Abstract
Background
This study assessed the predictive value of receptor-interacting protein kinase 3 (RIPK3) expression and its correlation with clinicopathological characteristics, disease-free survival, and overall survival of patients with cisplatin-based adjuvant chemotherapy after lung adenocarcinoma resection.
Methods
This study included 50 patients who underwent cisplatin-based adjuvant chemotherapy after lung adenocarcinoma (stage IB–IIIA) resection. Immunohistochemical analysis was performed by probing tumor tissue microarrays with anti-RIPK3 antibody.
Results
High RIPK3 expression was detected in 24 (48.0%) of the 50 patients. Moreover, high RIPK3 expression was associated with a prolonged disease-free survival (P=0.015) but not with a prolonged overall survival (P=0.109) of the patients who underwent cisplatin doublet therapy after lung adenocarcinoma resection. We also examined whether RIPK3 expression was associated with prognosis based on chemotherapeutic response and found that patients with low RIPK3 expression showed a higher tendency of developing a progressive disease [14/26 (53.8%) patients] than patients with high RIPK3 expression [6/24 (25.0%) patients] (P=0.03). Results of Cox univariate proportional hazards regression model showed that age, N stage, and high RIPK3 expression (P=0.04) were associated with the prolonged disease-free survival of the patients who underwent cisplatin-based adjuvant chemotherapy after lung adenocarcinoma resection.
Conclusions
These results suggest that RIPK3 overexpression is a potential biomarker to identify patients with lung adenocarcinoma who can benefit the most from cisplatin-based adjuvant chemotherapy after complete adenocarcinoma resection.
Keywords: Receptor-interacting protein kinase 3 (RIPK3), lung adenocarcinoma (LUAD), cisplatin, adjuvant chemotherapy
Introduction
Lung cancer is the leading cause of death worldwide (1). Surgical treatment is the best option for treating patients with early-stage (stage I or II) non-small cell lung cancer (NSCLC). However, the role of surgery in treating stage IIIA NSCLC remains controversial. The clinical approach for treating patients with stage IIIA NSCLC is often determined by a multidisciplinary team comprising thoracic surgeons who analyze the number of lymph nodes, tumor size, preoperative neoadjuvant therapy, and other challenging clinical problems (2). Even after complete tumor resection, patients are at a substantial risk of the recurrence of and death due to NSCLC. The use of adjuvant chemotherapy has shown survival benefits in patients who undergo complete resection of stage II–IIIA NSCLC. Moreover, advancements in cisplatin-based chemotherapy has widely increased its use as an adjuvant chemotherapy. A study by an NSCLC collaborative group compared the 5-year OS of patients undergoing surgery alone with that of patients undergoing surgery plus adjuvant chemotherapy and showed that the OS of the patients who underwent surgery plus adjuvant chemotherapy arm was longer than that of the patients who underwent surgery alone [hazard ratio (HR), 0.86; 95% confidence interval (CI), 0.81–0.92; P<0.0001] (3). Previous guidelines have attempted to prevent adverse effects such as toxicity in patients undergoing chemotherapy. However, toxicity remains a concern in patients undergoing chemotherapy, and not all patients benefit from chemotherapy. Therefore, it is important to determine predictive biomarkers of adjuvant chemotherapy to identify patients who will benefit the most from this therapy.
Receptor-interacting protein kinase 3 (RIPK3), a member of RIP serine/threonine family, is a key regulator of necroptosis. The human RIPK3 gene is located on chromosome 14q11.2 and is implicated in the tumorigenesis of several cancers (4,5). However, its clinical significance and potential role in the pathogenesis of lung cancer have been poorly studied. Recent studies have shown that treatment with hypomethylating agents restores silenced RIPK3 expression and increases sensitivity to chemotherapeutic agents in an RIPK3-dependent manner (6). Upregulation of silenced RIPK3 expression also restores cisplatin sensitivity, suggesting that RIPK3 contributes to cisplatin chemosensitivity (7). Based on these findings, we examined the role of RIPK3 as a potential predictive biomarker of cisplatin-based adjuvant chemotherapy in patients who previously underwent surgical resection of lung adenocarcinoma (LUAD).
