Abstract
Collagen is the most abundant fibrous protein in animal's body and is widely used for biomedical and pharmaceutical applications. The principal sources of this protein are bovine, porcine and fish skin and bones. In Colombia, bovine bones are waste from meat industry, this material have potential as an alternative source of collagen isolation. The aim of this study was to evaluate the composition and some properties of type I collagen (COL I) extracted of bovine bones of Zebu-Bos Primigenius Indicus and its use as textile finishing to modify two types of fabrics: first a taffeta weave and the second a single jersey knit, both 100% cotton. The extracted bone collagen showed the main characteristic bands of this material in the FTIR spectra, corresponding to amide A, I, II and III. Gel electrophoresis (SDS-PAGE) presented the main bands of α1 and α2 chains characteristic of COL I with a molecular weight of approximately 120 kDa and the amino acid profile of hydrolyzed protein evaluated by amino acid analysis showed 9.4% of hydroxyproline, 10.3% proline and 16.9% of glycine content. Two traditional methods of applying finished textiles were evaluated to modify both fabrics with COL I, exhibiting better attachment through PAD method compared with exhaustion method. These results suggest that bone is an alternative source for type I collagen extraction, which can be applied as a functional textile finishing for traditional fabrics for implementation in healthtech field.
Keyword: Materials science
1. Introduction
Collagen is the most abundant protein present in the body of vertebrates [1]. There are about 29 types of collagen differentiated into their constituent amino acids and each one of these has a specific function in tissues. For example, bone and tendon contains mainly fibrous type I collagen, cartilage contains type II, vascular system contains type I and III and basement membranes are constituted of primarily type IV collagen. The tissues also contain other minor amounts of other collagens types as V, VIII, IX, X, XI and XIV [2]. All collagen types are constituted by domains of tripeptide Gly-X-Y repetitions, where X is generally proline and Y is mainly hydroxyproline, therefore, is also known the presence of other amino acids as cysteine, tyrosine, histidine and hydroxylysine at low percentage [3].
Depending on the amino acid sequences, collagen has a wide range of applications, but type I collagen (COL I) is the most used in pharmaceutical and biomedical industry [4]. This protein has been extracted from different sources such as rat tail tendon [5] fish scales and bones [1], chick embryos [6] and bovine bone [7, 8] to develop functional materials [9].
Bovine bones are a promising source for COL I extraction, because of the organic matrix of this tissue is composed of 90 % of this protein and given its fibrillary characteristics, it provides the functional integrity and biomechanical properties of osseous tissue [10]. In Colombia livestock is one of the most sustainable economies, but animals are only exploited for their milk, meat and leather. The bones are an unexplored source, which is disposed in landfills contributing to soil erosion, in fact in Colombia 4,460 tons of bovine femur are buried per year [11].
COL I has been widely used as a biomaterial, since unlike of synthetic polymers, this biomaterial interacts with body cells during tissues and organs conformation [4, 12]. For biomedical applications, some remarkable properties of COL I are valuable as biodegradability, biocompatibility, versatility, and has high affinity with cells, allowing the manufacturing of scaffolds for cellular behaviour studies such as migration, proliferation, differentiation and phenotypic expression [3].
In biomedical industry, collagen has been employed to modify and functionalize different types of materials for wound treatments applications, due to collagen has shown properties such as adhesion and proliferation of human fibroblasts, which benefit the regeneration of macro and micro wounds [13, 14, 15]. Therefore, this work proposes the development of a functional textile finishing of COL I isolated from bovine femur to be applied on a traditional textile fabric, for potential wound dressing applications. These strategies allow progress in the development of functional fabrics for healthtech segment in textile industry, using traditional finishing process as pad-dry-cure (PAD) and exhaustion methods. The extracted collagen was characterized with FTIR, SEM, amino acid analysis and SDS-PAGE, moreover the modified fabrics was characterized by FTIR and SEM.
2. Materials and methods
2.1. Materials
The following reagents, used in this research, were analytical grade from Merk: sodium hydroxide, acetic acid, tris (hydroxymethyl) aminomethane and sodium chloride. EDTA- 4 Na, was industrial grade. For the collagen isolation, the femur bones of Zebu-Bos Primigenius Indicus were kindly supplied by a local butcher. Rat tail collagen from Sigma Aldrich was used for comparison purposes in characterization.
