Highlights
-
•
We identified a new approach to improve the prognostic value of EEG patterns.
-
•
Interrater agreement was evaluated and reported for each different EEG pattern.
-
•
Causes for discrepancy were elucidated to improve interrater concordance.
Keywords: Cardiac arrest, EEG pattern, Post anoxic coma, Prognosis, Interrater variability, SIRPIDs
Abstract
Objectives
To assess interrater variability and prognostic value of simple EEG features based on the recent American Clinical Neurophysiology Society (ACNS) classification in post cardiac arrest comatose patients.
Methods
All patients admitted for a resuscitated cardiac arrest in a university hospital were prospectively included in the study. EEG interpretation was made by 3 independent neurophysiologists (2 senior and 1 junior) blind to the outcome. Kappa score and prognostic performances were estimated for each EEG pattern and discrepancies were analyzed.
Results
122 cardiac arrest patients were admitted of whom 48 went through a full neurologic evaluation. Eighty-one percent had an unfavorable outcome. Burst suppression, paroxystic seizure activity, and non-reactive EEG were strongly associated with an unfavorable evolution. Kappa score between the senior neurophysiologists was excellent or substantial while it was only fair or slight between the junior and senior neurophysiologists. Reactivity, discontinuity and electrographic seizure were patterns particularly subject to discrepancy.
Conclusions
Bedside EEG is an excellent tool for predicting outcome of post-anoxic coma through simple EEG features. However, the interrater variability emphasizes the need to be well trained for the standardized methods of evaluating EEG parameters.
Significance
A second look at complex patterns seems mandatory. The development of automated tools could help to improve the reliability of EEG interpretation.
1. Introduction
Cardiac arrest is a major public health care problem both by its incidence and severity. It remains a frequent cause of admission to intensive care units. Every year, >250,000 cardiac arrests occur throughout the United States and Europe (Atwood et al., 2005, Mozaffarian et al., 2015). About two thirds of patients admitted alive into hospital die during their hospital stay (Nolan et al., 2007), mainly from the consequences of hypoxic-ischemic brain injury after a decision to withdraw life sustaining therapy (WLST) (Dragancea et al., 2013, Lemiale et al., 2013).
Predicting the outcome is a major issue and is of utmost importance in guiding decisions about the nature and duration of therapeutic interventions.
Recent recommendations (Ben-Hamouda et al., 2014, Sandroni et al., 2014, Nolan et al., 2015) proposed a strategy aimed at predicting the neurological outcome based on a multimodal approach using clinical examination, electrophysiology, biomarkers, and brain imaging.
Within this strategy, EEG is the most widely used prognostic tool to support clinical examination and is accessible in most hospitals (Friberg et al., 2015). It is recommended both for prognostication and for ruling out subclinical seizures. Several EEG patterns can effectively predict a poor outcome (Rossetti et al., 2010, Soholm et al., 2014, Sivaraju et al., 2015). However, uncertainties in the interpretation of some of these patterns (Alvarez et al., 2013, Amorim et al., 2015, Hofmeijer and van Putten, 2016) prevent predicting poor prognosis with a high degree of confidence because of interrater variability (Grant et al., 2014).
The American Clinical Neurophysiology Society (ACNS) has proposed a standardized critical care EEG terminology (Hirsch et al., 2013) to facilitate multicenter studies and maximize interrater reliability. This classification could allow intensivists to improve their ability to predict the outcome of post anoxic coma. However, despite this classification, interpretation of standard EEG remains difficult, in particular for some EEG patterns such as paroxysmal activity or reactivity (Westhall et al., 2015). Studies are still needed to identify the causes of discrepancies between EEG readers and to improve their ability to classify specific patterns that are essential to establish a reliable prognosis.
Our objective here was to analyze, in a prospective monocentric cohort of resuscitated cardiac arrest patients, the interrater variability and to assess the prognostic value of EEG features based on the recent American Clinical Neurophysiology Society (ACNS) standardized classification.
2. Patients and methods
We conducted a prospective monocentric observational study in a 12-bed medical intensive care unit in the University Hospital la Timone, Marseille, France.
2.1. Patients
All patients aged over 18 admitted for a resuscitated cardiac arrest between November 2012 and July 2014 who underwent therapeutic hypothermia and a full multimodal prognostic evaluation including an EEG were included in the study. Patients who failed to achieve 24 h of hypothermia, who died under sedation before any neurological examination, or who awoke normally immediately after discontinuation of sedation, were excluded.
