Abstract
Chronic stress has been associated with impairment of memory, learning, and social cognition. In animal studies, chronic stress has been shown to impair rodent sociability behaviour which mimics social withdrawal as observed in depression patients. The effect of chronic stress on social recognition, however, is uncertain. Moreover, with reference to spatial learning and memory, the effect of chronic stress is dependent on the type of behavioural task: an appetitively or aversively motivated tasks. The effect of chronic stress was consistent in impairing spatial learning and memory in the appetitive task; however, the effect was inconsistent in an aversive task like the Morris water maze. Thus, we aimed to investigate the effect of chronic restraint stress on sociability and social recognition by using a modified protocol of the three-chamber paradigm and the effect of chronic restraint stress on spatial learning and memory by using the Morris water maze test in young adult C57BL/6J male mice. The present report also describes a modified protocol of the three-chamber paradigm. Our modification is based on measurement of sniffing behaviour, which is a direct social interaction that represents sociability. We used the chronic restraint stress paradigm for 6 h/day for 21 days to induce depression-like symptoms in male C57BL/6J mice which were validated by forced-swim test. We observed that the stressed group had impairments in their sociability behaviour but that social recognition was not affected. Furthermore, we confirmed that chronic stress produced no significant impairment in spatial learning and memory of the mice in the water maze.
Keywords: learning, memory, recognition, social, stress
Introduction
Chronic stress, when it exceeds what a human’s body can take, has a detrimental effect on brain cognition such as memory and learning, social cognition, and emotional function. In rodent studies, the chronic stress model has been shown to produce a set of behavioural alterations that parallels the symptoms of depression, which makes it a more valid model of depression than any other models of depression, like acute stress or iatrogenic depression models [17].
Chronic stress has been shown to reduce social motivation and social interaction in a variety of sociability tests conducted in rodents, which reflect the social withdrawal behaviour in depressed patients [31, 32]. However, it has not been clear whether chronic stress also impairs social recognition, which is a condition more relevant to autism. Social recognition can be measured by conducting a social novelty preference task. In the present study, we aimed to investigate the effect of chronic stress on sociability and social recognition in mice by using a modified protocol of the sociability and social novelty preference test also known as the three-chamber paradigm. In this modified protocol, we measured sociability and social recognition in the aspect of sniffing behaviour of the test mice. The modification is presented and discussed in greater detail in the methodology and discussion sections.
The effect of chronic stress on spatial learning and memory in a rodent study is task-specific; that is, it depends on the nature of the behavioural task, such as either an aversively or appetitively motivated task. The Morris water maze (MWM) is an aversively motivated task and is commonly used to evaluate spatial learning and memory function. Most previous studies in rats have shown that chronic stress impairs spatial learning. However, the effect of chronic stress on spatial memory (long-term memory) is rather ambiguous, as some studies have found it to be insignificant, and a few have even reported that it facilitated spatial memory in the MWM test [7]. In addition, there are only a few studies that had been conducted in mice compared with rats. Thus, we examined the effect of chronic stress on spatial learning and memory of the C57BL/6J mice in the MWM test. In the present study, we used the chronic restraint stress (CRS) method to induce chronic stress in the test mice. Even with variations in the restraint period across previous studies, chronic stress restraint for 6 h per day for 21 days (6h/d/21d) has been found to be the most reliable and efficient way of inducing psychological stress in a test animal [19]. Therefore, we chose the CRS method for 6h/d/21d to induce chronic stress in our animal subjects.
Materials and Methods
Experimental animals
The experimental protocol adopted for the present study was approved by the Committee on Animal Research and Ethics, Universiti Teknologi Mara (UiTM) Selangor, Puncak Alam, Malaysia. The C57BL/6J mouse strain was used in this study. Male C57BL/6J mice aged of 6–7 weeks were purchased from Monash University, Malaysia. The animals were housed in a group of 3–4 animals in individually ventilated cages and maintained under 12:12 light/dark cycle conditions (lights on at 07:00). Food and drinking water were provided ad libitum except during the chronic stress treatment. All the behavioural tasks were performed during the light phase between 09:00 and 17:00.
Camera and software used
All the experiments performed in this study were recorded with a camera mounted on the ceiling located above the test apparatus. The video camera was connected to a computer in the test room. The ANY-maze video tracking software was used to automatically track and analyse the entire behavioural tasks performed in this study.
Chronic stress treatment
For the chronic stress treatment, the mice were restrained in a container for 6h/d/21d. The treatment was performed on mice aged 9–10 weeks. After 21 days of the treatment, the behavioural tasks were performed on the mice at the age of 12–13 weeks. During the chronic stress treatment, the control group was left undisturbed in their cages. The container was fabricated from steel by a local craftsman from the Faculty of Engineering, University of Malaya. It consisted of eight compartments which could be opened or closed by sliding doors. Each compartment was of the same size of 5.5 (L) × 5 (W) × 5 (H) cm. The bottom of the container was smooth and solid, while sturdy wire meshes made up the top. Impraboard was used to adjust the size of the compartment to fit the growing individual mouse during the treatment period. The impraboard was cut with a pair of scissors according to the compartment size. Cut pieces of impraboards were then stacked at the side of the compartment wall until the size of the compartment was tight enough to restrain the subject mouse from swivelling freely. We validated the ability of chronic restraint stress to cause depression-like symptoms by measuring the performance of the test mice in the forced-swim test (FST). The body weight of the test mice was recorded during the 21 days of chronic stress treatment and compared with that of control mice.
Behavioural tasks
A day after chronic stress treatment cessation, approximately 24 h after an FST was performed on test mice, and this was followed by a sociability and social novelty preference test performed on the following 4 days. The MWM experiment was carried out by using a separate set of mice from the set of mice utilized in the FST and the sociability tests. Likewise, the MWM test was started approximately 24 h after the cessation of chronic stress treatment.
Forced-swim test (FST): Briefly, a glass beaker (1 litter) was filled with 700 ml of tap water at 25–27°C. Each mouse was subjected to swimming for 6 min per trial. The mouse was released gently into the beaker, and the animal behaviour was recorded and analysed by the ANY-maze software. For the FST, the parameters measured were 1) total time of immobility, 2) total episode of immobility, 3) animal’s body rotations − clockwise and anticlockwise, and 4) latency to start of the first immobile episode.