2.2. Methods
2.2.1. Bone preparation
To extraction of collagen, the meatless bones were cut into small pieces and milled in a hammer grinder. The grinded bones were packed in polyethylene bags and storage at -80 °C for later uses.
2.2.2. Collagen isolation
The collagen was isolated using the method described by Li et al. [3]. Briefly, all the process was performed at c.a. 4 °C with continuous stirring. To remove the non-collagenous proteins, bones were immersed in a 0.1 M NaOH solution at a sample/alkali solution ratio of 1/10 (w/v) for 2 days, this alkali solution was changed every 6 h. Afterwards, the sample was washed with distilled water until neutral pH. Then, 0.5 M of EDTA-4Na (pH 7.5) was used to remove minerals, the solution was changed daily for 5 days. After that, a butanol solution was used at 10 wt % with a solid/solvent ratio of 1/20 (w/v), for two days with changes every 6 h to eliminate lipids. The samples were washed fully with distilled water. Defatted bone samples were left for three days in a solution of 0.5 M of acetic acid with a solid/solvent ratio of 1/15 (w/v). The solution was centrifuged at 9,000 rpm for 30 min at 4 °C, the filtrate was removed, both filtrates were combined with a 0.05 M tris (hydroxymethyl) aminomethane solution (pH 7.5) to solubilize collagen.
Finally, to precipitates the collagen, NaCl was added at final concentration of 2.6 M for 24 h. Precipitated collagen was then centrifuged, the pellet was solubilized in 0.5 M acetic acid solution. The resulting solution was dialyzed (Spectra/por, MWCO 12–14 kDa) with 0.1 M acetic acid and then with distilled water for two days, with changes of solution every 12 h. The isolated collagen was frozen with liquid nitrogen and freeze-dried under vacuum at 0.020 mBar for 36 h.
2.2.3. Bone collagen characterization methods
2.2.3.1. Attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR)
ATR-FTIR spectra was used to identify the amide bands and to compare the isolated collagen (BB) with a commercial collagen from rat tail (RT). The spectra were collected in a Nicolet 6700 spectrophotometer in 4000–400 cm−1 range using ATR with a diamond crystal. The spectra were recorded with a resolution of 4 cm−1 over 64 scans.
2.2.3.2. Gel electrophoresis sodium dodecyl sulfate polyacrylamide (SDS-PAGE)
Molecular weights of collagen polypeptides were measured to determine the presence of α1 and α2 chains, which are characteristics of type I collagen. SDS–PAGE was performed on a slab gel consisting of 4 % stacking gel and 7 % resolving gel. The protein sample was dissolved in 1 M acetic acid at a concentration of 3 mg/mL. Samples were heat for 5 min at 95 °C, to denature the collagen, and analyzed under reducing condition by the addition of 2-mercaptoethanol. The polypeptides migrated in a solution of Tris glycine after applying 100 volts for 120 minutes. After the migration, the gel was stained with a Coomassie Blue solution at 0.1 wt. % and washed overnight with agitation in an aqueous solution containing 5 vol. % methanol and 10 vol. % of acetic acid. The molecular weights of the bands in the protein sample were determined using standard markers Step TM Broad Ranger, Protein Marker.
2.2.3.3. Scanning electron microscope (SEM)
The morphology and microstructure of the isolated collagen was observed by SEM, using a JEOL JSM 6490 LV at high vacuum operated at 20 kV at the SIU Laboratories (Universidad de Antioquia). Prior to the observation, the freeze-dried samples were coated with gold using a Dentonvacum ion sputtering coater.
2.2.3.4. Amino acid analysis
The collagen composition was analyzed using the methods of Li et al. and Pati et al. [1, 3]. 50 mg of lyophilized sample was hydrolysed in HCl at 110 °C for 24 h. Then, it was vaporized and the residues were dissolved in citric acid buffer solution. In order to identify the amino acid composition of COL I, the sample was analyzed by high performance liquid chromatography (HPLC) on Hitachi instruments analyzer. This procedure was carried out by the Agricultural Experiment Station Chemical Laboratories (ESCL) from the University of Missouri.
2.2.4. Finishing methods
Two wet finishing methods were applied on two types of textile fabrics, one taffeta weave and other single jersey knit, both 100 % cotton. Samples for both types of textile fabrics, without modification were used for comparison. Textile samples were cut into 5 × 5 cm squares using industrial scissors. Before applying the finishing, sample fabrics were placed in an extraction soxhlet chamber with 250 mL petroleum ether and refluxed for 2 h to remove any non-fibrous material, such as waxes. Finally, the fabrics were dried in an oven for 2 hours at 50 °C.
