Abstract
Percutaneous coronary intervention (PCI) is main treatment for acute coronary syndrome (ACS). However, restenosis caused by PCI-induced injury influences the outcome of patients. Linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, has been reported to ameliorate intimal hyperplasia post vascular injury. The underlying mechanisms by which linagliptin protects against balloon injury are unclear and require to be explored. Herein, Wistar rats with carotid artery balloon injury were given 1, 2 or 3 mg/kg/day linagliprin for 6 weeks. We found that linagliptin attenuated vascular injury-mediated neointima formation in rats without affecting body weight and blood glucose levels. ELISA results indicated that linagliptin significantly reduced overproduction of cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 post balloon injury. By detecting the level of malondialdehyde (MDA) and the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), we found that linagliptin prevented balloon injury-induced oxidative stress. Additionally, linagliptin decreased the level of Kelch ECH-associating protein 1 (KEAP1) compared with injury group. Results of Western blots and electrophoretic mobility shift assay (EMSA) demonstrated that linagliptin augmented nuclear accumulation of nuclear factor-E2-related factor 2 (NRF2) and its binding ability to target genes in rats with balloon injury. Moreover, heme oxygenase-1 (HO-1) and NAD (P) H quinine oxidoreductase 1 (NQO1), two downstream targets of NRF2, were further up-regulated after linagliptin treatment compared with injury group. In conclusion, our data suggest that linagliptin protects carotid artery from balloon injury-induced neointima formation and activates the NRF2 antioxidant pathway.
Keywords: balloon injury, inflammatory factors, linagliptin, NRF2 antioxidant pathway, oxidative stress
Introduction
ACS is a disease characterized by the coronary artery stenosis-mediated insufficient blood supply, and it leads to severe health and economic burden on society[20]. PCI is the widely accepted treatment for ACS. Whereas, it cannot be ignored that PCI-induced vascular injury caused inflammatory response and oxidative stress and ultimately resulted in intimal hyperplasia, which had an undesirable effects on the outcomes of patients suffered ACS[11, 12, 25]. In view of these, agents with properties of preventing the PCI-induced vascular injury may give new insights for managing the ACS.
DPP-4 inhibitors are effective drugs for treating type 2 diabetes mellitus due to its ability of increasing the duration time of GLP-1 [6]. Nowadays, accumulative evidence has indicated that DPP-4 inhibitors also had vascular protective effects including attenuating the progression of carotid intima-induced thickness in type 2 diabetes [21]. A report from Lim et al. showed that treatment with stiaglipitn prevented carotid injury-mediated elevation of intima/media ratio in obese diabetic rats, and the protective effects of this DPP-4 inhibitor may be correlated with preventing inflammation and inhibiting vascular smooth muscle cells proliferation [19]. In addition, one in vivo study demonstrated that another DPP-4 inhibitor linagliptin ameliorated the neointima formation caused by endothelial denudation injury partially through attenuating the oxidative stress [27]. It also has been confirmed that linagliptin exhibited vascular protective effects in the Zucker diabetic fatty rats, indicating that linagliptin has beneficial effects on treating vascular injury [24]. However, the effects and underlying mechanisms of linagliptin on carotid balloon injury are still unclear.
NRF2 is a transcription activator, which is essential for the cellular redox homeostasis especially during the defense against oxidative stress-induced endothelial damage [3, 23]. Once being activated by oxidative stress, NRF2 were uncoupled from KEAP1 and then translocated to nucleus to bound with the antioxidant response element (ARE) of target genes to promote the transcription of these antioxidant genes such as HO-1 and NQO1[3, 5]. It has been reported that activation of the NRF2 antioxidant pathway suppressed the proliferation of smooth muscle cells in vitro and attenuated the intravascular oxidative stress in vivo, which were beneficial for alleviating the vascular injury[17]. Furthermore, a study from Choi et al. showed that gemigliptin inhibited the vascular damage and neointimal hyperplasia caused by ligation injury through regulating the NRF2 signaling pathway in smooth muscle cells [4]. As gemigliptin and liangliptin both are DDP-4 inhibitors, we has been inspired that linagliptin may exert its vascular protective effects via preventing the oxidative stress by regulating the NRF2 signaling cascade.
In our present study, we employed the rat carotid balloon injury model to investigate the effects of linagliptin on the intimal hyperplasia caused by vascular injury. Moreover, the role of NRF2 antioxidant pathway in the protective effects of linagliptin against vascular injury was also been examined.
Materials and Methods
Animals
All the animal experiments were conducted in strict accordance with the Guidelines for the Institutional Animal Care and Use Committee of Jining First People’s Hospital. Male Wistar rats (8 weeks old) were purchased from Beijing HFK Bioscience Co., Ltd. and housed in a standard laboratory environment (21 ± 1°C; 45–55% humidity; 12 h light/12 h dark cycle; free access to feed and water). The Wistar rats were randomly divided into five groups: sham operation group (Sham); carotid artery balloon injury group (Injury); balloon injury with daily administration of 1 mg/kg linagliptin (1 mg/kg linagliptin); balloon injury with daily administration of 2 mg/kg linagliptin (2 mg/kg linagliptin); balloon injury with daily administration of 3 mg/kg linagliptin (3 mg/kg linagliptin).