2.2. Standard of care
Therapeutic hypothermia (TH) was applied at admission in the intensive care unit to all patients who presented a cardiac arrest whatever the initial cardiac rhythm (shockable or not). The targeted temperature was 32–34 °C for 24 h and was achieved with infusion of cold saline or ice pack. During hypothermia, patients were sedated with continuous infusion of sufentanil and midazolam, and a neuromuscular blockage with a continuous infusion of cisatracurium was administered. Core body temperature was measured with a temperature probe in the urinary bladder. After 24 h, gradual rewarming to 37 °C in hourly increments of 0.5 °C was started. Neuromuscular blockage was interrupted at 35.5 °C and sedation was discontinued at 36 °C. Concomitant intensive care, cardiological and respiratory treatment followed standard practice.
2.3. Neurological evaluation
The neurological evaluation started 72 h after admission and was based on clinical neurological examination, including Glasgow Coma Scale (GCS), pupillary and corneal reflexes, somatosensory evoked potentials (SSEP), electroencephalogram (EEG) and biomarkers with dosing of NSE and SBeta100.
2.4. Electroencephalogram
EEG was performed in all patients still comatose after rewarming between 48 and 72 h after admission. It comprises a video EEG recording of 20 min with the NatusR® acquisition system. Twelve scalp electrodes were placed according to the international 10–20 system. Two non-cephalic electrodes were used to record the ECG and deltoid muscle activity, respectively. The recording was done with a monopolar assembly and a frontopolar reference Fpz. EEG signals were amplified with an online digital band-pass filter (0.5–200 Hz) and digitized at a rate of 512 kHz per channel. Cerebral activity was recorded spontaneously and EEG reactivity was then tested for all patients using intense auditory stimuli (loud noises, clapping, calling the patient’s name) and intense painful stimuli (pressure over the styloid process, the roots of the fingernails or the periosteal surfaces of bones) repeated three times with a five minutes interval.
Each EEG was analyzed offline in a standardized manner using the ACNS EEG terminology. Three neurophysiologists (AT and MG and MSD), interpreted the EEGs independently and were blinded to the clinical data and the outcome. Two of them (AT and MG) were senior neurophysiologists with >10 years of experience in reading EEG, and are experts in the Commission of EEG and evoked potentials at the French Society of Neurophysiology. MSD was a junior neurophysiologist with one year of experience but underwent high quality training in reading EEGs with full academic certification. All three were used to using the ACNS nomenclature as well as all the existing EEG classifications systems. However, none of these authors, who are neurophysiologists specialized in clinical neurophysiology with full academic certification, went through an official ACNS certification.
The inter-observer agreement rate for the studied EEG parameters was assessed using the kappa analysis. Kappa score was calculated between the 2 senior neurophysiologists (AT, MG) and between the junior and a senior neurophysiologist (MSD, AT). For the statistical analysis to evaluate prognostic ability of each EEG parameter, disagreements were resolved by consensus reading between neurophysiologists.
EEG features are based on the latest ACNS classification. Detailed definitions are presented in Table 1. Discrepancies were analyzed and detailed in the results section.
Table 1.
Definitions and criteria of EEG parameters adapted from the American Clinical Neurophysiology Society’s standardized critical care EEG 2012 Terminology.
| Background EEG | ||||
| Predominant background frequency | Beta Refers to frequencies >13 Hz |
Alpha Ranges from 8 to 13 Hz |
Theta Ranges from 4 to <8 Hz |
Delta Refers to frequencies <4 Hz |
| Voltage | Normal All activity ≥20 μV, measured in longitudinal bipolar with standard 10–20 electrodes from peak to trough |
Low Voltage Most or all activity <20 μV measured in longitudinal bipolar with standard 10–20 electrodes from peak to trough (Attenuation refers to low voltage EEG but with most or all >10 μV) |
Suppression refers to all activity <10 μV | Isoelectric All activity ≤2 μV. No activity of brain origin is detectable at a sensitivity of 2 μV/mm: electrocerebral silence |
| Continuity | Continuous Continuous normal or low-voltage EEG activity. Nearly continuous refers to continuous EEG activity, but with occasional (<10% of the record) periods of attenuation (>10 μV) or suppression (<10 μV) |
Discontinuous 10% to 49% of the record consisting of attenuation or suppression, as defined above. Burst attenuation or Burst-suppression: >50% of the record consisting of attenuation or suppression, as defined above, with bursts alternating with attenuation or suppression |
||