2.2.4.1. Method 1: Pad-dry-cure
10 mg of dry collagen was diluted in 2 ml 0.5 M acetic acid solution. The fabrics were immerse in this solution and were passed on a foulard with a pressure of 80 kg.f, then the fabrics were washed with distilled water until neutral pH. Finally, fabrics were transfer to a desiccator until completely dry.
2.2.4.2. Method 2: Exhaustion
Isolated collagen was centrifuged, and the pellet obtained was solubilized in 0.5 M acetic acid solution. The fabrics were immersed in the solution and dialyzed with 0.1 M acetic acid and then with distilled water for two days, with changes of solution every 12 h until reach a pH of 7. The fabrics were dried at the same conditions described above.
2.2.5. Textile finishing characterization methods
To verify the finishing deposition on the fabrics, ATR-FTIR spectroscopy (used to identify the presence of typical collagen bands) and SEM (observe changes on surface by deposition) were used at the same condition mentioned above. Textiles without modification were used for comparison.
3. Results and discussion
3.1. Bone collagen characterization
3.1.1. ATR-FTIR
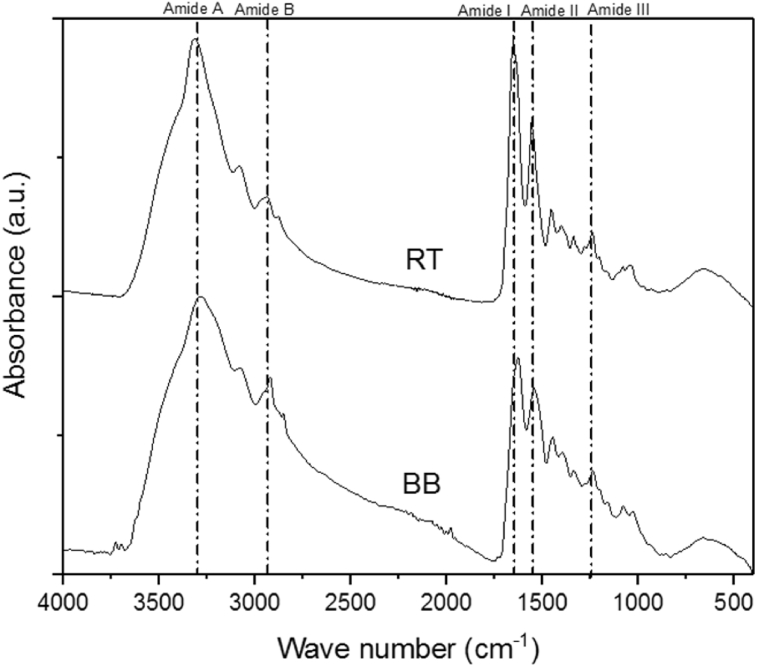
ATR-FTIR spectroscopy was used to compare the difference between the functional groups in collagen structure extracted from bovine bone (BB) and commercial collagen (RT). Infrared spectra of the BB and TR are shown in the Fig. 1, these spectra confirm that the BB bands are consistent with the TR ones, and the characteristic bands in both materials are amide A (3440 - 3400 cm−1), amide I (1700-1600 cm−1), amide II (1545 cm −1) and amide III (1200–1300 cm−1) [16,17].
Fig. 1.
FT-IR spectra of type I collagen extracted from bovine bone (BB) and the commercial collagen (RT).
The amide I band, was mainly associated with the stretching vibrations of the carbonyl groups (C=O bond) along the polypeptide backbone [18] and is related to the presence of secondary structure of proteins [16, 19, 20].
Comparing BB with RT, BB shows higher intensity band for amide III (1246 cm−1). Additionally, a strong intensity of 1246 cm−1 band indicates that the isolated sample has a high content of proline [21]. Moreover, the bands at c.a.1000-800 cm−1 are attributed to other amino acids as phenylalanine, which were much more intense in BB. Likewise, were found peaks c.a. 1450 cm−1 and c.a.1062 cm−1 in both samples, which confirm the existences of aspartic acid and glutamic acid, respectively [22].
