Summary
Whole-genome duplication (WGD) has been recognized as a significant evolutionary force in the origin and diversification of multiple organisms. Acropora, a speciose reef-building coral genus, is suspected to have originated by polyploidy. Yet, there is no genetic evidence to support this hypothesis. Using comprehensive phylogenomic and comparative genomic approaches, we analyzed six Acroporid genomes and found that a WGD event likely occurred ∼31 million years ago in the most recent common ancestor of Acropora, concurrent with a worldwide coral extinction. We found that duplicated genes were highly enriched in gene regulation functions, including those of stress responses. The functional clusters of duplicated genes are related to the divergence of gene expression patterns during development. Some proteinaceous toxins were generated by WGD in Acropora compared with other cnidarian species. Collectively, this study provides evidence for an ancient WGD event in corals, which helps explain the origin and diversification of Acropora.
Subject Areas: Genetics, Evolutionary Biology, Bioinformatics, Omics
Graphical Abstract
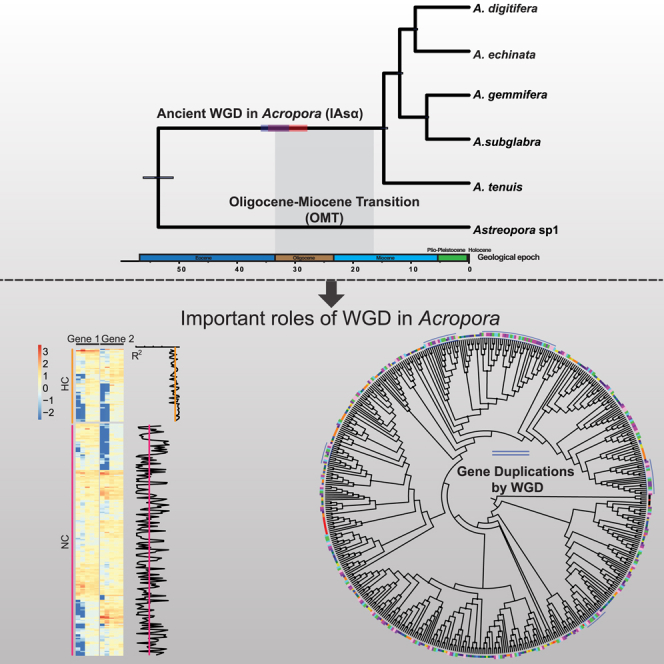
Highlights
-
•
An ancient genome duplication occurred in the most recent common ancestor of Acropora
-
•
This WGD event likely occurred between 28 and 36 mya in Acropora
-
•
The WGD event potentially contributes to the origin and diversification of Acropora
-
•
Duplications of toxic proteins were found in Acropora following the WGD
Genetics; Evolutionary Biology; Bioinformatics; Omics
Introduction
Reef-building corals contribute to tropical marine ecosystems that support innumerable marine organisms, but reefs are increasingly threatened because of recent increases in seawater temperatures, pollution, and other stressors (Ainsworth et al., 2016, Renema et al., 2016). The Acroporidae is a family of reef-building corals within the phylum Cnidaria, one of the basal phyla of the animal clade (Richards et al., 2013, Wallace, 2012, Wallace and Rosen, 2006). Astreopora (Anthozoa: Acroporidae) is the sister genus in the acroporid lineage according to fossil records and molecular phylogenetic evidence (Fukami et al., 2000, Suzuki and Nomura, 2013, Wallace, 2012). Importantly, Acropora (Anthozoa: Acroporidae), one of the most diverse genera of reef-building corals, including more than 150 species in the Indo-Pacific Ocean, is thought to have originated from Astreopora ∼60 mya with several species turnovers (Edinger and Risk, 1994, Renema et al., 2008, Wallace, 2012, Wallace and Rosen, 2006). Investigating the evolutionary history of this group importantly contributes to our understanding of coral reef biodiversity and conservation. Hybridization among Acropora species has been observed in the wild (Vollmer and Palumbi, 2002), and variable chromosome numbers have been determined in different Acropora lineages (Kenyon, 1997). In addition, gene duplications have been shown in several Acropora gene families (Gacesa et al., 2015, Hamada et al., 2013). Based on their unique lifestyle, variable chromosome numbers, and complicated reticular evolutionary history, Indo-Pacific Acropora likely originated via polyploidy (Gacesa et al., 2015, Hamada et al., 2013, Kenyon, 1997, Richards and Hobbs, 2015, Van Oppen et al., 2001, Vollmer and Palumbi, 2002, Willis et al., 2006). However, there is no direct molecular and genetic evidence to support this hypothesis.
Ancient whole (large-scale)-gene/genome duplication (WGD), or paleopolyploidy, has shaped the genomes of vertebrates, green plants, and other organisms, and is usually regarded as an evolutionary landmark in the origin and diversification of organisms (Soltis et al., 2015, Van de Peer et al., 2009, Van De Peer et al., 2017) (Figure S1). Two separate WGD events have been documented in the common ancestors of vertebrates (two rounds of WGD) (Dehal and Boore, 2005) and another major WGD has been reported in the last common ancestor of teleost fish (Christoffels et al., 2004, Glasauer and Neuhauss, 2014). Meanwhile, living angiosperms share an ancient WGD event (Jiao et al., 2011, Tiley et al., 2016), and many other WGD events have been reported in major clades of angiosperms (Soltis et al., 2009, Vanneste et al., 2014, Wang et al., 2018). In addition, two rounds of WGDs in the vertebrates are suggested to have occurred during the Cambrian Period, and some WGDs in plants are believed to have occurred during Cretaceous-Tertiary (Smith et al., 2013, Van De Peer et al., 2017, Vanneste et al., 2014). Thus, WGD is regarded as an important evolutionary way to reduce the risk of extinction (Van de Peer et al., 2009, Van De Peer et al., 2017, Vanneste et al., 2014). However, the study of WGD in Cnidaria has received less attention (Kenny et al., 2016, Li et al., 2018, Schwager et al., 2017, Van de Peer et al., 2009, Van De Peer et al., 2017).
Duplicated genes created by WGD have complex fates during diploidization (Sémon and Wolfe, 2007, Van de Peer et al., 2009). Usually, one of the duplicated genes is silenced or lost due to redundancy of gene functions, termed “nonfunctionalization.” However, retained duplicated genes provide important sources of biological complexity and evolutionary novelty due to subfunctionalization, neofunctionalization, and dosage effects (Conant et al., 2014, Jiao et al., 2011). Duplicated genes may develop complementary gene functions via subfunctionalization, evolve new functions through neofunctionalization, or are retained in complicated regulatory networks with different gene expressions due to dosage effects. For instance, duplicated MADS-Box genes are crucial for flower development and the origin of phenotypic novelty in plants (Van de Peer et al., 2009, Veron et al., 2006). Duplicated homeobox genes provide raw genetic material for vertebrate development (Canestro et al., 2013, Glasauer and Neuhauss, 2014). In addition, toxin diversification following gene duplications has been recognized as a mechanism to enhance adaptation in animals (Kondrashov, 2012, Kordiš and Gubenšek, 2000), especially in snake venoms (Hargreaves et al., 2014, Vonk et al., 2013). Interestingly, toxic proteins are involved in various important processes in corals, including prey capture, protection from predators, wound healing, etc (Armoza-Zvuloni et al., 2016, Ben-Ari et al., 2018), but it is still unclear how gene duplications of toxic proteins evolved in corals.
