Abstract
Bordetella (B.) bronchiseptica is primarily a zoonotic pathogen, which is often found in upper respiratory tract of various domestic and wild animals. Human infections are rarely reported in immunocompromised patients and are associated with a wide spectrum of presentation ranging from mild cough, tracheobronchitis to sepsis and death. Here, we describe a case of B. bronchiseptica pneumonia that led to the diagnosis of human immunodeficiency virus infection.
The diagnosis of B. bronchiseptica infection can be challenging, as there are no distinctive imaging features. This infection mimics Pneumocystis jiroveci infection and unless a detailed evaluation of an unusual presentation is done it may be missed, resulting in increased morbidity and mortality. This case emphasizes the importance of a systematic detailed investigation of patients with unusual pneumonia presentations.
Keywords: Bordetella bronchiseptica, Pneumonia, HIV
Introduction
Bordetella (B.) bronchiseptica is primarily a zoonotic respiratory pathogen, with close resemblance to B. pertussis, causative agent of whooping cough [1]. B. bronchiseptica is common inhabitant of upper respiratory tract of various domestic and wild animals including rodents, swine, household pets, voles, seals and captive koalas [1,2]. Human infections are rarely reported, despite frequent exposure to animals infected with this pathogen. Human infections are mostly reported in immunocompromised patients and are associated with a wide spectrum of presentation ranging from mild cough, tracheobronchitis to sepsis and death [3]. It is important to identify the pathogen timely to prevent morbidity and mortality associated with this infection. Here, we describe a case of B. bronchiseptica pneumonia that led to the diagnosis of human immunodeficiency virus infection.
Case report
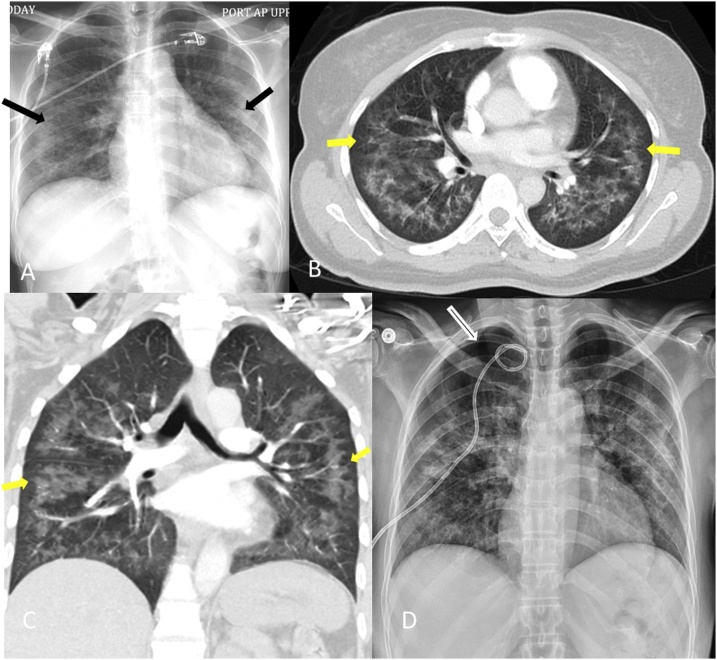
A 52-year-old lifetime non-smoker woman, with no significant past medical history, presented to the emergency department with worsening shortness of breath, fatigue and loss of appetite. She denied cough, fever or chills, recent change in environment, use of new chemicals or exposure to pets. The initial physical examination revealed normal body temperature, hypoxia with a SpO2 of 88% on room air, tachycardia (heart rate 110/min), tachypnea (respiratory rate 24/min) and normal blood pressure. There was no pallor, icterus, thrush, oral ulcer, skin rash or joint swelling. The pulmonary examination revealed bilateral coarse crackles with scattered wheezing. The cardiovascular, abdominal and neurological examinations were unremarkable. Laboratory investigations revealed a total leukocyte count of 5200 cells/μL, hemoglobin level of 13.2 g/dL, platelet count of 186,000/mm3. ESR was >130 mm/h and hsCRP was 22.6 mg/L. The liver function tests, electrolytes, renal panel and autoimmune screen were normal. Chest radiography revealed diffuse bilateral moderate interstitial and hazy alveolar opacities with relative sub-pleual sparing (Fig. 1A). Computed tomography (CT) chest angiography was negative for pulmonary embolism but demonstrated bilateral alveolar and interstitial ground-glass opacities with sparing of sub-pleural and peri-hilar regions (Fig. 1B and C).