3.1.2. SDS – PAGE
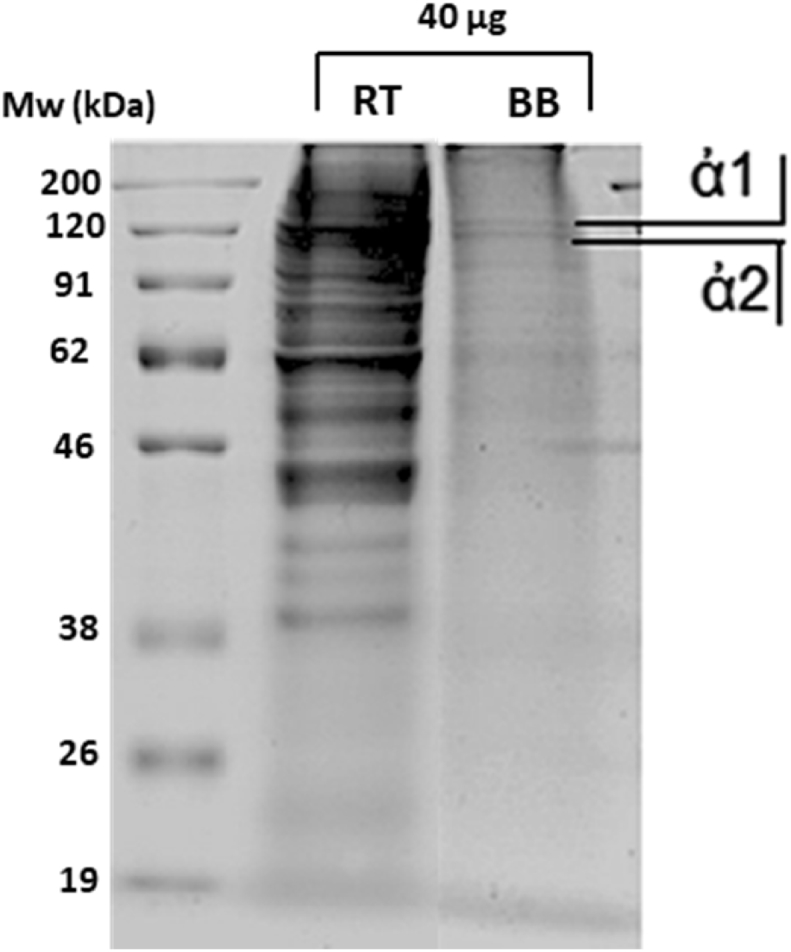
The electrophoretic separation of polypeptides on the gel is due to bands related to specific molecular weight regions, and to different intensities. The electrophoretic profile obtained for BB and RT is shown in Fig. 2, where are shown the α1 and α2 chains having a molecular weight of approximately 120 kDa. The migrate of bands is consistent with the molecular weight reported by other authors as Li et al [3].
Fig. 2.
SDS-PAGE for commercial collagen (RT) and collagen from bovine bone (BB). The unmodified figure of the SDS-PAGE is presented in Supplementary information Figure S1.
Other low molecular weight components between 100 and 55 kDa are recognized, probably for the slight degradation during extraction process [20] or other components in the sample [3]. This degradation and contamination can be also observed in commercial sample.
3.1.3. Amino acid analysis
Collagen is mainly composed by glycine, hydroxyproline and proline and, this was corroborated by the amino acid analysis (Table 1), in which the values obtained were 16.94%, 9.38% and 10.33%, respectively. However, the percentage of glycine is lower than in previous studies documented for other, where was found close to 30% glycine in their COL I (see Table 1) [1, 3]. This could indicate that BB contains relatively more non-collagenous sequences as it has been found for other researchers in the literature. That non-collagenous material with Mw between 40-70 kDa can be released under non-degradative extraction conditions and are too big to be eliminated by dialysis process, those components modify the total mass showing a decrease in the percentage of glycine in the sample [23, 24].
Table 1.