Isozyme electrophoresis and restriction fragment length polymorphism were used to identify gene duplications in polyploids a few decades ago (Fürthauer et al., 1999, Stuber and Goodman, 1983). In the past 10 years, next-generation sequencing has generated a wealth of genomic data at vastly decreased cost and reduced efforts (Goodwin et al., 2016, Hardwick et al., 2017). Three main methods were developed to identify WGD, based on (1) analysis of the rate of synonymous substitutions per synonymous site (dS) of duplicated genes within a genome (dS-based method) (Blanc et al., 2003, Lynch and Conery, 2000, Vanneste et al., 2014, Mao, 2019), (2) phylogenetic analysis of gene families among multiple genomes (phylogenomic analysis) (Blomme et al., 2006, Jiao et al., 2011), and (3) synteny block identification compared with sister lineages without WGD (synteny analysis) (Bowers et al., 2003, Dehal and Boore, 2005, Zhang et al., 2017). The dS-based method and phylogenomic analysis only require gene family information, without genome assembly. However, too ancient WGD cannot be detected by the dS-based method, while gene tree uncertainty usually causes bias in the phylogenomic analysis. Both methods rely heavily on gene family estimation and clustering. Inaccurate gene predictions (gene models) and rough gene family cluster algorithms can easily fail to detect WGD using either method. In contrast, the synteny analysis relies heavily on genome assembly quality. Poor assembly quality can hide the WGD signals, and some genomes with huge rearrangements cannot be used to detect WGD using synteny block identification. Therefore, the most credible conclusions depend on complementary evidence from different methods (Chen and Birchler, 2013, Soltis and Soltis, 2012, Tiley et al., 2016).
Here, using all three methods, we analyzed a genome of Astreopora (Astreopora sp1) as an outgroup, and five Acropora genomes (Acropora digitifera, Acropora gemmifera, Acropora subglabra, Acropora echinata, and Acropora tenuis) to address the following questions: (1) whether and when WGD occurred in Acropora, (2) what is the fate of duplicated genes in Acropora after the event, (3) what are the gene expression patterns of duplicated genes across five developmental stages in A. digitifera, and (4) what is the role of WGD in diversification of toxic proteins in Acropora (Figure S2).
Results
Calibration of the Acroporid Phylogenomic Tree
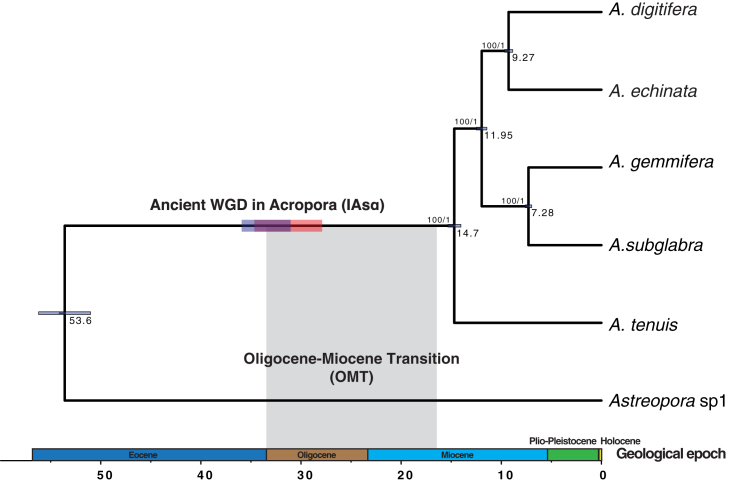
We clustered all homologs of the six Acroporid species and found that 6,520 gene families are shared among 19,760 gene families in total (Figure S3, see Data S1). Our previous gene family cluster analysis of the five Acropora species showed that each Acropora genome had very few unique gene families (<100) (Mao et al., 2018). Yet, we found that Astreopora sp1 had 836 unique gene families, suggesting that Astreopora sp1 is genetically divergent from the five Acropora species. A total of 3,461 single-copy orthologs selected from 6,520 shared gene families were concatenated to reconstruct a calibrated phylogenomic tree based on the reported divergence time of Acropora (Mao et al., 2018). We found that Astreopora sp1 splitted from Acropora ∼53.6 mya (95% highest posterior density: 51.02–56.21 Ma) (Figures 1 and S4). This result established a timescale to analyze the timing of the subsequent WGD.
Figure 1.
Ancient WGD in the Reef-Building Coral Acropora (IAsα)
A calibrated phylogenomic tree of six Acroporid species inferred from 3,461 single-copy orthologs using BEAST2. Horizontal bars on branches of the tree represent the timing of WGD in Acropora. The timing of IAsα was estimated at 35 mya (95% confidence interval: 31.18–35.7 mya) and 31 mya (95% confidence interval: 27.86–34.77 mya) by the dS-based method (horizontal purple bar) and phylogenomic analysis (horizontal orange bar), respectively. Gray shading represents the timing of one coral species turnover event, the Oligocene-Miocene transition (OMT), suggesting that IAsα is correlated with OMT. See also Figures S1, S3, and S4, Tables S5 and S6.
WGD Identification with the dS-Based Method
Synonymous substitution rate (dS) analysis has been widely used to infer WGD (Vanneste et al., 2012, Vanneste et al., 2014). We identified over 10,000 paralogous gene pairs, based on their sequence similarities, and over 10,000 anchor gene pairs, based on synteny information of each species (Table S1; see Methods). Using paralogous and anchor gene pairs, we calculated their dS values and reconstructed dS distributions for each species.
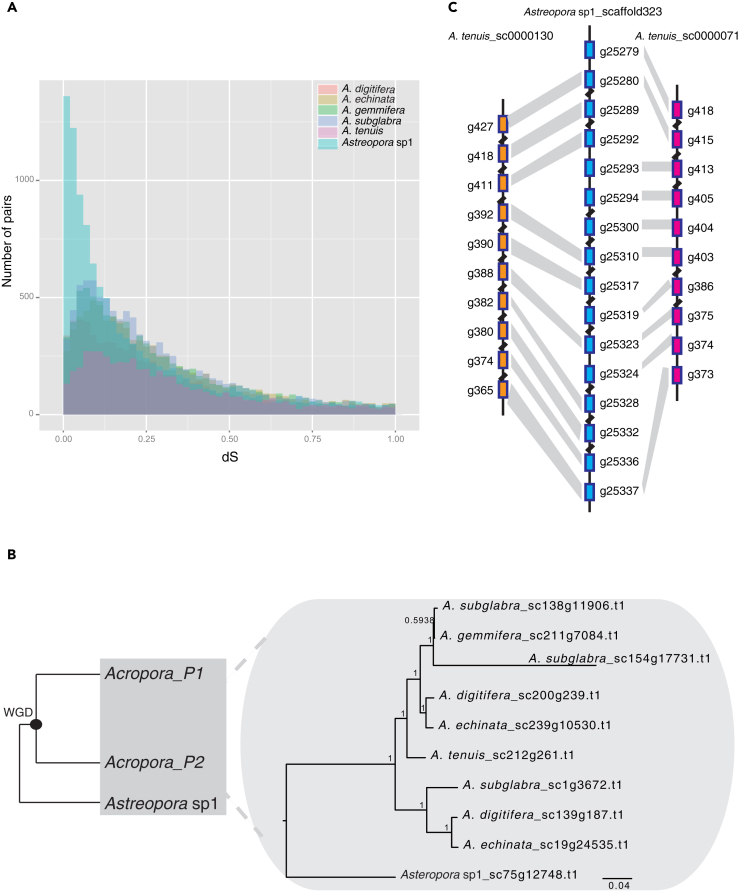
An “L-shaped” distribution was evident in both paralogous and anchor gene pair dS distributions of Astreopora sp1, illustrating that no WGD occurred in Astreopora sp1. However, all five Acropora species displayed a similar peak in dS distributions of both paralogous and anchor gene pairs (peak: 0–0.3), suggesting that WGD did occur in Acropora (Figures 2A, S5, and S6).