Carotid artery balloon injury model
For balloon injury, the rats were anaesthetized using isoflurane with an inspiratory concentration at 3 vol%. Besides, buprenorphine (0.05 mg/kg body weight) were injected percutaneously 30 min before the operation for analgesic rescue.
After disinfected with iodine, the skin was incised along anterior median raphe of the neck. Then the common carotid artery, internal carotid artery, and external carotid artery of left side were exposed. The distal end of the external carotid artery was ligated. Besides, the internal carotid and the proximal end of common carotid were closed with an arterial clip. After that, an incision was made in external carotid artery, and a balloon angioplasty catheter (Edwards Lifesciences Corp, Irvine, CA, USA) was inserted through the external carotid to the aortic arch. Subsequently, the balloon was inflated by 5 times atmospheric pressure for 30 seconds followed with gently withdrawn, and this procedure has been repeated for 3 times to induce vascular injury. After the balloon catheter been removed, the external carotid artery was ligated and the wound was closed. The rats were intramuscularly injected with penicillin (2 × 105 U, Jiangxi Jinkangjia Biochemical Pharmaceutical Co., Ltd., Jiangxi, China) for 3 d after operation to prevent infection. Besides, rats in sham group went through all the same procedures expect the balloon catheter insertion.
During present work, rats in different groups were oral administration of linagliptin (solved in 5% sodium carboxymethyl cellulose) or equal volume of vehicle solution (5% sodium carboxymethyl cellulose, Sigma-Aldrich, St. Louis, MO, USA) 2 weeks before the operation and continued until 4 weeks post-balloon injury. Additionally, the body weight of rats was measured every 2 weeks after firstly treatment with linagliptin, while the blood glucose levels were detected just before the rats were euthanized. After the rats have been euthanized 4 weeks after the operation, the lesion site of the common carotid artery about 2 cm was derived.
Hematoxylin and Eosin (H&E) staining
H&E staining was used to evaluate the morphology changes of the vascular tissue. Briefly, the carotid artery tissues were fixed in 10% neutral formalin (Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China) overnight followed with embedded in paraffin. Paraffin tissue was sliced into 5 µm transverse histological sections, and then stained with hematoxylin (Solarbio, Beijing, China) and eosin (Sangon, Shanghai, China) according to the standard protocol. The stained sections were observed using OLYMPUS BX53 microscope (Tokyo, Japan). The areas of intima, media and lumen were measured.
ELISA assay
The production of inflammatory factors was determined by ELISA assay. The carotid artery tissues were homogenized in ice-cold normal saline, and the supernatants were harvested for further experiments. The protein levels of carotid artery tissues were quantified using a BCA kit (Beyotime, Shanghai, China). Subsequently, the levels of TNF-α, IL-1β and IL-6 were analyzed using the ELISA kits (MultiSciences (Lianke) Biotech Co., Ltd., Hangzhou, China) according to the manufacturer’s instructions.
Detection of MDA, SOD and GSH-Px
The levels of the oxidative stress-related factors in carotid artery tissues were estimated using the commercial kit according to the manufacturer’s instructions. MDA level was assessed by TBA method using MDA assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). SOD activity was measured using Total Superoxide Dismutase (T-SOD) assay kit (Hydroxylamine method, Nanjing Jiancheng Bioengineering Institute). Glutathione peroxidase activity was determined by GSH-Px assay kit (Colorimetric method, Nanjing Jiancheng Bioengineering Institute).
EMSA assay
EMSA assay was performed to assess the binding ability of NRF2 to the ARE. In short, nuclear protein were obtained from carotid artery tissues using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime) and quantified by a BCA kit. EMSA was carried out using the NRF2 Electrophoretic mobility shift assay Kit (Viagene Biotech, Inc. Beijing, China) to evaluate the NRF2-DNA binding activity. Reactions were performed in present of the biotin-labeled DNA probe and nuclear extracts. Then the DNA-protein complexes were separated on a 6.5% polyacrylamide gel. The separated proteins were transferred onto membranes and fixed by UV crosslinking for 30 min. Subsequently, the membranes were incubated with streptavidin-HRP, and the bands were visualized using enhanced chemiluminescence (ECL).
Western blot
The unclear proteins and total proteins were extracted from artery tissues by Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime) or RIPA lysis buffer respectively as previously described. After quantified by the BCA Protein Assay Kit (Beyotime), the protein from different conditions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to PVDF membranes (Millipore, Boston, MA, USA). Subsequently, the membranes were blocked with 5% non-fat milk and incubated with primary antibodies against NRF2 (1:1,000; Abcam, Cambridge, MA, USA), KEAP1 (1:1,000; Abcam), HO-1 (1:1,000; Abcam), NQO1 (1:1,000; BOSTER, Wuhan, China), β-actin (1:500; Bioss, Beijing, China), Histone H3 (1:500; Bioss) at 4°C overnight. Following, membranes were incubated with HRP-secondary antibodies (Beyotime) at 37°C for 45 min. Finally, protein bands were visualized using an ECL reagent (Beyotime) and the relative levels of the proteins of interest were calculated by Gel-Pro-Analyzer.