Comparison of amino acids of different samples of COL I from different sources and the extracted bovine bone collagen.
| Aminoacids | Human dermis collagen [32] | Fish scales of Rohu collagen [1] | Fish scales of Catla collagen [1] | Bovine bone collagen (BB) |
|---|---|---|---|---|
| Taurine | ---- | ---- | ---- | 0.01 |
| Hydroxyproline | 8.6 | 8.3 | 8.4 | 9.38 |
| Aspartic Acid | 5.0 | 5.3 | 4.8 | 4.99 |
| Threonine | 2.0 | 1.7 | 1.7 | 1.67 |
| Serine | 4.0 | 2.8 | 2.6 | 2.37 |
| Glutamic Acid | 7.0 | 8.7 | 8.3 | 8.21 |
| Proline | 13 | 11.8 | 13 | 10.33 |
| Glycine | 33 | 36.1 | 35.3 | 16.94 |
| Alanine | 11 | 8.0 | 8.3 | 7.58 |
| Valine | 2 | 1.5 | 1.5 | 2.04 |
| Methionine | 0.6 | 0.9 | 0.9 | 0.73 |
| Isoleucine | 1 | 1.3 | 1.2 | 1.33 |
| Leucine | 2 | 2.8 | 2.6 | 2.89 |
| Tyrosine | 0.3 | 0.3 | 0.2 | 0.62 |
| Phenylalanine | 1 | 5.4 | 5.5 | 1.87 |
| Hydroxylysine | ---- | ---- | ---- | 0.87 |
| Ornithine | ---- | ---- | ---- | 0.32 |
| Lysine | 2 | 1.3 | 1.9 | 3.24 |
| Histidine | 0.5 | 0 | 0 | 0.64 |
| Arginine | 5 | 3.8 | 3.8 | 6.81 |
In FTIR spectra and amino acid analysis, the presence of additional amino acids such as phenylalanine, aspartic acid and glutamic acid was recognized, which could help to improve the performance of the finishing in healthtech application, due to those can act as a regenerator of the connective tissue, taking into account the influence of these on the elimination of toxins present in the tissue as is reported in the literature [25]. Also contributes to the synthesis of different amino acids, such as proline, hydroxyproline, ornithine and arginine, this could be of great benefit to the end use that is intended to applicate in the regeneration of connective tissue [25, 26, 27].
According with this, the comparison of COL I from different species (Table 1) with BB, shows the great similarity between most of the percentages reported for amino acid, which can be concluded the effectiveness of the extraction process. Furthermore, is important to know which amino acid constitute the collagen because of in many cases, this can improve the biocompatibility of materials [25, 28].
3.1.4. SEM
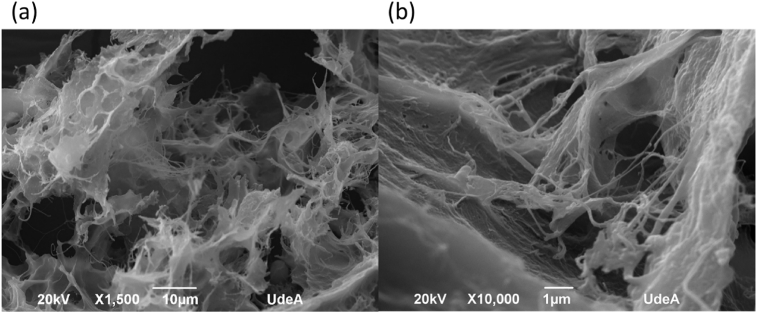
Fig. 3 (a) shows the microstructures of lyophilized BB, a porous and cancellous collagen structure is presented, which was left by the removal of water by the drying method [3, 29]. At higher magnification, in Fig. 3 (b) is possible to observe collagen fibrils that are characteristic of this type of collagen [6, 30, 31].
Fig. 3.
SEM images of extracted collagen; (a) image taken at low magnification 1,500X; (b) image taken at a high magnification 10,000X.