Sociability and social novelty preference test: The three-interconnected chamber apparatus used for this test was fabricated from a clear Plexiglas material. One of the outer chambers was designated the Social Chamber, the other outer chamber was designated Novelty Chamber, and the chamber at the centre was designated the Centre Chamber (Fig. 1). The apparatus (60 cm L × 40.5 cm W × 22 cm H) was divided by two removable partitions which formed three chambers of similar size. Both partitions had one central sliding door (4.0 cm W × 3.5 cm L) which could be lifted up to form a channel between the chambers. The outer chambers were divided virtually into two zones of the same size in the video tracking software: an Empty Zone and a Holding Zone. The two metal cylinder-shaped cages (11 cm D × 9 cm H) were placed in the centre of the Holding Zones to entrap two stranger mice, one in each Holding Zone. The cylinder cages had equal-sized vents (1 cm W) to allow perforation and limited interaction between animals. The Empty Zone was designated as the area in which the test mouse moves away from the entrapped Stranger mouse, while the Holding Zone was designated as the area in which the test mouse moves closer to the entrapped Stranger mouse. A schematic diagram of the three-chamber apparatus as viewed from the top and in three dimensions is depicted in Fig. 1.
Fig. 1.
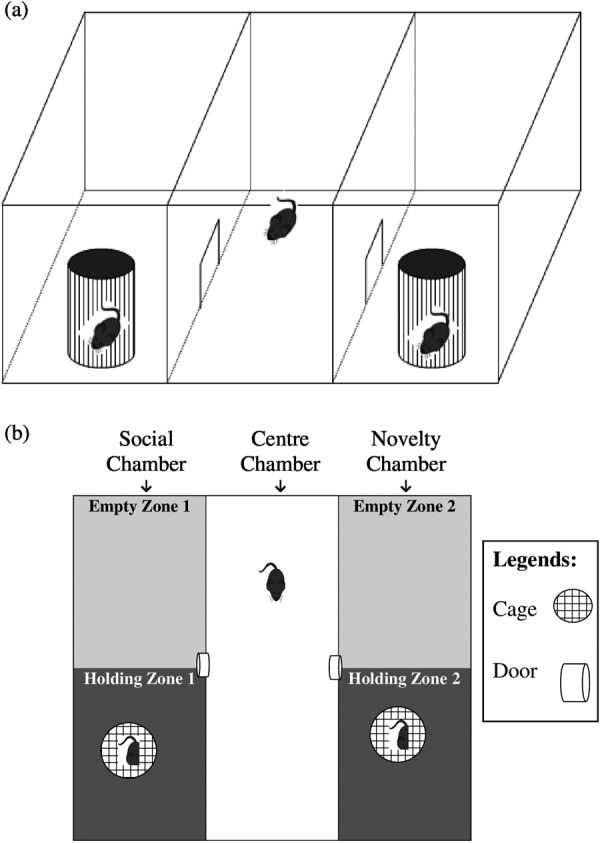
A three-interconnected chamber apparatus for sociability and social novelty preference tasks. (a) The apparatus consisted of 3 similar-sized chambers that were interconnected by two doors located in both dividers (3-D view). (b) Both sides of the chambers consisted of a Holding Zone and Empty Zone that were virtually divided in the video tracking software (top view from of the video tracking software). The sociability phase was started by the opening of the door which was connected Social Chamber to Centre Chamber, while the door which was connected to Novelty Chamber to Centre Chamber remained closed. During the sociability phase, the test mouse had a choice as to whether to socialize with stranger mouse 1 in Holding Zone 1 or to move away to either Empty Zone 1 or the Centre Chamber. For the social novelty preference task, which was performed after completion of the sociability phase, the trial started with the opening of both doors. In the social novelty preference task, the test mouse had a choice as to whether to socialize with the stranger mouse 1 (now familiar) in Holding Zone 1 or stranger mouse 2 (unfamiliar) in Holding Zone 2.
The sociability and social novelty preference test protocols were adopted from Moy [21] with slight modifications. Basically, the present test protocol had 3 phases that occurred in sequence: habituation, sociability, and social novelty preference. Before starting the test, Stranger mouse 1 and Stranger mouse 2 were acclimatized for 20 min in the cylinder cages located in Holding Zone 1 and Holding Zone 2, respectively. The stranger mice were kept in the cylinder cages during all three phases of the test. For every two complete tests, the two stranger mice were alternated between the outer chambers. The habituation phase was started by allowing the test mouse to habituate in the Centre Chamber for 5 min with the doors connected to both of the adjacent chambers closed. There was no recording in the habituation phase. After 5 min of habituation, the door that connected the Social Chamber to the Centre Chamber was lifted; meanwhile the door that was connected to the Novelty Chamber remained close.
The sociability phase was started immediately after the test mouse entered the Social Chamber, and the recording was then started. Entry was defined as when the centre of the mouse’s body crossed into an outer chamber from the Centre Chamber which was detected automatically by the video tracking software. The test mouse was allowed to explore the Centre Chamber and Social Chamber 1 for a duration of 5 min.
After undergoing the sociability phase, the test mouse then proceeded to the second phase, namely, the social novelty preference phase. The door connected to the Novelty Chamber was lifted, and the test mouse was gently transferred to Empty Zone 2 in the Novelty Chamber. Immediately following that, the social novelty preference phase was started, the duration of which was 5 min. The test mouse was allowed to explore all the three chambers. The sociability and social novelty preference test is illustrated in Fig. 1.
In the present study, the main and supporting parameters were obtained during both the sociability and social novelty preference phases. During the sociability phase, the main parameters obtained were sniffing behaviours of the test mouse toward Stranger Mouse 1; meanwhile, during the social novelty preference phase, the main parameters were sniffing behaviours of the test mouse to both Stranger Mouse 1 (now familiar) and Stranger Mouse 2 (unfamiliar). The sniffing behaviour of the test mouse toward the Stranger mouse was distinguished into three parameters, namely the number of sniffs, the intensity of sniffing, and the absolute intensity of sniffing. The number of sniffs was defined as the frequency of sniffing which can be interpreted as the frequency of social interaction that takes place. The intensity of sniffing was defined as the mean duration of direct sniffing, which can be interpreted as the motivation level to engage in the social interaction. The absolute intensity of sniffing was defined as the maximum duration of direct sniffing in a trial, which may reflect the highest motivation level to engage in the social interaction.
The sniffing behaviour was scored by a human observer using a computer in real time, with the number of keyboard pressed is equal to the number of sniffs, duration of keyboard pressing equal to the intensity of sniffing, and maximum duration of keyboard pressing equal to the absolute intensity of sniffing. The criteria required for a mouse’s behaviour to be counted as sniffing were that the mouse’s nose had to be within approximately within 1 cm of the wire cage and the mouse had to demonstrate a rhythmic inhalation and exhalation of air through the nose. As for supporting parameters, all the measurements were obtained automatically by the video tracking software during the sociability and social novelty phases. The purpose of the supporting parameters was to support the results from the main parameters.