Statistical analysis
The data were shown as mean ± SD, and GraphPad Prism 6 (Graph Pad Software, San Diego, CA, USA) was used to perform the statistical analysis. One-way analysis of variance (ANOVA) followed with Bonferroni test was carried out for analyzing statistical significance. P<0.05 was considered statistically significant.
Results
Effects of linagliptin on the body weight and blood glucose level
We firstly evaluated weather administration of linagliptin influenced the body weight and the levels of blood glucose in rats. As shown in Fig. 1A, even treatment with linagliptin at 3 mg/kg up to 6 weeks hardly affected the body weight. Further, there were no differences between the rats treated with or without linagliptin on blood glucose level (Fig. 1B).
Fig. 1.
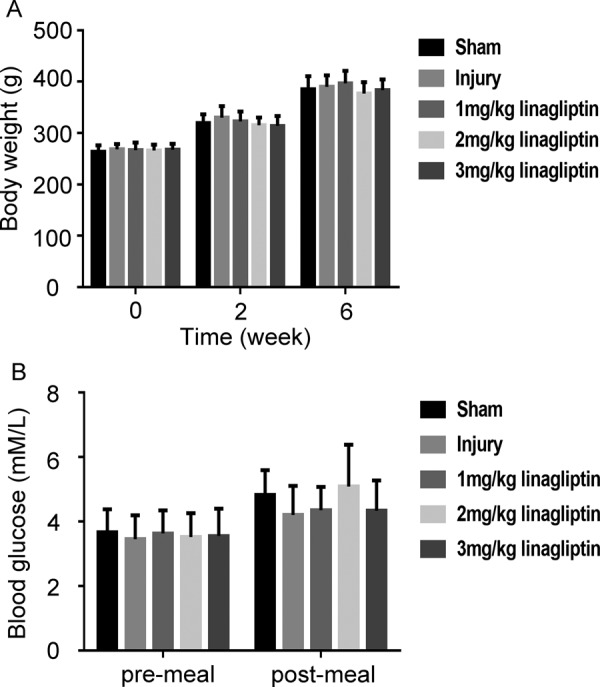
Linagliptin had no effects on body weight and blood glucose level in rats. The body weight (A) and the levels of blood glucose (B) in rats of different groups were detected. All data were presented as mean ± SD (n=6 for each group. one-way ANOVA followed by the Bonferroni test).
Effects of linagliptin on the neointima formation
H&E staining was used to assess the effects of linagliptin on neointima formation induced by balloon injury. As shown in Fig. 2A, the artery of rats in sham group showed normal morphology of vascular. The images of vascular showed obvious smooth muscle cell proliferation, irregular arrangement, intimal thickening and narrowing of lumen area in injury group, whereas administration of linagliptin reversed this phenomenon. The quantitative analysis were presented in Fig. 2B, there were a reduction of luminal area and an elevation of intimal area in injury group compared with sham group, and this trend was abrogated by linagliptin. There were no statistic differences on the medial area between sham, injury and linagliptin group. Whereas, I/M ratio was increased in injury group compared with sham group, and treatment with linagliptin attenuated the phenomenon. We further performed H&E staining to investigate the influence of linagliptin alone on the arterial morphology. As shown in Supplementary Fig. 1, the right carotid artery from different groups did not show difference in arterial morphology.
Fig. 2.
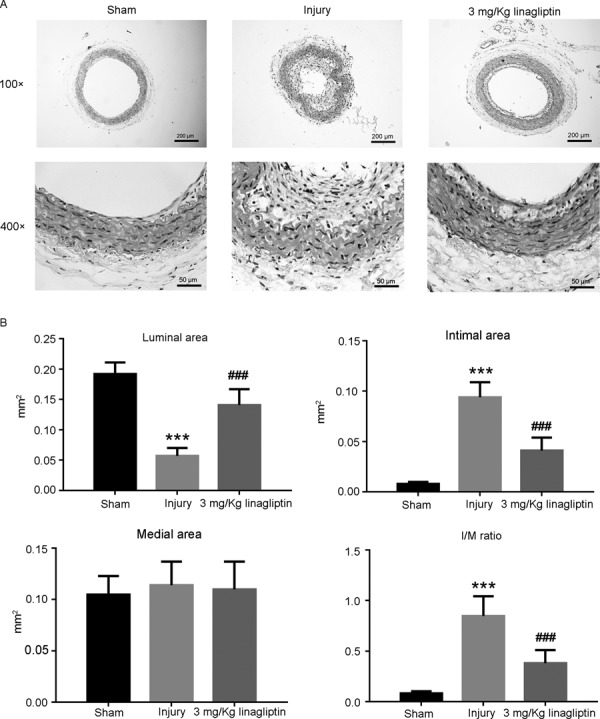
Linagliptin ameliorated neointima formation post carotid artery balloon injury. Histological changes of carotid arteries were evaluated by H&E staining (A), The area of lumen, intima, media and the I/M ratio were calculated (B). All data were presented as mean ± SD (n=6 for each group. ***P<0.001 compared with sham group; ###P<0.001 compared with injury group; one-way ANOVA followed by the Bonferroni test).