3.2. Finished fabrics characterization
3.2.1. FTIR
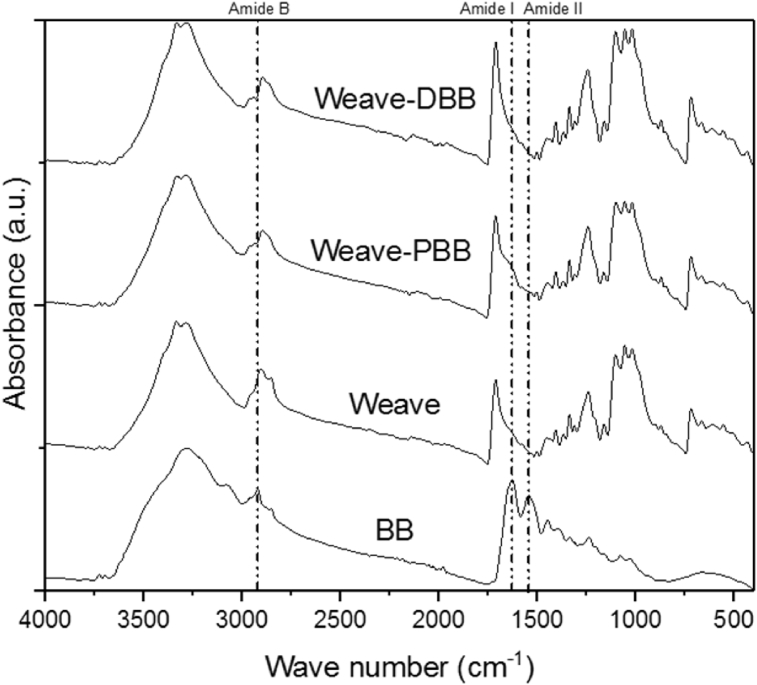
Figs. 4 and 5 show the spectra by FTIR of the different finishing methods (P: Pad and D: Dialysis). The characteristic peaks due to the cotton cellulose macromolecule match with some typical peaks of COL I, so that they are displayed in the spectra with highest intensity in the fabric finished (3334 cm−1 (OH stretch), 2893 cm−1 (stretching CH), 1337 cm−1 (CH bending) and 1046 cm−1 (stretching CO)).
Fig. 4.
FT-IR spectra of collagen (BB), plain weave dialysis (weave-DBB), plain weave dialysis, plain weave with Pad (weave-PBB) and plain fabric (weave).
Fig. 5.
FT-IR spectra of collagen (BB), knitting with dialysis (Knit-DBB), knitting with Pad (Knit-PBB) and knitting (Knit).
However, finished woven and knit fabrics spectra revealed shoulders founded in 2917, 1630 and 1540 cm−1, related with the presence of amine B, I and II compared with the untreated sample, which means that collagen was deposited on textile surface [3, 4]. Finally, was observed that the absorption areas for collagen characteristic peaks are higher in the samples processed by PAD, this may be because of more collagen are retained in this application process on construction fabric.
3.2.2. Fabrics scanning electron microscopy (SEM)
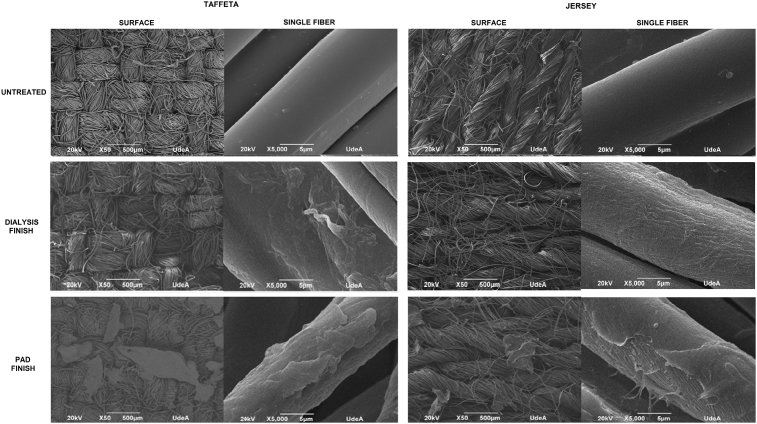
Imagens of taffeta weave and jersey knit fabrics without modification and treated by exhaustion and pad finishing are shown in Fig. 6. The surface in the exhaustion finishing fabrics do not exhibit significant changes compared with the untreated fabrics, this may be because the slight portion of collagen available in the dialysis solution. However, the images of single fibers show a coarse, rough and uneven coat on it, this is attributed to the deposition of collagen on the fibers.
Fig. 6.
SEM images of untreated cotton and finishing taffeta weave and jersey knit fabrics untreated and treated by dialysis and pad methods; fabric surface (X50) and magnification of single fibers (X5000) to the, upper: untreated fabrics, middle: dialysis finish, bottom: pad finish.
Finally, in the surface of pad finishing fabrics high degree of sediment residues can be seen compared to the exhaustion finishing. Therefore, it appears that in this process the increased concentration of COL I and pressure markedly influenced on appearance of collagen in both fabrics. In the illustrations of higher magnification (single fiber) are perceived to be uneven, rough and scaly areas, this may be due to the high concentration of collagen in the acid solution overlying on the textile substrates. The change in the original surface of the untreated cotton fibers is evident, thus setting the collagen solution was deposited on both fabrics.