Fig. 1.
A: Chest radiography revealed diffuse bilateral moderate interstitial and hazy alveolar opacities with relative sub-pleural sparing. B and C: Computed tomography (CT) chest angiography was negative for pulmonary embolism but demonstrated bilateral alveolar and interstitial ground-glass opacities with sparing of sub-pleural and peri-hilar regions. D: Right pneumothorax for which a chest tube was placed.
Bronchoscopy demonstrated normal bronchi bilaterally with patent segmental orifices and no excess secretions. The procedure was complicated by development of a right pneumothorax for which a chest tube was placed (Fig. 1D). Giemsa stain negative bronchoalveolar lavage (BAL) fluid had a leukocyte count of 56/μL with 67% lymphocytes. Culture of the BAL fluid grew B. bronchiseptica. Testing for Pneumocystis jiroveci, acid-fast bacilli and fungus were negative. Transbronchial biopsy showed cellular interstitial pneumonitis with poorly formed granulomas. Given these findings, the patient was started on levofloxacin. Human immunodeficiency virus (HIV) testing was positive with absolute CD4 count of 30/μL. She was started on highly active antiretroviral therapy (HAART) with prophylaxis for opportunistic infections. She completed 14 days treatment with levofloxacin and had improvement in her respiratory symptoms with resolution of pulmonary opacities.
Discussion
B. bronchiseptica is a primarily zoonotic infection which can cause severe infection in immunocompromised patients unless recognized early. This pleomorphic, gram-negative coccobacillus is an obligate aerobe with preferential adherence to respiratory epithelium of animals in contrast to other species like B. pertussis or B. parapertussis, which have predilection for ciliated human epithelium [3]. This infection has been rarely reported in patients with severe chronic obstructive pulmonary disease, lung cancer, previous lung transplantation, lymphoma, cystic fibrosis and HIV [2,4,5]. Although, majority of the patient present with respiratory tract infection, few of them can also present with peritonitis or meningitis [6,7]. As infection with B. bronchiseptica is rarely reported in immunocompetent host [8], it is very important to look for underlying immunocompromised status in any patient diagnosed with this pneumonia. Our patient was apparently healthy prior to presentation with no high risk factors except for a healthcare nurse. Identification of B. bronchiseptica in BAL fluid led us to screen the patient for HIV. Confirmation of HIV infection resulted in a marked change in her management and hospital course. The exact prognosis associated with this infection is difficult to establish because of scarcity of the literature. Wernli et al. reported mortality of 12.5% in his case series [1].
The diagnosis of B. bronchiseptica infection can be challenging, as there are no distinctive imaging features. Chest imaging may reveal lobar consolidation, diffuse ground glass opacities, interstitial or lobular infiltrations or, in rare instances, cavitation and necrosis [9]. This infection mimics Pneumocystis jiroveci infection and unless a detailed evaluation of an unusual presentation is done it may be missed, resulting in increased morbidity and mortality. Sputum gram stain and culture should be performed in all patients. However, the detection of microorganism depends on the sputum quality and laboratory skill. Our patient did not have cough and underwent bronchoscopy. Therefore, whenever, it is not feasible to induce sputum it is imperative to perform BAL for accurate sample collection. Both mass spectrometry and conventional biochemical staining and culture help in accurate identification of the pathogen. It has been proposed that B. bronchisepticainfection should be included as an opportunistic infection in HIV. Almost all of the reported cases have occurred in patients with CD4 counts <200/μL [4].
Little is known regarding the route of transmission. Some report suggest airborne transmission when in contact with animal with respiratory tract infection with B bronchiseptica, others suggest rare occurrence of airborne human-to-human transmission in hospital settings. Our patient denied any exposure to pets or farm animals. The possibility of these infections should be kept in mind even in patients with no animal contact.
Antimicrobial therapy is usually guided by antibiotic sensitivities. There are some reports describing sensitivity of this bacterium to fluoroquinolones, aminoglycosides, tetracyclines, co-trimoxazole and piperacillin [10]. They are reported with resistant to ampicillin and cephalosporins. The resistance is secondary to blaOXA-2 beta lactamase and decreased outer membrane permeability [11]. However, these reports are based on in vitro susceptibility of the bacterium. There are no Clinical and Laboratory Standards Institute (CLSI) guideline on antimicrobial treatment for human infection [12]. Similarly, there are currently no guidelines regarding duration of the treatment, although few reports suggest 2–4 weeks, depending on individual response to antimicrobials and underlying comorbidities [5]. We treated our patient with fluoroquinolones for 14 days, which resulted in improvement in clinical symptoms.