Effects of linagliptin on the production of inflammatory factors
We performed ELISA assay to investigate the influence of linagliptin on balloon injury-induced generation of inflammatory cytokines. As shown in Fig. 3, the levels of TNF-α, IL-1β and IL-6 in injury group were significantly increased compared with sham group, and treatment with linagliptin ameliorated this phenomenon dose dependently.
Fig. 3.
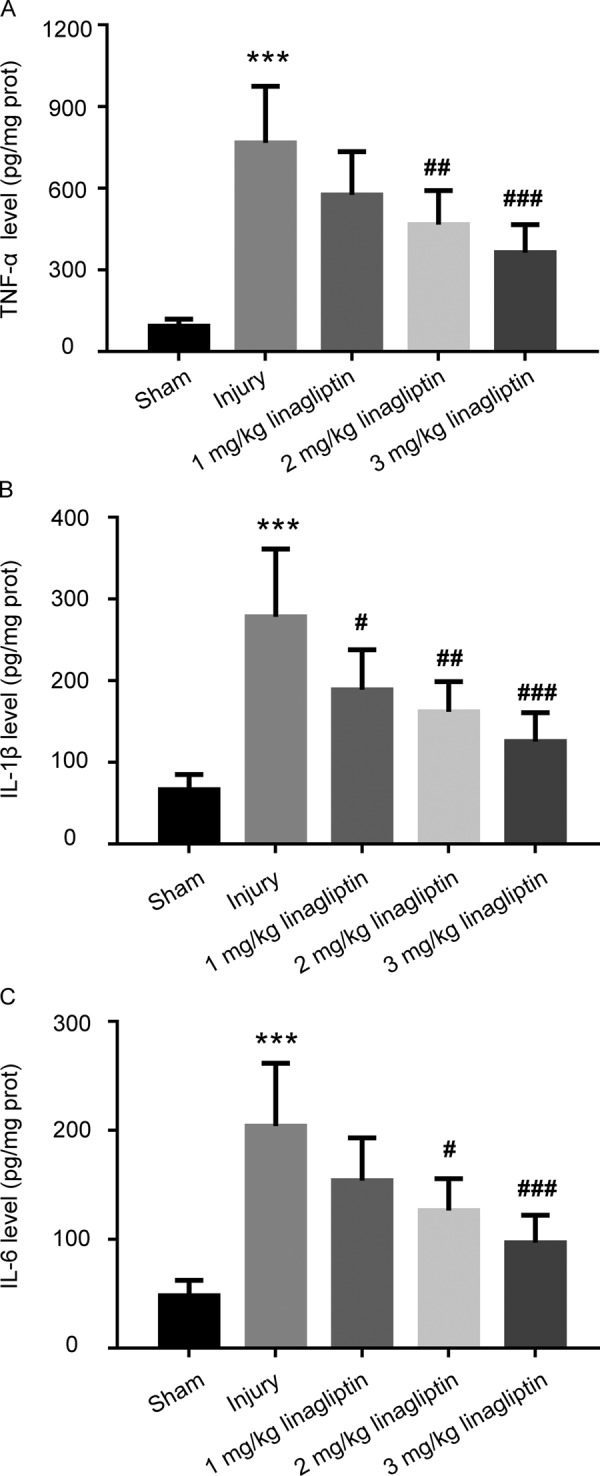
Linagliptin inhibited the generation of inflammatory factors induced by balloon injury. The production of TNF-α (A), IL-1β (B) and IL-6 (C) were measured by ELISA assay. The data were presented as mean ± SD (n=6 for each group. ***P<0.001 compared with sham group; #P<0.05, ## P<0.01, ### P<0.001 compared with injury group; one-way ANOVA followed by the Bonferroni test).
Effects of linagliptin on the oxidative stress
In order to evaluate the influence of linagliptin on the oxidative stress mediated by balloon injury, the level of MDA and the activities of SOD and GSH-Px have been assessed. As shown in Fig. 4A, linagliptin reversed the balloon injury-induced elevation of MDA level. Furthermore, the decreased activities of SOD and GSH-Px caused by balloon injury were attenuated by administration of linagliptin (Figs. 4B and C).
Fig. 4.