Figure 2.
Ancient WGD Identification (IAsα) and Timing of the Event in Acropora
(A) Frequency distribution of dS values for paralogous gene pairs in five Acropora and one Astreopora species showing that a WGD event occurred in Acropora. Similar peaks (dS value: 0.1–0.3) in dS distributions of the five Acropora lineages indicate that a WGD event occurred in Acropora. (Light red, A. digitifera; light yellow, A. echinata; light green, A. gemmifera; light blue, A. subglabra; light purple: A. tenuis; light cyan, Astreopora sp1).
(B) Hypothetical tree topology of duplicated genes in the Acroporidae and the phylogeny of one duplicated gene (alpha-protein kinase 1-like). The phylogenetic tree shows gene retention, loss, and duplications following WGD.
(C) Co-linear gene alignments of Astreopora sp1 and A. tenuis on scaffolds. The gray links show orthologs between Astreopora sp1 and A. tenuis.
See also Figures S5–S10 and Tables S1–S3.
dS values of orthologous gene pairs between two pairs of species (Astreopora sp1 and A. tenuis; A. tenuis, and A. digitifera) were estimated as the proxy of speciation time between them according to neutral evolution theory (Berthelot et al., 2014, Zhang et al., 2017). We combined the dS values of paralogous gene pairs of the five Acropora species and estimated the peak in the log dS distribution (modal value = −1.82). We also estimated the distribution of orthologous gene pairs between Astreopora sp1 and A. tenuis (modal value = −0.31) and between A. tenuis and A. digitifera (modal value = −3.46). The result indicates that the WGD occurred in Acropora before the split of A. tenuis and A. digitifera and after the split of A. tenuis and Astreopora sp1 (Figure S7). In other words, an ancient WGD event likely occurred in the most recent common ancestor of Acropora. Based on speciation time estimated in the calibrated phylogenomic tree and the dS-based method (Vanneste et al., 2014), we estimated that the WGD of Acropora occurred ∼35 mya (95% confidence interval: 31.18–35.7 Ma) (Table S2, see Methods). Here, we defined this event as invertebrate α event of WGD specifically in Acropora (IAsα).
Phylogenomic and Synteny Analysis of IAsα
If the proposed IAsα is deemed correct, the ohnologs of Acropora (paralogs created by IAsα) should form two clades from their orthologs in Astreopora sp1 by mapping IAsα onto phylogenetic trees (Jiao et al., 2011, Marcet-Houben and Gabaldón, 2015). In other words, the phylogenetic topology would be (((Acropora clade1) bootstrap1, (Acropora clade2) bootstrap2), Astreopora sp1), defined as gene duplication topology (Figure 2B).
We performed a phylogenomic analysis to further evaluate the proposed IAsα. Firstly, we defined orthogroups as clusters of homologous genes in Acropora derived from a single gene in Astreopora sp1. Each orthogroup contained at least seven homologous genes, including at least one gene copy in each Acropora species and only one gene copy in Astreopora sp1. We selected 883 orthogroups from 19,760 gene families, and reconstructed the phylogeny of 883 orthogroups using both maximum likelihood (ML) and Bayesian methods. We found that the phylogenetic topology of 205 orthogroups was consistent with gene duplication topology supporting IAsα. We further defined the 205 orthogroups as core-orthogroups (Table S3, see Data S1).
In particular, we found differential gene loss, retention, and duplication in Acropora lineages. For instance, the phylogeny of orthogroup 1370 (alpha-protein kinase 1-like) showed gene retention in A. subglabra, A. digitifera, and A. echinata; gene loss in A. tenuis; and an extra gene duplication in A. subglabra. This implies that diversification of duplicated genes may contribute to species complexity and evolutionary innovation in Acropora (Glasauer and Neuhauss, 2014) (Figure 2B).
To estimate the split time of the two Acropora clades that could be regarded as the timing of IAsα, we selected 154 high-quality core-orthogroups, with bootstrap values in both Acropora clades >70 in ML phylogeny, to reconstruct a time-calibrated phylogeny from the 205 core-orthogroups using BEAST2 (Jiao et al., 2011). However, we found that it was difficult for the parameters in MCMC to converge in 70 core-orthogroups, and we only successfully dated the phylogenetic trees of 135 high-quality core-orthogroups (see Data S1). Next, we estimated the distribution of inferred node ages between the two Acropora clades, and found that the peak value was estimated to be 31 Ma (95% confidence interval: 27.86–34.77 Ma), indicating that IAsα occurred at 31 Ma (Figure S8). This result is in coincidence with the timing of the IAsα estimated by the dS-based method.
Intergenomic co-linearity is often used to directly identify ancient WGD and to reconstruct ancestral karyotypes in vertebrates (Berthelot et al., 2014, Nakatani et al., 2007, Zhang et al., 2017). We performed intergenomic co-linearity and synteny analysis between Astreopora sp1 and A. tenuis to support IAsα. We found that synteny blocks of 21 scaffolds in Astreopora sp1 have at least two duplicated segments in A. tenuis (Figure S9). For example, two duplicated segments in scaffold 130 and scaffold 70 of A. tenuis corresponded to scaffold 323 in Astreopora sp1 (Figure 2C). In addition, we also observed that duplicated segments of the five Acropora species, which corresponded to the longest scaffold of Astreopora sp1, had good co-linearity (Figure S10).
In summary, we found evidence to support IAsα with the dS-based method, and phylogenomic and synteny analyses. Moreover, we suggest that IAsα probably occurred between 28 and 36 mya (Figure 1).
The Fate of Duplicated Genes Originating from IAsα
Duplicated genes provide substrates for diversification and evolutionary novelty, and most of them are regulators of gene networks in vertebrates and plants (Jiao et al., 2011, Kassahn et al., 2009, Zhang et al., 2017). We examined Gene Ontology (GO) for all genes among the 154 high-quality core-orthogroups to investigate their roles in IAsα and found that their molecular functions are enriched in specific categories: transporter, catalytic, binding, and receptor activity. Some of those are involved in gene regulation (Table 1).
Table 1.