Morris water maze: spatial learning and spatial memory assessment: The water pool used for the MWM test was an open circular pool painted white with an inside wall surface that was flat and smooth. The diameter of the pool was 179 cm, and it had a circular escape platform that was 9.5 cm in diameter, making the search-to-platform area ratio 355:1. The pool was half-filled with tap water at a temperature of 19–22°C and was stained with nontoxic white chalk to make it opaque. The pool was divided into 4 quadrants which were equal in size (N, North; S, South; E, East; and W, West). Four different images of shapes were placed on the inside of the pool wall at 3 cm above the water level as intentional cues. The circular escape platform was hidden by submerging it 0.5 cm below the water surface. Reflection of light on the water was avoided through the use of indirect room light.
A day before the test day, the experimental mice were placed in the behavioural testing room to acclimatise. The water maze protocol in this study was adapted from Vorhees and Williams [33]. There were 2 phases in this test: the acquisition and probe tests. In the acquisition phase, each mouse was trained for 5 days, with 3 trials for each acquisition phase. Each trial was limited to 90 s. The hidden platform was located at the centre of the S-W quadrant. During the acquisition phase, each mouse was released with its head towards the pool wall and was allowed to swim until it found the platform. There were 4 starting points for the mouse which was decided randomly (N, NW, E, and SE) for each day of acquisition phase. If the test mouse failed to find the hidden platform within 90 s, it was then guided gently to the hidden platform by hand and allowed to stay on the platform for 10–15 s. A condition was set in the tracking software so that each mouse needed to stay on the platform for 2 s to be counted as having successfully found the platform. This condition was important to avoid miscounting of mice crossing the hidden platform by accident. In order to avoid hypothermia, the mice were assigned to swim consecutively in the MWM pool in groups of 3–4 mice per trial; the inter-trial interval was 4–6 min. Thus, each mouse had around 4–6 min of rest before the starting the next trial. Total test duration, total distance travelled, swim speed, latency to first entry to a zone in which the hidden platform was located, and path efficiency to platform entry were analysed. The path efficiency is an index of the efficiency of the path taken by the animal to get from the first position in the test to the first position in the selected zone; a value of 1 indicates perfect efficiency (moving in a straight line), and a value of less than 1 indicates decreased efficiency.
The probe phase was started on the day after completion of the 5 days of the acquisition phase. In the probe phase, the hidden platform was removed from the water maze. The test mouse was released in the quadrant opposite to that in which the hidden platform had previously been located. Each trial in the probe phase was performed for 60 s. During the probe phase, the parameters obtained were the number of entries to the previous hidden platform, time spent in the quadrant in which the hidden platform had previously been located, path efficiency to platform entry, latency to first entry into the previous hidden platform zone, total distance travelled, and swim speed.
Statistics
The normality of data was determined by chi-square goodness of fit (data is not normally distributed if the P<0.05) and skewness and kurtosis values (data is normally distributed if the kurtosis and skewness values are in the range of −2 to 2). Results obtained from the FST were analysed by Mann-Whitney U test. Results from the sociability and social novelty preference test and mouse body weight changes were analysed by the two-tailed Student’s t-test with the treatment as an independent variable. Data from the mouse body weight changes and spatial learning were analysed by two-way repeated measures analysis of variance (ANOVA) with the day as the within-subjects factor and group as the between-subjects factors, followed by Bonferroni post hoc multiple comparisons where appropriate. Results obtained during the probe phase were analysed separately by one-way ANOVA (group as the between-subject factor). Microsoft Excel 2007 and SPSS 16.0 were used for all statistical analyses. A P<0.05 was accepted as a significant value for all data analyses. All the data presented are expressed as the mean ± SEM.
Results
Increased immobility indicates low escape behaviour in the chronic stress group
The distribution of the data for the FST results was not normal (skewness=2.065, kurtosis=4.776; goodness of fit x2(6)=37.15, P<0.001). Thus, the Mann-Whitney U test was favoured to analyse the FST data. A significant increase in immobility time was observed in the chronically restrained stressed animals when compared with the control group (U=9.0, Z (1)=1.98, P=0.048; Fig. 2a). Besides, the stressed group had significantly fewer body rotations than the control, which indicated a low motivation of the stressed group to escape (U=9.0, Z (1)=2.0, P=0.046; Fig. 2b).
Fig. 2.
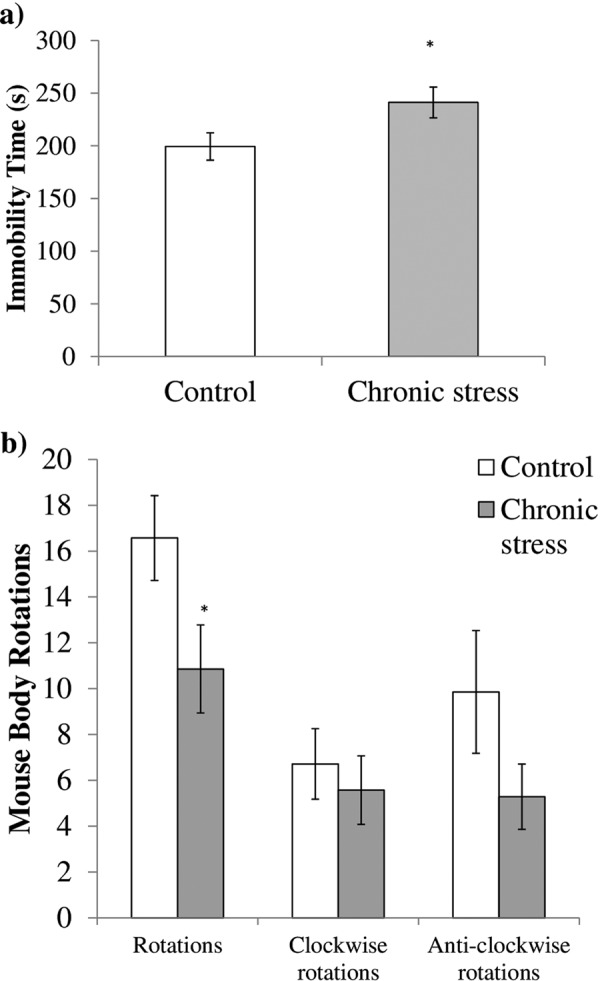
Forced-swim test performed on the stressed (n=7) and control (n=7) groups. (a) The stressed group showed a significant increase in the immobility time as compared with the control group, (b) Analysis of body rotations that occurred during the FST revealed that the stressed group had significantly fewer in overall body rotations compared with the control group. The data are expressed as the mean ± SEM. *P<0.05 vs. control.