| Reactivity | Reactive Change in cerebral EEG activity to intense auditory and/or noxious stimuli. This may include change in amplitude or frequency, including attenuation of activity. If the only form of reactivity is stimuli induced rhythmic or periodic discharges or appearance of only muscle activity or eye blink artifacts, does not qualify as reactive |
Non Reactive No change in cerebral EEG activity after intense auditory and painful stimuli |
||
| Paroxysmal EEG | ||||
| Rhythmic or Periodic patterns | Periodic Discharges Repetition of a waveform with relatively uniform morphology and duration, with a quantifiable interdischarge interval between consecutive waveforms and recurrence of the waveform at nearly regular intervals |
Rhythmic Activity repetition of a waveform with relatively uniform morphology and duration, and without an interval between consecutive waveforms |
||
| Epileptic Activity | Electrographic Seizure Generalized spike-wave discharges at 3 per second or faster; and clearly evolving discharges of any type that reach a frequency >4 per second, whether focal or generalized |
Epileptiform Discharge Non-rhythmic and Non-periodic sharp waves and spikes |
||
2.5. Outcome assessment
Outcome was classified according to the Cerebral Performance Category (CPC) Score measured at day 28. The reason for choosing day 28 to evaluate the prognosis is based on our observation that there was almost no difference or only a marginal one between the CPC score of patients at day 28 and at 3 or 6 months. Unfavorable outcome was defined as CPC 3-4-5 (severely disabled, comatose or vegetative state, or deceased). Favorable outcome was defined as CPC 1–2 (no or moderate disability). All patients who died before day 28 were categorized as CPC 5. If patients were discharged alive from the ICU before day 28, their clinical status was checked on the medical record of the hospital if they were still hospitalized, on the medical record of the long-term facility if they had been transferred, or on the outpatient consultation record if they were discharged home.
2.6. Data collection
Cardiac arrest characteristics, initial cardiopulmonary resuscitation with the initial rhythm, length of no flow (delay between onset of cardiac arrest and initiation of cardio-pulmonary resuscitation) and low flow (delay between initiation of cardio-pulmonary resuscitation and return to spontaneous circulation) were collected. Patients’ characteristics were registered and Sepsis-related Organ Failure Assessment (SOFA) (Vincent et al., 1996) score was calculated on admission.
2.7. Statistical analysis
Statistical analyses were based on the IBM SPSS statistics software version 20.0 (Inc.; IL; USA). A descriptive analysis for each variable was first performed. Comparison between groups with good (CPC1-2) and bad (CPC 3-4-5) outcomes was carried out using the Pearson Chi2 test for qualitative variables and the Mann Whitney test for quantitative variables. For each variable and EEG feature, sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) for predicting an unfavorable outcome were calculated. We also performed a multivariate analysis by logistic regression to determine which EEG patterns could predict neurological outcome. A maximum of five EEG patterns were included in each model tested.
The concordance strength between EEG readers was based on the kappa score nomenclature of Landis and Koch (Landis and Koch, 1977) which defines the agreement according to the kappa value (<0: Poor; 0–0.2: Slight; 0.21–0.40: Fair; 0.41–0.60: Moderate; 0.61–0.80: Substantial; 0.81–1: Almost perfect) (ref).
3. Results
3.1. Outcomes and demographic characteristics
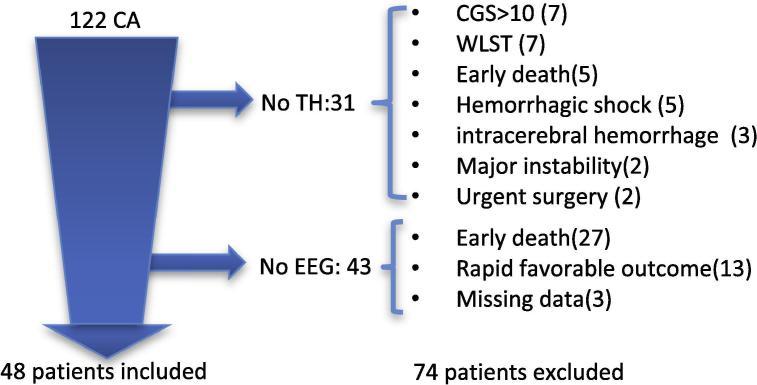
During the study period, 122 cardiac arrest (CA) patients were admitted into intensive care. Thirty-one did not receive TH mainly due to a contraindication. Forty-three patients were not explored with an EEG because of early death (27), rapid favorable evolution (13) or missing data (3) [Fig. 1]. Forty-eight patients went through a full neurologic evaluation after being treated by TH and were included in the study. According to the neurological outcome, 9 patients (19%) had a favorable evolution (CPC1-2), and 39 (81%) had an unfavorable outcome (CPC3-4-5). Among these 39 patients, 35 (73%) died, 54% of whom were in a Withdrawal and limitation of life-sustaining treatments (WLST) procedure. The cause of death was post-anoxic encephalopathy in 83% of these cases. None of the patients were discharged alive from the ICU with a CPC 4. The main demographics and initial clinical features are depicted in Table 2. Both prognostic groups were similar in terms of age and sex, with a median age of 65 years old and a predominance of males (71%). The length of stay in the hospital was significantly longer in the favorable outcome group. In the unfavorable outcome, the duration of “no flow” was longer, while the proportion of cardiac cause and initial shockable rhythm was lower. There was a trend towards a higher hyperlactatemia in the unfavorable group.