Functional Annotation Clustering on the GO Terms of 154 High-Quality Core-Orthogroups
| Annotation Cluster | P Value |
|---|---|
| Transmembrane | 1.90 × 10−6 |
| Death domain | 3.10 × 10−5 |
| G-protein-coupled receptor | 1.20 × 10−4 |
| VIT domain | 3.30 × 10−3 |
| Protein kinase-like domain | 1.90 × 10−2 |
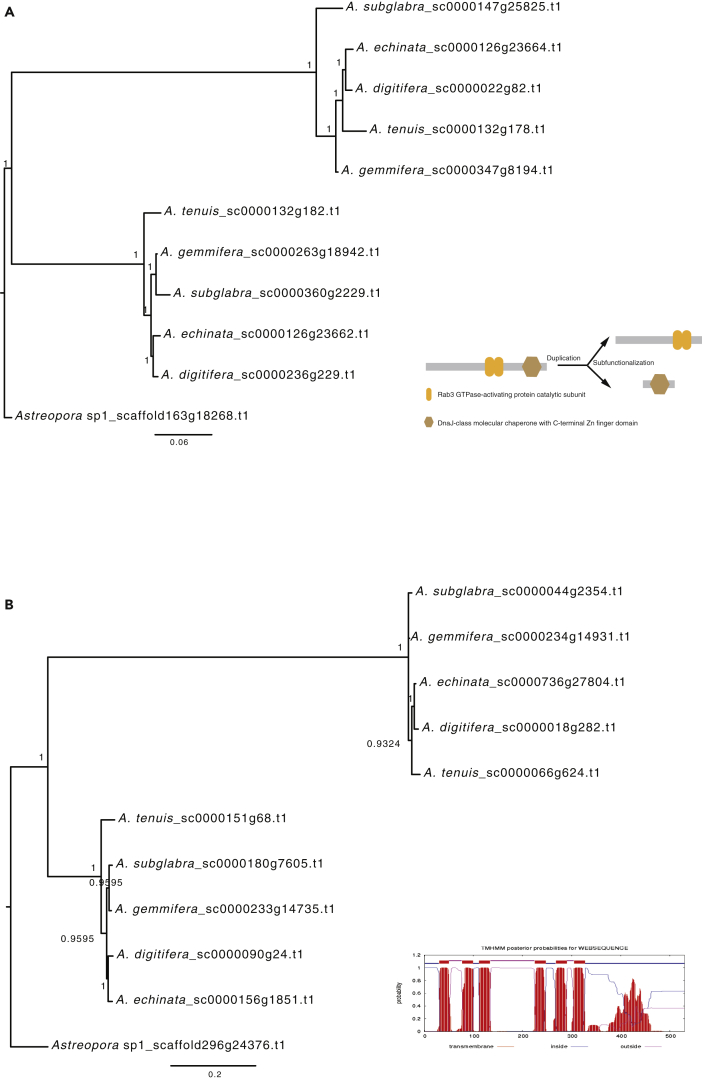
Furthermore, we identified some duplicated genes under subfunctionalization and neofunctionalization, possibly contributing to stress responses of corals. dnaJ homolog subfamily B member 11-like (DNAJB) protein was shown to be involved in heat stress responses in marine organisms (Fujikawa et al., 2010, Wang et al., 2014). Orthogroup 1247 (DNAJB) has two main domains (Ras and Dnaj domains) in Astreopora sp1 representing the ancient state. Each of the two domains was independently lost in the duplicated genes, resulting in complementary functions of the duplicated genes after IAsα (Figures 3A and S11). In addition, excitatory amino acid transporters may be related to symbiotic interactions in Acropora (Bertucci et al., 2015). Orthogroup 1244 (excitatory amino acid transporter 1-like) was predicted as a six transmembrane protein, and a high number of mutations have accumulated in both untransmembrane and transmembrane regions, suggesting that new functions would be generated (Figures 3B and S12). These examples suggest that IAsα might participate in stress responses and symbiotic interactions of Acropora. Together, these results agree with previous patterns of the fate of duplicated genes in vertebrates and plants (Jiao et al., 2011, Soltis et al., 2015, Van De Peer et al., 2017, Zhang et al., 2017), indicating that the IAsα possibly contributes to the species complexity and diversification in Acropora.
Figure 3.
Duplicated Genes under Subfunctionalization or Neofunctionalization are shown with phylogenetic trees.
(A) The phylogeny of orthogroup 1247 (dnaJ homolog subfamily B member 11-like) reconstructed with MrBayes shows a duplicated gene under subfunctionalization. Bayesian posterior probabilities are shown at each node. The bottom right panel shows that two domains are in Astreopora sp1, but each domain was independently lost in duplicated genes under subfunctionalization in orthogroup 1247.
(B) The phylogeny of orthogroup 1244 (excitatory amino acid transporter 1-like) reconstructed with MrBayes shows a duplicated gene under neofunctionalization. Bayesian posterior probabilities are shown at each node. Six transmembrane helices prediction is shown at the bottom right.
See also Figures S11 and S12.
Gene Expression Patterns of Duplicated Genes across Five Developmental Stages in A. digitifera
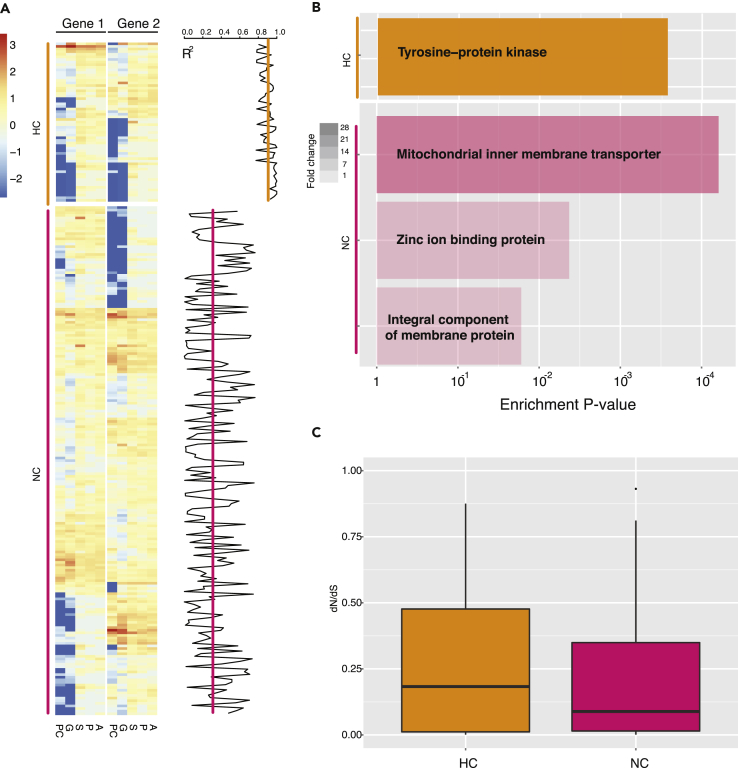
To better understand the evolution of duplicated genes, gene expression analysis across five developmental stages in A. digitifera (blastula, gastrula, postgastrula, planula, and adult polyps) was carried out (Reyes-Bermudez et al., 2016). We identified 236 ohnologous pairs in A. digitifera from 883 ML phylogeny (see Methods) and found that these ohnologous pairs presented an interesting gene expression profiling. We divided 236 ohnologous pairs into two clusters based on pairwise correlation of gene expression during development (high correlation [HC]: p < 0.05; no correlation [NC] p ≥ 0.05; Pearson's correlation test): 25% (25/236) ohnologous pairs in HC and 75% (211/236) ohnologous pairs in NC (Figure 4A). Ohnologous pairs in the HC cluster are enriched in protein kinase, whereas ohnologous pairs in the NC cluster are enriched in membrane transporter and ion-binding proteins (Figure 4B). This result indicates that the two clusters of ohnologous pairs potentially evolved into different gene functions. In addition, we compared dN/dS values to investigate selective pressure on HC and NC clusters (Figure 4C), but there is no significant difference between the two clusters (Mann-Whitney-Wilcoxon test, p = 0.51).