Reduced body weight gain in the chronic stress group over 21 days
Two-way repeated measures ANOVA with a Greenhouse-Geisser correction revealed a significant interaction between day and group (F1.8, 25.3=5.92, P=0.009) for mouse body weight changes during the whole 21 days of treatment (Fig. 3). Testing of the within-subjects effect of day showed significant differences between four days of treatment (F13.3, 25.3=5.20, P=0.015), while there was no significant difference in the between-subject effects of group. Post-hoc comparison revealed that mice treated with chronic stress showed significantly reduced weights between day 14 and day 21 (P<0.001). The significant increase in immobility during the FST and reduced body weight gain in the chronic stress group indicates depression-like symptoms.
Fig. 3.
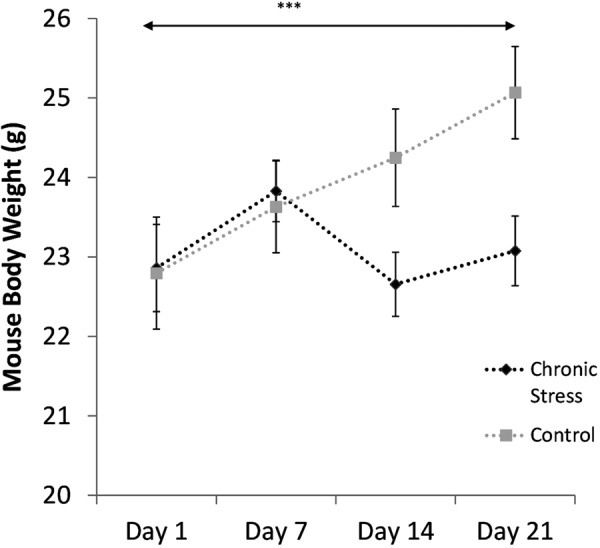
Comparison of mouse body weight changes during the 21 days of chronic stress treatment between the stressed (n=7) and control (n=7) groups. Data are presented as the mean ± SEM. ***P<0.001 vs. control.
Chronic stress impairs sociability but not social recognition
During the sociability phase, the stressed group showed a significant decrease in the number of sniffs (t12=−3.549, P=0.004), intensity of sniffing (t12=−4.847, P<0.001), and absolute intensity of sniffing (t12=−2.810, P=0.016) towards the Stranger mouse as compared with the control group (Figs. 4a–c). A decrease in the sniffing behaviours of the stressed group toward the Stranger mouse in Holding Zone 1 indicates impairment in the sociability parameter.
Fig. 4.
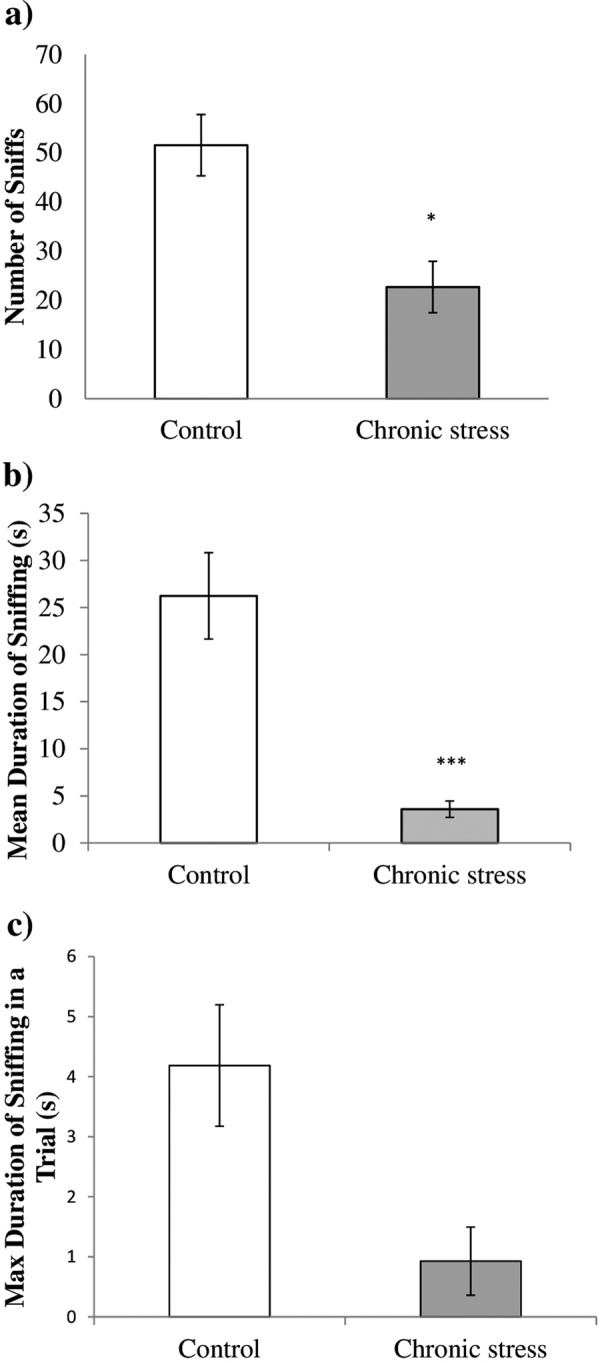
Comparison of sociability between the stressed group (n=7) and control group (n=7). (a) The stressed group showed a significantly low in number of sniffs toward stranger mouse 1 compared with the control. (b) The duration of sniffing toward stranger mouse 1 was significantly low in the stressed group compared with the control, which indicates a low intensity of sniffing. (c) The stressed group showed a significantly low maximum duration of sniffing as compared with the control, which indicates a low absolute intensity of sniffing. Data are presented as the mean ± SEM. *P<0.05 vs. control; **P≤0.01 vs. control; ***P≤0.001 vs. control.
With regard to the supporting parameters obtained during the sociability phase, the stressed group spent significantly less time and travelled a significantly shorter than the control group in Holding Zone 1. Moreover, there was no significant difference in the time spent and distance travelled in both Empty Zone 1 and the Centre Chamber between the control and stressed groups, which indicates no significant effect of new area exposure on time spent preference. The changes in the supporting parameters obtained during the sociability phase are summarized in Table 1. A significant decrease in time spent and distance travelled in the Holding Zone 1 for the stressed group supported the results seen in the main parameter of sniffing behaviour.