Fig. 1.
Study Flow chart *CA: Cardiac Arrest; WLST: Withdrawal of Life Sustaining Therapy; TH: Therapeutic Hypothermia; CGS: Coma Glasgow Score.
Table 2.
Patients and Cardiac Arrest characteristics.
| cohort | Global n = 48 | CPC 3–4-5n = 39 | CPC 1–2n = 9 | p |
|---|---|---|---|---|
| Age | 65 (54–75) | 64 (53–72) | 70 (48–79) | ns |
| Sex (male) | 34 (71%) | 28 (82%) | 6 (18%) | ns |
| Length of stay (days in intensive care) | 5 (3–9) | 5 (3–8) | 9 (6–14) | ns |
| Length of hospitalization (days) | 5 (4–17) | 5 (3–8) | 27 (18–51) | <0.001 |
| *“No flow” (min) | 5 (0.5–10) | 5 (2–10) | 1 (0–4) | <0.05 |
| **”Low flow” (min) | 15 (10–22) | 15 (10–25) | 17(3–20) | ns |
| Admission pH | 7.25 (7.19–7.36) | 7.24 (7.19–7.36) | 7.32 (7.23–7.47) | ns |
| PaO2/FiO2 | 217 (130–422) | 211 (128–352) | 335 (187–527) | ns |
| Admissions Lactates (mmol/l) | 5 (2–8) | 5 (3–8) | 2 (1.4–6) | 0.058 |
| OHCA (out hospital cardiac arrest) | 37 (77%) | 30 (77%) | 7 (78%) | ns |
| Initial rhythm VF/VT | 14 (29%) | 6 (15%) | 8 (89%) | <0.001 |
| CA of cardiac origin | 18 (37%) | 10 (26%) | 8 (89%) | <0.01 |
CA: Cardiac Arrest; VF: Ventricular Fibrillation; VT: Ventricular Tachycardia *delay between onset of cardiac arrest and initiation of cardio-pulmonary resuscitation; **delay between initiation of cardio-pulmonary resuscitation and return to spontaneous circulation.
3.2. Frequency and prognostic value of EEG parameters
Prognostic characteristics of the EEG parameters are summarized in Table 3, Table 4. Analysis of background frequency shows that an alpha rhythm is always associated with a favorable outcome. Theta frequency occurring in 52.1% of the cohort was the most frequent rhythm found. In 33.3% of the cohort, determination of background frequency was not possible because patients presented a flat pattern (with an amplitude <10 µV), exclusive burst suppression or a continuous epileptic activity. In this case background frequency was qualified as Indeterminate.
Table 3.
Prevalence of various EEG parameters according to neurological evolution.
| EEG parameters | Global n = 48 | CPC 3-4-5 (n = 39) | CPC 1–2 (n = 9) | p |
|---|---|---|---|---|
| Background frequency, n (%) | 0.00028 | |||
| Alpha | 3 (6.2) | 0 | 3 | |
| Theta | 25 (52.1) | 21 (43.8) | 4 (8.3) | |
| Delta | 4 (8.3) | 2 (4.2) | 2 (4.2) | |
| Undetermined | 16 (33.3) | 16 (33.3) | 0 | |
| No reactivity, n (%) | 30 (62.5) | 29 (60.4) | 1 (2.1) | 0.001 |
| Voltage | 0.713 | |||
| Suppression <10 μV, n (%) | 15 (31.2) | 13 (27.1) | 2 (4.2) | |
| Attenuation: 10–20 μV, n (%) | 21 (43.8) | 16 (33.3) | 5 (10.4) | |
| Low voltage: <20 μV, n (%) | 36 (75) | 29 (60.4) | 7 (14.6) | |
| Normal: >20 μV, n (%) | 12 (25) | 10 (20.8) | 2 (4.2) | |
| Discontinuous, n (%) | 11 (22.9) | 10 (20.8) | 1 (2.1) | 0.327 |
| Burst suppression, n (%) | 9 (18.8) | 9 (18.8) | 0 | 0.126 |
| Paroxysmal EEG seizure, n (%) | 8 (16.7) | 8 (16.7) | 0 | 0.163 |
| Paroxysmal EEG periodic or rhythmic pattern, n (%) | 19 (39.6) | 16 (33.3) | 3 (6.2) | 0.488 |
| Epileptiform discharge, n (%) | 16 (33.3) | 15 (31.2) | 1 (2.1) | 0.117 |
Table 4.