Conclusion
This case emphasizes the importance of a systematic detailed investigation of patients with unusual pneumonia presentations. The finding of B. bronchiseptica in bronchial culture should prompt an investigation for an immunosuppressed state given the critical implications for treatment. With increase in use of immunosuppressive therapy for various diseases, the incidence of this rare infection is expected to increase. Therefore, it is important that physicians are well aware of these human infections, which if left undiagnosed and untreated can result in worse outcome.
Grant support
None.
Conflict of interest
None of the authors have any conflict of interest.
All authors had access to the data and a role in writing the manuscript.
Patient’s consent was obtained for publication.
Contributor Information
Sonali Gupta, Email: gupta.sonali2706@gmail.com.
Pradeep Goyal, Email: pradeepgoyal78@gmail.com.
Joseph Mattana, Email: joseph.mattana@ascension.org.
References
- 1.Wernli D., Emonet S., Schrenzel J., Harbarth S. Evaluation of eight cases of confirmed Bordetella bronchiseptica infection and colonization over a 15-year period. Clin Microbiol Infect. 2011;17:201–203. doi: 10.1111/j.1469-0691.2010.03258.x. [DOI] [PubMed] [Google Scholar]
- 2.Mattoo S., Cherry J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolfrey B.F., Moody J.A. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev. 1991;4(3):243–255. doi: 10.1128/cmr.4.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yacoub A.T., Katayama M., Tran J., Zadikany R., Kandula M., Greene J. Bordetella bronchiseptica in the immunosuppressed population—a case series and review. Mediterr J Hematol Infect Dis. 2014;6:e2014031. doi: 10.4084/MJHID.2014.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz D.M., Bechara R.I., Wolfenden L.L. An unusual cause of cough and dyspnea in an immunocompromised patient. Chest. 2007;131(5):1599–1602. doi: 10.1378/chest.06-1541. [DOI] [PubMed] [Google Scholar]
- 6.Dlamini N.R., Bhamjee A., Levick P., Uniacke E., Ismail H., Smith A. Spontaneous bacterial peritonitis and pneumonia caused by Bordetella bronchiseptica. J Infect Dev Ctries. 2012;6:588–591. doi: 10.3855/jidc.2074. [DOI] [PubMed] [Google Scholar]
- 7.Belen O., Campos J.M., Cogen P.H., Jantausch B.A. Postsurgical meningitis caused by Bordetella bronchiseptica. Pediatr Infect Dis J. 2003;22(4):380–381. [PubMed] [Google Scholar]
- 8.Llombart M., Chiner E., Senent C. Necrotizing pneumonia due to Bordetella bronchiseptica in an immunocompetent woman. Arch Bronconeumol. 2006;42(5):255. doi: 10.1016/s1579-2129(06)60457-6. [DOI] [PubMed] [Google Scholar]
- 9.Patel A.K., Prescott-Focht J.A., Kunin J.R., Essmyer C.E., Rosado-de-Christenson M.L. Imaging findings in human Bordetella bronchiseptica pneumonia. J Thorac Imaging. 2011;26(4):W146–9. doi: 10.1097/RTI.0b013e31820209a1. [DOI] [PubMed] [Google Scholar]
- 10.García-de-la-Fuente C., Guzmán L., Cano M.E., Agüero J., Sanjuán C., Rodríguez C. Microbiological and clinical aspects of respiratory infections associated with Bordetella bronchiseptica. Diagn Microbiol Infect Dis. 2015;82(1):20–25. doi: 10.1016/j.diagmicrobio.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Kadlec K., Wiegand I., Kehrenberg C., Schwarz S. Studies on the mechanisms of beta-lactam resistance in Bordetella bronchiseptica. J Antimicrob Chemother. 2007;59:396–402. doi: 10.1093/jac/dkl515. [DOI] [PubMed] [Google Scholar]
- 12.Wayne P.A. CLSI. Performance standards for antimicrobial susceptibility testing. Twenty-Fifth Informational Supplement. 2015 [Google Scholar]