Table 1. Changes in time spent and distance travelled of the stressed group as compared with the control group during the sociability phase test.
| Parameters | Holding Zone 1 | Empty Zone 1 | Center Chamber | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=7) | Stress (n=7) | *P-value | Control (n=7) | Stress (n=7) | P-value | Control (n=7) | Stress (n=7) | P-value | |
| Time Spent | 218.571 | 158.829 | 0.001 | 20.529 | 30.986 | 0.337 | 41.986 | 60.043 | 0.245 |
| Distance Travelled | 13.934 | 7.375 | <0.001 | 1.699 | 1.889 | 0.735 | 3.629 | 3.463 | 0.893 |
The stressed group showed a significantly lower time spent and distance travelled in Holding Zone 1 which held a stranger mouse, as compared with the control group. No significant changes were seen in Empty Zone 1 or Centre Chamber, which indicates that the differences in the examined parameters between the control and stressed groups only happened in Holding Zone 1. *P<0.05 is significant. The two-tails t-test was performed with the assumpation of unequal variances. Abbreviation. n, number of samples.
During the social novelty preference phase, both the control and the stressed groups showed a significant increase in preferences in terms of the number of sniffs (stressed, t10=−5.12, P=0.0004; control, t9=−4.68, P=0.001; Fig. 5a) and intensity of sniffing (stressed, t7=−4.24, P=0.0038; control, t7=−4.44, P=0.003; Fig. 5b) toward the unfamiliar mouse (in Holding Zone 2) compared with the familiar mouse (in Holding Zone 1). However, regarding the absolute intensity of sniffing, unlike the control group (t7=3.70, P=0.008; Fig. 5c), the stressed group did not show a significant preference (t6=1.66, P=0.149) for the unfamiliar mouse compared with the familiar mouse. A statistically significant increase in preference in terms of the number of sniffs and intensity of sniffing toward the unfamiliar mouse indicates that the stressed group showed no impairment in social recognition.
Fig. 5.
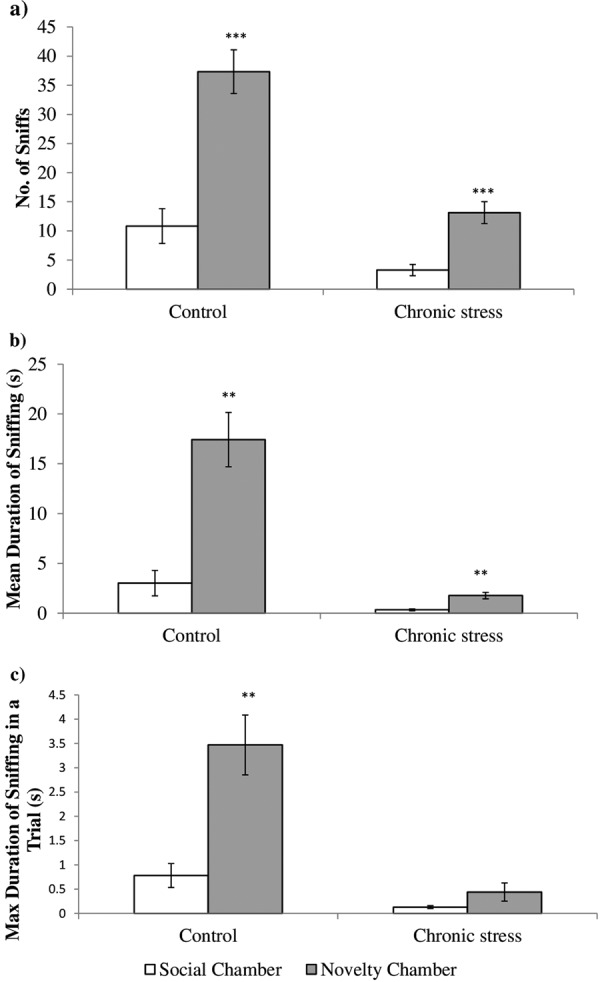
Comparison between the control (n=7) and stressed (n=7) groups in preference towards an unfamiliar mouse (Novelty Chamber) over a familiar mouse (Social Chamber). (a) Both groups showed higher preference in terms of the number of sniffs toward the unfamiliar mouse over the familiar mouse. (b) Both groups showed higher preference in terms of the intensity of sniffing toward the unfamiliar mouse over the familiar mouse. (c) The control group showed higher preference in terms of the absolute intensity of sniffing toward the unfamiliar mouse over the familiar mouse. However, the stressed group did not show significant changes in the absolute intensity of sniffing. Data are presented as the mean ± SEM. *P<0.05 vs. control; **P≤0.01 vs. control; ***P≤0.001 vs. control.
With reference to the supporting parameters obtained during the social novelty preference phase, both the control and stressed groups showed a significantly higher preference for time spent in Holding Zone 2 as compared with Holding Zone 1 (stressed, t12=8.20, P<0.0001; control, t9=2.63, P=0.027). Besides, the control and stressed groups spent more time and travelled longer distances in Holding Zones 1 and 2 (HZ1 and HZ2) as compared with Empty Zones 1 and 2 (EZ1 and EZ2), respectively, as shown in Table 2. The results in Table 2 indicate no significant effect of new chamber exposure for either the control or stressed group. Besides, results from supporting parameters indicate that the stressed group preferred to spend more time with the unfamiliar mouse in HZ2 than the familiar mouse in HZ1, thus supporting the results from main parameters which implied no impairment of social recognition function in the stressed group.
Table 2. Changes in the time spent and distance travelled between Empty Zone 1 and Holding Zone 1 and between Empty Zone 2 and Holding Zone 2 in both the control and stressed groups during the social novelty preference test.
| Groups | Parameters | Social Chamber | Novelty Chamber | ||||
|---|---|---|---|---|---|---|---|
| EZ1 | HZ1 | *P-value | EZ2 | HZ2 | *P-value | ||
| Control (n=7) | Time Spent | 15.057 | 83.914 | 0.003 | 23.743 | 139.271 | 0.004 |
| Distance Travelled | 1.145 | 4.99 | 0.009 | 1.67 | 7.069 | 0.018 | |
| Stress (n=7) | Time Spent | 24.757 | 59.829 | 0.005 | 29.843 | 132.371 | <0.001 |
| Distance Travelled | 1.123 | 2.948 | 0.001 | 1.876 | 5.761 | <0.001 | |
Both the control and stressed groups spent more time and travelled a longer distances in Holding Zones (HZ1, HZ2) than Empty Zones (EZ1, EZ2). *P<0.05 is significant. The two-tails t-test was performed with the assumption of unequal variances. Abbreviations. EZ, Empty Zone; HZ, Holding Zone; n, number of samples.