Prognostics performance of EEG parameters.
| EEG parameter | Se (IC 95%) | Sp (IC95%) | PPV | NPV | LR+ | LR− | False positives |
|---|---|---|---|---|---|---|---|
| Background frequency | |||||||
| Alpha* | 33% (12–64) | 100% (91–100) | 100% | 87% | – | 0.67 (0.42–1.06) | 0% |
| Theta | 54% (38–68) | 55% (27–81) | 84% | 22% | 1.21 (0.55–02.66) | 0.83 (0.42–1.6) | 16% |
| Delta | 5% (10–17) | 78% (45–94) | 50% | 16% | 0.23 (0.04–1.42) | 1.22 (0.85–1.7) | 50% |
| Indeterminate | 41% (27–56) | 100% (70–100) | 100% | 28% | – | 0.59 (0.45–0.76) | 0 |
| No reactivity | 74% (59–85) | 89% (56–98) | 97% | 44% | 6.69 (1.04–42.86) | 0.28 (0.16–0.51) | 3% |
| Voltage | |||||||
| Suppression <10 μV | 33% (20–49) | 78% (45–94) | 87% | 21% | 1.5 (0.41–5.51) | 0.86 (0.57–1.3) | 13% |
| Attenuation: 10–20 μV | 41% (27–56) | 44% (19–73) | 76% | 15% | 0.74 (0.34–1.5) | 1.33 (0.61–2.89) | 24% |
| Low voltage: <20 μV | 74% (59–85) | 22% (6.3–54) | 80% | 17% | 0.95 (0.64–1.4) | 1.15 (0.3–4.38) | 19% |
| Normal: >20 μV* | 22% (6–55) | 74% (59–85) | 16% | 80% | 0.87 (0.23–3.3) | 1.05 (0.70–1.55) | 80% |
| Discontinuous | 25% (14–41) | 89% (56–98) | 91% | 21% | 2.31 (0.34–15.8) | 0.84 (0.62–1.12) | 9% |
| Burst suppression | 23% (12–38) | 100% (70–100) | 100% | 23% | – | 0.77 (0.65–0.91) | 0% |
| Paroxysmal EEG seizure | 20% (11–35) | 100% (70–100) | 100% | 22% | – | 0.79 (0.68–0.93) | 0% |
| Paroxysmal EEG periodic or rhythmic pattern | 41% (27–56) | 67% (35–88) | 84% | 21% | 1.23 (0.45–3.34) | 0.88 (0.52–1.5) | 16% |
| Epileptiform Discharges | 38% (25–54) | 89% (56–98) | 93% | 25% | 3.46 (0.52–22.9) | 0.69 (0.49–0.97) | 6% |
Se: sensibility; Sp: specificity; PPV: Positive Predictive value; NPV: Negative predictive value; LR+: Positive likelihood ratio; LR−: Negative likelihood ratio
Thirty patients (62.5%) had a non-reactive EEG, of whom 29 had an unfavorable outcome. A non-reactive EEG was significantly associated with a bad outcome.
A low voltage (<20 μV) was observed in 36 patients (75% of the cohort) of whom 29 had a bad outcome and 7 a favorable evolution. A suppression EEG was found in 15 patients, 13 of them having a bad outcome. Ten of the 11 patients having a discontinuous EEG tracing had an unfavorable outcome. The presence of burst suppression is constantly associated with an unfavorable prognosis with 100% specificity.
All 8 patients with paroxysmal EEG seizure had an unfavorable outcome. Prognostics performances of periodic or rhythmic activity and epileptiform discharges are detailed in table 4.
Stimulus Induced Rhythmic and Periodic or Ictal Discharges (SIRPIDs).
After consensus reading, 7 patients were classified as having SIRPIDs. The prevalence in our cohort was 14.5%. All these patients were primarily categorized as paroxysmal periodic or rhythmic pattern. None of them had burst suppression, and one was considered to correspond to a discontinuous pattern, and another one to an electrographic seizure.
Multivariate analysis by logistic regression (Table 5).
Table 5.