Methods
Patients and clinical specimens
In all, 143 frozen tumor tissue specimens that were surgically resected from patients who underwent lobectomies for primary LUAD from 2009 to 2015 were provided by the Biobank of Pusan National University Yangsan Hospital. All the tumor specimens were histologically examined and were classified using the 2015 World Health Organization International Classification of Lung Tumors (8). Tumor tissue microarrays (TMAs) were constructed using two tissue cores (diameter, 1 mm) per tumor, specifically tissues obtained from central and peripheral tumor areas, after performing the histological examination of the 143 LUAD specimens at the Pathology Department of the Pusan National University Yangsan Hospital. As a part of staging work-up, all the patients underwent contrast-enhanced computed tomography (CT), molecular resonance brain metastasis scans, and positron emission-CT within 1 month before the surgery. Patients with invasive adenocarcinoma, including lepidic, acinar, papillary, mucinous, and solid adenocarcinomas, were included in a conventional adenocarcinoma group. Lymphatic and vascular invasion of tumor cells was observed in lymphatic and vascular vessel lumens, respectively. The following clinical characteristics of the patients were analyzed to determine their effect on patient survival: age, sex, smoking history, and tumor differentiation degree. The clinical and pathological data of all the patients were integrated. Of the 143 patients who underwent surgical resection for LUAD, 50 patients received a cisplatin-based regimen for at least three cycles (Table 1). CT scans were performed to evaluate the efficacy of the chemotherapy regimen after two to four cycles. This study was approved by the Institutional Review Board (05-2017-162) of the Pusan National University Yangsan Hospital.
Table 1. Adjuvant chemotherapy regimen in RIPK3 protein high- and low- expression groups.
| Regimen | RIPK3 | P value | |
|---|---|---|---|
| High (n=24) | Low (n=26) | ||
| Paclitaxel/CDDP | 20 | 22 | 0.845 |
| Vinorelbine/CDDP | 4 | 4 | |
CDDP, cis-diamminedichloroplatinum (cisplatin).
Western blotting analysis
The lung tissue samples of the patients were homogenized and lysed using PRO-PREPTM Protein Extraction Solution (iNtRON Biotechnology, Korea). The lung homogenates then were centrifuged, and supernatant obtained was collected. Protein concentration of the supernatant was determined using a protein assay kit (Bio-Rad, Hercules, CA). Next, equal amounts of protein present in the supernatant were resolved by performing SDS-PAGE and were transferred onto PVDF membranes for performing immunoblotting analysis. The membranes were blocked with 5% skimmed milk in tris-buffered saline containing Tween-20 and were incubated with primary antibodies against RIPK3 (Cell Signaling Technology, Danvers, MA) and β-actin (Santa Cruz Biotechnology, Dallas, TX). The bound antibodies were detected using a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Dallas, TX), and antigen-antibody complexes were visualized using WesternBright ECL HRP substrate (Advansta Inc., Menlo Park, CA). Densitometric analysis was performed using FluorChem SP AlphaEase® FC (version 6.0.0) software (Alpha Innotech, San Leandro, CA), and data obtained were normalized using those obtained for β-actin.
Immunohistochemical analysis
Immunohistochemical analysis of the lung tumor specimens was performed using an anti-RIPK3 antibody (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s instructions. The tissue sections were counterstained with Mayer’s hematoxylin, and immunostaining was visualized using cellSens standard (DP21; Olympus Life Sciences, Tokyo, Japan). Staining intensity in each slide was scored on a scale of 0 to 3+. RIPK3 expression was evaluated based on the staining intensity score, with a score of 0 to 1+ indicating low RIPK3 expression and a score of +2 to +3 indicating high RIPK3 expression. In case of discrepancy in the staining intensity scores, the tissue sections were re-examined by another pathologist.
The Cancer Genome Atlas data analysis
In the present study, The Cancer Genome Atlas (TCGA) mRNA sequencing datasets of 506 patients with LUAD, which are available on cBioPortal online platform, were analyzed to determine whether RIPK3 expression level affected the prognosis of patients with LUAD.
Statistical analysis
OS and disease-free survival (DFS) were analyzed by obtaining Kaplan-Meier plots showing survival probability curves with different strata. Cox univariate proportional hazard regression models were fitted to identify the effect and significance of each factor of interest on survival patterns. In the univariate analysis, HRs with 95% CIs were determined for each clinical characteristic of the patients.