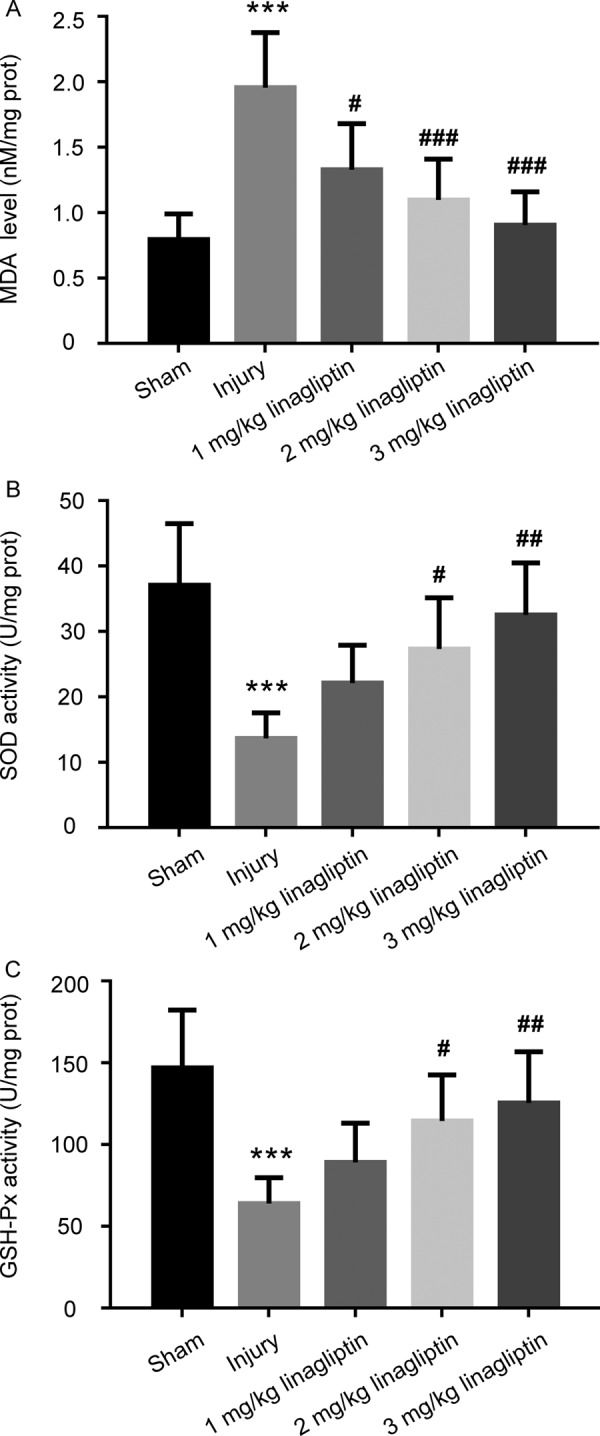
Linagliptin suppressed the oxidative stress mediated by balloon injury. The content of MDA (A) and the activities of SOD (B) and GSH-Px (C) in the carotid arteries tissues were detected. The data were presented as mean ± SD (n=6 for each group. ***P<0.001 compared with sham group; #P<0.05, ## P<0.01, ### P<0.001 compared with injury group; one-way ANOVA followed by the Bonferroni test).
Effects of linaglitin on the NRF2 antioxidant pathway
The expression of KEAP1, nuclear NRF2, HO-1 and NQO1 were determined by western blots. As shown in Fig. 5A, the levels of NRF2, HO-1 and NQO1 were increased significantly in linagliptin group compared with injury group, while the level of KEAP1 was decreased. Besides, the EMSA results indicated that administration of linagliptin enhanced the binding ability of NRF2 to ARE compared with balloon injury group (Fig. 5B).
Fig. 5.
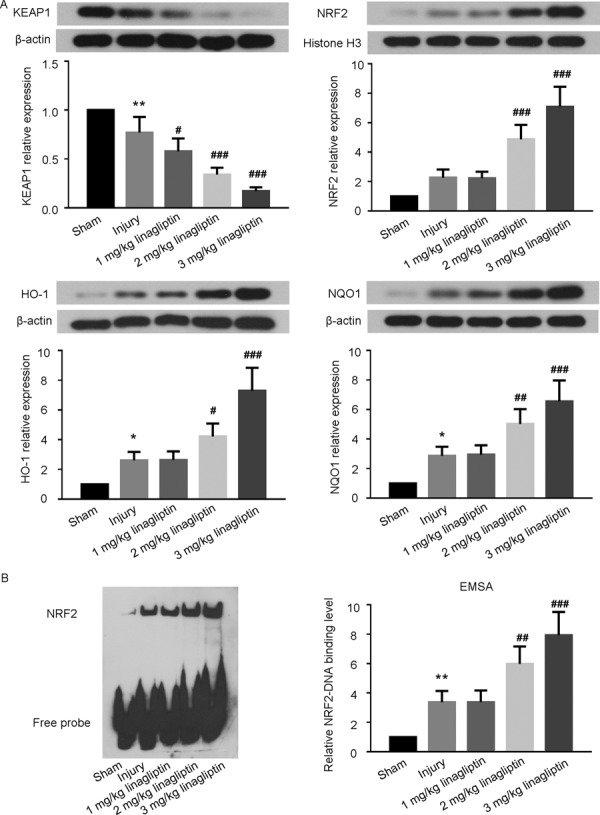
Linagliptin activated the NRF2 antioxidant pathway. The expression levels of the KEAP1, nuclear NRF2, HO-1 and NQO1 were determined by Western blot (A). The binding capacity of NRF2 to ARE was assessed by EMSA (B).The data were presented as mean ± SD (n=6 for each group. *P<0.05, **P<0.01, compared with sham group; #P<0.05, ## P<0.01, ### P<0.001 compared with injury group; one-way ANOVA followed by the Bonferroni test).