Model of multivariate analysis with logistic regression.
| EEG patterns | Adjusted OR | CI 95% | p |
|---|---|---|---|
| SIRPIDs | 0.543 | 0.040–7.308 | 0.645 |
| Reactivity | 0.077 | 0.007–0.853 | 0.037 |
| Discontinuous | 0.289 | 0.021–4.056 | 0.357 |
| Background frequency | 2.643 | 0.548–12.742 | 0.226 |
| Voltage | 1.607 | 0.180–14.321 | 0.671 |
Different models with 5 EEG patterns allowing valid analysis with 48 patients were tested. Whatever the choice of the model, only reactivity was independently associated with good outcome in our cohort (see Table 5).
3.3. Concordance
Kappa score for all the EEG features between the junior neurophysiologist and the senior AT was slight or fair and always under 0.4. The concordance strength for identifying the background frequency and burst suppression was slight (κ = 0.16 and κ = 0.15 respectively). Reactivity (κ = 0.35), voltage (κ = 0.21), paroxysmal seizure (κ = 0.38), or periodic and rhythmic patterns (κ = 0.22), had a fair strength of agreement.
Between the 2 senior neurophysiologists (AT and MG), inter-observer agreement was almost perfect for background frequency (κ = 0.83), reactivity (κ = 0.87), and epileptiform discharge (κ = 0.82); and substantial for voltage (κ = 0.75), burst suppression (κ = 0.61), paroxysmal seizure (κ = 0.67), periodic or rhythmic activity (κ = 0.79). Inter-observer agreement was moderate for continuity (κ = 0.54). Results of inter-observer agreement are summarized in table 6.
Table 6.
Inter-observer agreement for Interpretation of various EEG parameters.
| EEG parameters | κ 1(AT)-2(MSD)* | p | Strength of agreement | κ 1(AT)-3(MG)* | p | Strength of agreement |
|---|---|---|---|---|---|---|
| Background frequency | 0.16 | ns | Slight | 0.83 | <0.05 | Almost perfect |
| Reactivity | 0.35 | <0.05 | Fair | 0.87 | <0.05 | Almost perfect |
| Voltage | 0.21 | <0.05 | Fair | 0.75 | <0.05 | Substantial |
| Discontinuous | – | – | 0.54 | <0.05 | Moderate | |
| Burst suppression | 0.15 | ns | Slight | 0.61 | <0.05 | Substantial |
| Paroxysmal EEG seizure | 0.38 | <0.05 | Fair | 0.67 | <0.05 | Substantial |
| Paroxysmal EEG periodic or rhythmic pattern | 0.22 | ns | Fair | 0.79 | <0.05 | Substantial |
| Epileptiform discharge | 0.32 | <0.05 | Fair | 0.82 | <0.05 | Almost perfect |
AT and MG are senior neurophysiologists, MSD is a junior neurophysiologist.
4. Discussion
This study allowed us to identify a homogenous cohort of comatose patients who underwent therapeutic hypothermia after cardiac arrest. Thirty nine of the 48 patients (81%) had an unfavorable outcome, which is comparable with other published data (Lemiale et al., 2013, Amorim et al., 2015, Azabou et al., 2016). Usual mortality-associated factors such as no flow duration, non-shockable rhythm, and non-cardiac origin were found in the cohort.
There is important interrater variability between junior and senior neurophysiologists. The main difference between the junior and the senior neurophysiologists is the level of experience. This emphasizes the need to be trained at interpreting EEG on the basis of the 2012 ACNS classification. Using this nomenclature per se does not seem sufficient to improve interrater reliability of EEG interpretation between the junior and senior neurophysiologists. In contrast, it is interesting to note that between the experienced neurophysiologists there is substantial or excellent correlation in EEG interpretation based on ACNS terminology.
Absence of EEG reactivity was significantly associated with poor outcome, with good sensitivity (74%), specificity (89%), PPV (97%) and 3% of FPR. Only one patient had a good outcome with an unreactive EEG. This patient had his EEG recorded the day after discontinuation of sedation which was ongoing for 5 days because of severe aspiration pneumonia. He finally woke up after several days of coma, probably because of residual sedation rather than from post anoxic encephalopathy. This case emphasizes the need to rule out any confounding factor while recording EEG. Our results are in accordance with other studies (Rossetti et al., 2010, Crepeau et al., 2013, Oddo and Rossetti, 2014, Azabou et al., 2016). Limitation to accurate extrapolation is due to the lack of standardization regarding stimulation modality, and interpretation of various type of reactivity which results in lower specificity (70%) and higher FPR (6%), as recently reported in one multicenter study (Westhall et al., 2016).
We aimed to investigate discrepancies between neurophysiologists concerning the interpretation of reactivity. We found that the main cause for disagreement was the presence of a spectrum of stimulation-induced electroencephalographic patterns as SIRPIDs. These patterns were first described by Hirsch et al. in 2004 (Hirsch et al., 2004). Their prognostic value in cardiac arrest remains poorly investigated. One small cohort (Alvarez et al., 2013) was published suggesting that SIRPIDs were associated with poor outcome when present during the hypothermic period but not when exclusively seen in the post-hypothermia period.