Results
RIPK3 expression status in patients with LUAD
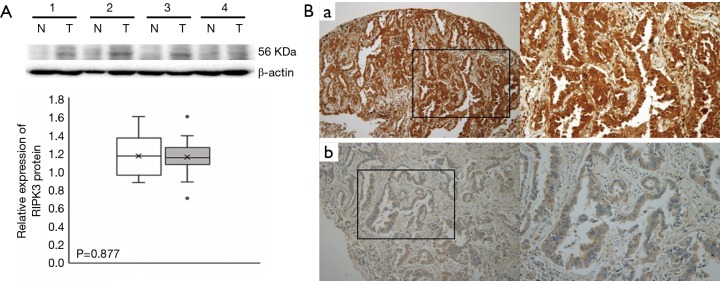
The predictive significance of RIPK3 was determined by performing western blotting analysis. No significant differences were observed between RIPK3 protein levels between the tumor tissues and paired normal tissues (n=11, P=0.877; Figure 1A). However, a variation in RIPK3 expression levels was observed in the TMAs constructed from the tumor specimens obtained from the 143 patients with LUAD (high RIPK3 expression, 53 patients; low RIPK3 expression, 90 patients; Table 2). The correlations between the clinical characteristics of the patients with LUAD and RIPK3 expression are summarized in Table 2.
Figure 1.
RIPK3 expression in human LUAD tissues and adjacent normal epithelial tissues. (A) Western blotting analysis of RIPK3 expression levels. Proteins obtained from 11 adenocarcinoma tissue (T) specimens and pair-matched normal tissue (N) specimens were examined by performing Western blotting analysis. β-Actin was used as an internal control. The experiment was repeated three times independently and provided similar results. Values are shown as mean ± SD (n=11 replicates). RIPK3 expression levels in the N and T specimens are shown in a box-whisker plot. Significance was calculated using Mann-Whitney U test (P=0.877). (B): (a) representative image showing high RIPK3 expression, which was observed in the cytoplasm and nucleus of cancer cells; (b) RIPK3 staining was absent or minimal in the tissues showing low RIPK3 expression. Magnification: ×100 in the left panels and ×200 in the right panels. LUAD, lung adenocarcinoma.
Table 2. RIP3 immunohistochemical data for lung adeno-NSCLC: association of RIPK3 score with clinical characteristics.
| Clinical factors | RIPK3 | P value | |
|---|---|---|---|
| High (n=53) | Low (n=90) | ||
| Age | 0.794 | ||
| ≤65 | 31 | 55 | |
| >65 | 22 | 35 | |
| Sex | 0.096 | ||
| Female | 17 | 43 | |
| Male | 36 | 47 | |
| Smoking Hx | 0.375 | ||
| Current | 16 | 18 | |
| Former | 8 | 17 | |
| Never | 29 | 55 | |
| TNM stage | 0.370 | ||
| Ia | 21 | 35 | |
| Ib | 14 | 15 | |
| IIa | 5 | 13 | |
| IIb | 5 | 4 | |
| IIIa | 3 | 13 | |
| IV | 5 | 10 | |
NSCLC, non-small cell lung cancer.
High RIPK3 expression levels are associated with good clinical outcomes of patients undergoing cisplatin-based adjuvant chemotherapy after complete tumor resection
To confirm the differential RIPK3 expression and its correlation with the clinical characteristics of the patients with LUAD, we performed immunohistochemical analysis of the TMAs constructed from the tumor specimens obtained from the 50 patients who underwent cisplatin-based adjuvant chemotherapy after LUAD (stage IB–IIIA) resection. Each adenocarcinoma tissue specimen was classified into high (RIPK3 staining intensity score of 2+ or 3+) or low (RIPK3 staining intensity score of <2+) RIPK3 expression groups (Figure 1B). High RIPK3 expression was detected in 48.0% (24/50) TMAs and low RIPK3 expression was detected in 52.0% (26/50) TMAs (Table 3).
Table 3. Clinical characteristics of patients for RIPK3 protein expression.