Chronic stress did not impair spatial learning and memory in the morris water maze
Over the 5 days of the spatial acquisition phase, no significant group effect or significant interaction between group and day effect was observed between the control and stressed groups in any of the five parameters tested (Figs. 6a–e). During the probe phase, there was no significant difference in performance between the control and stressed groups in previous platform entries, path efficiency to platform entry, or latency to first entry into the previous platform zone. In addition, there was no significant difference in swim speed and distance travelled during the 60 s trials in the probe phase between the control and stressed groups.
Fig. 6.
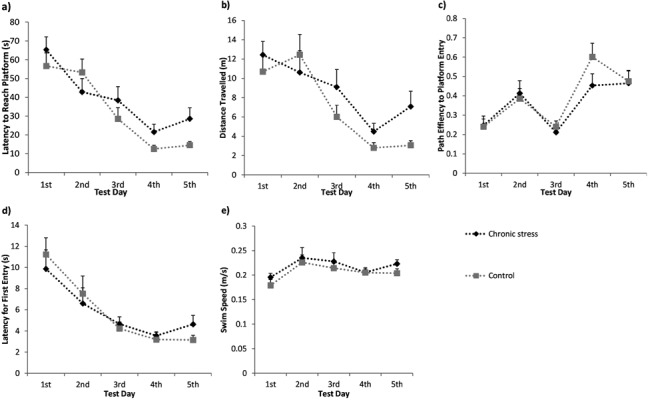
Effects of chronic stress on mouse acquisition performance in the MWM for five test days. (a) Total time required for the test mice to reach the hidden platform. (b) Total distance travelled by the test mice during the search for the hidden platform. (c) Path efficacy to platform entry. (d) Latency for first entry into the East South zone in which the hidden platform was located. (e) Swim speed of the test mice. Data are presented as the mean ± SEM. Control, n=8; Stressed, n=8.
Discussion
In this study, we made modifications to the previous protocol for the sociability and social novelty preference test. We validated the depression mouse model by performing the forced-swim test and measuring mouse body weight changes during the chronic stress treatment period. The effect of chronic stress was then evaluated in the modified protocol for the sociability and social novelty preference test. Our results confirmed that the chronic restraint stress treatment in the young adult mice impaired the sociability but not social novelty preference (social recognition). In addition, our results in the MWM confirmed that chronic stress did not impair spatial learning and memory during the acquisition and probe phases of the MWM test.
Originally, the three-chamber paradigm was designed with the purpose of testing for the autistic mouse model in terms of sociability and social recognition in a single procedure [21, 22]. Currently, the paradigm is widely used for studies associated with social deficits [2, 27, 32]. Nevertheless, the test procedure for the original protocol is relatively time-consuming (40 min per mouse), which also makes it laborious, especially for a single experimenter [13]. Besides, the sociability factor in the original protocol was assessed by indirect social interaction represented by the ‘time a mouse spent in the chamber’ rather than a direct or ‘true’ social interaction such as sniffing. Sniffing is commonly manifested in rodents during events that motivate them [6, 34] or as an act of social behaviour [8, 35]. It was also suggested that sniffing in rodents plays a role in their social communication [26]. In the original protocol for the three-chamber paradigm, it was shown that there was a strong correlation between the ‘time mouse spent in a chamber’ and sniffing duration of a test mouse toward a Stranger mouse [22]. Unfortunately, most of the subsequent studies that utilized this paradigm omitted measurement of direct social interaction and assumed that the ‘time spent in a chamber’ was always correlated with a direct social interaction in any conditions tested. Such an assumption raises concern about whether test conditions also affect mouse behaviours like locomotion, which may confound the results. Therefore, we measured the sniffing behaviour of the test mice instead of the ‘time a mouse spent in a chamber’, which has led to the modification performed in the present study. Nonetheless, the present study modification based on the sniffing behaviour measurement as a main parameter is not novel, as there was a previous study that also used ‘time spent sniffing a stranger mouse (wire cage)’ along with ‘time spent in chamber’ as main parameters in the sociability and social novelty paradigm [3]. However, the advantage of the current method is the experimental time was shorter (20 min) than the previous studies (40 min) per mouse including the cleaning time; the shorter time was achieved by the modification in the paradigm workflow.
There is a limitation in the present protocol for the three-chamber paradigm. The scoring method used for sniffing behaviour required the full concentration of the experimenter during the tasks (5 min for sociability and 5 min for social novelty preference); thus, it could be laborious at some point. Nonetheless, there is another method to measure sniffing behaviour, that is, measurement of intranasal pressure transients in mice during the behavioural task by using a pressure transducer [5, 34]. The advantage of this method is that scoring of sniffing behaviour does not involve a direct human observation, which makes the output more reliable. However, the disadvantage of the method is that it requires a surgery under anaesthesia to implant the intranasal cannula, which may cause unwanted stress in the test mice.
In the three-chamber paradigm, the sociability phase is used to examine the mouse’s ability to socialize with its conspecific, while the social novelty preference phase is used to examine the ability of a test mouse to distinguish and remember the unfamiliar and familiar conspecifics, which also indicates social recognition ability. However, the social novelty preference test has a subtle difference from the simple social recognition test because it gives the animal the option of choosing either to socialize with the unfamiliar or familiar mice. It was suggested that the social novelty preference test is more relevant to the symptoms observed in autism spectrum disorder [21].
Results of the present work in the three-chamber paradigm supported a previous study performed in the rat which showed that chronic restraint stress for 6h/d/21d caused impairment in the sociability test [32]. However, in contrast to our findings, the previous study reported that social recognition was also impaired. Such a difference supports the notion that the mouse is not simply a smaller version of a rat [10]. It must be noted, however, that the previous study measured the sociability parameter based on indirect social interaction, whereas the present study assessed sociability based on direct social interaction. The social interaction test which is based on direct social interaction like sniffing has been suggested to be a better way to access social deficits in the autism model of rodents [28]. In another previous study, which utilized a single prolonged stress to model post-traumatic stress disorder, it was found that the stress treatment impaired social recognition but not sociability of rat in the three-chamber paradigm [9], which was the opposite of the present results. However, it must be noted that the treatment to induce stress in the previous study was extremely rigorous and was over after a short period of time (2 h of restraint, followed by 20 min forced swimming in groups and then exposure to diethyl ether until the animal became unconscious) which was different from the chronic restraint stress treatment, which required a larger number of days. Nonetheless, these comparisons suggested that the nature of stress in affecting sociability and social recognition in rodents is not simply a one-way effect but may also depend on the regime of stress treatment applied. Besides, it also suggests that sociability and social recognition domains are independent of each other, particularly in terms of the stress effect on the domains.