Figure 4.
Gene Expression Profiling Reveals Evolution of Duplicated Genes in A. digitifera
(A) Gene expression profiling across five developmental stages (blastula, PC; gastrula, G; postgastrula, S; planula, P; and adult polyps, A) in A. digitifera. Two clusters of gene expression of ohnologous gene pairs: HC, high correlation: p < 0.05; NC, no correlation: p ≥ 0.05 (Pearson's correlation test). Pearson's correlation coefficients between two ohnologous gene pairs are presented in the right panel, and lines represent average values of correlation coefficients in each cluster.
(B) Significant functional enrichments of two clusters of ohnolog gene pairs (p < 0.05, Fisher's exact test) indicate that divergence of gene expression is associated with gene functions. Colors of the bar represent fold change values in enrichments.
(C) Boxplot of dN/dS values of ohnologous gene pairs shows no significant difference between the two clusters (p = 0.51, Mann-Whitney test).
Evolution of Toxic Proteins in Cnidaria
Next, we investigated the role of IAsα in the diversification of toxins in Acropora. We identified ∼200 putative toxic proteins in each of the five Acropora species, and we clustered them with the putative toxic proteins of Astreopora sp1 and other six Cnidarian species (Hydra magnipapillata, Nematostella vectensis, Montastraea cavernosa, Porites australiensis, Porites astreoides, and Porites lobata) into 24 gene families (Table S4, See Methods). Based on the gene family phylogeny, each of which contains at least 15 genes (See Data S1), we found that toxic proteins have undergone widespread gene duplications in Cnidaria, and most of the gene duplications occurred in individual species lineages, except for Acropora (Figures 5 and S13–S20). Gene duplications occurred in the most recent common ancestor of Acropora in 9 of 15 gene families, potentially caused by WGD (IAsα). For example, in gene family 1 (coagulation factor X), each species contains ∼50 genes, except H. magnipapillata and P. astreoides. Gene duplications occurred frequently in individual species lineages: Astreopora sp1, M. cavernosa, N. vectensis, and P. australiensis. Yet, five gene duplications are inferrred to have occurred in the most recent common ancestor of Acropora by WGD (Figure 5). These results indicated that IAsα potentially contributed to the diversification of proteinaceous toxins in Acropora.
Figure 5.
Diversification of Toxic Proteins via Gene Duplications in Cnidaria
Phylogenetic analysis of Coagulation factor X in 12 cnidarian species shows wide gene duplications. Gene duplication occurred in individual species lineages (red arrows), and gene duplications by WGD in Acropora are indicated with blue arches. Outer color strips represent 12 cnidarian species, and black strip represents non-cnidarian species. Bootstrap values greater than 50 are shown with black dots at nodes. See also Figures S13–S20 and Table S4.
Discussion
Ancient WGD is considered as a significant evolutionary factor in the origin and diversification of evolutionary lineages (Soltis et al., 2015, Van De Peer et al., 2017), but much work remains to be done to definitively identify WGD and to understand its consequences in different evolutionary lineages. Staghorn corals of the genus Acropora, which constitute the foundation of modern coral reef ecosystems, are hypothesized to have originated through polyploidization (Kenyon, 1997, Renema et al., 2016, Willis et al., 2006). However, there is no genetic evidence to support this assertion. To that end, we analyzed the genomes of one Astreopora and five Acropora species to address the possibility of WGD in Acropora and the functional fate of duplicated genes from that event.
To the best of our knowledge, this is the first study to report genomic-scale evidence of WGD in corals (IAsα). We find that large numbers of ohnologs are retained in Acropora species and hundreds of gene families display phylogenetic duplication topology among the five Acropora species. Meanwhile, our synteny analysis between Astreopora sp1 and A. tenuis directly supports IAsα. However, reconstruction of the ancestral karyotype will necessitate the assemblage of genomes to the chromosome level to fully understand gene fractionation and chromosome rearrangements in Acropora under IAsα (Smith and Keinath, 2015, Smith et al., 2013).
Ancient WGD is usually inferred using the dS-based method, but artificial signals in dS distributions have been reported in previous studies, because of dS saturation (dS value >1) or using poorly annotated genomes or allelic variations or low retention rates (Rabier et al., 2014, Tiley et al., 2016, Vanneste et al., 2012, Mao, 2019). There is an extra peak in the dS distribution of anchor gene pairs in A. digitifera and A. tenuis (Figure S6). One possible explanation is that the extra peak is artifactual because few anchor gene pairs were used in the analysis. However, this could also indicate a second WGD event in Acropora. We found few orthogroups with topologies that fit the two proposed WGD events (Figure S21). In addition, a new ML phylogeny modeling approach was recently developed to overcome shortcomings of the dS-based method (Rabier et al., 2014, Tiley et al., 2016). We used it to test whether a second WGD occurred in Acropora. The result showed that one WGD event is the best model in Acropora and that it occurred 30.69–34.69 mya (Tables S5 and S6; see Methods). Moreover, we applied the dS-based method on two recently released genomes by other groups (Acropora tenuis and Acropora millepora) (see Methods). We found similar peaks on these two dS distributions of paralogous gene pairs (Figure S22). Together, we have genome-scale evidence to support IAsα, yet, there is no conclusive evidence to support a second WGD in Acropora.
It is crucial to accurately estimate the timing of a WGD event to understand its evolutionary consequences (Jiao et al., 2011, Vanneste et al., 2014). Our study has clearly estimated the timing of IAsα using both phylogenomic analysis and the dS-based method. We suggest that IAsα probably occurred between 28 and 36 mya (Figure 1). Interestingly, species turnover events usually occurred with extinctions (Jackson and Sax, 2010), and one species turnover event in corals (Oligocene-Miocene transition [OMT]) was suggested to have occurred between 15.97 and 33.7 mya (Edinger and Risk, 1994). The timing of IAsα may correspond to a massive extinction of corals created by OMT. This finding supports the hypothesis that WGD may enable organisms to escape extinction during drastic environmental changes (Van De Peer et al., 2017) (Figure 1).