In this study, we found that SIRPIDs were associated with a poor outcome (PPV 89%) but with a high rate of false positives (14%). The prevalence in our cohort was in line with this previous study. In post anoxic encephalopathy, it appears that classifying SIRPIDs as an independent category will reduce interrater variability and improve the reliability of reactivity to predict outcome. Still, intrinsic prognostic value of SIRPIDs remains unclear and needs further study to evaluate its capability to predict outcome.
Burst suppression (BS) has recently been defined on the basis of >50% of the recording being with a suppression voltage ≤10 μv with alternating bursts. In our cohort, the 9 patients who presented this EEG parameter had a poor outcome with 100% specificity, 0% FPR and a low sensitivity of 23%. Timing of EEG recording was done at least 72 h after return of spontaneous circulation (ROSC) for all patients. These results are consistent with other reports (Sivaraju et al., 2015, Westhall et al., 2016). Five patients out of the 9 who presented BS had their ocular reflex preserved, which suggests that EEG had additional prognostic value over clinical examination.
Regarding interrater variability, difficulties with interpretation of this pattern come from the quantification of discontinuity. As indicated in the ACNS definition, discontinuous pattern corresponds to a proportion of EEG suppression comprised between 10 and 49% whereas burst suppression corresponds to a proportion of suppression of >50% with alternating bursts. Differences in the proportion of suppression evaluated by the interpreters (between 10 and 49% or >50%) lead to disagreement in the interpretation.
EEG quantification tools should be helpful for an accurate classification of such cases.
Eight patients presented unequivocal seizures according to the ACNS definition. They all had a bad outcome. Only one of them had clinical myoclonus, suggesting that EEG is useful for detecting non-clinical epileptic seizure. electrographic seizures have a low sensitivity of 20%. Epileptiform discharge was found in 16 patients with a 38% sensitivity, PPV of 93% and a 6% FPR, corresponding to only one patient having a good outcome with a continuous and reactive EEG.
Within the category of paroxysmal EEG, we found discrepancies in the interpretation between electroencephalographic seizure and rhythmic pattern. In some patients these EEGs are more difficult than others to interpret. This is in keeping with a previous observation that reports a higher variance due to subjects than to readers, suggesting that variability in EEG interpretation depends to a substantial extent on the nature of the EEG findings being interpreted (Grant et al. 2014).
To distinguish seizure from rhythmic pattern is important not only for prognosis but also for the indication of an anti-epileptic medication, keeping in mind that periodic elements could represent an ‘‘unstable interictal–ictal continuum (Pohlmann-Eden et al., 1996).
Three patients that had an alpha rhythm with reactive EEG had a good outcome. One of these 3 patients had a discontinuous EEG and another one of them had a suppression EEG. This emphasizes the need to be cautious with regard to the prognostic value of amplitude or continuity alone. In addition EEG could also be an interesting tool for predicting good outcome when identifying a continuous and reactive feature.
As in most studies we cannot rule out the effect of self-fulfilling prophecy in decisions of WLST. However all decisions of WLST were made after a close multimodal approach including evoked potential, and biomarkers.
Among experienced neurophysiologists, ACNS terminology provides a reliable tool for EEG interpretation and prognostication in comatose patients after cardiac arrest. Specific training is mandatory before using ACNS classification, notably for junior neurophysiologists. Before considering EEG in prognostication, it is crucial that clinician intensivists be aware of the level of experience of the neurophysiologist interpreting the EEG. Our results highlight the importance of proper training and of the level of experience in reading EEG using the ACNS nomenclature.
Prognostication cannot rely on a single tool, but on a multimodal approach integrating EEG, evoked potential, clinical signs, biomarkers and brain imaging.
