Prevalence of Atrial Fibrillation and Antithrombotic Therapy in Hemodialysis Patients: Cross-Sectional Results of the Vienna InVestigation of AtriaL Fibrillation and Thromboembolism in Patients on HemoDIalysis (VIVALDI). PLoS One. 2017;12(1):e0169400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy-Chaudhury P, Tumlin JA, Koplan BA, et al. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney international. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27(10):3816–3822. [DOI] [PubMed] [Google Scholar]
- 12.Airy M, Chang TI, Ding VY, et al. Risk profiles for acute health events after incident atrial fibrillation in patients with end-stage renal disease on hemodialysis. Nephrol Dial Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129(11):1196–1203. [DOI] [PubMed] [Google Scholar]
- 14.Shen JI, Montez-Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes After Warfarin Initiation in a Cohort of Hemodialysis Patients With Newly Diagnosed Atrial Fibrillation. Am J Kidney Dis. 2015;66(4):677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih CJ, Ou SM, Chao PW, et al. Risks of Death and Stroke in Patients Undergoing Hemodialysis With New-Onset Atrial Fibrillation: A Competing-Risk Analysis of a Nationwide Cohort. Circulation. 2016;133(3):265–272. [DOI] [PubMed] [Google Scholar]
- 16.Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetmore JB, Mahnken JD, Rigler SK, et al. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney international. 2012;81(5):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vazquez E, Sanchez-Perales C, Garcia-Garcia F, et al. Atrial fibrillation in incident dialysis patients. Kidney international. 2009;76(3):324–330. [DOI] [PubMed] [Google Scholar]
- 19.Shen CH, Zheng CM, Kiu KT, et al. Increased risk of atrial fibrillation in end-stage renal disease patients on dialysis: A nationwide, population-based study in Taiwan. Medicine (Baltimore). 2016;95(25):e3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21(3):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perl J, Wald R, McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol. 2011;22(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6(11):2642–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. Journal of clinical epidemiology. 1994;47(7):731–737. [DOI] [PubMed] [Google Scholar]
- 24.Hanson JA, Hulbert-Shearon TE, Ojo AO, et al. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19(6):625–633. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Unruh M, Zager PG, et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64(2):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obi Y, Streja E, Rhee CM, et al. Incremental Hemodialysis, Residual Kidney Function, and Mortality Risk in Incident Dialysis Patients: A Cohort Study. Am J Kidney Dis. 2016;68(2):256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buiten MS, de Bie MK, Rotmans JI, et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart. 2014;100(9):685–690. [DOI] [PubMed] [Google Scholar]
- 28.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115(2):135–143. [DOI] [PubMed] [Google Scholar]
- 29.Marron B, Remon C, Perez-Fontan M, Quiros P, Ortiz A. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl. 2008(108):S42–51. [DOI] [PubMed] [Google Scholar]
- 30.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Difference in select baseline characteristics between eligible vs excluded patients.
Association of dialysis modality and incident AF over 3 years.
Association of the first modality and incident AF from subdistribution hazard model.
Influence analysis of single confounders driving the differences in cause-specific hazard ratios between models 2 and 3.