The occurrence of IAsα raises the question of what impact it may have had upon coral evolution (Conant et al., 2014, Willis et al., 2006). We performed GO analysis on duplicated genes and examined duplicated gene families, showing that duplicated genes following IAsα indeed provided raw genetic material for Acropora to diversify and are potentially crucial for stress responses. In particular, toxin diversification in Acropora was mainly generated by WGD. In addition, we focused on expression patterns of duplicated genes in A. digitifera, showing that expressions of duplicated protein kinases are likely to be correlated during development. A possible explanation may be that protein kinases are probably retained in complex signal transduction pathways via subfunctionalization or dosage effects (Conant et al., 2014, Glasauer and Neuhauss, 2014). However, expressions of duplicated membrane proteins are likely uncorrelated because these proteins may have developed different functions via neofunctionalization, such as excitatory amino acid transporters (orthogroup 1244). However, more work is needed to be done to investigate molecular mechanisms of duplicated genes in order to examine these hypotheses in the diversification of Acropora (Yasuoka et al., 2016). For instance, previous gene functional studies have demonstrated that voltage-gated sodium channel gene paralogs, duplicated in teleosts, contributed to the acquisition of new electric organs via neofunctionalization in both mormyroid and gymnotiform electric fishes (Arnegard et al., 2010, Zakon et al., 2006).
Our previous study proposed that adaptive radiation in Acropora was probably driven by introgression (Mao et al., 2018); thus Acropora is the first invertebrate lineage reported to have undergone both WGD and introgression. Meanwhile, both introgression and WGD have also been reported in cichlid fish lineages (Berner and Salzburger, 2015), a famous model of adaptive radiation in vertebrates (Berner and Salzburger, 2015, Seehausen et al., 2014). Both WGD and introgression are regarded as significant forces in adaptive radiation of organisms (Berner and Salzburger, 2015, Van De Peer et al., 2017), but we still do not understand the relationship between WGD and introgression in adaptive radiations (Soltis and Soltis, 2009).
In conclusion, this study identified an ancient WGD shared by Acropora species (IAsα) that not only provides new insights into the evolution of reef-building corals but also expands a new model of WGD in animals.
Limitation of the Study
Small-scale gene duplication continually occurs within the evolution of organisms (Maere et al., 2005), but large-scale gene/genome duplication or entire genome duplication was regarded as a rare evolutionary event in the animals. With advanced increase of genomic data, we observed more and more WGD in animals (Van De Peer et al., 2017), such as vertebrates (Berthelot et al., 2014, Dehal and Boore, 2005, Kenny et al., 2016), insects (Li et al., 2018), and corals (this study). Yet, it is hard to distinguish large-scale gene/genome duplication from entire genome duplication using the dS-based method and phylogenomic and synteny analyses without precise genomic data. For example, the second-round WGD has been argued to be large-scale genome duplication rather than entire genome duplication (Smith and Keinath, 2015). Hence, in this study, we defined WGD as large-scale gene/genome duplication. Our evidence from different analyses supports the fact that WGD occurred in the common ancestor of Acropora, but there is still lack of sufficient evidence to support the fact that the WGD is generated by entire genome duplication. In addition, the distribution of inferred age nodes (Figure S8) was not shown as a standard normal distribution, one possible reason for which is that there is a lack of data in the phylogenomic approach.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was supported by OIST (Internal Fund to N.S.), Japan and was funded by JSPS grant (No. 17J00557 to Y.M.), Japan. We thank Dr. Chuya Shinzato for providing the genomic data in this study. We thank Dr. Evan P Economo, Dr. Douglas E Soltis, and Dr. Pamela S Soltis for their comments and insights into the draft manuscript. We thank Dr. Steven D. Aird and Hong Huat Hoh for editing the manuscript.
Author Contributions
Y.M. and N.S. conceived the study. Y.M. performed all analysis in this study. Y.M. and N.S. wrote the initial manuscript and edited the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: March 29, 2019
Footnotes
Supplemental Information includes Transparent Methods, 22 figures, 6 tables, and one data file and can be found with this article online at https://doi.org/10.1016/j.isci.2019.02.001.
Supplemental Information
1) Genome statistics of six Acroporid species, related to Figure 1.
2) Gene family clusters, related to Figure 1.
3) Orthogroup dataset, related to Figure 2.
4) Node ages of core-orthogroups inferred by BEAST2, related to Figure 2.
5) Gene expression data for ohnologous gene pairs, related to Figure 4.
6) Toxin gene family clusters, related to Figure 5.
References
- Ainsworth T.D., Heron S.F., Ortiz J.C., Mumby P.J., Grech A., Ogawa D., Eakin C.M., Leggat W. Climate change disables coral bleaching protection on the Great Barrier Reef. Science. 2016;352:338–342. doi: 10.1126/science.aac7125. [DOI] [PubMed] [Google Scholar]
- Armoza-Zvuloni R., Schneider A., Sher D., Shaked Y. Rapid Hydrogen Peroxide release from the coral Stylophora pistillata during feeding and in response to chemical and physical stimuli. Sci. Rep. 2016;6:21000. doi: 10.1038/srep21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnegard M.E., Zwickl D.J., Lu Y., Zakon H.H. Old gene duplication facilitates origin and diversification of an innovative communication system—twice. Proc. Natl. Acad. Sci. U S A. 2010;107:22172–22177. doi: 10.1073/pnas.1011803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari H., Paz M., Sher D. The chemical armament of reef-building corals: inter-and intra-specific variation and the identification of an unusual actinoporin in Stylophora pistilata. Sci. Rep. 2018;8:251. doi: 10.1038/s41598-017-18355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner D., Salzburger W. The genomics of organismal diversification illuminated by adaptive radiations. Trends Genet. 2015;31:491–499. doi: 10.1016/j.tig.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Berthelot C., Brunet F., Chalopin D., Juanchich A., Bernard M., Noël B., Bento P., Da Silva C., Labadie K., Alberti A. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 2014;5:3657. doi: 10.1038/ncomms4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci A., Foret S., Ball E.E., Miller D.J. Transcriptomic differences between day and night in Acropora millepora provide new insights into metabolite exchange and light-enhanced calcification in corals. Mol. Ecol. 2015;24:4489–4504. doi: 10.1111/mec.13328. [DOI] [PubMed] [Google Scholar]
- Blanc G., Hokamp K., Wolfe K.H. A recent polyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Res. 2003;13:137–144. doi: 10.1101/gr.751803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomme T., Vandepoele K., De Bodt S., Simillion C., Maere S., Van de Peer Y. The gain and loss of genes during 600 million years of vertebrate evolution. Genome Biol. 2006;7:R43. doi: 10.1186/gb-2006-7-5-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers J.E., Chapman B.A., Rong J., Paterson A.H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003;422:433–438. doi: 10.1038/nature01521. [DOI] [PubMed] [Google Scholar]