| Clinical factor | RIPK3 | P value | |
|---|---|---|---|
| High (n=24) | Low (n=26) | ||
| Age | 0.156 | ||
| ≤65 | 15 | 15 | |
| >65 | 9 | 11 | |
| Sex | 0.808 | ||
| Female | 6 | 9 | |
| Male | 18 | 17 | |
| Smoking Hx | 0.491 | ||
| Current | 8 | 5 | |
| Former | 5 | 6 | |
| Never | 11 | 15 | |
| TNM stage | 0.08 | ||
| Ib | 13 | 5 | |
| IIa | 2 | 6 | |
| IIb | 3 | 3 | |
| IIIa | 6 | 12 | |
| Degree of differentiation | 0.03 | ||
| Well | 1 | 1 | |
| Moderate/well | 1 | 7 | |
| Moderate | 11 | 15 | |
| Poor | 11 | 3 | |
| Lymphovascular invasion | 0.496 | ||
| Positive | 8 | 11 | |
| Negative | 16 | 15 | |
| Histologic subtype (predominant pattern) | 0.288 | ||
| Lepidic | 1 | 2 | |
| Acinar | 10 | 15 | |
| Papillary | 1 | 2 | |
| Solid | 7 | 6 | |
| Invasive mucinous | 5 | 1 | |
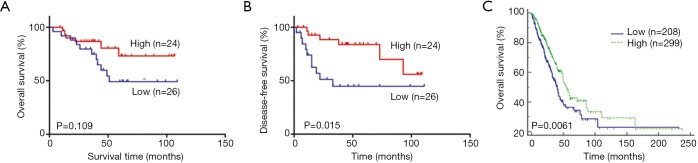
The Kaplan-Meier survival analysis with a log-rank test showed a correlation between high RIPK3 expression and the prolonged OS; however, the log-rank test of the curves showed no significant correlation (Figure 2A). Moreover, the Kaplan-Meier analysis showed that RIPK3 expression was significantly associated with DFS rate (log-rank test, P=0.015; Figure 2B). However, analysis of the TCGA mRNA sequencing data showed that the OS rate was higher in the patients showing high RIPK3 expression than in those showing low RIPK3 expression (P=0.0061; Figure 2C). These results suggest that increased RIPK3 expression levels are a good predictor of survival in patients who undergo cisplatin-based adjuvant chemotherapy after surgical LUAD resection.
Figure 2.
The Kaplan-Meier curves obtained using the TMA specimens. The OS (A) and DFS (B) curves of the patients showing high and low RIPK3 expression levels who underwent the cisplatin doublet chemotherapy; (C) the OS curves obtained using the TCGA mRNA sequencing data. OS, overall survival; DFS, disease-free survival.
High RIPK3 expression is associated with the positive outcomes of cisplatin-based adjuvant chemotherapy
To examine whether RIPK3 was associated with prognosis based on chemotherapeutic responses, we examined adenocarcinoma samples obtained from the 50 patients who underwent the cisplatin-based adjuvant chemotherapy after complete LUAD resection (Table 4). RIPK3 immunostaining was significantly more intense in the TMAs of patients showing complete remission, partial remission, and stable disease than in the TMAs of patients showing a progressive disease (PD). However, the frequency of developing a PD was higher in the patients showing low RIPK3 expression (14/26, 53.8%) than in those showing high RIPK3 expression (6/24, 25.0%). Results of the univariate analysis showed that the age (OS: HR, 1.09; 95% CI, 1.03–1.16; P=0.004, DFS: HR, 1.09; 95% CI, 1.03–1.16; P=0.003) and N stage (OS: HR, 1.71; 95% CI, 1.02–2.85; P=0.04, DFS: HR, 1.80; 95% CI, 1.09–2.99; P=0.02) of the patients with LUAD were associated with the prolonged OS and DFS, whereas the RIPK3 expression status of the patients with LUAD was only associated with the prolonged DFS (HR, 0.32; 95% CI, 0.11–0.98; P=0.04; Table 5). These results indicate that RIPK3 expression is significantly associated with the prolonged DFS of patient undergoing cisplatin-based adjuvant chemotherapy after LUAD resection.
Table 4. The relationship between RIPK3 protein expression and treatment responses.
| Response to chemotherapy | RIPK3 | P value | |
|---|---|---|---|
| High (n=24) | Low (n=26) | ||
| CR + PR + SD | 18 | 12 | 0.03 |
| PD | 6 | 14 | |
Table 5. Univariate analysis of clinical variables and RIPK3 in relation to the OS and DFS.