4. Conclusion
The extracted bone collagen showed similar characteristics to commercial type I collagen and others reported in literature. IR spectra showed the characteristic bands like amide A and amide B. In another point the SDS-PAGE shown the α1 and α2 chains with a molecular weight of approximately 120 kDa which confirms the isolation of type I collagen and the amino acid analysis, showed the main amino acid characteristics in COL I extracted.
The SEM images of both modified fabrics showed a finishing deposition on the surface by the two application methods, however, a greater amount is demonstrated in the finish applied by the PAD method, which is related to the additional pressure applied during the processing, in which the collagen penetrate more efficiently inside the fabrics.
These strategies allow advancing the development of functional fabrics for the health technology segment in the textile industry, in which the presence of collagen could provide bioactivity to fabrics, through properties like biocompatibility, cell adhesion and proliferation, facilitating its use in tissue regeneration aplications like wound treatment.
Declarations
Author contribution statement
Cortés M. Paola, Amya M. Camila: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Cañas Ana, Osorio Marlon, Castro Cristina: Conceived and designed the experiments; Analyzed and interpreted the data.
Sánchez Diego, Zuluaga Robin, Gómez Beatriz: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Universidad Pontificia Bolivariana (CIDI-UPB).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Pati F., Adhikari B., Dhara S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010;101(10):3737–3742. doi: 10.1016/j.biortech.2009.12.133. [DOI] [PubMed] [Google Scholar]
- 2.Bailey A.J., Paul R.G., Knott L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998;106(1–2):1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 3.Li Z.-R., Wang B., Chi C.-f., Zhang Q.-H., Gong Y.-d., Tang J.-J., Luo H.-y., Ding G.-f. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous Niphonius) Food Hydrocolloids. 2013;31(1):103–113. [Google Scholar]
- 4.Lee C., Singla a., Lee a. Y. Biomedical applications of collagen. Int. J. Pharm. 2001;221(1–2):1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 5.Goulam Houssen Y., Gusachenko I., Schanne-Klein M.C., Allain A.J.M. Monitoring micrometer-scale collagen organization in rat-tail tendon upon mechanical strain using second harmonic microscopy. J. Biomech. 2011;44(11):2047–2052. doi: 10.1016/j.jbiomech.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Birk D.E., Fitch J.M., Linsenmayer T.F. Organization of collagen types I and V in the embryonic chicken cornea. Invest. Ophthalmol. Vis. Sci. 1986;27(10):1470–1477. [PubMed] [Google Scholar]
- 7.Ferraro V., Gaillar-Martinie B., Sayd T., Chambon C., Anton M., Santé-Lhoutellier V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potentia. Int. J. Biol. Macromol. 2016;97:55–66. doi: 10.1016/j.ijbiomac.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 8.Yousefi M., Ariffin F., Huda N. An alternative source of type I collagen based on by-product with higher thermal stability. Food Hydrocolloids. 2017;63:372–382. [Google Scholar]
- 9.Nalinanon S., Benjakul S., Kishimura H., Osako K. Type I collagen from the skin of ornate threadfin bream (Nemipterus Hexodon): characteristics and effect of pepsin hydrolysis. Food Chem. 2011;125(2):500–507. [Google Scholar]
- 10.Stevens M.M. Biomaterials for bone tissue engineering. Mater. Today. 2008;11(5):18–25. [Google Scholar]
- 11.Fedegan . Medellín; 2013. National Cattle Inventory Statistics. [Google Scholar]
- 12.Jokinen J., Dadu E., Nykvist P., Käpylä J., White D.J., Ivaska J., Vehviläinen P., Reunanen H., Larjava H., Häkkinen L., Heino J. Integrin-mediated cell adhesion to type I collagen fibrils. J. Biol. Chem. 2004;279(30):31956–31963. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 13.Parenteau-Bareil R., Gauvina R., Cliche S., Gariépy C., Germain L., Berthod F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011;7(10):3757–3765. doi: 10.1016/j.actbio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Goddard J.M., Hotchkiss J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007;32(7):698–725. [Google Scholar]