Effects of chronic stress on impairment of spatial learning and memory have been mainly deduced from previous studies conducted in rats rather than mice. Most of the previous studies in rats reported that chronic stress impairs spatial learning during the acquisition phase of the MWM test. However, concern has been aroused as to whether the impairment was confounded by lack of motivation to escape or truly from impairment of spatial learning [4]. Here, we showed that the chronic stress paradigm for 6h/d/21days did not impair spatial learning and memory of the C57BL/6J mice tested in the MWM. Our results also did not appear to be confounded either by motivational or motor factors as indicated by no significant different in the distance travelled and swim speed during the acquisition phase between the control and stressed groups. Our results during the probe trial supported previous studies which showed that no significant difference was observed between control and stressed groups, which indicates that chronic stress did not affect spatial memory [14, 16, 36]. However, our results contradicted studies that showed chronic stress either impaired [1, 18, 20] or facilitated spatial memory [11] in the water maze test. It was suggested that, in aversive behavioural tasks like the MWM which invoke fear in the test mouse, chronic stress causes minimal impairment of spatial learning and memory [7]. Nonetheless, a recent study which utilized C57BL/6J mice reported that chronic restraint stress for 6h/d/21days produced spatial learning and memory deficits in the MWM test, which was in contrast to our results [15]. Unfortunately, no report either on swim speed, distance travelled, freezing, or immobility time was made, which raised a concern about these factors in confounding the results. Regardless, the main difference in our protocol is the intensity of the stressor. While the present study used a wire mesh container which resulted in limited immobility in which the mouse could still turn itself, the previous study used a well-ventilated conical tube which resulted in complete restraint of movement. The difference suggests that, in C57BL/6J mice in the CRS paradigm in particular, a higher intensity stressor than the commonly used one in rats is needed to induce robust spatial memory impairment in aversive motivated tests like the MWM. Thus, the differences in our results maintained previous reports which showed the intensity of a stressor may influence the behavioural response [4, 29] or plasma corticosterone changes [23, 25]. Moreover, regarding strain-related differences in particular, the C57BL/6J mouse strain was reported to be less vulnerable to the effect of stress in impairing memory and learning as compared with the BALB/c mouse strain [24].
The 6h/d/21days CRS paradigm utilized in the present study has been shown to be a reliable method to induce hippocampal CA3 apical dendritic retraction leading to spatial memory impairment of rats in an appetitive Y-maze behavioural task [19]. In terms of memory types, the hippocampal region has been found to be important in spatial memory and recognition memory; however, spatial memory is more dependent on the hippocampal region than recognition memory. Apart from the hippocampus, other brain regions that are important in social recognition include the olfactory bulb, amygdala, and septum [12, 30, 37]. Thus, the present results suggest that the effect of the CRS applied is not enough to impair social recognition memory; this may be due to the recognition memory being less dependent on the hippocampus region.
In conclusion, we described a modified protocol for the three-chamber paradigm for measurement of sociability and social recognition parameters which is based on direct social interaction measurement and is less time-consuming and less laborious compared with the previous protocol. By using this modified protocol, we confirmed that the chronic restraint stress for 6h/d/21d caused impairment in sociability but not social recognition function. We also conclude that chronic restraint stress for 6h/d/21d causes no significant impairment in spatial learning and memory in the MWM which indicates that stressed mice also learns and retain their spatial memory information.
Conflict of Interest
All authors report that they have no conflicts of interest.
Acknowledgments
We would like to express our appreciation to Professor Gavin P. Reynolds for his insightful suggestions during the development of the study. We would also like to offer special thanks to Associate Professor Dr. Vasudevan Mani for his supervision during the behavioural task experiments. We also thank staff of the Laboratory Animal Facility and Management (LAFAM), UiTM Selangor, Puncak Alam, Malaysia for their assistance during the conduct of this project.
The present study was supported by the University of Malaya postgraduate research grant, PG029-2014B, High Impact Research Grant UM.C/625/1/HIR/MOE/MED/05/01 and Institut Merieux grant, IF039-2017.
References
- 1.Abidin I., Yargiçoglu P., Agar A., Gümüslü S., Aydin S., Oztürk O., Sahin E.2004. The effect of chronic restraint stress on spatial learning and memory: relation to oxidant stress. Int. J. Neurosci. 114: 683–699. doi: 10.1080/00207450490430543 [DOI] [PubMed] [Google Scholar]
- 2.Azogu I., Plamondon H.2017. Inhibition of TrkB at the nucleus accumbens, using ANA-12, regulates basal and stress-induced orexin A expression within the mesolimbic system and affects anxiety, sociability and motivation. Neuropharmacology 125: 129–145. doi: 10.1016/j.neuropharm.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 3.Brunner D., Kabitzke P., He D., Cox K., Thiede L., Hanania T., Sabath E., Alexandrov V., Saxe M., Peles E., Mills A., Spooren W., Ghosh A., Feliciano P., Benedetti M., Luo Clayton A., Biemans B.2015. Comprehensive Analysis of the 16p11.2 Deletion and Null Cntnap2 Mouse Models of Autism Spectrum Disorder. PLoS One 10: e0134572. doi: 10.1371/journal.pone.0134572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buynitsky T., Mostofsky D.I.2009. Restraint stress in biobehavioral research: Recent developments. Neurosci. Biobehav. Rev. 33: 1089–1098. doi: 10.1016/j.neubiorev.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 5.Cheung M.C., Carey R.M., Wachowiak M.2009. A method for generating natural and user-defined sniffing patterns in anesthetized or reduced preparations. Chem. Senses 34: 63–76. doi: 10.1093/chemse/bjn051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke S., Trowill J.A.1971. Sniffing and motivated behavior in the rat. Physiol. Behav. 6: 49–52. doi: 10.1016/0031-9384(71)90013-8 [DOI] [PubMed] [Google Scholar]