| Variable | OS | DFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.09 (1.03–1.16) | 0.004 | 1.09 (1.03–1.16) | 0.003 | |
| Gender | 1.77 (0.58–5.40) | 0.31 | 1.39 (0.46–4.23) | 0.56 | |
| Smoking | 1.43 (0.56–3.72) | 0.45 | 1.50 (0.58–3.86) | 0.40 | |
| T stage | 0.86 (0.32–2.30) | 0.77 | 0.95 (0.36–2.54) | 0.92 | |
| N stage | 1.71 (1.02–2.85) | 0.04 | 1.80 (1.09–2.99) | 0.02 | |
| M stage | 0.42 (0.34–0.85) | 1.52 | 0.38 (0.33–1.56) | 1.46 | |
| TNM stage | 1.97 (0.76–5.11) | 0.16 | 2.32 (0.90–5.98) | 0.08 | |
| Lymphovascular invasion | 0.77 (0.30–1.95) | 0.57 | 0.93 (0.37–2.36) | 0.88 | |
| Differentiation | 1.02 (0.33–3.10) | 0.94 | 0.80 (0.26–2.44) | 0.70 | |
| RIPK3 | 0.45 (0.15–1.37) | 0.16 | 0.32 (0.11–0.98) | 0.04 | |
OS, overall survival; DFS, disease-free survival.
Discussion
Cisplatin-based adjuvant chemotherapy alleviates the risk of relapse after complete resection in patients with high-risk stage IB, II, and IIIA adeno-NSCLCs. International Adjuvant Lung Cancer Trial examined the effect of cisplatin-based adjuvant chemotherapy on the survival of patients undergoing complete NSCLC resection and reported a benefit of 4.1% in the 5-year OS of 1,867 randomized patients (9). Another clinical study (JBR.10 trial) assessed the benefit of cisplatin-based adjuvant chemotherapy on the 5-year OS of patients with early-stage IB or II NSCLC and reported that the 5-year OS of the patients receiving the cisplatin-based adjuvant chemotherapy improved by 15% compared with that of control patients (10). However, a long-term follow-up showed that the 5-year OS was not significantly different between patients who underwent surgical resection plus cisplatin-based adjuvant chemotherapy and those who underwent surgical resection alone (11). At present, no objective biomarkers besides clinical risk factors such as tumor size and lymphovascular invasion are available for selecting patients with stage IB adeno-NSCLC for administering adjuvant chemotherapy. Several biomarkers that predict the benefit of cisplatin-based adjuvant chemotherapy have been identified to date (12,13); however, none of these biomarkers are appropriate for clinical use. Therefore, there is a need for determining biomarkers that can identify patients who are most likely to benefit from adjuvant chemotherapy. In the present study, we elucidated the predictive significance of RIPK3 expression levels in the patients who underwent cisplatin-based adjuvant chemotherapy after the surgical resection of stage IB–IIIA NSCLCs.
Previous studies have shown that RIPK3 expression is markedly reduced in cancer tissues compared with that in normal tissues, which decreases necroptosis as an alternative to cell death in the presence of apoptosis resistance (14). Furthermore, the regulation of RIPK3 expression plays an important role in cancer survival by modulating the sensitivity of cancer cells to anticancer chemotherapy (15-18). The predictive role of RIPK3 by using clinical tissue analyses has not been published to date. The present study is the first to examine the RIPK3 expression status in LUAD tissues and adjacent normal tissues by using the TCGA mRNA sequencing data and to show no significant difference in RIPK3 expression levels between the LUAD tissues and adjacent normal tissues (data not shown). The results of immunohistochemical analysis performed in the present study are consistent with those obtained by analyzing the TCGA mRNA sequencing data (n=11, P=0.877; Figure 1A). A recent study also reported no statistical difference in RIPK3 expression between LUAD tissues and normal tissues (7). However, we observed a variation in RIPK3 expression level in the TMAs constructed using the surgical specimens obtained from the 143 patients with LUAD (Table 2).
We assessed the association of high and low RIPK3 expression with the clinical characteristics, DFS, and OS of the 50 patients with operative stage IB–IIIA adeno-NSCLC who underwent the cisplatin-based adjuvant chemotherapy. A recent study reported that mutant RIPK3 overexpression in esophageal cancer cells restores cisplatin sensitivity and that RIPK3 silencing is associated with a short OS rate (7). Another study suggested that RIPK3 is an important independent prognostic indicator of the OS and DFS of patients with colorectal cancer (19). The Kaplan-Meier curves obtained in the present study showed that high RIPK3 expression levels were associated with increased OS; however, the log-rank test of the curves showed no significant association (P=0.109; Figure 2A). Because the present study included a small number of patients with LUAD, the TCGA mRNA sequencing data were used to investigate the OS of patients with LUAD and its association with RIPK3 expression levels in these patients. Our results showed that the OS rate of the patients showing high RIPK3 expression was higher than that of the patients showing low RIPK3 expression (P=0.0061; Figure 2C). Moreover, our results showed that the DFS rate was significantly higher in the patients showing high RIPK3 expression than in those showing low RIPK3 expression (P=0.015; Figure 2B). We next analyzed whether RIPK3 expression was associated with the prolonged DFS rate by analyzing the chemotherapeutic responses of the patients and found that the patients with high RIPK3 expression showed lower incidence of PD than those with low RIPK3 expression (P=0.03; Table 4). In addition, the Cox univariate proportional hazards regression model indicated that age, N stage, and RIPK3 protein expression status were associated with the prolonged DFS rate of the patients with LUAD who underwent the cisplatin-based adjuvant chemotherapy after complete tumor resection (Table 5). These results suggest that RIPK3, at least partly, is a useful biomarker for predicting the response of patients with adeno-NSCLC to cisplatin-based adjuvant chemotherapy. However, additional large-scale studies should be performed to validate the results of our preliminary study and to assesses whether RIPK3 is a predictive biomarker associated with the long-term effectiveness of cisplatin-based adjuvant chemotherapy in patients with early-stage LUAD.