- 15.Ratner B., Hoffman A., Schoen F., Lemons J. ELSEVIER; 2012. Biomaterials Science: an Introduction to Materials in Medicine Medical Fibers and Biotextiles. [Google Scholar]
- 16.Muyonga J.H., Cole C.G.B., Duodu K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult nile perch (Lates Niloticus) Food Chem. 2004;86(3):325–332. [Google Scholar]
- 17.Singh P., Benjakul S., Maqsood S., Kishimura H. Isolation and characterisation of collagen extracted from the skin of striped catfish (pangasianodon hypophthalmus) Food Chem. 2011;124(1):97–105. [Google Scholar]
- 18.Wang L., An X., Yang F., Xin Z., Zhao L., Hu Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes Mentella) Food Chem. 2008;108(2):616–623. doi: 10.1016/j.foodchem.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Vidal B.D.C., Mello A.M.L.S. Collagen type I amide I band infrared spectroscopy. Micron. 2011;42(3):283–289. doi: 10.1016/j.micron.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Weng W., Zheng H., Su W. Characterization of edible films based on Tilapia (Tilapia Zillii) scale gelatin with different extraction pH. Food Hydrocolloids. 2014;41:19–26. [Google Scholar]
- 21.Nguyen T.T., Gobinet C., Feru J., Pasco S.B., Manfait M., Piot a. O. Characterization of type I and IV collagens by Raman microspectroscopy: identification of spectral markers of the dermo-epidermal junction. Adv. Biomed. Spectrosc. 2012;27(5-6):421–427. [Google Scholar]
- 22.Ikoma T., Kobayashi H., Tanaka J., Walsh D., Mann S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol. 2003;32(3–5):199–204. doi: 10.1016/s0141-8130(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 23.Chung E., Rhodes R.K., Miller E.J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem. Biophys. Res. Commun. 1976:1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- 24.Jander R., Rauterberg J., Voss B., Bassewitz D. B. v. A cysteine-rich collagenous protein from bovine placenta. Isolation of its constituent polypeptide chains and some properties of the non-denatured protein. Eur. J. Biochem. 1980:17–25. [PubMed] [Google Scholar]
- 25.Doolittle R.F. Springer US; 1989. Prediction of Protein Structure and the Principles of Protein Conformation; pp. 599–623. [Google Scholar]
- 26.Kim Y.A., Tarahovsky Y.S., Gaidin S.G., Yagolnik E.A., Muzafarov E.N. Flavonoids determine the rate of fibrillogenesis and structure of collagen type I fibrils in vitro. Int. J. Biol. Macromol. 2017;104(Part A):631–637. doi: 10.1016/j.ijbiomac.2017.06.070. [DOI] [PubMed] [Google Scholar]
- 27.Gauza-Włodarczyk M., Kubisz L., Włodarczyk D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017;104(Part A):987–991. doi: 10.1016/j.ijbiomac.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Ismail Y.M.B., Ferreira A.M., Bretcanu O., Dalgarno K., Haj A.J.E. Polyelectrolyte multi-layers assembly of SiCHA nanopowders and collagen type I on aminolysed PLA films to enhance cell-material interactions. Colloids Surfaces B Biointerfaces. 2017;159:445–453. doi: 10.1016/j.colsurfb.2017.07.086. [DOI] [PubMed] [Google Scholar]
- 29.Jeevithan E., Wu W., Nanping W., Lan H., Bao B. Isolation, purification and characterization of pepsin soluble collagen isolated from silvertip shark (Carcharhinus Albimarginatus) skeletal and head bone. Process Biochem. 2014;49(19):1767–1777. [Google Scholar]
- 30.Torres-Arreola W., Pacheco-Aguilar R., Sotelo-Mundo R., Rouzaud-Sández O., Ezquerra-Brauer J. Caracterización parcial del colágeno extraído a partir del manto, aleta y tentáculos de calamar gigante Dosidicus gigas. Cienc. Tecnol. Aliment. 2008;6(2):101–108. [Google Scholar]
- 31.Wu X., Liu Y., Liu A., Wang W. Improved thermal-stability and mechanical properties of type I collagen by crosslinking with casein, keratin and soy protein isolate using transglutaminase. Int. J. Biol. Macromol. 2017;98:292–301. doi: 10.1016/j.ijbiomac.2017.01.127. [DOI] [PubMed] [Google Scholar]
- 32.Schultz R.M., Liebman M. Proteins I: Composition and Structure. In: Devlin T., editor. Textbook of Chemistry with Chemical Correlations. fourth ed. Wiley-Liss; Pennsylvania, Philadelphia: 1997. p. 31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.