Discussion
To date, PCI is still the main mean for treating ACS. Unfortunately, the vascular injury caused by PCI and the following restenosis limits its efficacy [12]. Linagliptin, which widely used for treating diabetes, has been reported to have ability of inhibiting neointima formation recently [27]. It also showed properties of inhibiting the proliferation of smooth muscle cells in vitro and preventing inflammation in vivo [9]. The merits of our present work were to demonstrate that administration of linagliptin could protect rats from carotid balloon injury, and the NRF2 antioxidant pathway may be involved in the vascular protective effects of linagliptin.
Consideration of the therapeutic effects of DPP-4 inhibitors on diabetes and the report that administration of sitagliptin at concentration effective for attenuating intimal hyperplasia influenced the blood glucose levels in rats, we firstly evaluated the effects of linagliptin on the body weight and blood glucose levels [19, 26]. We found that neither carotid balloon injury nor orally administration of 3 mg/kg linagliptin daily for 6 weeks had any effects on body weight and blood glucose levels in rats. Our data were consistent with Terawaki’s study, which observed that treatment with linagliptin at 3 mg/kg/day ameliorated the intimal hyperplasia caused by vascular injury without affecting the concentrations of blood glucose [27].
In view of these, we chose 1, 2 and 3 mg/kg linagliptin to investigate its vascular protective effects on carotid balloon injury in present study. According to the results of H&E staining, we found that administration of linagliptin significantly reduced the elevation of intimal area and I/M ratio induced by balloon injury. The above results suggested that linagliptin may be a potential agent for treating PCI-induced intimal hyperplasia without influencing the body weight and glucose blood levels.
Furthermore, accumulative literatures have revealed that inflammation played an essential role in the neointima formation caused by balloon injury. Once causing iatrogenic trauma and damage during PCI, the platelets, macrophages and activated leukocytes in the damaged area secreted various inflammatory factors including IL-1, IL-6, MCP-1 and so on [7, 12]. These agents promoted the intimal hyperplasia via facilitating the SMC proliferation and migration, activating platelets and recruiting monocytes [16].
One in vivo study demonstrated that mangnolol ameliorated the intimal hyperplasia formation via suppressing the proliferation of vascular smooth muscle cells (VSMCs) through inhibiting the activation of NF-κB and the generation of TNF-α [14]. In addition, a report from Li et al. showed that anagliptin, the DPP-4 inhibitor, attenuated neointima formation in rats suffered balloon injury by preventing the production of inflammatory cytokines and chemokines [18]. In agreement with these studies, we found that the levels of TNF-α, IL-1β and IL-6 in the cervical aorta of rats were increased after balloon injury, and linagliptin inhibited the production of these inflammatory factors dose dependently. This phenomenon suggested that linagliptin may exert its vascular protective effects partially through preventing the inflammatory response. Moreover, consideration of the closely association between inflammation and PCI-induced SMC proliferation and the importance of SMC proliferation for intimal hyperplasia, we wonder if linagliptin could prevent the neointima formation by regulating SMC proliferation [22]. Further in vivo and in vitro studies will be carried out to confirm the hypothesis.
Additionally, ample studies revealed that DPP-4 inhibitors may also exerted its vascular protective effects via inhibiting oxidative stress, which was closely associated with pathogenesis and progress of PCI-mediated intimal hyperplasia [1]. It has been reported that the production of oxidative stress-related factors especially reactive oxygen species was significantly increased after PCI-induced injury, and led to SMCs proliferation, endothelial dysfunction, matrix remodeling and ultimately caused restenosis [13]. During the oxidative stress-related vascular injury, SOD and GSH-Px are important antioxidant enzymes to reduce oxidative stress injury, and MDA is the product of lipid oxidative damage [8]. Besides, it has been indicated that compound could suppress balloon injury-induced neointimal hyperplasia through attenuating oxidative stress by regulating the MAPK pathway [29]. Consist with these studies, we found that linagliptin reversed the increased level of MDA after balloon injury. It also attenuated the decrease of SOD and GSH-Px activities induced by carotid balloon injury. These results demonstrated that the property of anti-oxidative stress may contribute to the vascular protective effects of linagliptin against balloon injury.