- Canestro C., Albalat R., Irimia M., Garcia-Fernàndez J. Impact of gene gains, losses and duplication modes on the origin and diversification of vertebrates. Semin. Cell Dev. Biol. 2013;24:83–94. doi: 10.1016/j.semcdb.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Chen Z.J., Birchler J.A. John Wiley & Sons; 2013. Polyploid and Hybrid Genomics. [Google Scholar]
- Christoffels A., Koh E.G.L., Chia J.M., Brenner S., Aparicio S., Venkatesh B. Fugu genome analysis provides evidence for a whole-genome duplication early during the evolution of ray-finned fishes. Mol. Biol. Evol. 2004;21:1146–1151. doi: 10.1093/molbev/msh114. [DOI] [PubMed] [Google Scholar]
- Conant G.C., Birchler J.A., Pires J.C. Dosage, duplication, and diploidization: clarifying the interplay of multiple models for duplicate gene evolution over time. Curr. Opin. Plant Biol. 2014;19:91–98. doi: 10.1016/j.pbi.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Dehal P., Boore J.L. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:1700–1708. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger E.N., Risk M.J. Oligocene miocene extinction and geographic restriction of caribbean corals - roles of turbidity, temperature, and nutrients. Palaios. 1994;9:576–598. [Google Scholar]
- Fujikawa T., Munakata T., Kondo S., Satoh N., Wada S. Stress response in the ascidian Ciona intestinalis: transcriptional profiling of genes for the heat shock protein 70 chaperone system under heat stress and endoplasmic reticulum stress. Cell Stress Chaperon. 2010;15:193–204. doi: 10.1007/s12192-009-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami H., Omori M., Hatta M. Phylogenetic relationships in the coral family Acroporidae, reassessed by inference from mitochondrial genes. Zoolog. Sci. 2000;17:689–696. doi: 10.2108/zsj.17.689. [DOI] [PubMed] [Google Scholar]
- Fürthauer M., Thisse B., Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev. Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- Gacesa R., Chung R., Dunn S.R., Weston A.J., Jaimes-Becerra A., Marques A.C., Morandini A.C., Hranueli D., Starcevic A., Ward M. Gene duplications are extensive and contribute significantly to the toxic proteome of nematocysts isolated from Acropora digitifera (Cnidaria: Anthozoa: Scleractinia) BMC Genomics. 2015;16:774. doi: 10.1186/s12864-015-1976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasauer S.M.K., Neuhauss S.C.F. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genomics. 2014;289:1045–1060. doi: 10.1007/s00438-014-0889-2. [DOI] [PubMed] [Google Scholar]
- Goodwin S., McPherson J.D., McCombie W.R. Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016;17:333–351. doi: 10.1038/nrg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M., Shoguchi E., Shinzato C., Kawashima T., Miller D.J., Satoh N. The complex NOD-like receptor repertoire of the coral Acropora digitifera includes novel domain combinations. Mol. Biol. Evol. 2013;30:167–176. doi: 10.1093/molbev/mss213. [DOI] [PubMed] [Google Scholar]
- Hardwick S.A., Deveson I.W., Mercer T.R. Reference standards for next-generation sequencing. Nat. Rev. Genet. 2017;18:473. doi: 10.1038/nrg.2017.44. [DOI] [PubMed] [Google Scholar]
- Hargreaves A.D., Swain M.T., Hegarty M.J., Logan D.W., Mulley J.F. Restriction and recruitment—gene duplication and the origin and evolution of snake venom toxins. Genome Biol. Evol. 2014;6:2088–2095. doi: 10.1093/gbe/evu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.T., Sax D.F. Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends Ecol. Evol. 2010;25:153–160. doi: 10.1016/j.tree.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Jiao Y.N., Wickett N.J., Ayyampalayam S., Chanderbali A.S., Landherr L., Ralph P.E., Tomsho L.P., Hu Y., Liang H.Y., Soltis P.S. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–U113. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- Kassahn K.S., Dang V.T., Wilkins S.J., Perkins A.C., Ragan M.A. Evolution of gene function and regulatory control after whole-genome duplication: comparative analyses in vertebrates. Genome Res. 2009;19:1404–1418. doi: 10.1101/gr.086827.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny N.J., Chan K.W., Nong W., Qu Z., Maeso I., Yip H.Y., Chan T.F., Kwan H.S., Holland P.W.H., Chu K.H. Ancestral whole-genome duplication in the marine chelicerate horseshoe crabs. Heredity. 2016;119:388. doi: 10.1038/hdy.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J.C. Models of reticulate evolution in the coral genus Acropora based on chromosome numbers: parallels with plants. Evolution. 1997;51:756–767. doi: 10.1111/j.1558-5646.1997.tb03659.x. [DOI] [PubMed] [Google Scholar]
- Kondrashov F.A. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc. Biol. Sci. 2012;279:5048–5057. doi: 10.1098/rspb.2012.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordiš D., Gubenšek F. Adaptive evolution of animal toxin multigene families. Gene. 2000;261:43–52. doi: 10.1016/s0378-1119(00)00490-x. [DOI] [PubMed] [Google Scholar]
- Li Z., Tiley G.P., Galuska S.R., Reardon C.R., Kidder T.I., Rundell R.J., Barker M.S. Multiple large-scale gene and genome duplications during the evolution of hexapods. Proc. Natl. Acad. Sci. U S A. 2018;115:4713–4718. doi: 10.1073/pnas.1710791115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., Conery J.S. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- Mao Y. GenoDup Pipeline: a tool to detect genome duplication using the dS-based method. PeerJ. 2019;7:e6303. doi: 10.7717/peerj.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Economo E.P., Satoh N. The roles of introgression and climate change in the rise to dominance of Acropora Corals. Curr. Biol. 2018;28:3373–3382. doi: 10.1016/j.cub.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Maere S., De Bodt S., Raes J., Casneuf T., Van Montagu M., Kuiper M., Van de Peer Y. Modeling gene and genome duplications in eukaryotes. Proc. Natl. Acad. Sci. U S A. 2005;102:5454–5459. doi: 10.1073/pnas.0501102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet-Houben M., Gabaldón T. Beyond the whole-genome duplication: phylogenetic evidence for an ancient interspecies hybridization in the baker's yeast lineage. PLoS Biol. 2015;13:e1002220. doi: 10.1371/journal.pbio.1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y., Takeda H., Kohara Y., Morishita S. Reconstruction of the vertebrate ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Res. 2007;17:1254–1265. doi: 10.1101/gr.6316407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabier C.E., Ta T., Ane C. Detecting and locating whole genome duplications on a phylogeny: a probabilistic approach. Mol. Biol. Evol. 2014;31:750–762. doi: 10.1093/molbev/mst263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renema W., Bellwood D.R., Braga J.C., Bromfield K., Hall R., Johnson K.G., Lunt P., Meyer C.P., McMonagle L.B., Morley R.J. Hopping hotspots: global shifts in marine biodiversity. Science. 2008;321:654–657. doi: 10.1126/science.1155674. [DOI] [PubMed] [Google Scholar]