5. Conclusion
Our data show that bedside EEG is an excellent tool for predicting outcome of post-anoxic coma through simple EEG features. Burst suppression, epileptiform activity and non-reactive EEG are strongly associated with neurological outcome after cardiac arrest. Interrater variability is still a critical issue that is not abolished by the use of standardized terminology. It emphasizes the need for proper training in the standardized methods of evaluating EEG parameters, for having a second interpretation in complex patterns and for the development of automated tools to improve interrater concordance.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2018.12.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alvarez V., Oddo M., Rossetti A.O. Stimulus-induced rhythmic, periodic or ictal discharges (SIRPIDs) in comatose survivors of cardiac arrest: characteristics and prognostic value. Clin. Neurophysiol. 2013;124:204–208. doi: 10.1016/j.clinph.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Amorim E., Rittenberger J.C., Baldwin M.E., Callaway C.W., Popescu A. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–132. doi: 10.1016/j.resuscitation.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood C., Eisenberg M.S., Herlitz J., Rea T.D. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation. 2005;67:75–80. doi: 10.1016/j.resuscitation.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Azabou E., Fischer C., Mauguiere F., Vaugier I., Annane D., Sharshar T. Prospective cohort study evaluating the prognostic value of simple EEG parameters in postanoxic coma. Clin. EEG Neurosci. 2016;47:75–82. doi: 10.1177/1550059415612375. [DOI] [PubMed] [Google Scholar]
- Ben-Hamouda N., Taccone F.S., Rossetti A.O., Oddo M. Contemporary approach to neurologic prognostication of coma after cardiac arrest. Chest. 2014;146:1375–1386. doi: 10.1378/chest.14-0523. [DOI] [PubMed] [Google Scholar]
- Crepeau A.Z., Rabinstein A.A., Fugate J.E., Mandrekar J., Wijdicks E.F., White R.D. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80:339–344. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- Dragancea I., Rundgren M., Englund E., Friberg H., Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84:337–342. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Friberg H., Cronberg T., Dunser M.W., Duranteau J., Horn J., Oddo M. Survey on current practices for neurological prognostication after cardiac arrest. Resuscitation. 2015;90:158–162. doi: 10.1016/j.resuscitation.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Grant A.C., Abdel-Baki S.G., Weedon J., Arnedo V., Chari G., Koziorynska E. EEG interpretation reliability and interpreter confidence: a large single-center study. Epilepsy Behav. 2014;32:102–107. doi: 10.1016/j.yebeh.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch L.J., Claassen J., Mayer S.A., Emerson R.G. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia. 2004;45:109–123. doi: 10.1111/j.0013-9580.2004.38103.x. [DOI] [PubMed] [Google Scholar]
- Hirsch L.J., LaRoche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J. Clin. Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- Hofmeijer J., van Putten M.J. EEG in postanoxic coma: Prognostic and diagnostic value. Clin. Neurophysiol. 2016;127:2047–2055. doi: 10.1016/j.clinph.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lemiale V., Dumas F., Mongardon N., Giovanetti O., Charpentier J., Chiche J.D. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nolan J.P., Laver S.R., Welch C.A., Harrison D.A., Gupta V., Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC Case Mix Programme Database. Anaesthesia. 2007;62:1207–1216. doi: 10.1111/j.1365-2044.2007.05232.x. [DOI] [PubMed] [Google Scholar]
- Nolan J.P., Soar J., Cariou A., Cronberg T., Moulaert V.R., Deakin C.D. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41:2039–2056. doi: 10.1007/s00134-015-4051-3. [DOI] [PubMed] [Google Scholar]
- Oddo M., Rossetti A.O. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit. Care Med. 2014;42:1340–1347. doi: 10.1097/CCM.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Pohlmann-Eden B., Hoch D.B., Cochius J.I., Chiappa K.H. Periodic lateralized epileptiform discharges–a critical review. J. Clin. Neurophysiol. 1996;13:519–530. doi: 10.1097/00004691-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Rossetti A.O., Oddo M., Logroscino G., Kaplan P.W. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann. Neurol. 2010;67:301–307. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- Sandroni C., Cariou A., Cavallaro F., Cronberg T., Friberg H., Hoedemaekers C. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1816–1831. doi: 10.1007/s00134-014-3470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraju A., Gilmore E.J., Wira C.R., Stevens A., Rampal N., Moeller J.J. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015;41:1264–1272. doi: 10.1007/s00134-015-3834-x. [DOI] [PubMed] [Google Scholar]
- Soholm H., Kjaer T.W., Kjaergaard J., Cronberg T., Bro-Jeppesen J., Lippert F.K. Prognostic value of electroencephalography (EEG) after out-of-hospital cardiac arrest in successfully resuscitated patients used in daily clinical practice. Resuscitation. 2014;85:1580–1585. doi: 10.1016/j.resuscitation.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Vincent J.L. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- Westhall E., Rosen I., Rossetti A.O., van Rootselaar A.F., Wesenberg Kjaer T., Friberg H. Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin. Neurophysiol. 2015;126:2397–2404. doi: 10.1016/j.clinph.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Westhall E., Rossetti A.O., van Rootselaar A.F., Wesenberg Kjaer T., Horn J., Ullen S. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86:1482–1490. doi: 10.1212/WNL.0000000000002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.