- 7.Conrad C.D.2010. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 34: 742–755. doi: 10.1016/j.pnpbp.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 8.Doty R.L.1986. Odor-guided behavior in mammals. Experientia 42: 257–271. doi: 10.1007/BF01942506 [DOI] [PubMed] [Google Scholar]
- 9.Eagle A.L., Fitzpatrick C.J., Perrine S.A.2013. Single prolonged stress impairs social and object novelty recognition in rats. Behav. Brain Res. 256: 591–597. doi: 10.1016/j.bbr.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frick K.M., Stillner E.T., Berger-Sweeney J.2000. Mice are not little rats: species differences in a one-day water maze task. Neuroreport 11: 3461–3465. doi: 10.1097/00001756-200011090-00013 [DOI] [PubMed] [Google Scholar]
- 11.Gouirand A.M., Matuszewich L.2005. The effects of chronic unpredictable stress on male rats in the water maze. Physiol. Behav. 86: 21–31. doi: 10.1016/j.physbeh.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 12.Insel T.R., Fernald R.D.2004. How the brain processes social information: searching for the social brain. Annu. Rev. Neurosci. 27: 697–722. doi: 10.1146/annurev.neuro.27.070203.144148 [DOI] [PubMed] [Google Scholar]
- 13.Kaidanovich-Beilin O., Lipina T., Vukobradovic I., Roder J., Woodgett J.R.2011. Assessment of social interaction behaviors. J. Vis. Exp. 48: e2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasar M., Mengi M., Yildirim E.A., Yurdakos E.2009. Different effects of tianeptine pretreatment in rats exposed to acute stress and repeated severe stress. Methods Find. Exp. Clin. Pharmacol. 31: 157–163. doi: 10.1358/mf.2009.31.3.1362512 [DOI] [PubMed] [Google Scholar]
- 15.Kim D.M., Leem Y.H.2016. Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience 324: 271–285. doi: 10.1016/j.neuroscience.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 16.Kitraki E., Kremmyda O., Youlatos D., Alexis M., Kittas C.2004. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Ann. N. Y. Acad. Sci. 1018: 323–327. doi: 10.1196/annals.1296.039 [DOI] [PubMed] [Google Scholar]
- 17.Krishnan V., Nestler E.J.2011. Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 7: 121–147. doi: 10.1007/7854_2010_108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma W.P., Cao J., Tian M., Cui M.H., Han H.L., Yang Y.X., Xu L.2007. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci. Res. 59: 224–230. doi: 10.1016/j.neures.2007.06.1474 [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin K.J., Gomez J.L., Baran S.E., Conrad C.D.2007. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 1161: 56–64. doi: 10.1016/j.brainres.2007.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moosavi M., Naghdi N., Maghsoudi N., Zahedi Asl S.2007. Insulin protects against stress-induced impairments in water maze performance. Behav. Brain Res. 176: 230–236. doi: 10.1016/j.bbr.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 21.Moy S.S., Nadler J.J., Perez A., Barbaro R.P., Johns J.M., Magnuson T.R., Piven J., Crawley J.N.2004. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3: 287–302. doi: 10.1111/j.1601-1848.2004.00076.x [DOI] [PubMed] [Google Scholar]
- 22.Nadler J.J., Moy S.S., Dold G., Trang D., Simmons N., Perez A., Young N.B., Barbaro R.P., Piven J., Magnuson T.R., Crawley J.N.2004. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 3: 303–314. doi: 10.1111/j.1601-183X.2004.00071.x [DOI] [PubMed] [Google Scholar]
- 23.Natelson B.H., Ottenweller J.E., Cook J.A., Pitman D., McCarty R., Tapp W.N.1988. Effect of stressor intensity on habituation of the adrenocortical stress response. Physiol. Behav. 43: 41–46. doi: 10.1016/0031-9384(88)90096-0 [DOI] [PubMed] [Google Scholar]
- 24.Palumbo M.L., Zorrilla Zubilete M.A., Cremaschi G.A., Genaro A.M.2009. Different effect of chronic stress on learning and memory in BALB/c and C57BL/6 inbred mice: Involvement of hippocampal NO production and PKC activity. Stress 12: 350–361. doi: 10.1080/10253890802506383 [DOI] [PubMed] [Google Scholar]
- 25.Pitman D.L., Ottenweller J.E., Natelson B.H.1988. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol. Behav. 43: 47–55. doi: 10.1016/0031-9384(88)90097-2 [DOI] [PubMed] [Google Scholar]
- 26.Rennie S.M., Moita M.M., Mainen Z.F.2013. Social cognition in the rodent: nothing to be sniffed at. Trends Cogn. Sci. Regul. Ed. 17: 306–307. doi: 10.1016/j.tics.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 27.Rogers T.D., Anacker A.M.J., Kerr T.M., Forsberg C.G., Wang J., Zhang B., Veenstra-VanderWeele J.2017. Effects of a social stimulus on gene expression in a mouse model of fragile X syndrome. Mol. Autism 8: 30. doi: 10.1186/s13229-017-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato A., Mizuguchi M., Ikeda K.2013. Social interaction test: a sensitive method for examining autism related behavioral deficits. Protoc. exch .2013: 046. [Google Scholar]
- 29.Shors T.J., Servatius R.J.1997. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol. Learn. Mem. 68: 92–96. doi: 10.1006/nlme.1997.3763 [DOI] [PubMed] [Google Scholar]
- 30.Squires A.S., Peddle R., Milway S.J., Harley C.W.2006. Cytotoxic lesions of the hippocampus do not impair social recognition memory in socially housed rats. Neurobiol. Learn. Mem. 85: 95–101. doi: 10.1016/j.nlm.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 31.van der Kooij M.A., Fantin M., Kraev I., Korshunova I., Grosse J., Zanoletti O., Guirado R., Garcia-Mompó C., Nacher J., Stewart M.G., Berezin V., Sandi C.2014. Impaired hippocampal neuroligin-2 function by chronic stress or synthetic peptide treatment is linked to social deficits and increased aggression. Neuropsychopharmacology 39: 1148–1158. doi: 10.1038/npp.2013.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Kooij M.A., Fantin M., Rejmak E., Grosse J., Zanoletti O., Fournier C., Ganguly K., Kalita K., Kaczmarek L., Sandi C.2014. Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nat. Commun. 5: 4995. doi: 10.1038/ncomms5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vorhees C.V., Williams M.T.2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1: 848–858. doi: 10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wesson D.W., Donahou T.N., Johnson M.O., Wachowiak M.2008. Sniffing behavior of mice during performance in odor-guided tasks. Chem. Senses 33: 581–596. doi: 10.1093/chemse/bjn029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesson D.W.2013. Sniffing behavior communicates social hierarchy. Curr. Biol. 23: 575–580. doi: 10.1016/j.cub.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 36.Wright R.L., Conrad C.D.2008. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav. Brain Res. 187: 41–47. doi: 10.1016/j.bbr.2007.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young J.W., Powell S.B., Risbrough V., Marston H.M., Geyer M.A.2009. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol. Ther. 122: 150–202. doi: 10.1016/j.pharmthera.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]