- Renema W., Pandolfi J.M., Kiessling W., Bosellini F.R., Klaus J.S., Korpanty C., Rosen B.R., Santodomingo N., Wallace C.C., Webster J.M. Are coral reefs victims of their own past success? Sci. Adv. 2016;2:e1500850. doi: 10.1126/sciadv.1500850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Bermudez A., Villar-Briones A., Ramirez-Portilla C., Hidaka M., Mikheyev A.S. Developmental progression in the coral Acropora digitifera is controlled by differential expression of distinct regulatory gene networks. Genome Biol. Evol. 2016;8:851–870. doi: 10.1093/gbe/evw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards Z.T., Hobbs J.P.A. Hybridisation on coral reefs and the conservation of evolutionary novelty. Curr. Zool. 2015;61:132–145. [Google Scholar]
- Richards Z.T., Miller D.J., Wallace C.C. Molecular phylogenetics of geographically restricted Acropora species: implications for threatened species conservation. Mol. Phylogenet. Evol. 2013;69:837–851. doi: 10.1016/j.ympev.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Schwager E.E., Sharma P.P., Clarke T., Leite D.J., Wierschin T., Pechmann M., Akiyama-Oda Y., Esposito L., Bechsgaard J., Bilde T. The house spider genome reveals an ancient whole-genome duplication during arachnid evolution. BMC Biol. 2017;15:62. doi: 10.1186/s12915-017-0399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O., Butlin R.K., Keller I., Wagner C.E., Boughman J.W., Hohenlohe P.A., Peichel C.L., Saetre G.P., Bank C., Brannstrom A. Genomics and the origin of species. Nat. Rev. Genet. 2014;15:176–192. doi: 10.1038/nrg3644. [DOI] [PubMed] [Google Scholar]
- Sémon M., Wolfe K.H. Consequences of genome duplication. Curr. Opin. Genet. Dev. 2007;17:505–512. doi: 10.1016/j.gde.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Smith J.J., Keinath M.C. The sea lamprey meiotic map improves resolution of ancient vertebrate genome duplications. Genome Res. 2015;25:1081–1090. doi: 10.1101/gr.184135.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.J., Kuraku S., Holt C., Sauka-Spengler T., Jiang N., Campbell M.S., Yandell M.D., Manousaki T., Meyer A., Bloom O.E. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat. Genet. 2013;45:415–421. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis D.E., Albert V.A., Leebens-Mack J., Bell C.D., Paterson A.H., Zheng C., Sankoff D., Pamphilis C.W., Wall P.K., Soltis P.S. Polyploidy and angiosperm diversification. Am. J. Bot. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- Soltis P.S., Marchant D.B., Van de Peer Y., Soltis D.E. Polyploidy and genome evolution in plants. Curr. Opin. Genet. Dev. 2015;35:119–125. doi: 10.1016/j.gde.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Soltis P.S., Soltis D.E. The role of hybridization in plant speciation. Annu. Rev. Plant Biol. 2009;60:561–588. doi: 10.1146/annurev.arplant.043008.092039. [DOI] [PubMed] [Google Scholar]
- Soltis P.S., Soltis D.E. Springer; 2012. Polyploidy and Genome Evolution. [Google Scholar]
- Stuber C.W., Goodman M.M. Inheritance, intracellular localization, and genetic variation of phosphoglucomutase isozymes in maize (Zea mays L.) Biochem. Genet. 1983;21:667–689. doi: 10.1007/BF00498915. [DOI] [PubMed] [Google Scholar]
- Suzuki G., Nomura K. Species boundaries of Astreopora corals (Scleractinia, Acroporidae) inferred by mitochondrial and nuclear molecular markers. Zoolog. Sci. 2013;30:626–632. doi: 10.2108/zsj.30.626. [DOI] [PubMed] [Google Scholar]
- Tiley G.P., Ane C., Burleigh J.G. Evaluating and characterizing ancient whole-genome duplications in plants with gene count data. Genome Biol. Evol. 2016;8:1023–1037. doi: 10.1093/gbe/evw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer Y., Maere S., Meyer A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009;10:725–732. doi: 10.1038/nrg2600. [DOI] [PubMed] [Google Scholar]
- Van De Peer Y., Mizrachi E., Marchal K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017;18:411–424. doi: 10.1038/nrg.2017.26. [DOI] [PubMed] [Google Scholar]
- Van Oppen M.J., McDonald B.J., Willis B., Miller D.J. The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol. Biol. Evol. 2001;18:1315–1329. doi: 10.1093/oxfordjournals.molbev.a003916. [DOI] [PubMed] [Google Scholar]
- Vanneste K., Baele G., Maere S., Van de Peer Y. Analysis of 41 plant genomes supports a wave of successful genome duplications in association with the Cretaceous-Paleogene boundary. Genome Res. 2014;24:1334–1347. doi: 10.1101/gr.168997.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste K., Van de Peer Y., Maere S. Inference of genome duplications from age distributions revisited. Mol. Biol. Evol. 2012;30:177–190. doi: 10.1093/molbev/mss214. [DOI] [PubMed] [Google Scholar]
- Veron A.S., Kaufmann K., Bornberg-Bauer E. Evidence of interaction network evolution by whole-genome duplications: a case study in MADS-box proteins. Mol. Biol. Evol. 2006;24:670–678. doi: 10.1093/molbev/msl197. [DOI] [PubMed] [Google Scholar]
- Vollmer S.V., Palumbi S.R. Hybridization and the evolution of reef coral diversity. Science. 2002;296:2023–2025. doi: 10.1126/science.1069524. [DOI] [PubMed] [Google Scholar]
- Vonk F.J., Casewell N.R., Henkel C.V., Heimberg A.M., Jansen H.J., McCleary R.J.R., Kerkkamp H.M.E., Vos R.A., Guerreiro I., Calvete J.J. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. U S A. 2013;110:20651–20656. doi: 10.1073/pnas.1314702110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C.C. Acroporidae of the Caribbean. Geologica Belgica. 2012;15:388–393. [Google Scholar]
- Wallace C.C., Rosen B.R. Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: implications for the evolution of modern diversity patterns of reef corals. Proc. Biol. Sci. 2006;273:975–982. doi: 10.1098/rspb.2005.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-P., Yu J.-G., Li J., Sun P.-C., Wang L., Yuan J.-Q., Meng F.-B., Sun S.-R., Li Y.-X., Lei T.-Y. Two likely auto-tetraploidization events shaped kiwifruit genome and contributed to establishment of the actinidiaceae family. iScience. 2018;7:230–240. doi: 10.1016/j.isci.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Hui J.H.L., Chan T.F., Chu K.H. De novo transcriptome sequencing of the snail echinolittorina malaccana: identification of genes responsive to thermal stress and development of genetic markers for population studies. Mar. Biotechnol. (NY) 2014;16:547–559. doi: 10.1007/s10126-014-9573-0. [DOI] [PubMed] [Google Scholar]
- Willis B.L., van Oppen M.J.H., Miller D.J., Vollmer S.V., Ayre D.J. The role of hybridization in the evolution of reef corals. Annu. Rev. Ecol. Evol. Syst. 2006;37:489–517. [Google Scholar]
- Yasuoka Y., Shinzato C., Satoh N. The mesoderm-forming gene brachyury regulates ectoderm-endoderm demarcation in the coral Acropora digitifera. Curr. Biol. 2016;26:2885–2892. doi: 10.1016/j.cub.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Zakon H.H., Lu Y., Zwickl D.J., Hillis D.M. Sodium channel genes and the evolution of diversity in communication signals of electric fishes: convergent molecular evolution. Proc. Natl. Acad. Sci. U S A. 2006;103:3675–3680. doi: 10.1073/pnas.0600160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.Q., Liu K.W., Li Z., Lohaus R., Hsiao Y.Y., Niu S.C., Wang J.Y., Lin Y.C., Xu Q., Chen L.J. The Apostasia genome and the evolution of orchids. Nature. 2017;549:379–383. doi: 10.1038/nature23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1) Genome statistics of six Acroporid species, related to Figure 1.
2) Gene family clusters, related to Figure 1.
3) Orthogroup dataset, related to Figure 2.
4) Node ages of core-orthogroups inferred by BEAST2, related to Figure 2.
5) Gene expression data for ohnologous gene pairs, related to Figure 4.
6) Toxin gene family clusters, related to Figure 5.