With the objective of elucidating the underlying mechanisms of linagliptin against neointima formation mediated by carotid balloon injury, we evaluated the effects of linagliptin on the NRF2 pathway. As a transcription activator, NRF2 protects vascular tissues from oxidative stress injury via promoting the transcription of antioxidant genes such as HO-1 and NQO1 [3]. During this process, the interaction of NRF2-KEAP1 has been resolved and leads to the activation of NRF2 [2]. In present study, we observed that administration of lingaliptin further decreased the KEAP1 expression while increased the nuclear expression and the binding ability of NRF2 after balloon injury. Besides, the levels of HO-1 and NQO1, the downstream targets of NRF2, were also increased by lingaliptin treatment. The data suggested that the NRF2 antioxidant pathway may be involved in the vascular protective effects of lingaliptin against carotid balloon injury. Our current findings were in consistent with Kim’s study, which demonstrated that agent could ameliorate carotid artery injury-induced neointimal hyperplasia through activating the NRF2/HO-1 axis [15]. Furthermore, the slight activation of NRF2 antioxidant pathway after balloon injury may be associated with the defense against vascular damage by organism itself. It has been confirmed that various changes have been carried out in carotid to against oxidative stress after PCI-induced vascular injury. Activation of the NO signaling pathway to prevent the oxidative stress injury-induced neointimal formation was one of these changes [10]. Besides, Zhang and colleagues got similar results with us during their research, they observed that the NRF2 antioxidant pathway was activated after ox-LDL induced vascular damage [28]. In order to further clarify the role of NRF2 signaling pathway in the vascular protective effects of lingaliptin, the adenovirous-mediated silencing of NRF2 will be performed in our following studies.
In conclusion, the present study shows that linagliptin attenuates carotid balloon injury induced-intimal hyperplasia. The vascular protective effects of linagliptin may be correlated with inhibiting the production of cytokines, regulating the levels of oxidative stress-related factors, and activating the NRF2 antioxidant pathway. In view of these, linagliptin may be a promising agent for preventing and treating PCI-mediated vascular restenosis.
Supplementary Material
Acknowledgments
This study was supported by grants from the Science and Technology Development Project for Medicine and Health of Shandong Province (No. 2015WS0427) and the Jining Science and Technology Development Project (No. 2014jnwk04).
Reference
- 1.Avogaro A., de Kreutzenberg S., Fadini G.2014. Dipeptidyl-peptidase 4 inhibition: linking metabolic control to cardiovascular protection. Curr. Pharm. Des. 20: 2387–2394. doi: 10.2174/13816128113199990474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan H.K., Olayanju A., Goldring C.E., Park B.K.2013. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 85: 705–717. doi: 10.1016/j.bcp.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 3.Chen B., Lu Y., Chen Y., Cheng J.2015. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 225: R83–R99. doi: 10.1530/JOE-14-0662 [DOI] [PubMed] [Google Scholar]
- 4.Choi S.H., Park S., Oh C.J., Leem J., Park K.G., Lee I.K.2015. Dipeptidyl peptidase-4 inhibition by gemigliptin prevents abnormal vascular remodeling via NF-E2-related factor 2 activation. Vascul. Pharmacol. 73: 11–19. doi: 10.1016/j.vph.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 5.Cullinan S.B., Gordan J.D., Jin J., Harper J.W., Diehl J.A.2004. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24: 8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deacon C.F., Holst J.J.2013. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin. Pharmacother. 14: 2047–2058. doi: 10.1517/14656566.2013.824966 [DOI] [PubMed] [Google Scholar]
- 7.Delafontaine P.1998. Growth factors and vascular smooth muscle cell growth responses. Eur. Heart J. 19:(Suppl G): G18–G22. [PubMed] [Google Scholar]
- 8.El Assar M., Angulo J., Rodríguez-Mañas L.2013. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 65: 380–401. doi: 10.1016/j.freeradbiomed.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 9.Eriksson L., Röhl S., Saxelin R., Lengquist M., Kronqvist M., Caidahl K., Östenson C.G., Razuvaev A.2017. Effects of Linagliptin on Vessel Wall Healing in the Rat Model of Arterial Injury Under Normal and Diabetic Conditions. J. Cardiovasc. Pharmacol. 69: 101–109. [DOI] [PubMed] [Google Scholar]
- 10.Gallo G., Pierelli G., Forte M., Coluccia R., Volpe M., Rubattu S.2018. Role of oxidative stress in the process of vascular remodeling following coronary revascularization. Int. J. Cardiol. 268: 27–33. doi: 10.1016/j.ijcard.2018.05.046 [DOI] [PubMed] [Google Scholar]