Acknowledgements
The authors would like to thank Dr. YS Kim (Ajou University, Korea) for kindly gifting experimental supplies. The biospecimens and data used in this study were provided by the Biobank of the Pusan National University Yangsan Hospital. This study was supported by a grant from the Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, to DH Kim.
Ethical Statement: This study was approved by the Institutional Review Board (05-2017-162) of the Pusan National University Yangsan Hospital.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. [published 27 June 2018, accessed 9 July 2018]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 3.Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. 10.1016/S0140-6736(10)60059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 5.Moriwaki K, Chan FK. RIPK3: a molecular switch for necrosis and inflammation. Genes Dev 2013;27:1640-9. 10.1101/gad.223321.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo GB, Morgan MJ, Lee DG, et al. Methylation-dependent loss of RIPK3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res 2015;25:707-25. 10.1038/cr.2015.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Zhai L, Ma S, et al. Down-regulation of RIPK3 potentiates cisplatin chemoresistance by triggering HSP90-ERK pathway mediated DNA repair in esophageal squamous cell carcinoma. Cancer Lett 2018;418:97-108. 10.1016/j.canlet.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. 10.1056/NEJMoa031644 [DOI] [PubMed] [Google Scholar]
- 10.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completelyresected stage IB and II non-small- cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. 10.1200/JCO.2009.24.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol 2017;35:2960-74. 10.1200/JCO.2017.72.4401 [DOI] [PubMed] [Google Scholar]
- 12.Bell EH, Chakraborty AR, Mo X, et al. SMARCA4/BRG1 Is a Novel Prognostic Biomarker Predictive of Cisplatin-Based Chemotherapy Outcomes in Resected Non–Small Cell Lung Cancer. Clin Cancer Res 2016;22:2396-404. 10.1158/1078-0432.CCR-15-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma X, Le Teuff G, Lacas B, et al. Prognostic and Predictive Effect of TP53 Mutations in Patients with Non-Small Cell Lung Cancer from Adjuvant Cisplatin-Based Therapy Randomized Trials: A LACE-Bio Pooled Analysis. J Thorac Oncol 2016;11:850-61. 10.1016/j.jtho.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Jin Y, Yang W, et al. Necroptosis in cancer: An angel or a demon? Tumour Biol 2017;39:1010428317711539. 10.1177/1010428317711539 [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Xiao W, Huang L, et al. Shikonin induces apoptosis and necroptosis in pancreatic cancer via regulating the expression of RIP1/RIPK3 and synergizes the activity of gemcitabine. Am J Transl Res 2017;9:5507-17. [PMC free article] [PubMed] [Google Scholar]
- 16.He W, Wang Q, Srinivasan B, et al. A JNK-mediated autophagy pathway that triggers c-IAP degradation and necroptosis for anticancer chemotherapy. Oncogene 2014;33:3004-13. 10.1038/onc.2013.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Ma Y, Chen G, et al. Contribution of RIPK3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology 2016;5:e1149673. 10.1080/2162402X.2016.1149673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenabeele P, Declercq W, Van Herreweghe F, et al. The role of the kinases RIP1 and RIPK3 in TNF-induced necrosis. Sci Signal 2010;3:re4. 10.1126/scisignal.3115re4 [DOI] [PubMed] [Google Scholar]
- 19.Feng X, Song Q, Yu A, et al. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma 2015;62:592-601. 10.4149/neo_2015_071 [DOI] [PubMed] [Google Scholar]