- 11.Inoue T., Croce K., Morooka T., Sakuma M., Node K., Simon D.I.2011. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc. Interv. 4: 1057–1066. doi: 10.1016/j.jcin.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jukema J.W., Verschuren J.J., Ahmed T.A., Quax P.H.2011. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat. Rev. Cardiol. 9: 53–62. doi: 10.1038/nrcardio.2011.132 [DOI] [PubMed] [Google Scholar]
- 13.Juni R.P., Duckers H.J., Vanhoutte P.M., Virmani R., Moens A.L.2013. Oxidative stress and pathological changes after coronary artery interventions. J. Am. Coll. Cardiol. 61: 1471–1481. doi: 10.1016/j.jacc.2012.11.068 [DOI] [PubMed] [Google Scholar]
- 14.Karki R., Ho O.M., Kim D.W.2013. Magnolol attenuates neointima formation by inducing cell cycle arrest via inhibition of ERK1/2 and NF-kappaB activation in vascular smooth muscle cells. Biochim. Biophys. Acta 1830: 2619–2628. doi: 10.1016/j.bbagen.2012.12.015 [DOI] [PubMed] [Google Scholar]
- 15.Kim J.Y., Cho H.J., Sir J.J., Kim B.K., Hur J., Youn S.W., Yang H.M., Jun S.I., Park K.W., Hwang S.J., Kwon Y.W., Lee H.Y., Kang H.J., Oh B.H., Park Y.B., Kim H.S.2009. Sulfasalazine induces haem oxygenase-1 via ROS-dependent Nrf2 signalling, leading to control of neointimal hyperplasia. Cardiovasc. Res. 82: 550–560. doi: 10.1093/cvr/cvp072 [DOI] [PubMed] [Google Scholar]
- 16.Lee M.S., David E.M., Makkar R.R., Wilentz J.R.2004. Molecular and cellular basis of restenosis after percutaneous coronary intervention: the intertwining roles of platelets, leukocytes, and the coagulation-fibrinolysis system. J. Pathol. 203: 861–870. doi: 10.1002/path.1598 [DOI] [PubMed] [Google Scholar]
- 17.Levonen A.L., Inkala M., Heikura T., Jauhiainen S., Jyrkkänen H.K., Kansanen E., Määttä K., Romppanen E., Turunen P., Rutanen J., Ylä-Herttuala S.2007. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 27: 741–747. doi: 10.1161/01.ATV.0000258868.80079.4d [DOI] [PubMed] [Google Scholar]
- 18.Li Q., Wu X., Liu Y., Zhang M., Bai X., Chen C.2017. The effect of anagliptin on intimal hyperplasia of rat carotid artery after balloon injury. Mol. Med. Rep. 16: 8003–8010. doi: 10.3892/mmr.2017.7667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim S., Choi S.H., Shin H., Cho B.J., Park H.S., Ahn B.Y., Kang S.M., Yoon J.W., Jang H.C., Kim Y.B., Park K.S.2012. Effect of a dipeptidyl peptidase-IV inhibitor, des-fluoro-sitagliptin, on neointimal formation after balloon injury in rats. PLoS One 7: e35007. doi: 10.1371/journal.pone.0035007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makki N., Brennan T.M., Girotra S.2015. Acute coronary syndrome. J. Intensive Care Med. 30: 186–200. doi: 10.1177/0885066613503294 [DOI] [PubMed] [Google Scholar]
- 21.Mita T., Katakami N., Shiraiwa T., Yoshii H., Onuma T., Kuribayashi N., Osonoi T., Kaneto H., Kosugi K., Umayahara Y., Yamamoto T., Matsumoto K., Yokoyama H., Tsugawa M., Gosho M., Shimomura I., Watada H., Collaborators on the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE) Trial.2016. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the sitagliptin preventive study of intima-media thickness evaluation (SPIKE): a randomized controlled trial. Diabetes Care 39: 455–464. doi: 10.2337/dc15-2145 [DOI] [PubMed] [Google Scholar]
- 22.Ross R.1995. Cell biology of atherosclerosis. Annu. Rev. Physiol. 57: 791–804. doi: 10.1146/annurev.ph.57.030195.004043 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T., Yamamoto M.2015. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 88:(Pt B): 93–100. doi: 10.1016/j.freeradbiomed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Takai S., Sakonjo H., Jin D.2014. Significance of vascular dipeptidyl peptidase-4 inhibition on vascular protection in Zucker diabetic fatty rats. J. Pharmacol. Sci. 125: 386–393. doi: 10.1254/jphs.14052FP [DOI] [PubMed] [Google Scholar]
- 25.Tardif J.C., Grégoire J., L’Allier P.L.2002. Prevention of restenosis with antioxidants: mechanisms and implications. Am. J. Cardiovasc. Drugs 2: 323–334. doi: 10.2165/00129784-200202050-00005 [DOI] [PubMed] [Google Scholar]
- 26.Tella S.H., Rendell M.S.2015. DPP-4 inhibitors: focus on safety. Expert Opin. Drug Saf. 14: 127–140. doi: 10.1517/14740338.2015.977863 [DOI] [PubMed] [Google Scholar]
- 27.Terawaki Y., Nomiyama T., Kawanami T., Hamaguchi Y., Takahashi H., Tanaka T., Murase K., Nagaishi R., Tanabe M., Yanase T.2014. Dipeptidyl peptidase-4 inhibitor linagliptin attenuates neointima formation after vascular injury. Cardiovasc. Diabetol. 13: 154. doi: 10.1186/s12933-014-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H.P., Zheng F.L., Zhao J.H., Guo D.X., Chen X.L.2013. Genistein inhibits ox-LDL-induced VCAM-1, ICAM-1 and MCP-1 expression of HUVECs through heme oxygenase-1. Arch. Med. Res. 44: 13–20. doi: 10.1016/j.arcmed.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Chen J., Yang J., Xu C.W., Pu P., Ding J.W., Jiang H.2013. Resveratrol attenuates oxidative stress induced by balloon injury in the rat carotid artery through actions on the ERK1/2 and NF-kappa B pathway. Cell. Physiol. Biochem. 31: 230–241. doi: 10.1159/000343364 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


